Abstract
Fas-R is expressed constitutively in CD34+ cells of patients with chronic myelogenous leukemia (CML); Fas-R triggering results in decreased proliferation rate due to apoptosis of clonogenic cells. We have already shown that α-interferon (IFN-α) enhances Fas-R expression on CML progenitor cells, thus increasing their sensitivity to Fas-R agonists. Although it appears that IFN-α can prime CML cells for the effects of Fas, the response to IFN-α in vivo is not a constant feature in CML patients. We studied the mechanisms of Fas-mediated apoptosis in 11 patients suffering from CML in chronic phase and tried to see whether there was a correlation between in vitro inducibility of apoptosis in CD34+ CML cells after Fas-R triggering and the clinical response to IFN-α. After priming with IFN-α, Fas triggering resulted in in vitro suppression of hematopoietic cell growth in seven of eight patients who had optimal hematologic response to IFN-α; in the same conditions, no inhibitory response to Fas-R agonist was observed in cells from three of three patients who proved to be poor responders to IFN-α. In responders to IFN-α, Fas-R agonist induced dose-dependent apoptosis of CD34+ cells; this effect was associated with a decrease in the bcr/abl protein level. In cells derived from patients with a poor response to IFN-α, the rate of apoptosis in culture remained unchanged in the presence of Fas-R agonist and nobcr/abl downmodulation was observed. Finally, we measuredbcr/abl mRNA by quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) and found that decreased bcr/ablprotein after Fas triggering was not associated with decreased amounts of specific mRNA, a finding which is consistent with a posttranscriptional regulation of the bcr/abl protein expression. It appears that Fas-mediated downmodulation of p210bcr/abl restores susceptibility to apoptosis of CML cells; in addition, in vitro studies on CML cells may predict response to IFN-α treatment.
© 1998 by The American Society of Hematology.
RELATIVE RESISTANCE to apoptosis is thought to be one of the features of malignant cells. Many chemotherapeutic agents exert their anticancer activity through induction of apoptosis,1-4 and inhibition of this process, eg, through a blockade of the apoptotic pathway, can produce resistance to these agents.5-8 In chronic myelogenous leukemia (CML), the bcr/abl translocation and the resulting chimeric protein appear to play a central role in the pathophysiology of the disease.9,10 The ultimate effect of the bcr/ablproduct seems to be enhanced cell survival rather than a higher proliferation rate. The bcr/abl protein seems to exert antiapoptotic activity in CML cells, as shown in several experimental systems,9-18 with some discordant results.19
Under normal circumstances, programmed cell death may be induced by a variety of exogenous signals; it may also be the result of an intrinsic program through a totally endogenous pathway. Immune-mediated killing mechanisms also involve apoptosis, induced by tumor necrosis factor (TNF), interferons (IFNs), Fas-ligand, or the perforin/granzyme pathway.20-22 We have shown that Fas-receptor (Fas-R) is expressed on hematopoietic progenitor cells from CML patients and that Fas triggering transduces apoptotic signals to both normal23,24 and CML cells.25-27 Expression of Fas-R can be induced by TNF-α or IFN-γ, as well as by IFN-α on normal and malignant cells.23,24,28-36 In vitro induction of programmed cell death in CML cells by Fas indicates that thebcr/abl-mediated resistance to apoptosis is not absolute and can be overcome. Fas-R triggering may cause downmodulation ofbcr/abl17-19 or may exert other influences downstream in the sequence of events leading to apoptosis.16 Because resistance to chemotherapy can evolve through inhibition of drug-mediated apoptosis, it is possible that CML cells can also resist or acquire resistance to Fas-induced cell death. This process may be related to cell selection in the face of the pressure exerted by the immune system; such a hypothesis is supported by the observation of development of resistance to IFN-α37,38 or to a graft-versus-leukemia39-41effect in CML patients.
In view of the in vitro observation that IFN-α upmodulates Fas-R expression on CML cells,25-27 and of the variability of the in vivo response to IFN-α treatment, we hypothesized that Fas-mediated apoptosis may be related to modulations of thebcr/abl protein level. Therefore, we studied the mechanisms of apoptosis induced by Fas-R triggering on CML cells and attempted to establish a relationship between inducibility of apoptosis in vitro and clinical response to IFN-α in vivo.
MATERIALS AND METHODS
Patient specimen collection.
Bone marrow (BM) samples were obtained after informed consent from 28 patients with CML. In 16 of them, we evaluated only the expression of CD95 on CD34+ cells; in 12 patients, complete in vitro studies could be performed. Of the latter, 11 patients were in chronic phase; eight were studied before starting IFN-α treatment and three during IFN-α treatment; one patient was studied in blastic crisis. The diagnosis of CML was confirmed by the cytogenetic finding of the Ph chromosome and by the molecular finding of rearrangedbcr/abl. Patient characteristics are presented in Table 1. Our operational definition of the response to IFN-α treatment was as follows: optimal response = complete hematologic recovery after 1 month of treatment at the maximum tolerated dose (6 or 9 million units [MU]); poor response = persisting or increasing leukocytosis (> 80.000) after 1 month of treatment at the maximum tolerated dose (6 or 9 MU), leading to IFN-α treatment discontinuation. Karyotypic response was evaluated after 1 year of the therapy according to the proportion of residual Ph+metaphases: no response (Ph+ 100%), minimal response (Ph+ 99% to 66%), minor response (Ph+ 66% to 35%), and major response (Ph+ <33%).
BM cell separation.
BM was aspirated from the posterior iliac crest into syringes containing Iscove's modified Dulbecco's Medium (IMDM) supplemented 1:10 with heparin (O'Neil & Feldman, St Louis, MO). Mononuclear cells (BMMNC) were isolated by density gradient centrifugation using lymphocyte separation medium (Organon, Durham, NC). After washing with Hanks' Balanced Salt Solution (HBSS), cells were resuspended in IMDM supplemented with 5% fetal calf serum (FCS). HBSS, IMDM, and FCS were purchased from Life Technologies, Gaithersburg, MD.
Separation of CD34+ cells.
In some experiments, purified CD34+ cells were used for the analysis of Fas-R expression or for colony assay. CD34+cells were separated using affinity columns (Cellpro, Bothel, WA). Briefly, nonadherent BM cells were incubated at room temperature with streptavidin-conjugated murine antihuman CD34 IgM, washed with phosphate-buffered saline (PBS), and applied to an affinity column containing biotin-coated beads; after mechanical disruption, the CD34+ fraction was eluted with PBS. An aliquot of the eluted cells was stained with phycoerythrin (PE)-conjugated anti-CD34 HPCA-2 monoclonal antibody (MoAb) (Becton Dickinson, Mountain View, CA) for purity assessment; the average purity of the separated cell population was 87% for all purifications (70% to 95%).
Flow cytometry analysis.
A fluorescein isothiocyanate (FITC)-conjugated (Fab′) fragment of a murine antihuman CD95 (clone UB2; Amac, Westbrook, ME) was used to determine the expression of Fas-R on BM cells. For two-color analysis, PE-conjugated MoAb to CD34 (clone UCH11, Becton Dickinson, Mountain View, CA) was used in combination with FITC-conjugated CD95 MoAb. Proper isotypic controls were used in all experiments. BMMNC were resuspended in 100 μL of PBS (Life Technologies, Grand Island, NY) containing 2% FCS, incubated with 20 μL of MoAb, washed three times with PBS, and analyzed by flow cytometry (Epics, Coulter, Miami, FL). Fas expression on CD34+ cells was analyzed in a uniformly set blast gate.
Hematopoietic colony assay and suspension cultures.
Isolated CD34+ cells were plated in methylcellulose (Stem Cell Technologies, Vancouver, Canada) at a concentration of 1 × 103 cells/mL of medium (35-mm dishes; 1 mL of medium/dish). The growth factor cocktail consisted of 10 ng/mL interleukin-3 (IL-3), 50 ng/mL granulocyte colony-stimulating factor (G-CSF), 50 ng/mL GM-CSF, 20 ng/mL stem cell factor (SCF), and 2 U/mL erythropoietin (EPO) (Amgen, Thousand Oaks, CA). Human recombinant IFN-α (Hoffmann La Roche, Basel, Switzerland) and anti-Fas MoAb (clone CH11, Amac) was used at a concentration range of 20 to 1,000 U/mL and at 1 μg/mL, respectively. All cultures were performed in duplicate. Suspension cultures were performed in 24-well plates in IMDM containing 20% FCS and growth factors at the concentrations described above. All experimental procedures were performed in endotoxin-free plastic ware.
DNA fragmentation assay.
DNA fragmentation was measured after extraction of low molecular weight DNA from a constant number of cells as previously described.24 25 Briefly, 2 × 106 cells were resuspended in 900 μL 1X TRIS-EDTA buffer and lysed with 25 μL 20% sodium dodecyl sulfate (SDS). High molecular weight DNA fraction was precipitated for 6 hours in the presence of 5 mol/L NaCl and pelletted by high-speed centrifugation; the fragmented DNA was extracted from the aqueous phase with phenol and chloroform, and precipitated with ethanol. After resuspension in water, DNA was electrophoresed using 1.5% agarose gel and visualized by ethidium bromide staining.
Terminal deoxynucleotidyl transferase (TdT) assay for quantitation of apoptotic cells.
To estimate the number of cells undergoing apoptosis, cultured CD34+ cells were washed in PBS, fixed with 4% paraformaldehyde, and cytocentrifuged onto siliconized slides. Apoptotic cells were identified using the TdT method (Apotag; Oncor, Gaithersburg, MD). Endogenous peroxidase was first quenched with 0.5% hydrogen peroxide, and the cells were permeabilized using equilibration buffer supplied by the manufacturer. The 3′ OH ends of degraded DNA were reacted with TdT and labeled with digoxygenin-uvidine triphosphate (UTP) for 30 minutes. After washing with PBS, slides were reacted with peroxidase-conjugated antidigoxygenin MoAb, washed, and developed with 3.3′-diaminobenzidine tetrahydrochloride (Pierce, Rockford, IL). Stained cells were counted using a light microscope.
Detection and quantitation of bcr-abl hybrid mRNA by reverse transcription-polymerase chain reaction (RT-PCR).
Total RNA was extracted from a constant number of total BM CML cells using the acid guanidinium thiocyanate and phenol-chloroform method.42 RT-PCR analysis was performed as previously described.43 Reverse transcription was performed with the antisense primer 5′-TGTGATTATAGCCTAAGACCCGGAG-3′, which hybridizes to sequences of the second abl exon, and 100 U of Moloney murine leukemia virus reverse transcriptase (BRL, Bethesda, MD). For the amplification, the antisense primer (see above) and the sense primer 5′-GAAGAAGTGTTTCAGCTTCTCCC-3′, complementary to sequences of bcr exons b1 and b2 were used. PCR products were analyzed on 2% agarose gel containing ethidium bromide.
The absolute amount of the bcr/abl transcripts was quantified using a noncompetitive PCR technique.44,45 Briefly, the technique consists in the reverse transcription followed by PCR amplification of two aliquots (500 and 250 ng, respectively) of total RNA extracted from each sample. The conditions of the PCR reaction ensured a constant amplification. The reaction was terminated during the exponential phase of amplification allowing a log-log linear relation between the number of starting molecules of bcr/ablmRNA and the amount of PCR products. The limiting number of amplification cycles up to which each amplification proceeds with a constant efficiency was calculated by amplifying scalar dilutions from 500 to 5 ng of total RNA by RT-PCR using a decreasing number of cycles. After each experiment, logarithmically transformed data were analyzed by linear regression, and the highest number of cycles that ensured a log-log linear relationship between sample RNA dilutions and amplified products was used in the assays. The absolute amount of bcr/ablmRNA molecules was calculated by interpolating the amount of PCR products with the titration curve obtained by amplifying, in parallel with the samples, known amounts of a “synthetic” RNA molecule with the same sequence as that of the type of bcr/abl mRNA to be quantitated, as previously described.44 45 The reaction was repeated for a total of 18 cycles and the quantitative frame, ie, the linearity range of the assay under these conditions, was between 1.2 × 106 and 6 × 103 molecules.
Western blotting of bcr/abl protein.
Total BM cells derived from patients with CML were cultured in suspension without or with IFN-α and Fas triggering. After 48 hours, cells were washed in PBS and lysed in PBS-TDS (PBS containing 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS) for 10 minutes at 4°C. Cellular debris was removed by centrifugation, and the protein concentration of supernatants was determined by colorimetric assay (micro-BCA; Pierce, Rockford, IL) according to the manufacturer's specifications. A total of 70 μg of each whole-cell lysate, together with molecular weight markers (Amersham, Little Chalfont, UK) were fractionated by 7.5% and 10% SDS-polyacrylamide gel electrophoresis (PAGE) for p210 bcr/abl and actin, respectively. Equal protein loading was assessed by Coomassie-blue staining of SDS-PAGE gels. Gels were equilibrated in transfer buffer (125 mmol/L TRIS-base, 960 mmol/L glycine, 20% methanol) and electrically transferred to polyvinyl difluoride membrane filters (Millipore, Bedford, MA). Membranes were blocked in TBST-3% milk (10 mmol/L TRIS-HCL pH 8.0, 150 mmol/L NaCl, 0.5% Tween-20, 3% nonfat dry milk) for 1 hour at room temperature and incubated with 10 μg/mL of mouse anti-bcr/abl MoAb (Calbiochem, Cambridge, MA) or rabbit anti-actin polyclonal serum (Sigma, St Louis, MO) in TBST-0.5% milk overnight at 4°C. The reaction was developed by incubating filters with horseradish peroxidase–conjugated goat antirabbit antibodies (BioRad, Richmond, CA) and ECL (Amersham, Little Chalfont, UK) according to the manufacturer's specifications. Bands of 210 and 145 kD correspond to human bcr-abl and c-Abl proteins, respectively. Densitometer analysis of films was performed using a scanning densitometer (GS 300; Hoefer Scientific Instruments, San Francisco, CA).
RESULTS
Expression of Fas antigen on BM cells from CML patients.
The expression of CD95 on CD34+ BM cells was studied in a global population of 28 CML patients. On average, 25.5% ± 22% CD34+ BM cells from CML patients expressed CD95, as compared with 8.4% ± 6% in 40 normal controls. When the CML population was dissected according to cytoreduction by IFN-α treatment, the figure was 25.3% ± 16% in 21 patients with optimal response to IFN-α (P = .01 vnormal controls) and 11.3% ± 6.7% in seven patients with poor response (P = .26 v normal controls). When analyzed according to the cytogenetic response, the figure was 19.7% ± 14% in 8 patients with a major (<33% residual Ph+ mitoses) response and 22.0% ± 16% in 20 patients with absent, minimal, or minor response (P = .25). In 11 of these patients, it was possible to perform Western blotting for bcr/ablexpression, apoptosis assays, and methylcellulose cultures (Table 1).
Clinical Characteristics of CML Patients and Results of In Vitro Studies
| Case . | Sex/Age . | Phase . | % CD34+ Cells Bearing CD95 . | Effect of IFN-α 1,000 U + Fas Triggering . | Hematologic Response to IFN-α . | % Ph+ Mitoses at 12 mo-153 . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| % of Apoptotic Cells-151 . | CFU-C/103CD34+ Plated . | p210-152 . | ||||||||
| Untreated . | Treated . | Untreated . | Treated . | % of Untreated . | ||||||
| 1-150 | M/35 | CP | 42 | 5.6 | 20.0 | 78.5 | 16 | 30 | Optimal | 60 |
| 2-150 | F/28 | CP | 25 | − | + | 137 | 25 | 50 | Optimal | 16 |
| 3 | F/53 | CP | 8.3 | 7.3 | 18.5 | 95.5 | 27.5 | 25 | Optimal | 20 |
| 4 | F/26 | CP | 9.5 | − | + | ND | ND | 40 | Optimal | 33 |
| 5-150 | F/40 | CP | 49 | − | + | 86.5 | 17.5 | 35 | Optimal | 61 |
| 6 | M/59 | CP | 3.2 | 6.6 | 21.0 | 122.5 | 19 | 25 | Optimal | 42 |
| 7 | M/33 | CP | 52 | 6.5 | 25.0 | 132 | 17.5 | 10 | Optimal | 75 |
| 8 | M/48 | CP | 13.5 | 9.0 | 16.5 | 87 | 68 | 90 | Optimal | 16 |
| X ± SD | 40 ± 12 | 25.3 ± 19 | 7.0 ± 1 | 20.2 ± 3 | 105.5 ± 24 | 27.1 ± 18 | 38.1 ± 24 | |||
| 9 | M/40 | CP | 15.2 | 9.0 | 13.5 | 102.5 | 88 | 95 | Poor | 80 |
| 10 | F/40 | CP | 17.8 | 8.0 | 11.5 | 170 | 158 | 100 | Poor | 100 |
| 11 | M/63 | CP | 4.5 | 9.0 | 10.5 | 65 | 62 | 100 | Poor | 90 |
| X ± SD | 47.6 ± 13 | 12.5 ± 7 | 8.6 ± 0.6 | 11.8 ± 1 | 112.5 ± 31 | 102.6 ± 29 | 98.3 ± 1.6 | |||
| 12 | M/58 | BC | 1.1 | 9.5 | 12.5 | 77 | 65 | 100 | NA | NA |
| Case . | Sex/Age . | Phase . | % CD34+ Cells Bearing CD95 . | Effect of IFN-α 1,000 U + Fas Triggering . | Hematologic Response to IFN-α . | % Ph+ Mitoses at 12 mo-153 . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| % of Apoptotic Cells-151 . | CFU-C/103CD34+ Plated . | p210-152 . | ||||||||
| Untreated . | Treated . | Untreated . | Treated . | % of Untreated . | ||||||
| 1-150 | M/35 | CP | 42 | 5.6 | 20.0 | 78.5 | 16 | 30 | Optimal | 60 |
| 2-150 | F/28 | CP | 25 | − | + | 137 | 25 | 50 | Optimal | 16 |
| 3 | F/53 | CP | 8.3 | 7.3 | 18.5 | 95.5 | 27.5 | 25 | Optimal | 20 |
| 4 | F/26 | CP | 9.5 | − | + | ND | ND | 40 | Optimal | 33 |
| 5-150 | F/40 | CP | 49 | − | + | 86.5 | 17.5 | 35 | Optimal | 61 |
| 6 | M/59 | CP | 3.2 | 6.6 | 21.0 | 122.5 | 19 | 25 | Optimal | 42 |
| 7 | M/33 | CP | 52 | 6.5 | 25.0 | 132 | 17.5 | 10 | Optimal | 75 |
| 8 | M/48 | CP | 13.5 | 9.0 | 16.5 | 87 | 68 | 90 | Optimal | 16 |
| X ± SD | 40 ± 12 | 25.3 ± 19 | 7.0 ± 1 | 20.2 ± 3 | 105.5 ± 24 | 27.1 ± 18 | 38.1 ± 24 | |||
| 9 | M/40 | CP | 15.2 | 9.0 | 13.5 | 102.5 | 88 | 95 | Poor | 80 |
| 10 | F/40 | CP | 17.8 | 8.0 | 11.5 | 170 | 158 | 100 | Poor | 100 |
| 11 | M/63 | CP | 4.5 | 9.0 | 10.5 | 65 | 62 | 100 | Poor | 90 |
| X ± SD | 47.6 ± 13 | 12.5 ± 7 | 8.6 ± 0.6 | 11.8 ± 1 | 112.5 ± 31 | 102.6 ± 29 | 98.3 ± 1.6 | |||
| 12 | M/58 | BC | 1.1 | 9.5 | 12.5 | 77 | 65 | 100 | NA | NA |
Abbreviations: CP, chronic phase; BC, blastic crisis; ND, not done; NA, not applicable.
Patients studied while under IFN-α treatment.
Numerical values: detected by Tdt assay; ± detected qualitatively by DNA laddering in agarose gel.
Detected by densitometric scanning of Western blotting films.
At diagnosis, all patients showed 100% Ph+ mitoses.
IFN-α– and Fas-mediated apoptosis of BM progenitor cells derived from patients with CML in chronic phase. Correlation with bcr/abl product cell content.
In 7 of 11 patients tested, the addition of Fas agonist resulted in enhancement of apoptosis, as demonstrated by agarose gel electrophoresis of low molecular weight DNA extracted from total BM cells (Fig 1B and 2B) or by the Tdt assay (Table 1, Figs 1C,2C, 3A and B). Western blot performed on cell extracts derived from the same cells using identical protein concentration for each sample showed that this effect was associated with downmodulation of the bcr/abl protein (Fig 1A). The downmodulation, which was not caused by proteolysis, as p145 c-abland actin proteins remained unchanged, was enhanced by in vitro addition of IFN-α; the enhancement was dose-dependent in three cases and occurred only at the highest IFN-α concentration (1,000 U/mL) in four cases (data not shown). Addition of the partially blocking anti-Fas MoAb ZB4, serving as a Fas antagonist, significantly reduced the effect of IFN-α + Fas triggering on apoptosis, as well as on downmodulation of bcr/abl (Fig 2). These seven patients all showed a complete hematologic response after IFN-α treatment. In a single patient (Table 1, no. 8), who also showed an optimal response to IFN-α, the effect of Fas triggering in vitro was only marginal. By contrast, in 3 of 11 patients, Fas triggering failed to induce apoptosis in IFN-α–treated cells, as shown by characteristic DNA laddering in agarose gel electrophoresis and quantitative Tdt assay (Fig 3B), and no change in bcr/abl expression was observed; these patients had a poor response to IFN-α treatment and were switched to other types of treatment. In the only case of lymphoid blastic crisis studied, we could not document any effect of Fas triggering in vitro (Table 1, no. 12).
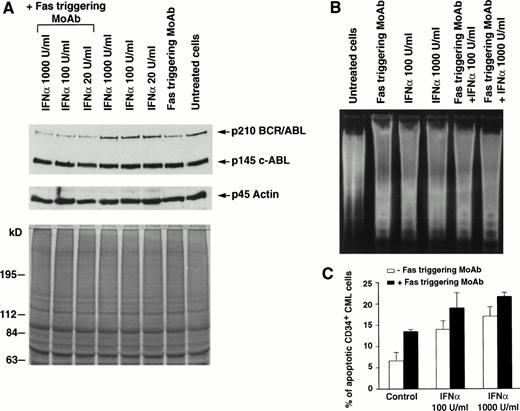
Effects of Fas triggering by anti-Fas MoAb CH11 on p210bcr/abl protein expression and on susceptibility to apoptosis of total and CD34+ BM cells from a representative patient with CML in chronic phase who had optimal response to IFN-α. (A) Immunoblotting of p210 from total BM cells (upper) and Coomassie-blue staining to document equal protein loading (lower). P145 c-abland actin proteins are used as controls for constant protein loading and absence of degradation. (B) Agarose gel stained with ethidium bromide after electrophoresis of low-molecular-weight DNA extracted from a constant number of total BM cells. Cell lysates and low-molecular-weight DNA were obtained from the same plates for both (A) and (B) experiments. (C) In situ TdT assay of CD34+cells treated as indicated; bars represent the mean number of apoptotic cells determined in triplicate experiments ± standard error of mean (SEM). Statistical analysis (paired t-test) showed P< .05 for control versus Fas triggering alone and after addition of any IFN-α concentration and for Fas triggering versus IFNα 1,000 U/mL + Fas triggering. Total BM and CD34+ cells were cultured for 48 hours in the presence of the indicated concentrations of IFN-α. MoAb CH11 was used at a concentration of 1 μg/mL.
Effects of Fas triggering by anti-Fas MoAb CH11 on p210bcr/abl protein expression and on susceptibility to apoptosis of total and CD34+ BM cells from a representative patient with CML in chronic phase who had optimal response to IFN-α. (A) Immunoblotting of p210 from total BM cells (upper) and Coomassie-blue staining to document equal protein loading (lower). P145 c-abland actin proteins are used as controls for constant protein loading and absence of degradation. (B) Agarose gel stained with ethidium bromide after electrophoresis of low-molecular-weight DNA extracted from a constant number of total BM cells. Cell lysates and low-molecular-weight DNA were obtained from the same plates for both (A) and (B) experiments. (C) In situ TdT assay of CD34+cells treated as indicated; bars represent the mean number of apoptotic cells determined in triplicate experiments ± standard error of mean (SEM). Statistical analysis (paired t-test) showed P< .05 for control versus Fas triggering alone and after addition of any IFN-α concentration and for Fas triggering versus IFNα 1,000 U/mL + Fas triggering. Total BM and CD34+ cells were cultured for 48 hours in the presence of the indicated concentrations of IFN-α. MoAb CH11 was used at a concentration of 1 μg/mL.
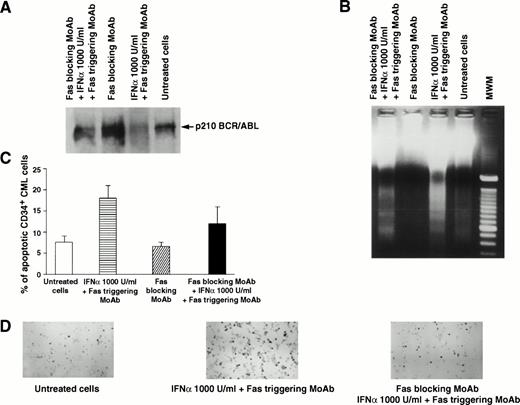
Effects of Fas blocking by MoAb ZB4 on Fas-mediated regulation of p210 and apoptosis of total and CD34+ BM cells from a CML patient in chronic phase who had optimal response to IFN-α. Total BM and CD34+ cells were cultured for 48 hours in the absence or presence of Fas triggering (by MoAb CH11) and/or Fas blocking (by MoAb ZB4), without or with IFN-α (1,000 U/mL). For (A), (B), and (C), see legend to Fig 1. (D), (E), and (F) show apoptotic CD34+ CML cells stained positively with peroxidase (black cells) after the indicated treatment. In (C), bars represent the mean number of apoptotic cells determined in triplicate experiments ± SEM. Statistical analysis (pairedt-test) showed P < .05 for untreated cells versus Fas triggering + IFN-α and for Fas blocking versus Fas blocking + IFN-α + Fas triggering, while there was no statistical difference between untreated and Fas blocking.
Effects of Fas blocking by MoAb ZB4 on Fas-mediated regulation of p210 and apoptosis of total and CD34+ BM cells from a CML patient in chronic phase who had optimal response to IFN-α. Total BM and CD34+ cells were cultured for 48 hours in the absence or presence of Fas triggering (by MoAb CH11) and/or Fas blocking (by MoAb ZB4), without or with IFN-α (1,000 U/mL). For (A), (B), and (C), see legend to Fig 1. (D), (E), and (F) show apoptotic CD34+ CML cells stained positively with peroxidase (black cells) after the indicated treatment. In (C), bars represent the mean number of apoptotic cells determined in triplicate experiments ± SEM. Statistical analysis (pairedt-test) showed P < .05 for untreated cells versus Fas triggering + IFN-α and for Fas blocking versus Fas blocking + IFN-α + Fas triggering, while there was no statistical difference between untreated and Fas blocking.
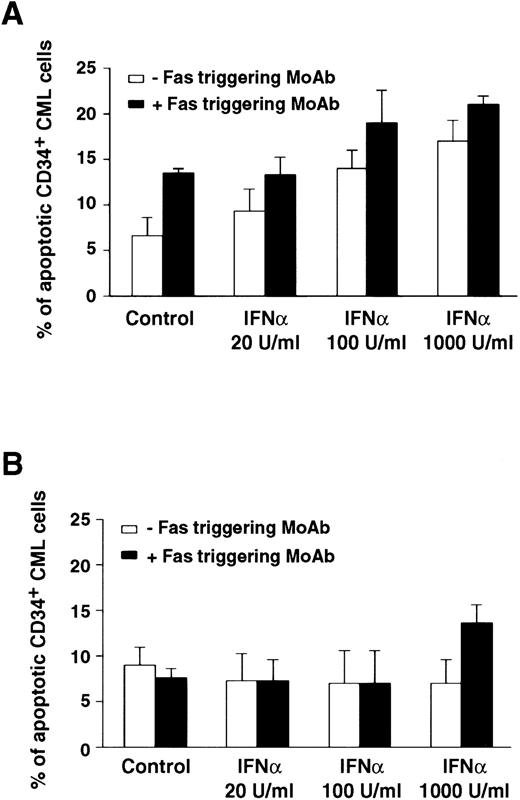
Comparison of apoptotic effect of Fas triggering on CD34+ BM cells from representative CML patients in chronic phase who showed optimal (A) or poor (B) response to IFN-α treatment. Values (mean ± SEM of triplicate measurements) represent percentage of apoptotic CD34+ BM CML cells evaluated by the Tdt assay. CD34+ cells were purified from CML BM and cultured in the presence of IFN-α and MoAb CH11. MoAb CH11 was used at a concentration of 1 μg/mL. TdT assay was performed as described in Materials and Methods. Each experiment was performed in triplicate. Statistical analysis (paired t-test) showed P < .05 only in the responder group, for control versus IFN-α 100 and 1,000 U/mL; control versus Fas triggering; any IFN-α concentration versus IFN-α + Fas triggering.
Comparison of apoptotic effect of Fas triggering on CD34+ BM cells from representative CML patients in chronic phase who showed optimal (A) or poor (B) response to IFN-α treatment. Values (mean ± SEM of triplicate measurements) represent percentage of apoptotic CD34+ BM CML cells evaluated by the Tdt assay. CD34+ cells were purified from CML BM and cultured in the presence of IFN-α and MoAb CH11. MoAb CH11 was used at a concentration of 1 μg/mL. TdT assay was performed as described in Materials and Methods. Each experiment was performed in triplicate. Statistical analysis (paired t-test) showed P < .05 only in the responder group, for control versus IFN-α 100 and 1,000 U/mL; control versus Fas triggering; any IFN-α concentration versus IFN-α + Fas triggering.
Posttranslational modulation of bcr/abl expression mediated by Fas triggering.
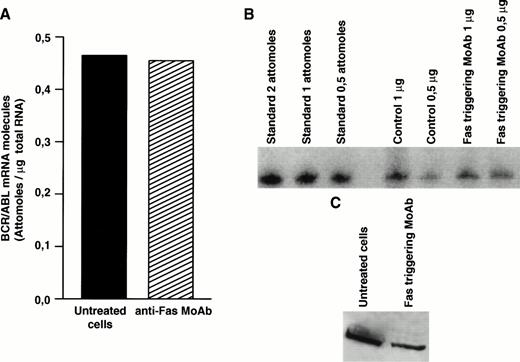
Decreased expression of the bcr/abl protein after Fas triggering could be related to inhibition of transcription, to decreased stability of bcr/abl mRNA, or to posttranslational regulation. To clarify this issue, we determined the level ofbcr/abl mRNA by a quantitative mRNA assay. In total CML cells susceptible to Fas-mediated apoptosis, the bcr/abl mRNA level was not influenced by Fas-R triggering (Fig4A and B). Nevertheless, when identical amounts of protein extract derived from total CML cell cultures from the same patient were blotted, Fas-triggered samples showed markedly decreased levels ofbcr/abl protein (Fig 4C), suggesting a posttranslational regulation.
Effect of Fas triggering on bcr/abl expression (mRNA and protein) from a CML patient in chronic phase who had optimal response to IFN-α and showed in vitro susceptibility to Fas-mediated apoptosis. Quantitative RT-PCR: (A) densitometry and (B) agarose gel. (C) Immunoblotting of p210 from total BM cells. Fas triggering induced downregulation of p210, but not of bcr/abl mRNA.
Effect of Fas triggering on bcr/abl expression (mRNA and protein) from a CML patient in chronic phase who had optimal response to IFN-α and showed in vitro susceptibility to Fas-mediated apoptosis. Quantitative RT-PCR: (A) densitometry and (B) agarose gel. (C) Immunoblotting of p210 from total BM cells. Fas triggering induced downregulation of p210, but not of bcr/abl mRNA.
Differential effects of IFN-α and Fas-L on hematopoietic colony formation of patients with CML. Relation with clinical response to IFN-α.
In previous studies, we showed that the activity of Fas triggering on hematopoietic progenitor cells derived from patients with CML is related to the levels of Fas-R induced by IFN-α.25 When proliferation of CD34+ CML progenitor cells was studied in methylcellulose colony assays, the inhibitory effect of Fas triggering on hematopoietic colony formation paralleled the induction of apoptosis by Fas triggering. We looked for a possible correlation between the differential susceptibility of CML cells to Fas triggering, as assessed by a Fas-mediated inhibition colony assay, and the hematologic response to IFN-α therapy. BM cells were treated with increasing concentrations of IFN-α to induce Fas-R expression, while the concentration of the Fas triggering agent was kept constant. BM cells derived from patients with an optimal response to IFN-α showed higher expression of Fas-R on CD34+ cells (Table 1) and a higher baseline sensitivity to Fas triggering than those derived from patients with a poor response (Table 2). The suppression of colony formation by IFN-α was dose-dependent in good responders, while poor responders showed virtually no IFN-α–mediated hematopoietic inhibition. Finally, in patients with an optimal response to IFN-α, there was synergism in the hematopoietic suppression by addition of Fas triggering to IFN-α–treated samples; no synergism was observed in patients who failed to respond to IFN-α therapy (Table 2). In parallel experiments, we evaluated whether there was a correlation between rate of apoptosis induced by Fas triggering in vitro and hematologic response to IFN-α (Table 3). CD34+ CML progenitor cells derived from good responders to IFN-α were suceptible to in vitro Fas-mediated apoptosis, while CD34+ cells derived from poor responder patients were resistant to Fas triggering, in agreement with the data obtained in methylcellulose cultures.
Effect of Fas Triggering on CFU-C Growth From CD34+ BM Cells of CML Patients Sensitive or Resistant to IFN-α
| . | Sensitive n = 7 . | Resistant n = 3 . | ||
|---|---|---|---|---|
| Control . | Fas Triggering . | Control . | Fas Triggering . | |
| Untreated | 100 | 63.6 ± 5 | 100 | 97 ± 1 |
| IFN-α 20 U/mL | 91.3 ± 4 | 51.7 ± 9 | 101 ± 6 | 88 ± 7 |
| IFN-α 100 U/mL | 82.8 ± 7 | 48.7 ± 13 | 106 ± 2 | 88 ± 6 |
| IFN-α 1,000 U/mL | 65.4 ± 5 | 25.8 ± 11 | 99.3 ± 6 | 80.3 ± 3 |
| . | Sensitive n = 7 . | Resistant n = 3 . | ||
|---|---|---|---|---|
| Control . | Fas Triggering . | Control . | Fas Triggering . | |
| Untreated | 100 | 63.6 ± 5 | 100 | 97 ± 1 |
| IFN-α 20 U/mL | 91.3 ± 4 | 51.7 ± 9 | 101 ± 6 | 88 ± 7 |
| IFN-α 100 U/mL | 82.8 ± 7 | 48.7 ± 13 | 106 ± 2 | 88 ± 6 |
| IFN-α 1,000 U/mL | 65.4 ± 5 | 25.8 ± 11 | 99.3 ± 6 | 80.3 ± 3 |
Values (mean ± SEM) represent colony formation as a percentage of untreated CML progenitors. For untreated cells, the mean number of CFU-C (erythroid and myeloid)/103 CD34+ plated was 105 ± 9 for responders and 112 ± 33 for nonresponders. Fas was triggered by the agonist MoAb CH11 used at the concentration of 1 μg/mL. Statistical analysis (paired t-test) showed P< .05 only in the responder group, for control versus IFN-α 100 and 1,000 U/mL; control versus Fas triggering; control + any IFN-α concentration versus control + any IFN-α concentration + Fas triggering.
Effect of Fas Triggering on Apoptosis of CD34+ BM Cells From CML Patients Sensitive or Resistant to IFN-α
| . | Sensitive n = 5 . | Resistant n = 3 . | ||
|---|---|---|---|---|
| Control . | Fas Triggering . | Control . | Fas Triggering . | |
| Untreated | 7 ± 1 | 14.3 ± 3 | 8.6 ± 1 | 7.8 ± 1 |
| IFN-α 20 U/mL | 9.3 ± 1 | 13.7 ± 4 | 6.5 ± 1 | 8.1 ± 1 |
| IFN-α 100 U/mL | 11 ± 3 | 17.7 ± 5 | 6 ± 1 | 7 ± 1 |
| IFN-α 1,000 U/mL | 16.6 ± 3 | 20.2 ± 1 | 6.8 ± 2 | 11.8 ± 2 |
| . | Sensitive n = 5 . | Resistant n = 3 . | ||
|---|---|---|---|---|
| Control . | Fas Triggering . | Control . | Fas Triggering . | |
| Untreated | 7 ± 1 | 14.3 ± 3 | 8.6 ± 1 | 7.8 ± 1 |
| IFN-α 20 U/mL | 9.3 ± 1 | 13.7 ± 4 | 6.5 ± 1 | 8.1 ± 1 |
| IFN-α 100 U/mL | 11 ± 3 | 17.7 ± 5 | 6 ± 1 | 7 ± 1 |
| IFN-α 1,000 U/mL | 16.6 ± 3 | 20.2 ± 1 | 6.8 ± 2 | 11.8 ± 2 |
Values (mean ± SEM) represent percentage of apoptotic CD34+ BM CML cells evaluated by the Tdt assay. The Fas agonist MoAb CH11 was used at the concentration of 1 μg/mL. Each experiment was performed in triplicate. Statistical analysis (pairedt-test) showed P < .05 only in the responder group, for control versus IFN-α 100 and 1,000 U/mL; control versus Fas triggering; control + any IFN-α concentration versus control + any IFN-α concentration + Fas triggering.
DISCUSSION
High response rates to IFN-α, evidence of a graft-versus-leukemia effect in transplanted patients, and the favorable effect of donor lymphocyte infusion in cytogenetic relapse of transplanted patients all imply that immunologic mechanisms play an important role in the pathophysiology of CML. We previously showed that Fas-R is expressed on CD34+ cells derived from CML patients and that it can be further upmodulated by IFN-α. Triggering of Fas-R induces inhibition of proliferation of these cells and its effect is potentiated in the presence of IFN-α.25-27 In the current study, we expand these observations and describe a possible mechanism by which IFN-α and Fas-L may act on the proliferation and survival of CML cells.
Our results show that CD34+ cells derived from CML patients who showed an optimal cytoreducing effect after IFN-α therapy undergo apoptosis upon Fas-R triggering, while those derived from patients with a poor response to IFN-α are almost completely resistant to Fas-mediated killing. Based on the reported antiapoptotic activity of bcr/abl, we hypothesized that the differential effect of Fas-R triggering could be related to modulation of bcr/ablprotein expression in CML cells. Western blotting confirmed this hypothesis: in seven of eight samples susceptible to Fas-induced apoptosis, we observed a decrease of the bcr/abl hybrid protein, whereas lack of apoptotic response to Fas-agonist was associated with unchanged bcr/abl protein level. The mechanism of the Fas-mediated bcr/abl downmodulation is, at present, only a matter of speculation. In principle, the differential effects of the Fas agonist could be due to the variability of Fas-R inducibility by IFN-α. However, because there was no difference in the effect of IFN-α alone on the proliferative capacity of CML cells between the two groups of patients and our previous studies showed that the effect of IFN-α on Fas-R upmodulation is reproducible in all patients studied, we conclude that an intrinsic mechanism related to the intracellular transduction pathway of Fas must be responsible for the inability of Fas agonists to induce apoptosis in patients with a poor response to IFN-α.
Our results are in agreement with other reports showing that downregulation of bcr/abl produces decreased resistance of CML cells to apoptosis induced by various agents.17-19 The presented experiments show that the decrease in bcr/abl protein levels caused by Fas triggering is related to a posttranscriptional modulation, as the levels of bcr/abl mRNA were not affected by Fas triggering in cells susceptible to Fas-mediated apoptosis. The lower levels of bcr/abl after Fas-R triggering cannot be due to unspecific degradation of cellular proteins by proteases activated in the process of Fas-mediated apoptosis, as documented by the constant amount of p145 c-abl and actin protein levels (Fig 1A).
A correlation between susceptibility to in vitro Fas-mediated inhibition and in vivo response to IFN-α has several implications. We have analyzed the hematologic response to IFN-α, which can be evaluated in a few months' time and is considered necessary, although not sufficient, for a major cytogenetic response, which, in turn, is associated with a better survival. Cytogenetic response analysis was precluded by the fact that poor responders to IFN-α were switched to other treatments. Enhanced Fas-R expression on CD34+ CML cells of patients with optimal cytoreduction by IFN-α may be related to the in vivo action of several cytokines, such as IFN-γ, IFN-α, and TNF-α, endogenously released as a part of the natural response to the expansion of the leukemic clone, and further suggests the involvement of the Fas-R/Fas-L system in the immunologic regulation of CML progenitor growth. In addition, although IFN-α may exert a direct effect on hematopoietic progenitor cells, its inhibitory effects may also be mediated by other factors, such as Fas-L supplied by T lymphocytes. In patients with Kaposi's sarcoma, IFN-α is effective only if the number of CD4+ T cells is greater than 500/μL, implying that at least in that disease, an efficient immune system is required for the antitumor effect of IFN-α.46In CML, the situation is even more complicated; in some patients, a poor response to IFN-α may be due to an intrinsic inability (resistance) of the CML cells to respond to Fas-L. At present, it is unknown whether such a defect is a specific feature of some subset of CML or whether the ineffective Fas-R transduction pathway in CML cells may develop as a secondary phenomenon. Indeed, the pressure exerted by the immune system may select for cells resistant to Fas-mediated apoptosis. In patients with blast crisis, we have seen a spectrum of Fas-R patterns: while in some patients, CML blasts showed a high level of Fas-R, in others, no Fas-R expression was detected (personal unpublished data, October 1997). In the case of lymphoid blastic crisis reported here, the bcr/abl protein level did not decrease upon Fas triggering, and we could not demonstrate Fas-mediated colony inhibition and induction of apoptosis. The inability to induce apoptosis via the Fas pathway in the reported case, as well as in other patients in blastic crisis (manuscript in preparation), suggests that different resistance mechanisms may operate in transformed cells. In acute myeloid leukemia, a correlation between Fas-R expression and response to initial induction chemotherapy has been reported; however, there was no correlation between inducibility of apoptosis and level of Fas-R expression.47-50 Because it is likely that the transduction pathways involved in apoptosis are convergent, ie, common effector mechanisms may be used,3,51-55 resistance to Fas-mediated killing may be associated with resistance to apoptosis induced by a variety of agents acting downstream.4,8 Interruption of the apoptotic response in CML cells may be related to properties of the p210bcr-abl hybrid protein; however, several putative effector genes such as phosphatidylinositol-3 kinase,56Shc,57 GRB-2,58 p21RAS,59p160BCR,60,61 Myc,62 and C-Myb63may also play a role in this process. Therefore, apoptosis could result either from signals counterbalancing the apoptotic drive or from a blockade in the apoptotic machinery. Even under normal circumstances, TNF or IFNs have been shown to induce upregulation of Fas-R on normal hematopoietic progenitor cells and subsequent Fas-R triggering results in apoptosis.23 24
Our study was designed to investigate the regulation of proliferation and survival of CML progenitors rather than the utility of IFN-α and Fas triggering in the elimination of the Ph+ clone. Further studies, some of which are currently in progress in our laboratories, are needed to assess whether exposure to IFN-α followed by Fas triggering can be of any help for eradicating the disease or for purging purpose.
ACKNOWLEDGMENT
We thank Dr Luigi Del Vecchio (Immunohematology Service, Cardarelli Hospital, Naples) for flow cytometry analysis.
Supported in part by Associazione Italiana Ricerca Cancro (A.I.R.C.), Milan, Associazione Italiana contro le Leucemie (AIL), Rome, and MURST, Rome, Italy.
Address reprint requests to Jaroslaw P. Maciejewski, MD, Department of Internal Medicine, University of Nevada, Reno, Howard Medical Bldg 320, Reno, NV 89557-0046.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal