Abstract
The development of an immune response towards factor VIII (fVIII) remains a major complication for hemophilia A patients receiving fVIII infusions. The design of a specific therapy to restore unresponsiveness to fVIII has been hampered by the diversity of the anti-fVIII antibody. Molecular analysis of the specific immune response is therefore required. To this end, we have characterized an fVIII-specific human IgG4κ monoclonal antibody (BO2C11) produced by a cell line derived from the memory B-cell repertoire of a hemophilia A patient with inhibitor. BO2C11 recognizes the C2 domain of fVIII and inhibits its binding to both von Willebrand factor (vWF) and phospholipids. It completely inhibits the procoagulant activity of native and activated fVIII, with a specific activity of approximately 7,000 Bethesda units/mg. vWF reduces the rate of fVIII inactivation by BO2C11. The antibody-fVIII association rate constant (kass ∼7.4 × 105M−1 s−1) is eightfold lower than that for vWF-fVIII association, whereas its dissociation rate constant (kdiss ≤1 × 10−5s−1) is 100-fold lower than that for the vWF-fVIII complex, which suggests that BO2C11 almost irreversibly neutralizes fVIII after its dissociation from vWF. BO2C11 is the first human monoclonal anti-fVIII IgG antibody that has been isolated and allows the study of fVIII inactivation at the molecular level.
HEMOPHILIA A IS AN X-linked bleeding disorder characterized by the absence or an insufficient amount of functional factor VIII (fVIII). The deficiency affects 1 in 10,000 males and results in bleeding in joints, muscles, and soft tissues. fVIII replacement therapy relies on the use of concentrates prepared from plasma or of recombinant fVIII (rfVIII). However, in both cases, such a treatment can trigger the production of specific antibodies,1,2 in particular in patients with severe forms of the disease.3-5 Circulating antibodies often preclude further use of human fVIII, because they immediately neutralize its function. Such patients, therefore, find themselves in a life-threatening situation for which there is no current specific treatment.
fVIII is a 330-kD glycoprotein produced by the liver as a single polypeptide chain of 2,332 amino acids that subsequently undergoes proteolytic processing.6 The circulating molecule consists of two chains. The heavy chain is constituted of the A1 and A2 domains and variable lengths of the B domain; the light chain contains the A3 domain and the C1 and C2 domains. fVIII contains a phospholipid (PL) binding site in the C2 domain, between amino acids 2302 and 2332.7,8 Within the same fVIII region, there is also a von Willebrand factor (vWF) binding site, which acts in conjunction with amino acid residues 1649 through 1689 in the A3 domain.9-11fVIII circulates complexed to vWF that protects fVIII from rapid degradation in plasma.12 Upon cleavage by thrombin, activated fVIII (fVIIIa) dissociates from vWF,13 binds to negatively charged PL, and participates as a cofactor to factor IXa in the factor X activating (tenase) complex.
The human immune response towards fVIII is highly heterogeneous.14,15 It is made of antibodies belonging to almost any isotype, with a preferential involvement of IgG4 antibodies.16 However, three major clusters of B-cell epitopes have been delineated using native or rfVIII fragments or mouse monoclonal antibodies (MoAbs) of defined specificity. Thus, heavy chain-specific inhibitors react primarily with the 18.3-kD amino-terminal segment of the A2 domain.15,17 Light chain-specific inhibitors recognize epitopes in the A3 domain18 and a major antigenic region in the C2 domain.9,10,19,20 Binding of polyclonal human fVIII antibodies to the C2 domain inhibits the fVIII procoagulant activity by either preventing the binding of fVIII to PL7 or reducing the dissociation rate of fVIII from vWF.21 In addition, antibodies binding to the carboxy-terminal end of the C2 domain can prevent fVIII binding to vWF.9-11 Interestingly, a proportion of anti-fVIII antibodies do not inhibit the procoagulant function of the molecule,18 but could possibly play a role in the clearance of fVIII from the circulation.
The complex interactions between fVIII, PL, vWF, and specific antibodies are far from being elucidated due to the heterogeneous nature of the human anti-fVIII antibody response. However, understanding these interactions in more detail may provide the molecular basis for the development of new forms of therapy. Thus, we and others have proposed that specific suppression of the production of certain anti-fVIII antibodies could possibly be achieved by anti-idiotypic antibodies.22-25 It has also been suggested that PL or vWF could efficiently compete with inhibitors for fVIII binding26-28 and that such a property could be exploited for replacement therapy with fVIII concentrates.
To get further insight into the mechanisms of fVIII neutralization and to establish rational grounds for a specific therapy of fVIII inhibitors, it was deemed necessary to analyze the human immune response to fVIII at the clonal level. We have therefore generated human MoAbs (hu-MoAbs) from cell lines derived from the B-memory cell repertoire of hemophilia A patients with inhibitors. We report here on the first of such antibodies, selected on the basis of its capacity to interfere with fVIII binding to both vWF and PL.
MATERIALS AND METHODS
Reagents and Buffers
Human rfVIII (specific activity, 4,000 IU/mg) was obtained from Hyland (Glendale, CA) as material for laboratory use only; plasma-derived fVIII-vWF complex, purified by ion exchange chromatography (specific activity, ±160 IU/mg protein; 15:1 vWF to fVIII wt/wt ratio), and purified fVIII-depleted vWF (lot 951016; vWF to fVIII wt/wt ratio 4,800:1) were obtained from the Belgian Red Cross (Brussels, Belgium); human rfVIII light and heavy chains were obtained by courtesy of Dr Mirella Ezban (Novo Nordisk, Copenhagen, Denmark); anti-fVIII mouse MoAbESH829 was obtained by courtesy of Dr Duncan Pepper (Edinburgh, UK), but is now commercialized by American Diagnostica Inc (Greenwich, UK). The anti-CD40 mouse MoAb8930 was a kind gift of J. Banchereau (Schering-Plough Research Institute, Dardilly, France). The fVIII-light chain specific mouse MoAb7,18mouse MoAbF15B12 towards fVIII A2 domain, and MoAb4H1D7 towards vWF (Arnout and Hoylaerts, unpublished data) were produced in our laboratory. Recombinant hirudin was a kind gift of Dr P. Close (Ciba-Geigy, Basel, Switzerland).
The following reagents were purchased: Caryoser from Biosepra (Villeneuve-la-Garenne, France); casein from Aldrich Chemicals (Milwaukee, WI); Ficoll-Hypaque from Nycomed Pharma AS (Oslo, Norway); Geneticin from Life Technologies (Bethesda, MD); human serum albumin (HSA) from the Belgian Red Cross; L-glutamin from Merck (Darmstadt, Germany); N-hydroxysuccinimide-LC (NHS) biotin from Pierce (Rockford, IL); ortho-phenylenediamine (OPD), L-α-phosphatidyl-L-serine (PS) prepared from bovine brain, and thimerosal from Sigma Chemical Co (St Louis, MO); and Tween-20 from Technicon (Tarrytown, NY).
The following buffers were used: glycine-buffered saline (GBS; 20 mmol/L glycine, 34 mmol/L NaCl, pH 9.2); HEPES-buffered saline (HBS; 10 mmol/L HEPES, pH 7.4, containing 300 mmol/L NaCl, 5 mmol/L CaCl2, and 0.05% Tween-20); imidazole-BSA (20 mmol/L imidazole, containing 150 mmol/L NaCl, 10 mmol/L CaCl2, and 1% bovine serum albumin); phosphate-buffered saline (PBS; 140 mmol/L NaCl, 67 mmol/L KCl, 20 mmol/L Na2HPO4, 4.4 mmol/L KH2PO4, pH 7.4); PBS-BSA (PBS containing 0.5% BSA); PBS-BSA 5% (PBS containing 5% BSA); PBS-Tween (PBS containing 0.1% Tween-20); Tris [10 mmol/L tris(hydroxymethyl)-aminomethane, pH 7.3, containing 150 mmol/L NaCl]; Tris-casein (Tris containing 0.5% casein and 0.02% thimerosal, pH 7.2); Tris-BSA (Tris containing 0.5% BSA; Tris-Tween (Tris containing 0.05% Tween-20).
Human Peripheral Blood Lymphocytes and Cell Lines
The LCD32 cell line stably expressing human FcγRII/CD3231was obtained from the American Type Culture Collection (Rockville, MD) by permission of J. Banchereau. The 3T6 cell line was stably transfected with an expression vector for human CD40 ligand (3T6-TRAP).32 Blood was collected after informed consent from a hemophilia A patient (BO) with inhibitor. This 49-year-old patient had an inhibitor to fVIII for more than 10 years, with titers ranging from 36 to 2,200 Bethesda units (BU). At the start of the study, the inhibitor titer was 575 BU. Peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll-Hypaque density centrifugation using standard methods.
All cell cultures were performed in Dulbecco's Modified Eagle Medium/Nutrient Mix F12 (Life Technologies) supplemented with 10% IgG-free horse serum, 1.5 g/L glucose, 4 mmol/L L-glutamine, 1% Caryoser, and 80 mg/L Geomycin. The 3T6-TRAP and LCD32 cell lines were maintained in culture medium supplemented with 200 μg/mL Geneticin.
Immortalization of Human PBMCs
PBMCs were immortalized according to described procedures.30 33 Briefly, 107 PBMCs were resuspended in 2 mL culture medium and incubated for 2 hours at 37°C with 200 μL Epstein-Barr virus (EBV) supernatant (B95-8 strain). Cells were then seeded at 300 to 24,000 cells/well in 96-well microtiter plates (Nunc, Roskilde, Denmark) containing LCD32 cells and 0.5 μg/mL anti-CD40 MoAb89. LCD32 cells had been irradiated (7,000 rads) or treated with mitomycin C (50 μg/mL) for 1 hour at 37°C and seeded in culture wells the day before EBV infection of PBMCs. Alternatively, mitomycin C-treated 3T6-TRAP cells were used as feeders instead of LCD32 cells. One hundred fifty microliters of culture supernatant was replaced every week by fresh culture medium. After 4 to 8 weeks, depending on growth rate in individual wells, culture supernatants were tested in enzyme-linked immunosorbent assay (ELISA) for the presence of anti-fVIII antibodies. Positive cell lines were transferred to 24-well plates and immediately cloned at 60 cells per 96-well plate without feeder cells.
Sequencing of Ig Rearranged Genes
The isolation of RNA from EBV-immortalized human B-cell lines was performed using TRIzol Reagent according to the manufacturer's instructions (Life Technologies). cDNA was synthesized with the SuperScript preamplification system for first-strand cDNA synthesis. The cDNA encoding the heavy chain variable region genes (VH) was amplified by polymerase chain reaction (PCR) using primers specific for the leader sequence of the VH families and for the first exon of the Cγ region, as described.34Annealing was performed at 60°C for 40 PCR cycles. PCR products of the appropriate size (460 bp) were isolated from 1.5% agarose gel and cloned using the TA Cloning Kit (Invitrogen BV, Leek, The Netherlands). A PCR screening using couples of primers corresponding to the VH gene family of interest was performed on cultures of randomly selected colonies. Plasmid DNA from positive colonies was isolated using Wizard Plus Minipreps (Promega, Menlo Park, CA) and sequenced in both directions with Sequenase (US Biochemical, Cleveland, OH), according to the manufacturer's instructions. Analysis of the variable gene sequences was made using the V BASE Sequence Directory (Tomlinson et al, MRC Centre for Protein Engineering, Cambridge, UK). The complete sequences of the VH and VL were submitted to the EMBL Nucleotide Sequence Database under the accession numbers AJ224083 and AJ224084, respectively.
Purification of Human IgG
Human MoAbs were purified by adsorption on immobilized protein A. One hundred milliliters of cell culture supernatant was passed through a high-TRAP protein A (Pharmacia, Uppsala, Sweden) at a flow rate of 1 mL/min. Bound IgG was eluted with citric acid 100 mmol/L, pH 3. After pH neutralization with Tris, pH 9, IgG was dialyzed against 150 mmol/L NaCl. The concentration of proteins was determined with the Bio-Rad assay (Bio-Rad, Hercules, CA). The purity of the final preparation was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 8% gel and unreduced proteins followed by Coomassie-blue staining, showing one major band corresponding to unreduced IgG and two minor bands at 45 and 60 kD, respectively. The IgG band represented 80% of the total protein content as determined using a Du-68 Spectrophotometer (Beckman-Coulter Inc, Fullerton, CA). Human polyclonal anti-fVIII antibodies were prepared from plasma by salt precipitation and gel filtration chromatography, as previously described.18
Recombinant DNA Fragments
DNA fragments encoding fVIII amino acid residues 1981-2222 (exons 19-24) and 2125-2332 (exons 23-26) were generated by PCR using primers bound by the restriction sites BamHI and NotI.10 Primers corresponding to fVIII amino acid residues 1981-2222 were 5′-CGTGGTGGATCCTCTGCAGACGGTGTTTTTGAGACAGTGGAAATG-3′ and 5′-TTCTCGACTTGCGGCCGCCTGAGGTCTCCAGGCATTACTCCTC-3′. Those corresponding to fVIII amino acid residues 2125-2332 were 5′-CGTCGTGGATCCTCTGCAGACGTCTTCTTTGGCAATGTGGATTCA-3′ and 5′-TTCTCGACTTGCGGCCGCGTAGAGGTCCTGTGCCTCGCAGCC-3′. Primers for fVIII 2125-2303 amino acid residues were 5′-CGTCGTGGATCCTCTGCAGACGTCTTCTTTGGCAATGTGGATTCA-3′ and 5′-TTCTCGACTTGCGGCCGCAGTCAGTAACGGTGGGTCTAGAGA-3′. The 5′ primer for the fragment corresponding to residues 2216-2332 was 5′-TCGAAACTAATACGACTCACTATAGGGAAGATGAAGCTTGGATCCAGTAATGCCTGGAGACCTCAGGTG-3′. The 5′ primer for the C2 domain gene (residues 2170-2332) with a site directed mutation coding for a glycine residue in place of the cysteine residue 2174 was 5′-TCGAAACTAATACGACTCACTATAGGGAAGATGAAGCTTGGATCCGATTTAAATAGTGGCAGCATGCCA-3′. The fragments were cloned in frame in pGEX4T-2 (Pharmacia) and controlled by sequencing in both directions with the T7 Sequencing kit (Pharmacia). The glutathione S-transferase (GST) fusion proteins were expressed in the DH5α Escherichia coli strain. Fusion proteins were solubilized with 1.5% Sarkosyl (Sigma) and 2% Triton X-100 and purified on a glutathione column, as described.35Purified proteins were evaluated by ELISA, SDS-PAGE, and Western blotting. The presence in the chimeric proteins of amino acid residues 2108-2121 or 2248-2312 was controlled using a rabbit polyclonal antiserum directed to fVIII amino acid residues 2108-2121 of the C1 domain (kind gift of M. Di Giambattista, Belgian Red Cross, Brussels, Belgium) or the anti-C2 MoAbESH8,21 29respectively.
Immunoassays
Detection of anti-fVIII antibodies.
Plasma-derived fVIII was insolubilized on microtitration plates through binding on a specific MoAb. Thus, plates were incubated overnight with 50 μL GBS containing 5 μg/mL of MoAb7.18 The plates were washed 4 times with PBS-Tween before the addition of 50 μL of plasma-derived fVIII diluted at 5 μg/mL in Tris-casein. Alternatively, 50 μL of rfVIII (1 μg/mL) was used as a substitute to plasma-derived fVIII. In some experiments, rfVIII was insolubilized by incubating plates for 2 hours at 4°C directly with 50 μL of rfVIII (1 μg/mL) diluted in GBS. The plates were washed as described above and 50 μL of culture supernatant was added for a further incubation of 2 hours at 4°C. After washing, 50 μL peroxidase-labeled antihuman Fcγ goat IgG (Sigma) diluted 1,000-fold in Tris-casein were added. After 2 hours at RT, the plates were washed again and supplemented with 100 μL OPD. The resulting OD was read at 492 nm in a Emax Microplate Reader (Molecular Devices, Menlo Park, CA). Negative and positive controls were culture medium and IgG purified from a high-titer inhibitor hemophilia A patient, respectively.
IgG subclass determination.
IgG subclasses in cell line supernatants were determined as described.18 Briefly, polystyrene microtitration plates (Nunc) were incubated for 2 hours at RT with 50 μL of an antimouse IgG1 rat MoAb (UCL, Brussels, Belgium) at a concentration of 5 μg/mL in GBS. After each step, the plates were washed four times with Tris-Tween. Fifty microliters of a mouse IgG1 MoAb diluted to 0.5 μg/mL in PBS-BSA and specific for either human IgG1 (Oxoid Ltd, Basingstoke, UK), IgG2, or IgG3 (Calbiochem, La Jolla, CA) was added and the plates were incubated for 2 hours at RT. For the evaluation of IgG4, 50 μL of a specific mouse MoAb (Calbiochem), diluted at a concentration of 5 μg/mL in GBS, was incubated directly on the plate for 2 hours at RT. The plates were washed and 50 μL of culture supernatant or of a calibrated reference serum diluted in PBS-BSA were added for an incubation of 2 hours at RT. Human antibody binding was detected by the addition of 50 μL peroxidase-labeled antihuman Fcγ goat IgG (Sigma) diluted 1,000-fold in Tris-casein. After 2 hours at RT, the plates were treated with OPD and read as described above.
The IgG subclass was confirmed by a second method in which the procedure used above to detect anti-fVIII antibodies after binding to fVIII-coated plates (see section “Detection of anti-fVIII antibodies”) was modified by substituting goat IgG specific for human Fcγ by subclass-specific mouse MoAbs. MoAb binding was then detected by addition of 50 μL of an antimouse Fcγ goat IgG conjugated with peroxidase (Sigma) and diluted 1,000-fold in Tris-casein, followed by washing and addition of OPD. All samples were assayed in duplicates.
Inhibition of fVIII binding to PS.
Antibodies interacting with the binding of fVIII to PS were detected using an assay system adapted from described methods.7 36Polystyrene plates (Maxisorb; Nunc) were incubated with 100 μL of PS dissolved at 5 μg/mL in methanol and allowed to dry overnight at RT. The plates were then saturated by incubation with 200 μL PBS-BSA 5% for 1 hour at RT. Fifty microliters of a 4 μg/mL rfVIII solution made in Tris-BSA were mixed with 50 μL of purified hu-MoAb diluted in the same buffer. The mixture was incubated for 30 minutes at 37°C and then added to the plate in 50-μL aliquots for a further incubation of 2 hours at RT. After washing with Tris-Tween, horseradish peroxidase (HRP)-coupled anti-A2 MoAbF15B12 was added at a 1/1,000 dilution in Tris-BSA for a further 1 hour of incubation. OPD was then added and the OD was measured at 492 nm. Negative controls included an hu-MoAb of irrelevant specificity. The concentration of fVIII used in this assay was just below the plate-saturating concentration and gave the highest signal to noise ratio.
Inhibition of fVIII binding to vWF.
Microtitration plates were incubated overnight at 4°C with the anti-vWF MoAb4H1D7 diluted at 4 μg/mL in GBS. The plates were then blocked with 120 μL of PBS-BSA 5% for 1 hour at RT. After washing the plates with Tris-Tween, 50 μL of a normal human plasma pool diluted 1/20 in the same buffer was added for 1 hour at RT. The plates were washed 3 times with PBS-Tween and then incubated for 30 minutes with 50 μL of 400 mmol/L CaCl2 to detach fVIII from vWF. Fifty microliters of rfVIII diluted at 0.4 μg/mL in PBS-BSA was mixed with 50 μL of purified hu-MoAb made at different dilutions in the same buffer. The mixture was incubated for 30 minutes at 37°C before adding a 50-μL aliquot to the plate for 2 hours of incubation at RT. After washing with PBS-Tween, bound fVIII was detected as in the PS assay described above. In preliminary experiments, fVIII bound to insolubilized vWF was detected by the addition of HRP-coupled mouse MoAb F15B12 before and after treatment of the plates with 400 mmol/L CaCl2. Such a treatment reduced absorbency by more than 90%, showing effective removal of fVIII from vWF.
Effect of vWF on fVIII binding to BO2C11.
Microtiter plates were coated with BO2C11 (2 μg/mL) in GBS and blocked as in previously described assays. rfVIII (0.2 μg/mL) in Tris-BSA 5% was preincubated for 30 minutes at 37°C in the presence or absence of vWF (1 to 20 μg/mL) before addition to BO2C11-coated plates. After an incubation of 5 minutes at 37°C, the wells were washed and the binding of fVIII to BO2C11 was detected as described above for inhibition of fVIII binding to PS by addition of the anti-A2 MoAb F15B12 for 1 hour at 4°C. The amount of fVIII bound to BO2C11 was determined by comparing the OD with those of a calibration curve established with known amounts of fVIII. To establish a calibration curve, 50 μL of different concentrations of rfVIII (0.2 to 200 ng/mL) were incubated at 4°C in microtitration plates coated with BO2C11. Supernatants were removed after 2 hours of incubation and the anti-A2 MoAb F15B12 was added to the wells for 1 hour at 4°C followed by OPD. Residual fVIII activity in the supernatants was determined in an fVIII chromogenic assay as described below. The quantity of bound fVIII was calculated by subtracting unbound fVIII from the total amount of fVIII added to wells. The low dissociation rate constant of fVIII from BO2C11 (see below) allowed us to relate the levels of fVIII captured by BO2C11, determined in the fVIII chromogenic assay, to the optical densities, recorded in ELISA after the addition of the anti-A2 MoAb F15B12.
Antibody-dependent dissociation of fVIII-vWF complex.
Microtitration plates were coated with anti-vWF MoAb4H1D7 and blocked by incubation with TBS-BSA. Fifty microliters of plasma-derived fVIII-vWF (15:1 vWF to fVIII wt/wt ratio), diluted at 1 IU/mL in Tris-BSA, was mixed with 50 μL of the same buffer containing or not containing human IgG (1 to 10 μg/mL). The mixture was preincubated for 1 hour at 37°C and a 50-μL aliquot was added to the plate for a further incubation of 2 hours at 4°C. After washing with Tris-Tween, bound fVIII was detected as in the inhibition of fVIII binding to PS. ODs recorded after the addition of OPD were used to determine the amount of fVIII bound to vWF, by comparison with a calibration curve obtained with known amounts of fVIII bound to vWF. To establish the calibration curve, 50 μL of different concentrations of plasma-derived fVIII-vWF (0.2 to 200 ng/mL) was incubated at 4°C in microtitration plates coated with MoAb4H1D7. After 2 hours of incubation at 4°C, the supernatants were removed and the anti-A2 MoAb F15B12 was added to the wells for 1 hour at 4°C. Bound fVIII was detected by the addition of OPD or in a functional assay. In the latter case, 30 μL of imidazole-BSA was added to the wells, and fVIII was measured in the fVIII chromogenic assay described below. The reference curve of the fVIII chromogenic assay was made with known amounts of soluble fVIII diluted in 30 μL imidazole-BSA. Preliminary experiments indicated that MoAb F15B12 did not interfere with fVIII activity in fVIII functional assays. A calibration curve was then established by plotting the amounts of fVIII bound to vWF, as measured in the fVIII chromogenic assay, and the OD obtained in ELISA.
Functional Assays
Kinetics of fVIII inactivation by BO2C11.
The functional inhibitory capacity of anti-fVIII hu-MoAbs was evaluated by using a modification of the DADE fVIII chromogenic assay (Dade AG, Switzerland), as described.18 In this assay, thrombin-activated fVIII accelerates the conversion of factor X into factor Xa in the presence of factor IXa, PL, and calcium ions; factor Xa activity is then assessed by hydrolysis of a p-nitroanilide substrate. Reagents, which were reconstituted according to the manufacturer's instruction, comprised bovine factor X (1 mmol/L), factor IXa (0.3 mmol/L), thrombin (0.3 mmol/L), CaCl2 (30 mmol/L), PL (60 mmol/L), a chromogenic factor Xa substrate (CH3OCO-D-CHG-gly-Arg-pNA.AcOH; 3.4 mmol/L), and a thrombin inhibitor (L-amidinophenylalanine piperidine). One vol of rfVIII (240 ng/mL) in imidazole-HSA was mixed with 1 vol of purified vWF (12 μg/mL) or buffer and incubated for 30 minutes at 37°C. The mixtures were then kept at 4°C. Aliquots containing rfVIII or rfVIII-vWF complexes were mixed with an equal volume of purified hu-MoAb (concentration varying from 170 ng to 170 μg/mL) and incubated during 2 to 60 minutes at 37°C. Aliquots of 30 μL were then retrieved and displayed in microtitration plates; 30 μL of the factor X and factor IXa/thrombin reagents were added sequentially. After 90 seconds, 60 μL of the chromogenic substrate was added and the incubation was extended for 10 minutes at 37°C. The reaction was then blocked by the addition of 30 μL citric acid (1 mol/L), and OD was measured at 405 nm. The residual fVIII activity was determined by comparing the OD405nm of test samples with that obtained with rfVIII solutions of known concentrations. Preliminary experiments had shown that there was no significant difference between the OD405nm of rfVIII as compared with rfVIII-vWF complexes. The rfVIII concentration (120 ng/mL) used for mixing experiments with hu-MoAb was selected as the highest concentration that could still be measured in the chromogenic assay without further diluting the sample. The residual fVIII activity was expressed as the percentage of activity measured in rfVIII aliquots handled and diluted exactly as test samples throughout the entire experiment.
Inhibition of activated fVIII by hu-MoAbs.
fVIII was activated by thrombin by mixing 1 vol fVIII (10 IU/mL) with 1 vol thrombin (0.2 IU/mL) at 37°C. After 30 seconds of incubation, thrombin activity was inhibited by the addition of 1 vol of a 60 μg/mL hirudin solution. fVIIIa was measured in the chromogenic assay described above, except that hirudin was added to the reagents before the assay. Thus, a 30-μL aliquot of thrombin-activated fVIII was added to a microtitration plate and incubated with 80 μL of a solution made by mixing 30 μL factor X and factor IXa reagents, 10 μL hirudin (60 μg/mL), and 10 μL imidazole-HSA containing or not containing inhibitor antibody. After 90 seconds, the generation of factor Xa was evaluated by the addition of a chromogenic substrate for a further 5 minutes of incubation. The OD was read at 405 nm and the results are expressed as percentages of the OD measured in the absence of inhibitor antibody.
Coagulation assays.
fVIII inhibitor titers were measured by the Bethesda method in which normal pooled plasma was used as an fVIII source. After an incubation of 2 hours with antibody, the residual fVIII activity was measured by a one-stage clotting assay according to Kasper et al37 with the modifications of Verbruggen et al.38
Measurement of Surface Plasmon Resonance (SPR)
Real-time kinetic interaction between fVIII and hu-MoAbs was analyzed using a Pharmacia Biosensor BIAcore TM instrument (Pharmacia Biosensor AB). Purified BO2C11 (20 μg/mL in 10 mmol/L sodium acetate buffer, pH 5.0) was immobilized on the activated surface of a CM5 sensor chip, according to the manufacturer's instructions. All binding experiments were performed in HBS at a constant flow rate of 10 μL/min. fVIII in HBS was infused at various concentrations over the ECR-immobilized sensor chip surface. At the end of each cycle, the surface was regenerated by flushing HCl, pH 2, for 36 seconds. The amount of fVIII molecule bound per BO2C11 molecule was determined by assuming that the molecular weight of FVIII and BO2C11 are 330.000 and 170.000, respectively, and that the response in RU corresponding to the binding of one molecule is proportional to the molecular weight.
Association and dissociation rate constants were determined by nonlinear fitting of individual sensorgram data39 using the BIA evaluation 2.1 software (Pharmacia Biotech, Uppsala, Sweden). Values of kass and kdiss were determined by averaging the values obtained for individual curves established with various analyte concentrations. Values of kdiss were determined from the individual curves obtained with only the highest analyte concentration to reduce bias due to rebinding of the analyte to free immobilized ligand. All data were analyzed after correction of the baseline by subracting the response observed before injection of the analyte (rfVIII) from the response values obtained during the association and dissociation phases.
Data corresponding to the dissociation phase were fitted to the following equation: R = R0e − (kdisst),39 where R is the SPR signal in RU at time t and R0 is the SPR signal at the start of the dissociation phase. The equation used for nonlinear regression analysis of the association phase was as follows: R = (kassCRmax[1 − e − (kassC + kdiss)t])/(kassC + kdiss), where C is the concentration of the analyte and Rmax is the maximum analyte binding capacity in RU.
RESULTS
Production and Characterization of the Anti-fVIII BO2C11 hu-MoAb
PBMCs of a hemophilia A patient with inhibitor (BO) were immortalized by EBV transformation. Five hundred cell line supernatants were screened by ELISA for the presence of antibodies towards fVIII and rfVIII C2 domain. Five supernatants contained anti-fVIII antibodies. Two supernatants containing anti-C2 antibodies were further tested for their ability to inhibit fVIII activity in a chromogenic assay and to prevent the binding of fVIII to PS and/or to vWF in an ELISA system. The BO2C11 cell line was selected because it produced an IgG antibody that fulfilled these characteristics. BO2C11 was then cloned by limiting dilution. Clonality was verified by two independent reverse transcription-PCR amplifications of mRNA coding for the variable part of the antibody heavy chain: a single sequence was obtained from the 12 clones of PCR products (data not shown). The complete sequences of the VH genes of BO2C11 were determined. BO2C11 VH gene was most homologous to DP-5, a member of the VH-1 gene family, and the J segment was most homologous to JH3b. Sequencing of the cloned light chain gene identified the VL as a VκIII and the J segment as a Jκ5. Purified antibodies were obtained by passage of the BO2C11 cell culture supernatant on protein-A Sepharose. An ELISA performed with IgG subclass- and light chain-specific antibodies identified BO2C11 as an IgG4κ.
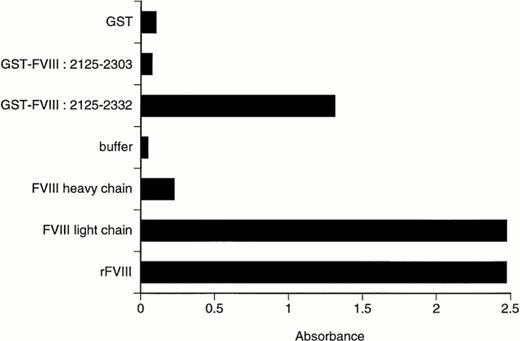
As shown in Fig 1, BO2C11 binds to full-length rfVIII, to its isolated light chain, and to the chimeric fVIII fragment encompassing residues 2125 to 2332, corresponding to the carboxy-terminal end of C1 (residues 2125-2172) and the full C2 domain (residues 2173-2332). Control experiments showed no binding to assay reagents other than fVIII or fVIII fragments, and preincubation with soluble rfVIII prevented the binding to fVIII-coated plates, confirming the binding specificity (data not shown). Minimal binding to the isolated fVIII heavy chain was observed (Fig 1), which can be accounted for by contamination of the heavy chain by 5% (wt/wt) light chain (unpublished data). Furthermore, no binding was observed to chimeric GST-fVIII fragments corresponding to the A1, A2, or A3 domains; to the small acidic regions 1, 2, and 3; or to residues 1981-2222, corresponding to the carboxy-terminal part of the A3 domain, the C1 domain, and the amino-terminal part of the C2 domain (data not shown). BO2C11 and the MoAb ESH8 did not compete with each other for the binding to fVIII (data not shown); the fVIII epitope recognized by ESH8 has been mapped to residues 2248 to 2285.21 In addition, BO2C11 did not bind to a chimeric fVIII fragment corresponding to residues 2125 to 2303 (Fig 1) or to residues 2216-2332 (data not shown). Both of the latter deletion fragments have lost a cysteine residue, 2326 and 2174, respectively, involved in the intradomain disulphide bridge. However, the absence of binding of BO2C11 to these fragments cannot be accounted for solely by the disruption of the disulfide bridge for the following three reasons: (1) treatment of fVIII for 24 hours with β2-mercaptoethanol in the presence of 8 mol/L urea, followed by alkylation with iodoacetamide, did not significantly reduce BO2C11 binding in ELISA (data not shown); (2) BO2C11 also recognized reduced fVIII in Western blot (data not shown), and (3) in addition, a mutant chimeric GST-fVIII C2 domain, corresponding to residues 2170 to 2332 and in which cysteine 2174 was replaced by a glycine, was recognized by BO2C11 (data not shown). Taken together, these results suggest that the formation of the epitope recognized by B02C11 requires residues located between amino acid residues 2303 and 2332 and between residues 2170 and 2216. However, this does not exclude the fact that amino acid residues located elsewhere in the fVIII C2 domain may contribute to the constitution of the BO2C11 epitope.
BO2C11 binding to full-length rfVIII or recombinant fragments and domains. Microtitration plates coated with the indicated purified proteins or polypeptides were incubated with 5 μg/mL of BO2C11 for 2 hours at 4°C. The binding of BO2C11 was detected by addition of a HRP-labeled mouse MoAb specific for human Fcγ. GST-fVIII: 2125-2332 includes exon 23 and exons 24 to 26 forming the C2 domain.
BO2C11 binding to full-length rfVIII or recombinant fragments and domains. Microtitration plates coated with the indicated purified proteins or polypeptides were incubated with 5 μg/mL of BO2C11 for 2 hours at 4°C. The binding of BO2C11 was detected by addition of a HRP-labeled mouse MoAb specific for human Fcγ. GST-fVIII: 2125-2332 includes exon 23 and exons 24 to 26 forming the C2 domain.
Inhibition of fVIII Function by BO2C11
BO2C11 inhibits fVIII functional activity.
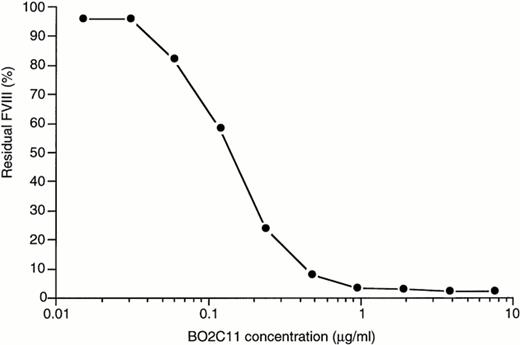
BO2C11 capacity to inhibit plasma fVIII activity was tested in a coagulation assay. As shown in Fig 2, when a solution containing 0.14 μg/mL BO2C11 was incubated with plasma as in a conventional Bethesda assay, fVIII activity was reduced by 50%. The BO2C11 specific activity is therefore approximately 7,000 BU/mg protein.
Inhibition of fVIII functional activity in coagulation assays. Equal volumes of BO2C11 and of a pool of normal plasma were incubated for 2 hours at 37°C. BO2C11 concentrations before mixing with plasma were as indicated. The residual fVIII activity was measured in an one-stage coagulation assay and was expressed as the percentage of the activity obtained in the absence of antibody.
Inhibition of fVIII functional activity in coagulation assays. Equal volumes of BO2C11 and of a pool of normal plasma were incubated for 2 hours at 37°C. BO2C11 concentrations before mixing with plasma were as indicated. The residual fVIII activity was measured in an one-stage coagulation assay and was expressed as the percentage of the activity obtained in the absence of antibody.
BO2C11 inhibits fVIII binding to PS.
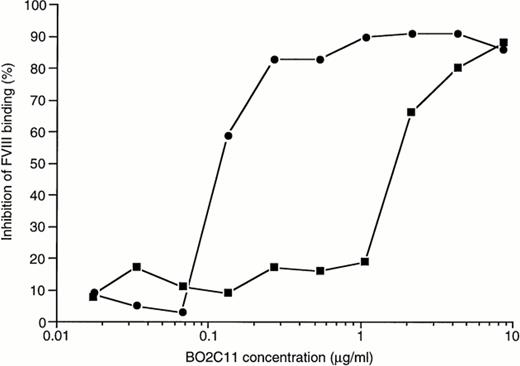
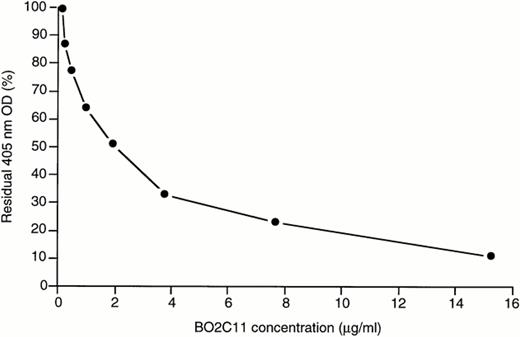
The capacity of BO2C11 to inhibit the binding of fVIII to PS-coated plates was investigated by ELISA. As shown in Fig 3, a dose-dependent inhibition was observed. The BO2C11 concentration yielding 50% inhibition (IC50) of fVIII binding is 1.6 μg/mL.
BO2C11-dependent inhibition of the binding of fVIII to PS and vWF. To assess the capacity of BO2C11 to inhibit the binding of fVIII to PS (▪) or to vWF (•), rfVIII at 2 μg/mL or 0.2 μg/mL final concentration, respectively, was mixed for 30 minutes at 37°C with different concentrations of BO2C11 before addition to PS- or vWF-coated plates, as appropriate. In both cases, the plates were then incubated for 2 hours at RT and the binding of fVIII was detected by the addition of the anti-A2 MoAbF15B12.
BO2C11-dependent inhibition of the binding of fVIII to PS and vWF. To assess the capacity of BO2C11 to inhibit the binding of fVIII to PS (▪) or to vWF (•), rfVIII at 2 μg/mL or 0.2 μg/mL final concentration, respectively, was mixed for 30 minutes at 37°C with different concentrations of BO2C11 before addition to PS- or vWF-coated plates, as appropriate. In both cases, the plates were then incubated for 2 hours at RT and the binding of fVIII was detected by the addition of the anti-A2 MoAbF15B12.
BO2C11 inhibits the binding of fVIII to vWF.
The capacity of BO2C11 to inhibit the binding of fVIII to vWF was assessed in ELISA. Figure 3 shows that BO2C11 inhibits the binding of fVIII to vWF in a dose-dependent manner. The concentration of BO2C11 required to achieve 50% inhibition (IC50) of fVIII binding is 0.12 μg/mL. The difference in BO2C11 concentrations required to inhibit the binding of fVIII to PS or to vWF is related to the difference in fVIII concentrations in the two assay systems. Thus, on a molar basis, the ratios of BO2C11 IC50 to fVIII concentration in the PS- and vWF-binding assays are 0.8 and 0.6, respectively.
Inhibition of BO2C11-fVIII Interaction by vWF
vWF inhibits the binding of fVIII to BO2C11.
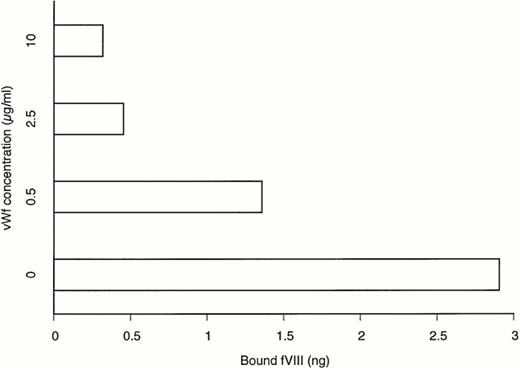
The inhibitory property of BO2C11 on the binding of fVIII to vWF prompted us to determine whether physiological concentrations of vWF could compete with BO2C11 for fVIII binding. This was assessed in an ELISA system in which the binding of free or vWF-bound fVIII to BO2C11 was measured. As shown in Fig 4, when fVIII was complexed to vWF at a ratio similar to that found in plasma (50:1 vWF to fVIII wt/wt ratio) and incubated for 5 minutes at 37°C with insolubilized BO2C11, the binding to fVIII was only 10% of that obtained in the absence of vWF. The protective effect of vWF was concentration-dependent. However, when the incubation time in the above system was prolonged, the protective effect of vWF progressively disappeared (data not shown). This suggested that BO2C11 could displace fVIII from its vWF-binding site.
Effect of vWF on fVIII binding to BO2C11. rfVIII (1 μg/mL) was incubated for 30 minutes at 37°C in the absence or presence of different concentrations of vWF before the addition to BO2C11-coated plates. The binding of fVIII to BO2C11 was detected by the addition of the anti-A2 MoAbF15B12 after 5 minutes of incubation at 37°C. Bound fVIII was calculated by comparison with a calibration curve established with known levels of rfVIII bound to BO2C11 (see the Materials and Methods).
Effect of vWF on fVIII binding to BO2C11. rfVIII (1 μg/mL) was incubated for 30 minutes at 37°C in the absence or presence of different concentrations of vWF before the addition to BO2C11-coated plates. The binding of fVIII to BO2C11 was detected by the addition of the anti-A2 MoAbF15B12 after 5 minutes of incubation at 37°C. Bound fVIII was calculated by comparison with a calibration curve established with known levels of rfVIII bound to BO2C11 (see the Materials and Methods).
BO2C11 displaces fVIII from vWF.
To test this hypothesis, we incubated plasma-derived fVIII (0.5 U/mL) complexed to vWF (15:1 vWF to fVIII wt/wt ratio) for 60 minutes at 37°C in the presence or absence of BO2C11 (5 μg/mL). We then evaluated the proportion of fVIII that remained bound to vWF by capturing fVIII-vWF complexes on a microtiter plate onto which an anti-vWF MoAb had been insolubilized. fVIII was then detected by the addition of the anti-A2 MoAbF15B12. BO2C11 reduced the amount of fVIII bound to vWF by more than 90% within the time frame of the experiment, whereas the addition of a pool of normal donor gammaglobulins had no effect (Fig 5). To determine whether this BO2C11 activity was representative of the inhibitory activity of the patient's inhibitor antibodies, polyclonal IgG antibodies were purifed from the plasma of the patient from whom the BO2C11 cell line was derived. The latter polyclonal IgG antibodies also strongly reduced the amount of fVIII bound to vWF (Fig 5). Similar results were obtained when complexes made of biotinylated fVIII and vWF were used and the binding of biotinylated fVIII was evaluated by the addition of HRP-labeled avidine instead of anti-A2 MoAbF15B12 (data not shown).
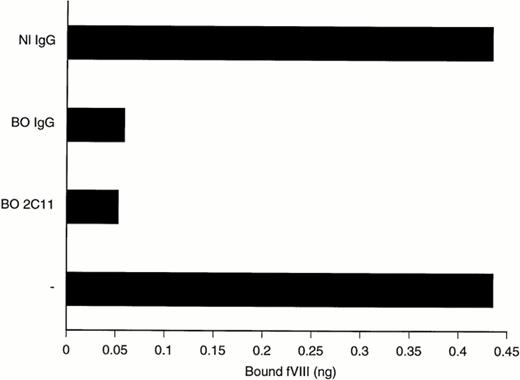
Antibody-dependent dissociation of fVIII from vWF. Plasma-derived fVIII (0.5 U/mL) complexed to vWF (15:1 vWF to fVIII wt/wt ratio) was incubated for 60 minutes at 37°C in the presence of 5 μg/mL normal donor's polyclonal IgG (Nl IgG), of 0.5 μg/mL BO2C11 hu-MoAb (BO2C11), of 5 μg/mL patient BO's polyclonal IgG (BO IgG), or of buffer (-). The proportion of fVIII that remained bound to vWF was evaluated by capture of the fVIII-vWF complexes on a microtitration plate coated with the anti-vWF MoAb4H1D7. fVIII was then detected by the addition of the HRP-labeled anti-A2 MoAbF15B12. Control experiments showed that BO2C11 and BO's polyclonal IgG did not inhibit MoAbF15B12 binding to fVIII. The amounts of bound fVIII were calculated by comparison with a calibration curve established with known levels of insolubilised fVIII (see the Materials and Methods).
Antibody-dependent dissociation of fVIII from vWF. Plasma-derived fVIII (0.5 U/mL) complexed to vWF (15:1 vWF to fVIII wt/wt ratio) was incubated for 60 minutes at 37°C in the presence of 5 μg/mL normal donor's polyclonal IgG (Nl IgG), of 0.5 μg/mL BO2C11 hu-MoAb (BO2C11), of 5 μg/mL patient BO's polyclonal IgG (BO IgG), or of buffer (-). The proportion of fVIII that remained bound to vWF was evaluated by capture of the fVIII-vWF complexes on a microtitration plate coated with the anti-vWF MoAb4H1D7. fVIII was then detected by the addition of the HRP-labeled anti-A2 MoAbF15B12. Control experiments showed that BO2C11 and BO's polyclonal IgG did not inhibit MoAbF15B12 binding to fVIII. The amounts of bound fVIII were calculated by comparison with a calibration curve established with known levels of insolubilised fVIII (see the Materials and Methods).
vWF Prevents fVIII Inactivation by BO2C11 in a Functional Assay
The relevance of these findings to the fVIII cofactor activity in the tenase complex formation was assessed in a functional assay system. A purified vWF preparation was mixed with rfVIII at a wt/wt ratio close to that in plasma. The vWF/fVIII complex was then incubated with BO2C11 for different lengths of time, after which the residual fVIII activity was measured in a chromogenic assay. vWF effectively reduced fVIII inactivation by BO2C11 (Fig 6). However, the protective effect was progressively reduced during later stages of the experiment and, after 1 hour, the activity of fVIII incubated with BO2C11 in the presence of vWF was only slightly higher than that observed in the assay performed with fVIII alone. This phenomenon could be ascribed to dissociation of fVIII from vWF, allowing the progressive binding to BO2C11. Indeed, the association (kass) and dissociation (kdiss) rate constants of the fVIII-vWF complex are 5.9 ± 1.9 × 106mol/L−1 s−1 and 1.6 ± 1.2 × 10−3 s−1 (mean ± SD), respectively,40 which implies that half of the fVIII molecules dissociates from vWF every 7 to 13 minutes.40 41It is likely that BO2C11 remains bound to fVIII after thrombin activation, thereby preventing fVIIIa interactions with PL. By contrast, the protective effect of vWF was no longer observed when fVIII-vWF complexes were incubated with a large excess of BO2C11. As shown in Fig 6, BO2C11 at a final concentration of 85 μg/mL (ie, 600 BU/mL) rapidly inhibited fVIII in the presence of vWF. The absence of vWF protection in the presence of high concentrations of BO2C11 suggests that, in addition to inactivating a major fraction of rfVIII during the time-course experiment, BO2C11 could inhibit the residual fVIII activity during the chromogenic assay used to determine this activity. In the latter case, BO2C11 would bind to fVIIIa, once it has dissociated from vWF upon cleavage of the fVIII light chain by thrombin and before fVIIIa binding to PL. To test the validity of these hypotheses, real-time kinetics of BO2C11 binding to native fVIII was studied by SPR technology, and the ability of BO2C11 to interfere with fVIIIa was tested in a chromogenic assay.
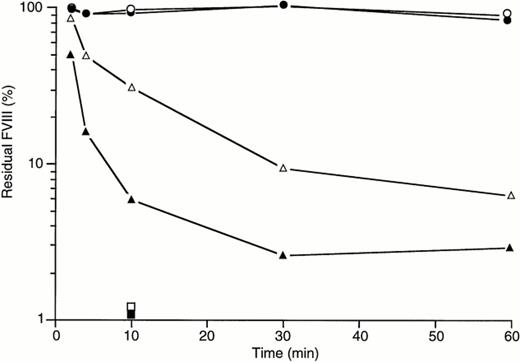
Time-dependent effect of vWF on fVIII inactivation by BO2C11. rfVIII alone (solid symbols) or rfVIII complexed with purified vWF (open symbols) was mixed with BO2C11 at either one of two concentrations (see below). The mixture was then incubated at 37°C for various periods of time, from 2 to 60 minutes, after which the residual fVIII activity was evaluated in a chromogenic assay. Results are expressed as the percentage of the activity observed in the absence of antibody. All values are given as final concentrations in the assay system. (•) rfVIII at 60 ng/mL (0.22 nmol/L); (○) rfVIII with vWF at 3 μg/mL (12 nmol/L); (▴) rfVIII + BO2C11 at 85 ng/mL (0.57 nmol/L); (▵) rfVIII-vWF + BO2C11 at 85 ng/mL (0.57 nmol/L); (▪) rfVIII + BO2C11 at 85 μg/mL (570 nmol/L); and (□) rfVIII-vWF + BO2C11 at 85 μg/mL (570 nmol/L).
Time-dependent effect of vWF on fVIII inactivation by BO2C11. rfVIII alone (solid symbols) or rfVIII complexed with purified vWF (open symbols) was mixed with BO2C11 at either one of two concentrations (see below). The mixture was then incubated at 37°C for various periods of time, from 2 to 60 minutes, after which the residual fVIII activity was evaluated in a chromogenic assay. Results are expressed as the percentage of the activity observed in the absence of antibody. All values are given as final concentrations in the assay system. (•) rfVIII at 60 ng/mL (0.22 nmol/L); (○) rfVIII with vWF at 3 μg/mL (12 nmol/L); (▴) rfVIII + BO2C11 at 85 ng/mL (0.57 nmol/L); (▵) rfVIII-vWF + BO2C11 at 85 ng/mL (0.57 nmol/L); (▪) rfVIII + BO2C11 at 85 μg/mL (570 nmol/L); and (□) rfVIII-vWF + BO2C11 at 85 μg/mL (570 nmol/L).
Kinetics of fVIII-BO2C11 Association
The rate of native fVIII association and dissociation to BO2C11 was measured by SPR. BO2C11 was covalently bound to the sensor chip surface and the binding of rfVIII was measured in real time (Fig 7). Control experiments ensured that fVIII bound only to BO2C11. Thus, rfVIII did not bind to the sensor chip in the absence of BO2C11, and preincubation of rfVIII with soluble BO2C11 before addition to the chip completely prevented fVIII binding (data not shown). The resonance signal of the antibody immobilized on the sensor surface in Fig 7 was 7,144 RU. The calculated maximal binding of FVIII to the antibody was 926 RU (SD = 358), which on a molar basis corresponds to 1 fVIII molecule for 12 antibody molecules. Taking into account that the purity of the preparation is 80%, the figure is now 1 fVIII molecule for 10 antibody molecules. The dissociation rate constant of fVIII from BO2C11 was below the detection level, namely, the kdiss was ≤1 × 10−5 s−1. However, analysis of the association phase was impeded by binding heterogeneity as indicated by a nonrandom distribution of the difference between the experimental and calculated data for each point in the fit as a function of time. When heterogeneity was ignored and when assuming a kdissof 1 × 10−5 s−1, the association rate constant (kass) determined by averaging the kass derived from the sensorgrams corresponding to various concentrations of rfVIII (Fig 7) was 2 × 105 mol/L−1 s−1 (SD = 1.86; χ2 = 50 to 300).
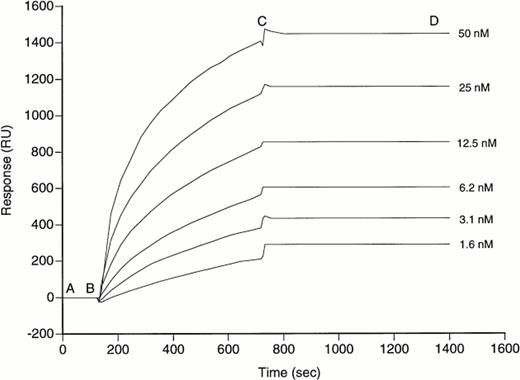
Kinetics of fVIII/BO2C11 association. BO2C11 was bound to the sensor chip surface by covalent coupling. Real-time binding was measured for rfVIII at the concentrations indicated. Between each experiment, surface-bound rfVIII was washed off by incubation with HCl, pH 2.0, for 36 seconds. The SPR response gives the amount of surface-bound component at each stage of the reaction, namely (A and B) baseline resonance signal; (B and C) association phase; and (C and D) dissociation phase.
Kinetics of fVIII/BO2C11 association. BO2C11 was bound to the sensor chip surface by covalent coupling. Real-time binding was measured for rfVIII at the concentrations indicated. Between each experiment, surface-bound rfVIII was washed off by incubation with HCl, pH 2.0, for 36 seconds. The SPR response gives the amount of surface-bound component at each stage of the reaction, namely (A and B) baseline resonance signal; (B and C) association phase; and (C and D) dissociation phase.
A likely explanation for the low number of rfVIII bound per BO2C11 molecule and for the poor fit of the association phase with a simple bimolecular model is that a proportion of antibody molecules were altered or rendered inaccessible by the coupling procedure. We therefore analyzed the binding of rfVIII to BO2C11 bound to a polyclonal antibody specific for human IgG and covalently coupled to the sensor chip surface. This approach allowed the analysis of the binding of fVIII to BO2C11 but not that of the dissociation phase, because BO2C11 dissociated faster from the goat anti-hu Ig antibody (kdiss = 1.6 × 10−4s−1) than rfVIII from BO2C11 (as determined above). When 60 nm of rfVIII was injected and the reaction allowed to reach equilibrium, 0.7 fVIII molecule was bound per BO2C11 molecule. This seemingly low fVIII/BO2C11 ratio may be due to a negative cooperativity for the occupation of the second-antigen binding site of BO2C11 once the first site is already occupied. This would be in keeping with the reduced flexibility of the hinge region of IgG4 antibodies.42 Nonlinear regression analysis of the data provided an association rate constant of fVIII for BO2C11 of 7.4 × 105 s−1 mol/L−1(SD = 0.6), with a very good fit to a simple second-order association model (χ2 < 5). The latter kass is 3.7 times faster than the association rate constant determined when BO2C11 was covalently coupled to the sensor chips (kass ∼2 × 105mol/L−1 s−1). The calculated apparent dissociation constant (KD = kdiss/kon) derived from the above-cited data was 1.4 × 10−11 mol/L−1, in agreement with the KD value of 2.5 × 10−11 (SD = 1.7) derived from Scatchard analysis of FVIII inhibition by BO2C11 (unpublished data).
These data indicate that the dissociation rate constant of the BO2C11-fVIII complex is at least 100-fold lower than that of the vWF/fVIII complex.40,41 By contrast, the association rate constant of BO2C11 to fVIII is about eightfold lower than that of the binding of fVIII to vWF.40 Taking into account the high dissociation rate of the fVIII-vWF complex, a speculative model of fVIII inactivation is that BO2C11 progressively displaces fVIII from vWF to form inactive BO2C11-fVIII complexes, which are extremely stable over time.
BO2C11 Inhibits fVIIIa
Because increasing the concentration of BO2C11 resulted in a loss of the protective effect of vWF on fVIII inactivation (see Fig 6), we postulated that BO2C11 could inhibit fVIIIa. This hypothesis was tested in a chromogenic assay. As shown in Fig 8, BO2C11 is able to inhibit thrombin-activated fVIII in a dose-dependent manner. Because fVIII is unstable at physiological pH after thrombin activation,43 BO2C11 was not preincubated with fVIII but was added directly to the reagents of the chromogenic assay, before the addition of fVIIIa. The final concentration of BO2C11 required to inhibit 50% of fVIII activity in this assay system was 2 μg/mL (∼30 BU/mL).
BO2C11 inhibits fVIIIa. Plasma-derived fVIII (5 IU/mL; 1 μg/mL) was incubated with bovine thrombin (0.1 U/mL) for 30 seconds at 37°C, and the reaction was stopped by the addition of hirudin. fVIIIa was then mixed for 90 seconds at 37°C with factor IXa, factor X, PL, and CaCl2, in the absence or presence of different concentrations of BO2C11. The final concentration of fVIII in the assay was 1 IU/mL (200 ng/mL). After 90 seconds, the generation of factor Xa was evaluated by the addition of a chromogenic substrate for a further 5 minutes of incubation. OD was read at 405 nm and results were expressed as percentages of OD measured in the absence of BO2C11.
BO2C11 inhibits fVIIIa. Plasma-derived fVIII (5 IU/mL; 1 μg/mL) was incubated with bovine thrombin (0.1 U/mL) for 30 seconds at 37°C, and the reaction was stopped by the addition of hirudin. fVIIIa was then mixed for 90 seconds at 37°C with factor IXa, factor X, PL, and CaCl2, in the absence or presence of different concentrations of BO2C11. The final concentration of fVIII in the assay was 1 IU/mL (200 ng/mL). After 90 seconds, the generation of factor Xa was evaluated by the addition of a chromogenic substrate for a further 5 minutes of incubation. OD was read at 405 nm and results were expressed as percentages of OD measured in the absence of BO2C11.
DISCUSSION
The emergence of an immune response towards fVIII remains a major complication for hemophilia A patients treated by infusions of fVIII. Available therapies to restore hemostasis in those patients are based on the administration of porcine fVIII or of fVIII bypassing agents, such as activated prothrombin complex concentrates or activated recombinant factor VII.44,45 Alternatively, restoring fVIII unresponsiveness can be attempted by administering high doses of fVIII, in conjunction or not in conjunction with extracorporeal removal of IgG, cytostatic agents, or IV infusions of pooled human gammaglobulins.23 However, all of these methods have shown disadvantages, such as inconstant results and high costs. The development of a novel therapy has been hampered by the lack of understanding of the mechanisms by which antibodies interact with fVIII, which is mainly due to the diversity of the human antibody response to this coagulation factor.
Because such an understanding could open novel therapeutic approaches, we decided to analyze the human anti-fVIII immune response at the clonal level. To this end, we have produced anti-fVIII hu-MoAbs by immortalization of peripheral blood memory B cells. This approach was preferred to genetic engineering of antibodies so as to obtain antibodies of physiological relevance. We report here on the properties of the first of these antibodies: (1) BO2C11 is a high-affinity IgG4κ antibody derived from B cells of a hemophilia A patient with high titer of inhibitors; (2) BO2C11 binds to the fVIII C2 domain and inhibits the binding of vWF and of PS to fVIII; (3) BO2C11 captures and neutralizes fVIII in an almost irreversible way when the latter is released from its vWF binding site; and (4) BO2C11 has the capacity to inhibit the binding of fVIIIa to PS, thereby interfering with the tenase complex formation.
The complete sequences of the V genes of BO2C11 were determined. BO2C11 VH gene is a member of the VH-1 gene family and VL is a VκIII. When more hu-MoAbs become available, it will be possible to determine whether the immune response towards FVIII is associated to a restriction in gene usage, although such a restriction in gene usage would perhaps be more readily expected with auto-antibodies to FVIII.33 46
The affinity of BO2C11 for fVIII is high, due to a very low dissociation rate constant. This is illustrated by the observation that greater than 50% inhibition of the procoagulant activity of fVIII can be obtained with only a slight molar excess of BO2C11 over fVIII, whenever the reaction is allowed to run until equilibrium, as is the case in the routine evaluation of fVIII inhibitors in the Bethesda assay. BO2C11 completely inhibits the binding of fVIII to PS, suggesting that this is the mechanism by which it interferes with fVIII activity. Its specific activity is approximately 7,000 BU/mg; it can therefore be calculated that an inhibitor titre of 1,000 BU/mL would correspond to the inhibitory activity of 140 μg/mL of BO2C11.
Epitope mapping using recombinant fVIII fragments indicated that BO2C11 interaction with the C2 domain requires the presence of amino acids within the 2303-2332 carboxy-terminal region, as well as within residues 2170-2215. By contrast, the disulfide bridge between cysteine residues 2174 and 2326 is not required for BO2C11 binding. Interestingly, the structural requirements for the binding of the C2 domain to BO2C11 are reminiscent of those required for the binding of vWF A1 domain residues 508-704 to platelet membrane glycoprotein Ib. Indeed, reduction/alkylation of the vWF domain does not prevent binding to GPIb, whereas reduction in conjunction with deletion of residues flanking either side of the disulfide bridge severely impedes the binding to GPIb.47 Further epitope mapping of BO2C11 should be based on complementary strategies, such as the use of C2 variants generated by site-directed mutagenesis or by homolog recombination between human and porcine FVIII.48
The capacity of BO2C11 to prevent fVIII binding to both vWF and PL fits well with the localization of the epitope in the C2 domain.7-11 A major cluster of epitopes has been mapped to this fVIII region15 at the level of amino acid residues 2248-2312.20 Antibodies binding to that region inhibit the function of fVIII by two distinct mechanisms. One type of inhibitor reduces the dissociation of fVIII from vWF, thereby preventing indirectly the binding of fVIIIa to PL.21 A second type of inhibitors bind to epitopes overlapping the vWF- and PL-binding site encompassing amino acid residues 2303-2332.9-11,20 These latter antibodies presumably act in vivo by blocking access of fVIIIa to PL and by interfering with the ability of fVIII to bind to and be stabilized by vWF in the circulation.10 However, the polyclonal human antibodies described in the above-mentioned studies were unable to displace fVIII from vWF to any significant extent once the latter had already bound fVIII.10 In contrast, BO2C11 has the capacity to progressively displace vWF from fVIII, suggesting that such a mechanism is likely to play a significant role in severe hemophilia A patients with this type of inhibitor.
Precisely how vWF and human inhibitors compete for their binding to fVIII has not been clearly defined. In some cases, vWF appears to restrict the binding of antibodies to fVIII,28 although patients can present with high titers of inhibitors despite the fact that their antibodies exclusively recognize the vWF- and PL-binding sites.9,19 Our studies with BO2C11 provide a potential explanation for these seemingly contradictory results. In the presence of vWF, the capture and inactivation of fVIII by BO2C11 is reduced. However, this protection is limited in time, a finding that can be ascribed to the dissociation of fVIII from vWF and nearly irreversible capture of fVIII by BO2C11. Indeed, the dissociation rate constant of the fVIII-vWF complex indicates that half of the fVIII molecules dissociate from vWF every 10 minutes.40 41 The free fVIII molecules are then exposed to inactivation by BO2C11. Analysis of the kinetics of interaction between fVIII and BO2C11 by SPR endorsed such an interpretation: the dissociation rate constant of the BO2C11-fVIII complex is at least 100-fold lower than that of the vWF-fVIII complex, whereas the fVIII association rate constant is eightfold lower for BO2C11 than for vWF. However, in the presence of large excess of soluble BO2C11, vWF was unable to prevent fVIII inactivation. This was attributed to the combination of two phenomena, binding to fVIII after its dissociation from vWF and inhibition of fVIIIa after thrombin-induced dissociation of the fVIII-vWF complex.
BO2C11 shares properties with the polyclonal antibody populations analyzed so far. Thus, BO2C11 recognizes an epitope located in the C2 domain, which is known to contain 1 of the 3 main fVIII regions offering a cluster of B-cell epitopes.15,18,20 Inhibition of fVIII binding to both vWF and PL is a recurrent observation made with polyclonal anti-fVIII antibody preparations.9,11,19,20Moreover, BO2C11 belongs to the IgG4 isotype, another common property of human anti-fVIII antibodies.16 The latter also indicates that the cell line producing BO2C11 is derived from the population of memory B cells, which are the only lymphocytes spontaneously producing IgG antibodies in vitro.49 50 Hence, BO2C11 appears to be representative of a population of FVIII inhibitors, indicating that the EBV transformation technique is efficient to generate hu-MoAbs corresponding closely to patients' pathogenic antibodies.
An important issue with respect to interactions between FVIII, vWF, and inhibitors binding to C2 was brought about by the determination of the association and dissociation rate constants of BO2C11 for FVIII. It clearly shows that, depending on the relative affinity of antibodies for FVIII, the actual effect of anti-C2 antibodies on FVIII activity can be expected to vary dramatically, independently of the epitope specificity.
An original aspect of BO2C11's mechanism of action is its ability to progressively displace FVIII from vWF. This activity is also observed in the polyclonal antibodies of the patient from whom the B-cell line producing BO2C11 was derived. However, given that the patient's polyclonal antibodies recognize a series of antigenic determinants on the FVIII molecule,18 it would have been impossible to formally demonstrate by using the patient's polyclonal antibodies that the dissociation of the FVIII-VWF complex was due to a single type of antibody binding to the C2 domain. It is expected that the isolation of additional human MoAbs will allow for a better understanding of the human polyclonal inhibitor population and their epitope specificity.
ACKNOWLEDGMENT
The authors thank Jean-Jacques Pin and Drs Serge Lebecque and Jacques Banchereau for their invaluable help in the production of human MoAbs.
Supported in part by Grant No. BR1/4-255/138 of the Institut pour l'Encouragement de la Recherche Scientifique dans l'Industrie et l'Agriculture. J.V. is holder of the Dr Jean Choay Chair for Hemostasis Research. M.B. is a postdoctoral fellow of the F.W.O. (Grant No. 295.332.0871).
Address reprint requests to Marc G. Jacquemin, MD, Center for Molecular and Vascular Biology, Katholieke Universiteit Leuven, Campus Gasthuisberg, O&N, Herestraat 49, B-3000 Leuven, Belgium; e-mail:marc.jacquemin@med.kuleuven.ac.be.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal