Abstract
The c-myc oncoprotein accelerates programmed cell death (apoptosis) after growth factor deprivation or pharmacological insult in many cell lines. We have shown that max, the obligate c-myc heterodimeric partner protein, also promotes apoptosis after serum withdrawal in NIH3T3 fibroblasts or cytokine deprivation in interleukin-3 (IL-3)-dependent 32D murine myeloid cells. We now show that c-myc– and max-overexpressing 32D cells differ in the nature of their apoptotic responses after IL-3 removal or treatment with chemotherapeutic compounds. In the presence of IL-3, c-myc overexpression enhances the sensitivity of 32D cells to Etoposide (Sigma, St Louis, MO), Adriamycin (Pharmacia, Columbus, OH), and Camptothecin (Sigma), whereas max overexpression increases sensitivity only to Camptothecin. Drug treatment of c-myc–overexpressing cells in the absence of IL-3 did not alter the spectrum of drug sensitivity other than to additively accelerate cell death. In contrast, enhanced sensitivity to Adriamycin, Etoposide, and Taxol (Bristol-Meyers Squibb, Princeton, NJ) was revealed in max-overexpressing cells concurrently deprived of IL-3. Differential rates of apoptosis were not strictly correlated with the ability of the drugs to promote G1 or G2/M arrest. Ectopic expression of Bcl-2 or Bcl-XL blocked drug-induced apoptosis in both cell lines. In contrast, whereas Bcl-2 blocked apoptosis in both cell lines in response to IL-3 withdrawal, Bcl-XL blocked apoptosis in max-overexpressing cells but not in c-myc–overexpressing cells. These results provide mechanistic underpinnings for the idea that c-myc and max modulate distinct apoptotic pathways.
© 1998 by The American Society of Hematology.
THE c-myc ONCOPROTEIN plays important roles in cellular transformation, proliferation, and differentiation.1-4 More recently, a role for c-myc in programmed cell death (apoptosis) in primary fibroblasts and in cytokine-dependent hematopoietic cell lines has been documented where, after the removal of these survival factors, the cells undergo apoptosis at a greatly accelerated pace.5-8
The molecular mechanisms underlying c-myc–mediated apoptosis in response to growth factor withdrawal are incompletely understood. The process seems to be at least partly dependent on the p53 tumor suppressor9,10 and can also be prevented by overexpression of the Bcl-2 oncoprotein.11-13 c-myc–mediated apoptosis also requires that the protein dimerize with max,7 which, like c-myc, is a member of the basic-helix-loop-helix-leucine zipper family.14,15 It is believed that c-myc–max heterodimers represent the state in which c-myc is able to recognize its specific DNA binding sites in vivo and activate the expression of adjacent genes.16 17
max consists of two major protein isoforms of either 151 or 160 amino acids, respectively.14,15 These length differences are attributable to a nine–amino acid insertion/deletion between amino acids 12 and 13. Recently, we have shown that stable ectopic expression of the longer max isoform (max[L]) results in reduced proliferation, decreased sensitivity to growth factors, and accelerated apoptosis after serum deprivation in NIH3T3 fibroblasts or interleukin-3 (IL-3) withdrawal in the 32D murine myeloid cell line.18 In contrast, overexpression of the shorter isoform results in slightly faster proliferation, increased sensitivity to growth factors, and no appreciable effect on apoptosis in response to cytokine removal.
The more robust demise of c-myc and max(L)-overexpressing cells in response to cytokine deprivation raises the question of whether the biochemical pathways leading to this response are identical or distinct. In the latter case, it might then be possible to define these pathways functionally based on whether they can be modulated by other known proapoptotic or antiapoptotic factors.
In the present study, we have examined the sensitivity of c-myc and max(L)-overexpressing cells to apoptotic induction by several pharmacological agents commonly used in cancer chemotherapy. The agents were chosen based on their differing modes of action, although all eventually promote cellular death through apoptosis. 32D cells were used to test the effects of c-myc and max(L) overexpression because they are a nontumorigenic, euploid cell line that express wild-type p53 protein, a requirement for a normal apoptotic response in several systems.19 20 We show here that c-myc and max(L) alter the apoptotic response of 32D cells to pharmacological insult in distinctly different ways. The ectopic expression of either Bcl-2 or the closely related protein Bcl-XL was able to prevent apoptosis in either cell line in response to drug treatment. In contrast, whereas Bcl-2 was able to block apoptosis in both cell lines after IL-3 withdrawal, Bcl-XL blocked apoptosis only in max(L)-overexpressing cells. Taken together, our results argue that c-myc and max(L) likely affect different, although overlapping, apoptotic pathways.
MATERIALS AND METHODS
Plasmids.
The construction of pSVLneo-max(L) and pSVLneo-c-myc have been previously described.18 pMEP4-Bcl-2 and the empty pMEP4 parental vector21 were kindly provided by Dr Yusuf Hannun (Duke University Medical Center, Durham, NC). A full-length human Bcl-XL cDNA22 in a pBluescript vector was provided by Dr Craig Thompson (Pritzker School of Medicine, The University of Chicago, IL). The cDNA was excised with NotI andEcoRV, made blunt-ended with the Klenow fragment of DNA polymerase and ligated into the blunt-ended EcoRI site of the pAPuro expression vector.23
Cell lines.
32D c13 cells19 were obtained from Dr Seth Corey (Children's Hospital of Pittsburgh). The derivation of cells overexpressing max(L) (hereafter referred to as 32D-max) and c-myc (hereafter referred to as 32D-c-myc) has been previously described.18 Briefly, cells were transfected by electroporation with NdeI-linearized pSVLneo-max(L) or pSVLneo-c-myc plasmid DNAs and selected in G-418 (GIBCO-BRL, Grand Island, NY).24 Pooled G-418-resistant populations of cells were used to eliminate clonal variability as an explanation for observed differences in subsequent biological behavior. A population of control cells was also obtained after stable transfection with the empty pSVLneo vector and is referred to as 32D-neo. All cell lines were maintained in RPMI medium (GIBCO-BRL) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 10% conditioned medium from the IL-3–producing murine WEHI 238 lymphoblastoid cell line, penicillin, streptomycin, 2 mmol/L glutamine, and 400 μg/mL G-418 (absolute concentration) (GIBCO-BRL).
To obtain cell lines stably overexpressing Bcl-2, 32D-neo, 32D-max, and 32D-c-myc cells were transfected by electroporation withBgIII-linearized pMEP4-Bcl-2 and selected in 250 μg/mL Hygromycin (GIBCO-BRL). Control cell lines, transfected with the empty pMEP4 vector, were derived in parallel. Cell lines overexpressing Bcl-XL were obtained by electroporation withNotI-linearized pAPuro-Bcl-XL and selected in 1 μg/mL Puromycin (Sigma, St Louis, MO). Control cell lines were obtained by stable transfection with the NotI-linearized pAPuro parental vector. All cell lines were maintained continuously in the above stated concentrations of the appropriate antibiotic.
Western blotting.
Western blotting was performed with 50 μg of total cell lysate from each cell line. Briefly, logarithmically growing cells were pelleted by centrifugation, washed twice in phosphate-buffered saline (PBS), and lysed in standard 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) lysis buffer. Protein concentrations were determined using the Pierce BCA Protein Determination Assay (Pierce, Rockford, IL) and were then verified by Coomassie blue staining of electrophoresed aliquots. After SDS-PAGE, proteins were transferred to Immobilon-P membranes (Millipore, Bedford, MA) using a semi-dry blotting apparatus (Owl Scientific, Cambridge, MA). All pre-incubations and incubations with antibodies were performed in PBS-T + 5% nonfat dry milk.25 Blots were first incubated for 2 hours at room temperature followed by an overnight incubation at 4°C with a 1:1,000 dilution of rabbit anti-Bcl-2 (#15616E) or Bcl-X (#65186E) antibodies (Pharmingen, San Diego, CA). After exhaustive washing in PBS-T, the blots were incubated for 2 hours at room temperature with a 1:1,000 dilution of horseradish peroxidase-conjugated goat anti-rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA) followed by washing with PBS-T and development of the blots using the “Supersignal” enhanced chemiluminescence kit (Pierce) according to the supplier's directions.
Apoptosis studies.
32D cell lines (>95% viability) were seeded at 2 × 105 cells/mL in 6- or 12-well tissue culture plates and allowed to resume growth for 6 to 8 hours before the addition of Adriamycin (ADR; Pharmacia, Columbus, OH), Etoposide (VP-16; Sigma), Camptothecin (CPT; Sigma), Cis-platinum (CDDP; Sigma), Taxol (Bristol-Meyers Squibb, Princeton, NJ), or Nitrogen mustard (N-Mus; Merck, West Point, PA) to the indicated final concentrations. All drugs were dissolved in tissue culture medium as stock solutions 100-fold more concentrated than the highest concentrations used here and were frozen in small aliquots. In some experiments, IL-3 was withheld to allow the combined effects of drug treatment and cytokine deprivation to be determined. At various times after the addition of drugs, aliquots of cells were removed and viability was assessed using the trypan blue exclusion assay. Total DNA was extracted as previously described18 and analyzed by electrophoresis in 2% agarose gels followed by staining with ethidium bromide.
Cell cycle studies.
Logarithmically growing cells (approximately 5 × 105/mL) were treated with chemotherapeutic agents for 16 hours. Propidium iodide staining of isolated nuclei was then performed as previously described.26 Cell cycle analyses were performed on a Becton Dickinson (Mountain View, CA) FACSTAR fluorescence-activated cell sorter. For each assay, 2 × 104 cells were analyzed. Quantitation was performed using single histogram statistics.18
RESULTS
c-myc and max differentially affect apoptosis in response to pharmacological agents.
For this study, we used the 32D myeloid cell line which is diploid, untransformed, and expresses wild-type p53 protein.19,20 We tested six structurally unrelated and mechanistically diverse pharmacological compounds commonly used in cancer chemotherapy.27-29 ADR is a DNA intercalating agent and inhibitor of topoisomerase II, CPT is a non-DNA binding inhibitor of topoisomerase I, VP-16 is a nonintercalating topoisomerase II inhibitor, CDDP is a DNA cross-linking agent, N-Mus is an alkylating agent, and Taxol is a microtubule inhibitor. Because growth factors have been previously shown to protect against apoptosis in several settings,30 31 a potential antiapoptotic role for IL-3 in c-myc– and max-mediated apoptosis was also investigated.
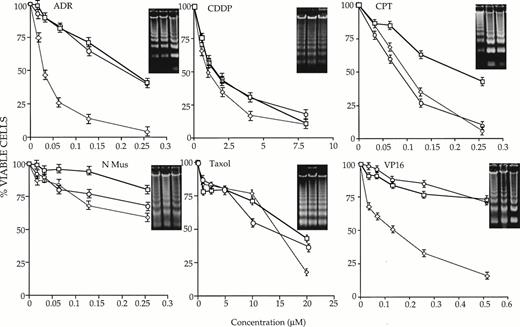
32D-neo, 32D-c-myc, and 32D-max cells were first incubated continuously in IL-3–supplemented medium containing different concentrations of each chemotherapeutic compound. At various times thereafter, the fraction of apoptotic cells was assessed by trypan blue exclusion. As shown in Fig 1, each of the tested cell lines manifested distinct behaviors in response to the drugs. In comparison to 32D-neo cells, 32D-c-myc cells were more sensitive to ADR, CPT, and VP-16. Depending on the concentration of drug used and the times at which apoptosis was assessed, these differences ranged between 3- and 20-fold. In contrast, 32D-max cells were indistinguishable from 32D-neo cells in response to all agents except CPT. In this case, 32D-max and 32D-c-myc cells showed equivalent degrees of increased sensitivity to the agent. From these studies we conclude that c-myc overexpression increases the chemotherapeutic sensitivity of 32D cells to ADR, CPT, and VP-16, whereas max overexpression increases the sensitivity of 32D cells only to CPT.
Susceptibility of 32 D-neo (□), 32D-max (○), and 32D-c-myc (◊) cells to six different antineoplastic agents in the presence of IL-3. Cells of >95% viability were plated in the presence of IL-3 and the indicated concentrations of drug as described in Materials and Methods. At various times thereafter, viability was determined by trypan blue staining of a 40-μL aliquot. The results shown here depict survival curves at 40 hours for ADR; 41 hours for CDDP, CPT, and N-Mus; 16 hours for Taxol; and 48 hours for VP-16. Each graph is representative of three or more experiments (±1 SE. The percent values shown have been normalized to those of cells grown in the absence of any drug for the equivalent length of time. The inserts show the results of electrophoresis of apoptotic DNAs from cells grown in the highest concentration of each drug for the times indicated above. All inserts show DNAs from 32D-neo, 32D-max cells, and 32D-c-myc (left to right).
Susceptibility of 32 D-neo (□), 32D-max (○), and 32D-c-myc (◊) cells to six different antineoplastic agents in the presence of IL-3. Cells of >95% viability were plated in the presence of IL-3 and the indicated concentrations of drug as described in Materials and Methods. At various times thereafter, viability was determined by trypan blue staining of a 40-μL aliquot. The results shown here depict survival curves at 40 hours for ADR; 41 hours for CDDP, CPT, and N-Mus; 16 hours for Taxol; and 48 hours for VP-16. Each graph is representative of three or more experiments (±1 SE. The percent values shown have been normalized to those of cells grown in the absence of any drug for the equivalent length of time. The inserts show the results of electrophoresis of apoptotic DNAs from cells grown in the highest concentration of each drug for the times indicated above. All inserts show DNAs from 32D-neo, 32D-max cells, and 32D-c-myc (left to right).
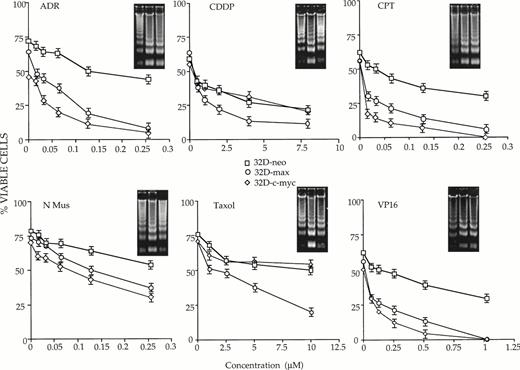
Additional differences among the three cell lines were evident when the above experiments were performed in the absence of IL-3 (Fig2). As expected, a more rapid rate of killing was observed in all cases due to the combined proapoptotic effects of IL-3 withdrawal and drug treatment. Most importantly, the removal of IL-3 did not reveal any new differential drug sensitivities in the 32D-c-myc cell line, which remained more sensitive than 32D-neo cells to killing only by ADR, CPT, and VP-16. In addition, the enhanced killing afforded by IL-3 depletion was additive rather than synergistic, with the differences in cell killing between the 32D-neo and 32D-c-myc cell lines remaining in the 3- to 10-fold range.
32D-neo (□), 32D-c-myc (◊), and 32D-max (○) cells were treated as described in Fig 1 except that they were also maintained in IL-3–depleted medium for the duration of the experiment. Viability was then determined at 18 hours for ADR, CPT, VP-16, and CDDP; 14 hours for N-Mus; and 16 hours for Taxol. The results shown here are the average of three experiments ±1 SE. Note that the viability of the cells in the absence of drug is not 100% due to cell death caused by the absence of IL-3. Viability was again scored by trypan blue exclusion. Apoptotic DNAs are again depicted as in Fig 1.
32D-neo (□), 32D-c-myc (◊), and 32D-max (○) cells were treated as described in Fig 1 except that they were also maintained in IL-3–depleted medium for the duration of the experiment. Viability was then determined at 18 hours for ADR, CPT, VP-16, and CDDP; 14 hours for N-Mus; and 16 hours for Taxol. The results shown here are the average of three experiments ±1 SE. Note that the viability of the cells in the absence of drug is not 100% due to cell death caused by the absence of IL-3. Viability was again scored by trypan blue exclusion. Apoptotic DNAs are again depicted as in Fig 1.
A much different type of behavior was observed in IL-3–deprived 32D-max cells where, in the absence of the cytokine, increased sensitivity to ADR, Taxol, and VP-16 was now observed in cells that previously were indistinguishable from control 32D-neo cells. A tendency for 32D-max cells to be more sensitive to CDDP was also seen but the differences were less than the twofold which we define as significant.
From the above studies, we conclude that IL-3 does not protect 32D-c-myc cells against killing by any of the agents tested, whereas the cytokine does protect 32D-max cells against Adriamycin, Taxol, and VP16-mediated cytotoxicity.
To confirm the results of many other groups that cell death resulting from IL-3 withdrawal and drug treatment was apoptotic in nature, total DNAs were extracted from each cell line and examined for evidence of apoptotic “laddering.” As shown in the insets in Figs 1 and 2, there was, in general, an excellent correlation between cell death determined by trypan blue exclusion and the degree of DNA fragmentation.
Cell cycle analyses.
One possible explanation for the observed differential effects on cell killing (Fig 1) is that c-myc and max altered the ability of 32D cells to undergo cell cycle arrest after treatment with chemotherapeutic drugs.32 33 Therefore, we determined the cell cycle distribution of each of the three cell lines either in the absence of any drug or after treatment with each of the six previously tested chemotherapeutic compounds. As seen in Table1, the overexpression of c-myc and max did, in some cases, alter the degree to which these agents caused cell cycle arrest. For example, in comparison with 32D-neo cells, both 32D-c-myc and 32D-max cells showed a reduced tendency to arrest in G0/G1 after treatment with ADR and CPT. However, in general there was no consistency between the ability of an agent to induce arrest at a particular phase of the cell cycle and the extent of the subsequent apoptotic response. From these results we conclude that although c-myc and max were clearly capable of influencing the efficiency of cell cycle arrest, this did not necessarily correlate with the ability of the cells to undergo subsequent apoptotic death.
Cell Cycle Profiles After Drug Exposure
| Cell Line . | Drug . | % of Cells in Phase . | ||
|---|---|---|---|---|
| G0/G1 . | S . | G2/M . | ||
| 1. 32D-neo | None | 48 | 30 | 22 |
| 2. 32D-c-myc | None | 45 | 31 | 24 |
| 3. 32D-max | None | 42 | 36 | 22 |
| 4. 32D-neo | ADR | 31 | 14 | 55 |
| 5. 32D-c-myc | ADR | 16 | 7 | 77 |
| 6. 32D-max | ADR | 22 | 8 | 70 |
| 7. 32D-neo | CDDP | 22 | 35 | 43 |
| 8. 32D-c-myc | CDDP | 26 | 25 | 49 |
| 9. 32D-max | CDDP | 39 | 36 | 25 |
| 10. 32D-neo | CPT | 32 | 13 | 55 |
| 11. 32D-c-myc | CPT | 9 | 16 | 75 |
| 12. 32D-max | CPT | 15 | 30 | 55 |
| 13. 32D-neo | N-Mus | 39 | 22 | 39 |
| 14. 32D-c-myc | N-Mus | 26 | 24 | 50 |
| 15. 32D-max | N-Mus | 36 | 32 | 32 |
| 16. 32D-neo | Taxol | 8 | 15 | 77 |
| 17. 32D-c-myc | Taxol | 7 | 14 | 79 |
| 18. 32D-max | Taxol | 16 | 21 | 63 |
| 19. 32D-neo | VP16 | 38 | 22 | 40 |
| 20. 32D-c-myc | VP16 | 41 | 27 | 32 |
| 21. 32D-max | VP16 | 50 | 23 | 27 |
| Cell Line . | Drug . | % of Cells in Phase . | ||
|---|---|---|---|---|
| G0/G1 . | S . | G2/M . | ||
| 1. 32D-neo | None | 48 | 30 | 22 |
| 2. 32D-c-myc | None | 45 | 31 | 24 |
| 3. 32D-max | None | 42 | 36 | 22 |
| 4. 32D-neo | ADR | 31 | 14 | 55 |
| 5. 32D-c-myc | ADR | 16 | 7 | 77 |
| 6. 32D-max | ADR | 22 | 8 | 70 |
| 7. 32D-neo | CDDP | 22 | 35 | 43 |
| 8. 32D-c-myc | CDDP | 26 | 25 | 49 |
| 9. 32D-max | CDDP | 39 | 36 | 25 |
| 10. 32D-neo | CPT | 32 | 13 | 55 |
| 11. 32D-c-myc | CPT | 9 | 16 | 75 |
| 12. 32D-max | CPT | 15 | 30 | 55 |
| 13. 32D-neo | N-Mus | 39 | 22 | 39 |
| 14. 32D-c-myc | N-Mus | 26 | 24 | 50 |
| 15. 32D-max | N-Mus | 36 | 32 | 32 |
| 16. 32D-neo | Taxol | 8 | 15 | 77 |
| 17. 32D-c-myc | Taxol | 7 | 14 | 79 |
| 18. 32D-max | Taxol | 16 | 21 | 63 |
| 19. 32D-neo | VP16 | 38 | 22 | 40 |
| 20. 32D-c-myc | VP16 | 41 | 27 | 32 |
| 21. 32D-max | VP16 | 50 | 23 | 27 |
The indicated cell lines, in log-phase growth, were exposed for 16 hours to ADR (0.125 μmol/L), CDDP (1 μmol/L), CPT (0.125 μmol/L), N-Mus (0.1 μmol/L), Taxol (0.5 μmol/L), or VP16 (0.1 μmol/L). Nuclei were then stained with propidium iodide, and their cell cycle distribution determined by fluorescence-activated cell sorting as previously described.18 Preliminary experiments indicated that the distributions shown were reached by 12 hours after drug exposure and did not change significantly before the onset of detectable apoptosis. None of the conditions used here induced more than 5% to 10% apoptosis as determined by the presence of subdiploid nuclear populations and trypan blue staining.
Differential effects of Bcl-2 and Bcl-XL on c-myc– and max-mediated apoptosis.
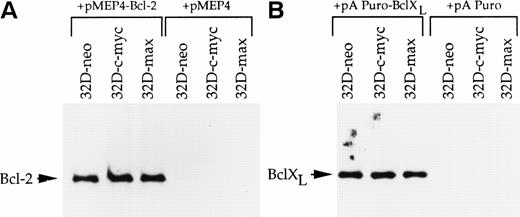
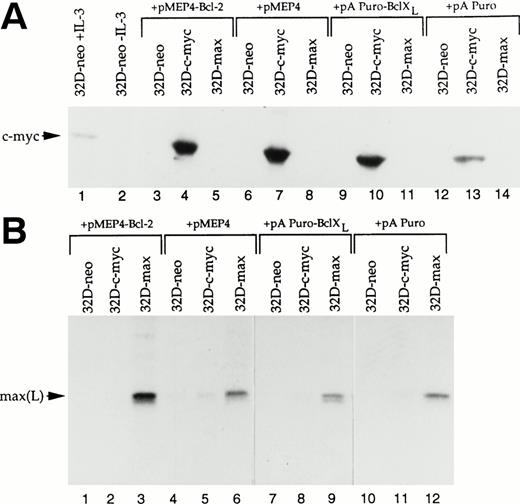
Both Bcl-2 and the related protein Bcl-XL exert protective effects against a wide variety of apoptotic stimuli including cytokine deprivation and chemotherapeutic agents.34-39 In addition, Bcl-2 overexpression has been shown to protect several different cell types against the accelerated apoptosis mediated by c-myc after cytokine withdrawal.11-13 To investigate the role of each of these proteins in abrogating apoptosis after IL-3 deprivation or drug treatment, 32D-neo, 32D-c-myc, and 32D-max cells were transfected with expression vectors for Bcl-2, Bcl-XL, or the corresponding parental vectors alone. Pools of transfected clones were again used to ensure that any observed responses were not the result of clonal variability. Western blotting experiments confirmed that each protein was expressed at equivalent levels in all three cell lines (Fig3).
Western blot analysis of 32D cell lysates. (A) 32D-neo, 32D-c-myc, and 32D-max cells were electroporated with either the linearized pMEP4-Bcl-2 expression vector or the empty pMEP4 parental vector. Stable clones were selected in hygromycin and pooled. From each cell line 50 μg of total cell lysate was subjected to SDS-PAGE and Western blotting using an anti–Bcl-2 antibody. (B) 32D-neo, 32D-c-myc, and 32D-max cell lines were stably transfected with either the pAPuro-Bcl-XL vector or the pAPuro parental vector. SDS-PAGE and Western blotting were performed as described in (A) except that an anti–Bcl-X antibody was used.
Western blot analysis of 32D cell lysates. (A) 32D-neo, 32D-c-myc, and 32D-max cells were electroporated with either the linearized pMEP4-Bcl-2 expression vector or the empty pMEP4 parental vector. Stable clones were selected in hygromycin and pooled. From each cell line 50 μg of total cell lysate was subjected to SDS-PAGE and Western blotting using an anti–Bcl-2 antibody. (B) 32D-neo, 32D-c-myc, and 32D-max cell lines were stably transfected with either the pAPuro-Bcl-XL vector or the pAPuro parental vector. SDS-PAGE and Western blotting were performed as described in (A) except that an anti–Bcl-X antibody was used.
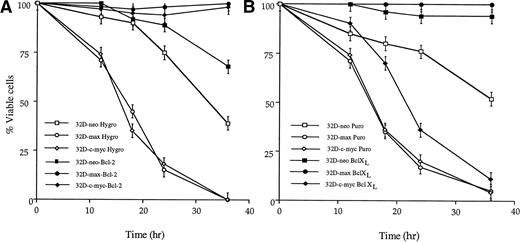
We first examined the effects of enforced Bcl-2 and Bcl-XLexpression on apoptosis in response to IL-3 withdrawal. As shown in Fig4A, overexpression of Bcl-2 conferred nearly complete protection against cell death in all three cell lines. In contrast, whereas overexpression of Bcl-XL also protected 32D-neo and 32D-max cells, it provided minimal protection (<twofold) against apoptosis in 32D-c-myc cells (Fig 4B). From these results, we conclude that Bcl-XL exerts a more restrictive antiapoptotic effect in these cell lines.
Effects of Bcl-2 and Bcl-XL overexpression on apoptosis in response to IL-3 withdrawal. Bcl-2–overexpressing (A) or Bcl-XL–overexpressing (B) 32D cell lines, or their vector-transfected control counterparts, were washed free of IL-3 and incubated in fresh, IL-3–depleted medium for the times indicated. The fraction of viable cells at each point was determined as in Figs 1 and2. The results shown are representative of triplicate experiments ±1 SE.
Effects of Bcl-2 and Bcl-XL overexpression on apoptosis in response to IL-3 withdrawal. Bcl-2–overexpressing (A) or Bcl-XL–overexpressing (B) 32D cell lines, or their vector-transfected control counterparts, were washed free of IL-3 and incubated in fresh, IL-3–depleted medium for the times indicated. The fraction of viable cells at each point was determined as in Figs 1 and2. The results shown are representative of triplicate experiments ±1 SE.
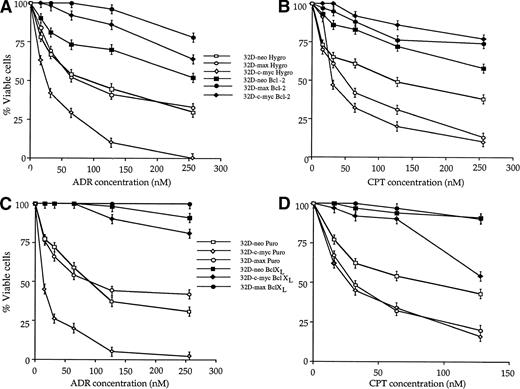
The above cell lines were also used to determine whether Bcl-2 and Bcl-XL could protect 32D cells against apoptosis mediated by chemotherapeutic agents. As shown in Fig5, both Bcl-2 and Bcl-XLprotected all three cell lines against both ADR and CPT. In some cases, the protection afforded was complete; for example, Bcl-2 and Bcl-XL completely spared 32D-max cells. In other cases such as 32D-c-myc cells exposed to CPT, only exposure to low doses of drug were associated with a high degree of protection.
Effects of Bcl-2 and Bcl-XL on drug-mediated apoptosis in 32D cell lines. 32D-neo, 32D-c-myc, and 32D-max cells were stably transfected with Bcl-2 or Bcl-XL expression plasmids or with the respective empty parental vectors. The resulting pooled clones were then tested for their ability to undergo apoptosis in response to treatment with either ADR or CPT. (A) Effects of ectopic Bcl-2 expression on the response to ADR treatment. The results shown (±1 SE) are those obtained after a 42-hour exposure to the indicated concentrations of the drug. (B) Effects of ectopic Bcl-2 expression in response to a 48-hour exposure to the indicated concentrations of CPT. (C) Effects of ectopic Bcl-XL expression in response to a 48-hour exposure to the indicated concentrations of CPT. The results shown are representative of three experiments ±1 SE.
Effects of Bcl-2 and Bcl-XL on drug-mediated apoptosis in 32D cell lines. 32D-neo, 32D-c-myc, and 32D-max cells were stably transfected with Bcl-2 or Bcl-XL expression plasmids or with the respective empty parental vectors. The resulting pooled clones were then tested for their ability to undergo apoptosis in response to treatment with either ADR or CPT. (A) Effects of ectopic Bcl-2 expression on the response to ADR treatment. The results shown (±1 SE) are those obtained after a 42-hour exposure to the indicated concentrations of the drug. (B) Effects of ectopic Bcl-2 expression in response to a 48-hour exposure to the indicated concentrations of CPT. (C) Effects of ectopic Bcl-XL expression in response to a 48-hour exposure to the indicated concentrations of CPT. The results shown are representative of three experiments ±1 SE.
It was possible that the overexpression of Bcl-2 or Bcl-XLaltered the levels of transfected c-myc or max proteins, either by directly altering their in vivo half-lives, or by affecting the rates of transcription of their mRNAs. To examine this, we compared the levels of c-myc and max proteins in 32D-neo, 32D-c-myc, and 32D-max with the levels in the same cell lines transfected with either Bcl-2 or Bcl-XL expression vectors. As seen in Fig6, neither Bcl-2 nor Bcl-XLsignificantly affected the levels of c-myc or max proteins. In comparison to endogenous c-myc, all cell lines derived from 32D-c-myc expressed 2 to 3 times as much exogenous protein. As expected, the levels of c-myc in each of the 32D-c-myc cell lines were unaffected by the removal of IL-3 (Fig 6A). In the case of cultures derived from the starting 32D-max cell line, all expressed 5 to 10 times more max protein in comparison to untransfected controls (Fig 6B). The levels of max in these lines were also unaffected by the coexpression of Bcl-2 or Bcl-XL.
Levels of c-myc and max proteins in 32D cell lines. (A) c-myc levels. From the indicated cell lines, 50 μg of protein was resolved by SDS-PAGE, Western blotted, and probed with a polyclonal anti–c-myc antibody.40 Lane 1 shows the level of endogenous c-myc protein in logarithmically growing 32D-neo cells. Lane 2 shows the disappearance of c-myc in the same cells after a 6-hour deprivation of IL-3. In lanes 3 through 14, the indicated cultures were deprived of IL-3 for 6 hours to deplete endogenous c-myc stores. Note the complete absence of endogenous c-myc protein in IL-3–deprived 32D-neo and 32D-max cells. Lanes 4, 7, 10, and 13 show that c-myc arising from the transfected expression plasmid was expressed at levels 2 to 3 times that of endogenous c-myc and was not downregulated after IL-3 deprivation. (B) max levels. The indicated cell lines were labeled with 35S-Translabel as previously described.18 After lysis in triple detergent radioimmunoprecipitation buffer, max proteins were immunoprecipitated with a polyclonal rabbit anti-max antibody, resolved by SDS-PAGE, and subjected to autoradiography. The arrow indicates the position of endogenous and overexpressed max(L).
Levels of c-myc and max proteins in 32D cell lines. (A) c-myc levels. From the indicated cell lines, 50 μg of protein was resolved by SDS-PAGE, Western blotted, and probed with a polyclonal anti–c-myc antibody.40 Lane 1 shows the level of endogenous c-myc protein in logarithmically growing 32D-neo cells. Lane 2 shows the disappearance of c-myc in the same cells after a 6-hour deprivation of IL-3. In lanes 3 through 14, the indicated cultures were deprived of IL-3 for 6 hours to deplete endogenous c-myc stores. Note the complete absence of endogenous c-myc protein in IL-3–deprived 32D-neo and 32D-max cells. Lanes 4, 7, 10, and 13 show that c-myc arising from the transfected expression plasmid was expressed at levels 2 to 3 times that of endogenous c-myc and was not downregulated after IL-3 deprivation. (B) max levels. The indicated cell lines were labeled with 35S-Translabel as previously described.18 After lysis in triple detergent radioimmunoprecipitation buffer, max proteins were immunoprecipitated with a polyclonal rabbit anti-max antibody, resolved by SDS-PAGE, and subjected to autoradiography. The arrow indicates the position of endogenous and overexpressed max(L).
DISCUSSION
Numerous reports have established that deregulated c-myc expression promotes apoptosis in various settings, including those resulting from serum deprivation of primary fibroblasts and the removal of cytokines from hematopoietic cells.5,6,10 c-myc overexpression has also been reported to alter the sensitivities of hematopoietic cell lines to select chemotherapeutic agents.31,41 Recently, we have shown that overexpression of the 160 amino acid isoform of max, the obligate c-myc heterodimeric partner, can also promote apoptosis in NIH 3T3 fibroblasts and in 32D myeloid cells in response to serum or IL-3 deprivation, respectively.18 The proapoptotic effect of max is quite strong and rivals or even exceeds that of c-myc.
Despite the similar proapoptotic phenotypes imparted to 32D cells by c-myc and max, several indirect lines of evidence suggest that they arise through distinct pathways. For example, although it has been reported that apoptosis mediated by c-myc requires dimerization with max,7 the converse does not seem to be true. This is based on our observations that after the removal of IL-3 from all 32D cell lines, the levels of endogenous c-myc transcripts and protein rapidly decline to undetectable levels within 3 to 6 hours, long before apoptosis becomes evident (Fig 6A) (H.Z. and E.V.P., unpublished observations).
In the current work, the differential apoptotic responses of 32D-c-myc and 32D-max cells to chemotherapeutic agents lend further support to the notion that c-myc and max operate through separate, although possibly overlapping, biochemical pathways (Fig 2). The ability of IL-3 to protect 32D-max cells against all agents tested (except CPT) is not seen in the case of 32D-c-myc cells. Instead, these behave much like control 32D-neo cells on which the effects of drug treatment and IL-3 deprivation are additive. More direct evidence to support the existence of distinct c-myc and max apoptotic pathways is provided by our observation that they are differentially affected by the overexpression of Bcl-2 and Bcl-XL. Whereas Bcl-2 completely protected all cell lines against the proapoptotic effects of both IL-3 deprivation and drug treatment, Bcl-XL provided virtually no protection against IL-3 removal in 32D-c-myc cells, despite protecting them as well as Bcl-2 against drug-mediated apoptosis.
In 32D-c-myc and 32D-max cells, cellular demise in response to IL-3 withdrawal can be viewed as the sum of two apoptotic events, the first of which, the “basal” event, is that provided by IL-3 removal itself (and defined as that occurring in control 32D-neo cells), and the second of which is that imposed by overexpressed c-myc or max (the “c-myc” or “max” event). The inability of Bcl-XL to provide significant protection against apoptosis after IL-3 removal in 32D-c-myc cells suggests that the overexpression of c-myc affects the basal event in such a way as to make it unresponsive to what should otherwise be a fully protective antiapoptotic stimulus.
Perhaps the most important outcome of this study is that it now provides us with the ability to order Bcl-2 and Bcl-XLalong the c-myc and max apoptotic pathways. Thus, in the simplest of models, Bcl-2 exerts its effect downstream of both c-myc and max to protect against apoptosis after either IL-3 withdrawl or drug treatment. Similarly, Bcl-XL seems to function downstream of c-myc and max to prevent apoptosis in response to cytotoxic drug treatment. In contrast, Bcl-XL appears to act downstream of max but upstream of c-myc in cells deprived of IL-3. Although a number of biochemical and biological properties of Bcl-2 and Bcl-XL have been described,21,38,39 42-44 it is not yet clear which of these, if any, is important for the apoptotic events we have studied here. Nevertheless, this work provides a framework within which it should be possible to use these and other modulators of apoptosis to begin to dissect the cell death pathways used by c-myc, max, and other members of the c-myc oncoprotein family.
ACKNOWLEDGMENT
We are grateful to Yusuf Hannun, Dan Johnson, and Craig Thompson for providing plasmids; to Dan Johnson for advice; and to Don Wojchowski for reading the manuscript.
Supported by NIH Grant No. HL33741 to E.V.P.
Address reprint requests to Edward V. Prochownik, MD, PhD, Section of Hematology/Oncology, Children's Hospital of Pittsburgh, 3705 Fifth Ave, Pittsburgh, PA 15213; e-mail: edward_prochownik@poplar.chp.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal