Abstract
The addition of thrombopoietin (TPO) to HEL cells, cultured in a chemically defined serum-free medium, induced a rapid and dose-dependent phosphorylation of the transcription factor CREB on serine133 (PSer133), as detected by Western blot analysis. TPO also significantly increased the transactivation of CRE-dependent promoter, as determined in transient transfection experiments. On the other hand, neither erythropoietin (Epo; 1 to 10 U) nor hemin (10−7 mol/L) were able to significantly stimulate CREB-PSer133 or to activate CRE-promoter in HEL cells. Although pharmacological inhibitors of protein kinase C (chelerytrine and BIM) and protein kinase A (H-89) failed to block the TPO-mediated CREB phosphorylation, a specific inhibitor of the mitogen-activated protein kinases (PD98059) completely blocked the ability of TPO to stimulate CREB-PSer133. Moreover, PD98059 significantly decreased the ability of TPO to upregulate the surface expression of the αIIbβ3 megakaryocytic marker in HEL cells. In parallel, primary CD34+ hematopoietic cells were seeded in liquid cultures supplemented with 100 ng/mL of TPO and examined by immunofluorescence for the coexpression of αIIbβ3 and CREB-PSer133 at various time points. High levels of nuclear CREB-PSer133 were unequivocally demonstrated in αIIbβ3+cells, including morphologically recognizable megakaryocytes. Taken together, these data suggest that CREB plays a role in modulating the expression of genes critical for megakaryocyte differentiation and that the TPO-mediated CREB phosphorylation seems to be regulated via mitogen-activated protein kinases.
THROMBOPOIETIN (TPO), the recently cloned ligand for c-Mpl receptor1 is a polypeptide with a predicted molecular mass of 35,000 kD and a novel two-domain structure.2 The amino-terminal domain shows homology with erythropoietin (Epo), the lineage-specific growth factor for red blood cell production, whereas the carboxyl-terminal domain is rich in serine, threonine, and proline residues and contains seven potential N-linked glycosylation sites.2 It has been demonstrated by several groups of investigators that TPO acts on both the proliferative and maturative pools of megakaryocytopoiesis, stimulating the growth and differentiation of megakaryocyte progenitors as well as the release of functional platelets in vitro.3-7
Several studies have begun to elucidate the intracellular pathways activated in response to TPO/c-Mpl interactions. Although c-Mpl, similarly to other receptors for hematopoietic growth factors, does not contain consensus sequence motifs for tyrosine or serine-threonine kinases,1 TPO stimulation of target cells or platelets results in tyrosine phosphorylation and activation of several proteins, including Tyk2, Jak2, Shc, Sos, Vav, c-Cbl, and the p85 regulatory subunit of PI-3 kinase.8-14 From all these studies, it is clearly emerging that TPO can activate several signaling pathways: a Tyk2/Jak2/STAT3 pathway, a Shc/Vav/Ras/Raf-1/MAPK pathway, and possibly another pathway involving c-Cbl and PI 3-kinase.
Previous studies have also shown that the addition in culture of phorbol esters, which are potent activators of protein kinase C (PKC), enforces megakaryocyte differentiation in a number of leukemic cell lines and enhances the yield of megakaryocyte colonies achievable from normal progenitors.15-18 On the other hand, cAMP/protein kinase A (PKA) signaling pathway seems to be involved in the terminal phase of megakaryocyte differentiation, which leads to the release of functional platelets.19,20 Both PKC and PKA serine/threonine kinases are able to activate members of a complex superfamily of transcriptional transactivator proteins that specifically bind to the CRE motif: 5′-TGACGTCA-3′. The best studied CRE binding protein is a 43-to 46-kD protein termed cyclic AMP responsive element binding protein (CREB), whose activation results from phosphorylation of Ser133.21
The purpose of this study was to investigate whether agonists inducing megakaryocyte differentiation were able to modulate the phosphorylation levels of the transcription factor CREB in hematopoietic progenitor cells. To address this issue, we performed experiments on the biphenotypic (erythroid/megakaryocytic) HEL cell line, which expresses significant levels of Epo22 and c-Mpl23receptors, and can be induced to differentiate along both the megakaryocytic lineage, by phorbol esters, and the erythroid lineage, by hemin.15-18 To establish the physiological significance of the data obtained on HEL cell line, some experiments were also performed on freshly isolated primary CD34+ hematopoietic progenitor cells induced to differentiate along the megakaryocytic lineage in liquid cultures supplemented with TPO (100 ng/mL).
MATERIALS AND METHODS
Growth factors, chemicals, and antibodies.
Human recombinant TPO was from Genzyme (Cambridge, MA), whereas Epo was from Cilag (Milan, Italy). Phorbol myristate acetate (PMA) and hemin were purchased from Sigma Chemicals (St Louis, MO). BIM [2-(1-(3-dimethylaminopropyl)-1H-indol-3-yl)-3-(1H-indol-3-yl)-maleimide], chelerytrine, H-89, forskolin, and PD98059 were purchased from Calbiochem (La Jolla, CA). Stock solutions of PMA, hemin, BIM, chelerythrine, H-89, forskolin (FK), and PD98059 were initially dissolved in dimethyl sulfoxide (DMSO) and further diluted in culture medium.
Monoclonal antibodies (MoAbs) to CD34 and CD8, either unconjugated or directly conjugated to fluorescein isothiocyanate (FITC), were from Becton Dickinson (San José, CA), whereas MoAb to CD41a, either unconjugated or directly conjugated to FITC, was from Serotec (Oxford, UK). Polyclonal rabbit anti-CREB serum or serum directed against the phosphorylated Ser133 form of CREB (CREB-PSer133) were from Upstate Biotechnology Inc (Lake Placid, NY). Anti-CREB-PSer133 serum was obtained from rabbits immunized with a phosphopeptide corresponding to amino acids 123 to 136 of CREB. Goat antimouse IgG conjugated to tetramethyl rhodamine isothiocyanate (GAM-TRIC), goat antirabbit IgG conjugated to fluorescein isothiocyanate (GAR-FITC), and peroxidase-conjugated antirabbit IgG were from Sigma Chemicals (St Louis, MO).
HEL cell line and primary CD34+ hematopoietic progenitor cells.
HEL cells, obtained from American Type Culture Collection (Rockville, MD), were maintained in Iscove's modified Dulbecco's medium (IMDM; GIBCO Laboratories, Grand Island, NY) supplemented with 10% fetal calf serum (GIBCO) at an optimal cell density of 3 × 105 to 1.5 × 106 cells/mL.
For the isolation of primary CD34+ cells, informed consent to the study was obtained according to the Helsinki declaration of 1975 from 10 normal blood donors. Mononuclear cells were isolated from leukapheresis units by Ficoll-Paque (d = 1.077 g/mL; Pharmacia, Uppsala, Sweden), rinsed, and adherence-depleted overnight. After removal of adherent cells, CD34+ cells were isolated using a magnetic cell sorting program Mini-MACS (Miltenyi Biotec, Auburn, CA) and the CD34 isolation kit in accordance with the manufacturer's recommendations. The purity of CD34-selected cells was determined for each isolation by FACScan using an MoAb that recognizes a separate epitope of the CD34 molecule (HPCA-2; Becton Dickinson) directly conjugated to fluorescein. CD34+ cells averaged about 90% to 98%.
To avoid the interference of serum, both HEL and purified CD34+ cells were resuspended in a chemically defined serum-free medium: IMDM, containing 10−4 mol/L BSA-adsorbed cholesterol, nucleosides (10 μg/mL each), 0.5% bovine serum albumin (fraction V Chon), 10 μg/mL insulin, 200 μg/mL iron-saturated transferrin, 5 × 10−5 mol/L 2-β-mercaptoethanol (all purchased from Sigma). Cells were adjusted to an optimal cell density (5 × 105/mL for HEL, 5 × 104/mL for primary CD34+ cells) and treated with various agonists for short (0 to 60 minutes) or longer time periods (up to 6 days). For Western blotting experiments, CD34+ cells were pooled from 4 blood donors and either immediately processed or examined after 6 days of liquid culture in the presence of 100 ng/mL of TPO.
In blocking experiments performed with the pharmacological inhibitors BIM, chelerythrine, H-89, and PD 98059, cells were pretreated for 1 hour at 37°C with various doses of each inhibitor diluted in serum-free medium, whereas control cells were treated with DMSO diluted in medium. In some experiments using the PD 98059 inhibitor of the mitogen-activated kinase kinases (MAPKK), HEL cells were treated at various times before or after the addition of TPO.
Western blotting.
Samples containing approximately 100 μg of proteins were obtained from 1 to 1.5 × 106 of (1) HEL cells, (2) freshly isolated CD34+ cells, or (3) primary megakaryocytic cells obtained after 6 days of liquid culture with 100 ng/mL TPO. Proteins were migrated in 10% acrylamide gels and blotted onto nitrocellulose filters. Blotted filters were blocked for 30 minutes in a 3% suspension of dried skimmed milk in phosphate-buffered saline (PBS) and incubated overnight at 4°C with 1:1,000 of rat anti-CREB or anti-CREB-PSer133 serum. Filters were washed and further incubated for 1 hour at room temperature with 1:1,500 peroxidase-conjugated antirat IgG in 1% BSA. Specific reactions were shown with the ECL Western blotting detection reagent (Amersham Corp, Arlington Heights, IL).
Indirect immunofluorescence staining shown by fluorescence microscopy or flow cytometry.
For the fluorescence microscopy analysis, primary CD34+cells were first spun on coverslips, fixed in 4% paraformaldehyde/PBS for 10 minutes at room temperature, washed twice with IMDM, incubated with IMDM for an additional 15 minutes to quench the remaining paraformaldehyde, and saturated/permeabilized in NET gel solution (150 mmol/L NaCl, 5 mmol/L EDTA, 50 mmol/L Tris-HCl, pH 7.4, 0.05% NP-40, 0.25% Carrageenan Lambda gelatin, 0.02% Na azide) for 30 minutes at room temperature. After two washings with NET gel solution, cells were treated with anti-CREB-PSer133 (1:50) serum and/or anti-CD41a MoAb (1:350), diluted in NET gel solution, for 60 minutes at room temperature. After two washings with NET gel solution, GAR-FITC (1:150) and/or GAM-TRIC (1:150), diluted in NET gel solution, were added to the cells and incubated for 45 minutes at room temperature. After three additional washings (2 in NET gel solution and 1 in PBS), the nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI; Sigma) and mounted in DABCO (Sigma)-glycerol-PBS. The negative controls consisted of normal rabbit serum (1:50), anti-CREB-PSer133 (1:50) pretreated with saturating concentrations (1 μg) of the KRREILSRRPpSYRK specific phosphopeptide, synthesized, and purified by high-performance liquid chromatography at the Biotechnology Department of the University of Ferrara (Ferrara, Italy) or the isotype-matched anti-CD8 MoAb (1:350) followed by identical second layer labeling as described above. The positive control consisted of HEL cells treated with 100 ng/mL TPO for 30 minutes. The cells were finally analyzed by means of an Axyophot Zeiss fluorescence microscopy.
For the flow cytometry analysis, samples containing 5 × 105 HEL cells were analyzed by FACScan for the expression of αIIbβ3 (Lysis II program; Becton Dickinson). Staining was performed in 200 μL of PBS containing 1% BSA, 0.1% azide, FITC-conjugated CD41a MoAb (at 1:20 dilution). Autofluorescence was assessed using cells treated only with 20% normal mouse serum for 10 minutes to block potentially unoccupied binding sites. Nonspecific fluorescence was assessed by using an isotype-matched control: anti-CD8 directly conjugated to FITC.
Plasmids and transfection experiments.
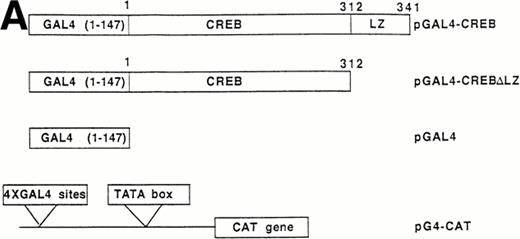
The following plasmids were a generous gift of Dr Enzo Lalli (IGBMC, Strasbourg, France): (1) pCRE-CAT (pSom-CAT) plasmid,24which represents the CRE motif of the rat somatostatin promoter cloned in front to the chloramphenicol acetyl-transferase gene in pBLCAT2 vector; (2) pBLCAT2 backbone vector; (3) pGAL4 encoding for amino acids 1 to 147 of GAL4 yeast protein (DNA binding domain); (4) pGAL4-CREBΔLZ, containing CREB protein fused to the COOH-terminus of GAL4 (1 to 147) except the COOH-terminal 29 amino acids that include the leucine repeat dimerization motif (LZ)25; (5) pGAL4-CREBΔLZM1, representing the pGAL4-CREBΔLZ with the Ser133 to Ala mutation; and (6) pG4-CAT,26containing 4 x GAL4 sites cloned in front of herpes simplex virus tymidine kinase promoter.
Transient transfection experiments were performed using the dethylaminoethyl (DEAE)-dextran method, as described previously.27 In single transfection experiments, 107 HEL cells were maintained in serum-free medium for at least 48 hours and then transfected with 10 μg of CRE-CAT or pBLCAT2 in 500 μg DEAE-dextran for 90 minutes. Twelve hours after transfection, cells were treated with various agonists or left untreated. Twenty-four hours after treatment, HEL cells were lysated and assayed for CAT activity using volumes of extract corresponding to equal protein amounts. Protein determination was performed by Bradford assay (Bio-Rad, Richmond, CA).
In cotransfection experiments, 107 HEL cells cultured in IMDM plus 10% FCS were transfected with 10 μg each of pG4-CAT plus pGAL4 or pGAL4-CREBΔLZ or pGAL4-CREBΔLZM1 in 500 μg DEAE-dextran for 90 minutes. Twelve hours after transfection, cells were treated with the same agonists described above for 24 hours and assayed for CAT activity.
Mitogen-activated protein kinase (MAPK) assay.
In some experiments, the kinase catalytic activity of MAP was analyzed in whole homogenates obtained from HEL cells treated for different time points with TPO (100 ng/mL) or Epo (4 IU/mL). For this purpose, the Biotrak kit p42/p44 MAPK enzyme assay system was used according to the manufacturer's instructions (Amersham). Radioactivity was counted in a liquid scintillation counter and expressed in cpm.
Statistical analysis.
Statistical analysis was performed using the two tailed Student'st-test.
RESULTS
Dose-dependence and time course of TPO-induced CREB-PSer133 in HEL cells.
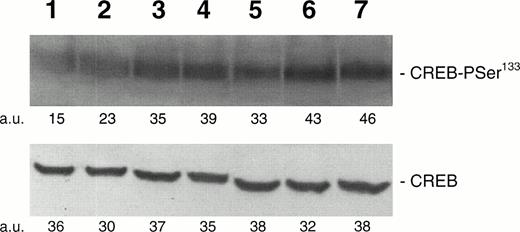
In the first group of experiments, we were interested in evaluating whether TPO was able to modulate the levels of CREB phosphorylation in the HEL hematopoietic cell line, which expresses the high-affinity TPO receptor, c-Mpl.23 For this purpose, HEL cells were cultured in a chemically defined serum-free medium for at least 72 hours, before adding TPO, to decrease the high levels of endogenous CREB-PSer133 observed in serum-containing cultures. HEL cells were then stimulated with increasing concentrations of TPO for 30 minutes, and cell homogenates were subjected to Western blot analysis with anti-CREB-PSer133 or anti-CREB sera. As shown in Fig 1, TPO induced a dose-dependent phosphorylation of a 43- to 46-kD band, corresponding to CREB-PSer133. Maximal CREB phosphorylation levels were reached at the TPO concentration of 100 ng/mL. Moreover, both FK (10−5 mol/L) and PMA (10−7 mol/L), which are known to activate PKA- and PKC-dependent pathways, respectively, produced a sharp induction of CREB-PSer133in HEL cells (Fig 1). When the membranes were stripped and reprobed with an anti-CREB polyclonal serum, no significant modifications in the total amounts of CREB were noticed upon TPO treatment. The intensity of the immunoreactive bands were quantified by densitometric analysis and expressed in arbitrary units (a.u.).
Western blot analysis of PSer133-CREB and total CREB protein performed in HEL cells stimulated for 30 minutes with increasing concentrations of TPO. Cells were treated with 0 (negative control, lane 1), 1 (lane 2), 10 (lane 3), 100 (lane 4), or 500 (lane 5) ng/mL of TPO; 10 μmol/L forskolin (lane 6); or 10−7 mol/L PMA (lane 7). A representative experiment with the densitometric analysis expressed in arbitrary units (a.u.) is shown. The mean PSer133-CREB densitometric values ± SD of five separate experiments were 13 ± 3.5 (lane 1), 21 ± 4.8 (lane 2), 36.5 ± 4.4 (lane 3), 41.5 ± 5.8 (lane 4), 32 ± 4.3 (lane 5), 45.6 ± 5.8 (lane 6), and 47.3 ± 5.1 (lane 7).
Western blot analysis of PSer133-CREB and total CREB protein performed in HEL cells stimulated for 30 minutes with increasing concentrations of TPO. Cells were treated with 0 (negative control, lane 1), 1 (lane 2), 10 (lane 3), 100 (lane 4), or 500 (lane 5) ng/mL of TPO; 10 μmol/L forskolin (lane 6); or 10−7 mol/L PMA (lane 7). A representative experiment with the densitometric analysis expressed in arbitrary units (a.u.) is shown. The mean PSer133-CREB densitometric values ± SD of five separate experiments were 13 ± 3.5 (lane 1), 21 ± 4.8 (lane 2), 36.5 ± 4.4 (lane 3), 41.5 ± 5.8 (lane 4), 32 ± 4.3 (lane 5), 45.6 ± 5.8 (lane 6), and 47.3 ± 5.1 (lane 7).
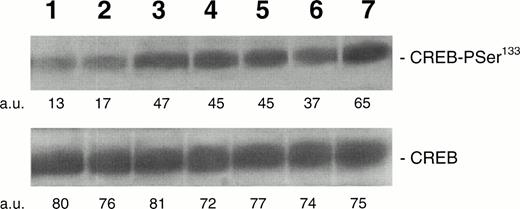
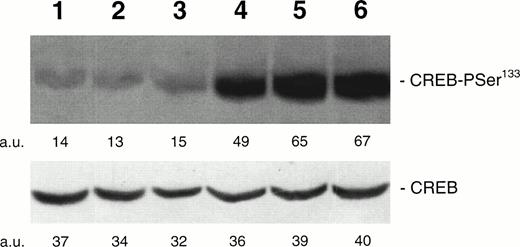
To determine the kinetics of CREB-PSer133 phosphorylation in response to TPO, we next performed a Western blot analysis and time course with HEL cells. TPO (100 ng/mL) induced a rapid increase of CREB-PSer133 phosphorylation, reaching the maximum level after 15 minutes and showing a decline towards basal levels after 60 minutes (Fig 2). In parallel experiments, it was possible to demonstrate that neither Epo (at concentrations up to 10 U/mL) nor hemin (0.1 μmol/L), two inducers of erythroid differentiation of HEL cells,22 28 were able to significantly stimulate CREB-Pser133 phosphorylation (Fig 3).
Western blot analysis of PSer133-CREB and total CREB protein performed in HEL stimulated with 100 ng/mL of TPO for various time points. Cells were left untreated (negative control, lane 1) or treated with 100 ng/mL of TPO for 1 (lane 2), 5 (lane 3), 15 (lane 4), 30 (lane 5), or 60 minutes (lane 6) or with 10−7 mol/L PMA for 30 minutes (lane 7). A representative experiment with the densitometric analysis expressed in arbitrary units (a.u.) is shown. The mean PSer133-CREB densitometric values ± SD of four separate experiments were 12.4 ± 2.9 (lane 1), 18.3 ± 3.9 (lane 2), 46.1 ± 5.1 (lane 3), 47.9 ± 6.3 (lane 4), 46 ± 5.2 (lane 5), 35.9 ± 5.2 (lane 6), and 62.3 ± 7.4 (lane 7).
Western blot analysis of PSer133-CREB and total CREB protein performed in HEL stimulated with 100 ng/mL of TPO for various time points. Cells were left untreated (negative control, lane 1) or treated with 100 ng/mL of TPO for 1 (lane 2), 5 (lane 3), 15 (lane 4), 30 (lane 5), or 60 minutes (lane 6) or with 10−7 mol/L PMA for 30 minutes (lane 7). A representative experiment with the densitometric analysis expressed in arbitrary units (a.u.) is shown. The mean PSer133-CREB densitometric values ± SD of four separate experiments were 12.4 ± 2.9 (lane 1), 18.3 ± 3.9 (lane 2), 46.1 ± 5.1 (lane 3), 47.9 ± 6.3 (lane 4), 46 ± 5.2 (lane 5), 35.9 ± 5.2 (lane 6), and 62.3 ± 7.4 (lane 7).
Western blot analysis of PSer133-CREB and total CREB protein performed in HEL stimulated with agonists inducing differentiation along either the megakaryocytic or erythroid lineages. Cells were either left untreated (negative control, lane 1) or treated for 30 minutes with 10 U/mL Epo (lane 2), 10−7 mol/L hemin (lane 3), 100 ng/mL TPO (lane 4), 10 μmol/L forskolin (lane 5), or 10−7 mol/L PMA for 30 minutes (lane 6). Results from one experiment representative of three separate experiments are shown. The densitometric analysis is expressed in arbitrary units (a.u.).
Western blot analysis of PSer133-CREB and total CREB protein performed in HEL stimulated with agonists inducing differentiation along either the megakaryocytic or erythroid lineages. Cells were either left untreated (negative control, lane 1) or treated for 30 minutes with 10 U/mL Epo (lane 2), 10−7 mol/L hemin (lane 3), 100 ng/mL TPO (lane 4), 10 μmol/L forskolin (lane 5), or 10−7 mol/L PMA for 30 minutes (lane 6). Results from one experiment representative of three separate experiments are shown. The densitometric analysis is expressed in arbitrary units (a.u.).
TPO inducibility of reporter genes with CRE-CAT or GAL4-CREB constructs in HEL cells.
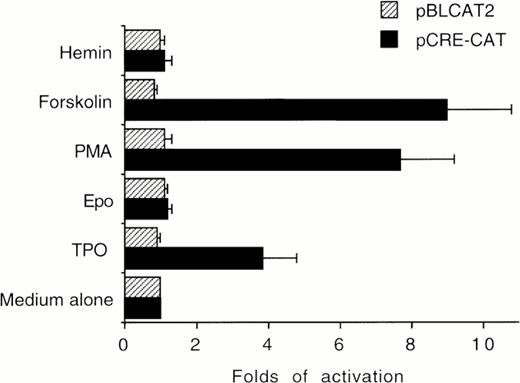
The ability of TPO to stimulate CRE-CAT expression in HEL cells was next evaluated in transient transfection experiments. TPO (100 ng/mL), FK (10−5 mol/L), and PMA (10−7mol/L), which induced phosphorylation of CREB, also induced a significant (P < .05) increase of CRE-dependent gene expression with respect to untreated HEL cells. On the other hand, Epo and hemin failed to significantly induce CRE-CAT transactivation (Fig 4).
HEL cells were cultured in the presence of medium alone (negative control), TPO (100 ng/mL), Epo (2 U/mL), hemin (10−7 mol/L), forskolin (10−5 mol/L), or PMA (10−7 mol/L) for 30 minutes. CRE-CAT promoter activity was measured as folds of activations with respect to HEL cells left untreated. Data are reported as the means ± SD of four independent transfection experiments performed in duplicate.
HEL cells were cultured in the presence of medium alone (negative control), TPO (100 ng/mL), Epo (2 U/mL), hemin (10−7 mol/L), forskolin (10−5 mol/L), or PMA (10−7 mol/L) for 30 minutes. CRE-CAT promoter activity was measured as folds of activations with respect to HEL cells left untreated. Data are reported as the means ± SD of four independent transfection experiments performed in duplicate.
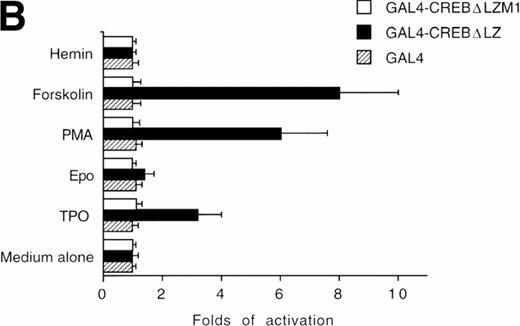
The ability of CREB to contribute to TPO signaling was further investigated by using a gene that encodes a chimeric protein in which CREB is fused at its amino terminus to the DNA-binding and dimerization domain of the yeast transcription factor, GAL41-147. We used a specific GAL4-CREB mutant (GAL4-CREBΔLZ) in which the CREB leucine zipper region was deleted while the GAL4 DNA-binding and dimerization domains remained intact. This mutant was used to rule out possible interactions of full-length CREB with cellular proteins containing leucine zipper regions. To determine the functional relevance of CREB Ser133 phosphorylation, we also performed transfection experiments using GAL4-CREBΔLZM1 construct, in which Ser133 was mutated to Ala. It has been previously shown that this specific mutation does not affect the stability of CREB protein or its nuclear localization.25 All cotransfection experiments were performed by using a CAT reporter plasmid (pG4-CAT) containing 4 GAL4 binding sites (Fig 5). In the presence of GAL4-CREBΔLZ, CAT reporter gene expression showed a threefold induction in HEL cells upon addition in culture of TPO and a sixfold to eightfold induction upon treatment with TPA or FK, whereas Epo and hemin failed to induce a significant increase of GAL4-CREBΔLZ transcription. In the presence of GAL4-CREBΔLZM1 mutant, all agonists failed to induce a significant activation of CAT expression. Moreover, TPO failed to induce transcription of the reporter gene if cells were transfected with only GAL41-147. Thus, TPO induction of pG4-CAT reporter gene required the CREB moiety of the GAL4-CREBΔLZ fusion protein.
Analysis of GAL4-CREB fusions by cotransfection assay. (A) Schematic structure of GAL4-CREB fusion constructs and reporter gene construct. (B) HEL cells were cotransfected with the pG4-CAT reporter gene and a gene encoding GAL4(1-147), GAL4-CREB▵LZ, or GAL4-CREB▵LZM1 and then treated with medium alone (negative control), TPO (100 ng/mL), Epo (2 U/mL), hemin (10−7 mol/L), forskolin (10−5 mol/L), or PMA (10−7 mol/L) for 30 minutes. CAT promoter activity was measured as folds of activations with respect to HEL cells left untreated (medium alone). Data are reported as the means ± SD of three independent transfection experiments performed in duplicate.
Analysis of GAL4-CREB fusions by cotransfection assay. (A) Schematic structure of GAL4-CREB fusion constructs and reporter gene construct. (B) HEL cells were cotransfected with the pG4-CAT reporter gene and a gene encoding GAL4(1-147), GAL4-CREB▵LZ, or GAL4-CREB▵LZM1 and then treated with medium alone (negative control), TPO (100 ng/mL), Epo (2 U/mL), hemin (10−7 mol/L), forskolin (10−5 mol/L), or PMA (10−7 mol/L) for 30 minutes. CAT promoter activity was measured as folds of activations with respect to HEL cells left untreated (medium alone). Data are reported as the means ± SD of three independent transfection experiments performed in duplicate.
The TPO-mediated induction of CREB-PSer133 in HEL cells is independent of both PKC and PKA pathways, but requires a functional MAPK pathway.
In the next series of experiments, some pharmacological inhibitors were used to explore whether PKC or PKA signal transduction pathways elicited by TPO/c-Mpl interactions were involved in the TPO-induced CREB phosphorylation.
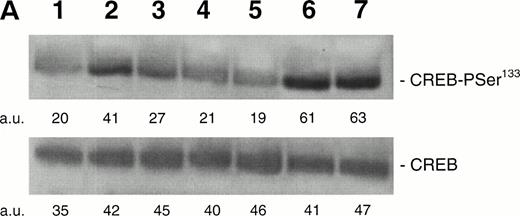
BIM and chelerytrine, on one hand, and H-89, on the other hand, were used at doses that efficiently blocked the PMA- and FK-mediated CREB-PSer133 phosphorylation in HEL cells (Fig 6). Pretreatment of HEL cells with BIM (10−6 mol/L), chelerytrine (10−4mol/L), or H-89 (10−6 mol/L) did not show any significant inhibition of the TPO-mediated induction of CREB-PSer133. This suggested that neither PKC nor PKA was involved in the intracellular signaling pathways evoked by TPO to stimulate the phosphorylation of CREB.
Western blot analysis of PSer133-CREB and total CREB protein, performed in HEL cells stimulated with 100 ng/mL TPO (lanes 1 through 4), 10−5 mol/L FK (lanes 5 and 6), 10−7 mol/L PMA (lanes 7 through 10), or left untreated (lane 11) in the absence (lanes 1, 5, 7, and 9) or presence of 10−6 mol/L BIM (lanes 2 and 8), 10−4 mol/L chelerythrine (lanes 3 and 10), or 10−6 mol/L H89 (lane 6). Results from one representative experiment of three separate experiments are shown. The densitometric analysis is expressed in arbitrary units (a.u.).
Western blot analysis of PSer133-CREB and total CREB protein, performed in HEL cells stimulated with 100 ng/mL TPO (lanes 1 through 4), 10−5 mol/L FK (lanes 5 and 6), 10−7 mol/L PMA (lanes 7 through 10), or left untreated (lane 11) in the absence (lanes 1, 5, 7, and 9) or presence of 10−6 mol/L BIM (lanes 2 and 8), 10−4 mol/L chelerythrine (lanes 3 and 10), or 10−6 mol/L H89 (lane 6). Results from one representative experiment of three separate experiments are shown. The densitometric analysis is expressed in arbitrary units (a.u.).
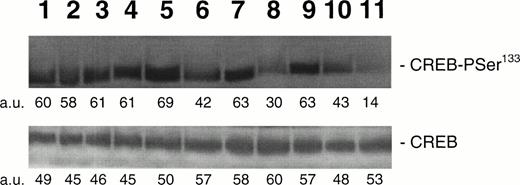
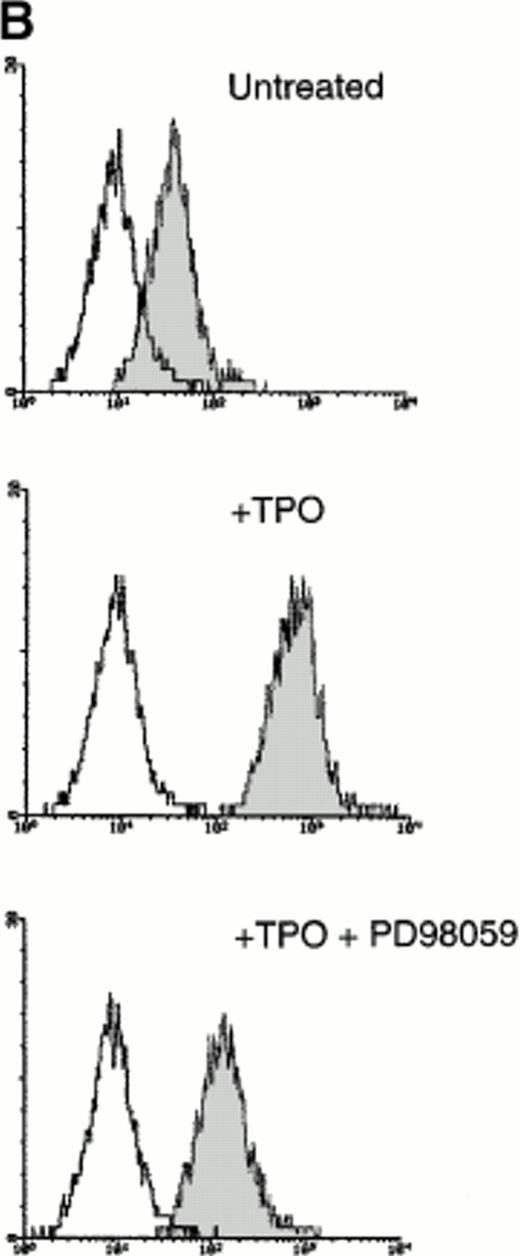
Because it has been demonstrated that CREB appears downstream from a variety of intracellular signal transduction pathways that activate tyrosine kinases and the Ras/Raf/MAPK pathway,9-12 in other experiments HEL cells were pretreated with PD98059 (10−5 mol/L to 5 × 10−5mol/L), a specific inhibitor of MAPKK, the upstream enzyme involved in the MAPK activation. At both concentrations, PD98059 specifically inhibited the TPO-mediated phosphorylation of CREB (Fig 7A). Moreover, in the presence of 5 × 10−5 mol/L PD98059, the TPO-mediated upregulation of surface αIIbβ3 in HEL cells was reduced to less than 50% of the level observed after TPO stimulation in the absence of the inhibitor (Fig 7B). As also reported by other investigators on different hematopoietic cell lines,29-31 PD98059 had no toxic effects on HEL cell viability and growth rate (data not shown). In agreement with previous findings of other investigators,31 these data indicate that the transcriptional induction of megakaryocyte markers require MAPK activation.
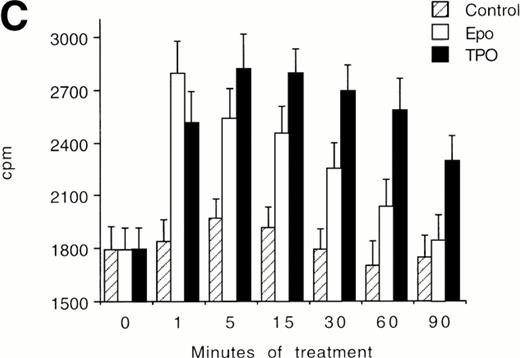
(A) Western blot analysis of PSer133-CREB and total CREB protein performed in HEL cells before (lane 1) and after stimulation with 100 ng/mL of TPO (lanes 2 through 5) or FK (lanes 6 and 7) in the absence (positive control, lane 2) or presence of 10−5 mol/L (lanes 3 and 7) or 5 × 10−5mol/L (lanes 4 and 5) of the specific MAPK inhibitor, PD 98059. Results from one representative experiment of three separate experiments are shown. The densitometric analysis is expressed in arbitrary units (a.u.). (B) Flow cytometric analysis of integrin αIIbβ3 receptors at the plasma membrane of HEL cells left untreated or treated for 48 hours with 100 ng/mL TPO in the absence or presence of 5 × 10−5 mol/L PD98059, which was readded in culture every 8 hours. Results from one representative experiment of three separate experiments are shown. X axis: relative αIIbβ3 (CD41a) fluorescence intensity. Y axis: relative cell number. (C) Assay of MAPK catalytic activity performed in HEL cells treated with TPO (100 ng/mL) or Epo (2 U/mL) for various time points. Data are expressed in cpm as the means ± SD of four separate experiments performed in duplicate.
(A) Western blot analysis of PSer133-CREB and total CREB protein performed in HEL cells before (lane 1) and after stimulation with 100 ng/mL of TPO (lanes 2 through 5) or FK (lanes 6 and 7) in the absence (positive control, lane 2) or presence of 10−5 mol/L (lanes 3 and 7) or 5 × 10−5mol/L (lanes 4 and 5) of the specific MAPK inhibitor, PD 98059. Results from one representative experiment of three separate experiments are shown. The densitometric analysis is expressed in arbitrary units (a.u.). (B) Flow cytometric analysis of integrin αIIbβ3 receptors at the plasma membrane of HEL cells left untreated or treated for 48 hours with 100 ng/mL TPO in the absence or presence of 5 × 10−5 mol/L PD98059, which was readded in culture every 8 hours. Results from one representative experiment of three separate experiments are shown. X axis: relative αIIbβ3 (CD41a) fluorescence intensity. Y axis: relative cell number. (C) Assay of MAPK catalytic activity performed in HEL cells treated with TPO (100 ng/mL) or Epo (2 U/mL) for various time points. Data are expressed in cpm as the means ± SD of four separate experiments performed in duplicate.
In an additional set of experiments, we found that TPO stimulated the catalytic activity of MAPK differentially from Epo (Fig 7C). In fact, the stimulation of MAPK peaked after 1 minute in Epo-treated cells and then rapidly decreased to the basal levels. On the other hand, MAPK activity was maximally stimulated between 5 and 15 minutes in TPO-treated cells and then showed a slow decline, maintaining significantly (P < .05) higher levels than in Epo-treated cells at 30 to 90 minutes.
TPO-mediated CREB phosphorylation in primary megakaryocytic cells derived in culture from CD34+ hematopoietic progenitors.
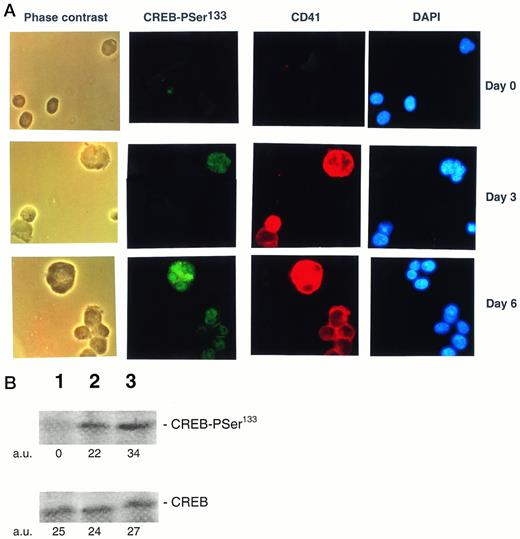
The TPO effect on CREB-PSer133 was also evaluated in freshly isolated CD34+ cells and primary megakaryocytes. CD34+ hematopoietic progenitor cells showed an absence or very low immunoreactivity to the anti-CREB-PSer133 serum by both in situ immunocytochemistry (Fig8A) and Western blot (Fig 8B) analyses. On the other hand, the addition of 100 ng/mL of TPO, which drives a unilineage differentiation along the megakaryocytic pathway in liquid culture suspension cultures,32 induced the appearance of CREB-PSer133 specific immunofluorescence in a progressively increasing number of cells (Fig 8A). At early culture times (day 3), megakaryocytic cells staining positively for αIIbβ3 also showed a clear-cut nuclear positivity for CREB-PSer133. It is noteworthy that morphologically recognizable megakaryocytes with polylobated nuclei, appearing in culture at later time points (from day 6 onwards), stained positively for both cytoplasmic αIIbβ3 and nuclear CREB-PSer133 (Fig 8A). At this time point, an immunoreactive band corresponding to CREB-PSer133 was also noticed at Western blot analysis (Fig 8B).
(A) Analysis of PSer133-CREB and αIIbβ3 by double (anti-PSer133-CREB polyclonal rabbit serum + anti-CD41a MoAb followed by GAR-FITC + GAM-TRIC) immunofluorescence in primary CD34+hematopoietic progenitor cells stimulated for various days with 100 ng/mL of TPO. CD34+ cells were analyzed immediately after purification (day 0) and after 3 or 6 days of liquid culture in the presence of 100 ng/mL of TPO by either phase contrast or fluorescence microscopy. Green nuclear fluorescence corresponds to PSer133-CREB positivity, red membrane and cytoplasmic fluorescence corresponds to CD41a positivity, and blue fluorescence corresponds to nuclear counterstaining with DAPI. Results from one representative experiment of three separate experiments are shown. (B) Western blot analysis of PSer133-CREB and whole CREB protein performed in CD34+ hematopoietic progenitor cells immediately after purification (lane 1) or after 6 days of liquid culture in the presence of 100 ng/mL of TPO (lane 2). The positive control is represented by HEL cells (lane 3) treated with TPO for 30 minutes. The densitometric analysis is expressed in arbitrary units (a.u.).
(A) Analysis of PSer133-CREB and αIIbβ3 by double (anti-PSer133-CREB polyclonal rabbit serum + anti-CD41a MoAb followed by GAR-FITC + GAM-TRIC) immunofluorescence in primary CD34+hematopoietic progenitor cells stimulated for various days with 100 ng/mL of TPO. CD34+ cells were analyzed immediately after purification (day 0) and after 3 or 6 days of liquid culture in the presence of 100 ng/mL of TPO by either phase contrast or fluorescence microscopy. Green nuclear fluorescence corresponds to PSer133-CREB positivity, red membrane and cytoplasmic fluorescence corresponds to CD41a positivity, and blue fluorescence corresponds to nuclear counterstaining with DAPI. Results from one representative experiment of three separate experiments are shown. (B) Western blot analysis of PSer133-CREB and whole CREB protein performed in CD34+ hematopoietic progenitor cells immediately after purification (lane 1) or after 6 days of liquid culture in the presence of 100 ng/mL of TPO (lane 2). The positive control is represented by HEL cells (lane 3) treated with TPO for 30 minutes. The densitometric analysis is expressed in arbitrary units (a.u.).
DISCUSSION
The CRE consensus sequence (5′-TGACGTCA-3′) is recognized by a family of DNA binding proteins, the CREB/ATF superfamily, consisting of a dozen or so different proteins, which share a carboxyl-terminus containing a leucine zipper dimerization domain juxtaposed to a DNA binding domain rich in basic amino acids. With this group, CREB heterodimerizes with activating transcription factor 1 (ATF-1) and CRE modulator protein (CREM).33 CREB, CREM, and ATF-1 are strongly related in sequence and form a subgroup within the CREB/ATF superfamily of transcription factors. The best studied CRE binding protein is the 43- to 46-kD protein termed CREB, whose expression is constitutive. In fact, CREB activation results from posttranslational modifications such as phosphorylation of Ser133 in situ. This phosphorylation step is critical for trans-activation of a CRE-dependent promoter.21
In this study, we have demonstrated that PMA and FK, two pharmacological inducers of megakaryocyte differentiation,15-20 as well as TPO stimulate the rapid phosphorylation of CREB on Ser133 in the HEL cell line. High levels of CREB-PSer133 were also observed in primary megakaryocytes derived from CD34+ hematopoietic progenitor cells cultured in the presence of 100 ng/mL TPO, supporting the hypothesis that phosphorylation events of transcription factors such as CREB mediate signaling pathways of forskolin, PMA, and TPO in hematopoietic cells. The increased functional activity of CREB-PSer133 in HEL cells stimulated with PMA, forskolin, or TPO was then demonstrated by transient transfection experiments with CRE-CAT and GAL4-CREBΔLZ plasmid constructs. Experiments performed with pharmacological inhibitors showed that, whereas PMA15-18 and forskolin19,20 stimulate megakaryocyte differentiation through PKC and PKA pathways, respectively, the PKC inhibitors BIM and chelerytrine and the PKA inhibitor H-89 failed to show any inhibition on the TPO-induced CREB phosphorylation. These results clearly suggested that CRE binding transcription factors may have an important regulatory role in mediating the biological activities of TPO via a mechanism that is not PKC or PKA dependent. Although the role of CREB in mediating transcriptional activation induced by PKA is well established,21 recent studies performed in both neuronal and hematopoietic cells have shown that CREB appears downstream from a variety of intracellular signal transduction pathways that activate receptor tyrosine kinases. In particular, a novel RSK2/CREB kinase has been recently identified downstream of the Ras/Raf/MAPK pathway.34 In this respect, it is noteworthy that binding of TPO to its high-affinity c-Mpl receptor results in a specific activation of this pathway.9-11 Moreover, Whalen et al31 have recently demonstrated that constitutive activation of MAPK selectively induced megakaryocyte differentiation in the pluripotent K562 hematopoietic cell line.
Nevertheless, it has also been shown that Epo is able to activate a MAPK pathway in several Epo-dependent cell lines.35,36 We likewise found that both TPO and Epo were able to stimulate the catalytic activity of MAPK in HEL cells, although the activation of MAPK was delayed and more prolonged in the presence of TPO. Beside TPO, interleukin-3 (IL-3), a cytokine with a well-known pleiotropic activity on various hematopoietic lineages, also stimulates CREB-PSer133 phosphorylation in the TF-1 hematopoietic cell line.37 Thus, neither MAPK activation nor CREB-PSer133 phosphorylation can be considered specific of the TPO signaling. However, the ability of both pharmacological and physiological inducers of megakaryocyte differentiation to stimulate CREB-PSer133 phosphorylation clearly suggests that the regulation of the transcriptional activity of CREB and/or of other members of the CREB/ATF family plays a potential physiological role in megakaryocyte development. In this respect, another relevant conclusion of our study is that neither Epo nor hemin, which induce erythroid differentiation in HEL cells,22 28 was able to significantly stimulate CREB-PSer133 phosphorylation.
It has also been claimed that Epo can promote megakaryocyte development, but its role on megakaryocytopoiesis and platelet production remains controversial. Despite some in vitro evidence suggesting that Epo induces megakaryocyte maturational effects,38,39 only transient increases in platelets have been noticed in vivo both in animal models40 and in patients with chronic renal failure treated in a large phase III Epo study.41 Moreover, it has been reported that a chronic treatment with high doses of Epo caused thrombocytopenia in mice,42 possibly through the recruitment of common erythroid/megakaryocyte progenitors towards the erythroid differentiation pathway.43
Our findings are particularly remarkable in light of a tight linkage between the megakaryocyte and erythroid lineages. In fact, all human leukemic cell lines described as erythroleukemic or megakaryoblastic or even primary erythroleukemic cells express erythroid and megakaryocytic-specific markers at a single cell level,44,45 and a common bipotent megakaryocytic and erythroid progenitor has been recently characterized in human bone marrow.43 Moreover, several transcription factors, such as GATA-1, Tal-1, and NFE-2, are shared by both lineages.46-48Thus, a CREB-dependent activation of promoters showing the CRE-containing motif induced by TPO through MAPK or even through PKC or PKA activators might be a means by which megakaryocyte commitment is enforced in bipotent erythroid/megakaryocyte progenitors.
Supported by the “Blood Project” of the Italian Ministry of Health and by AIRC.
Address reprint requests to Giorgio Zauli, MD, PhD, Institute of Human Anatomy, University of Ferrara, Via Fossato di Mortara 66, 44100 Ferrara, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal