Abstract
Protein kinase C (PKC) activity has a recognized role in mediating apoptosis. However, the role of individual PKC isoforms in apoptosis is poorly defined. Therefore, we investigated the translocation of individual PKC isoforms during radiation-induced apoptosis with and without rescue from apoptosis by granulocyte-macrophage colony-stimulating factor (GM-CSF) in the human erythroleukemia cell line TF-1. PKCα was translocated from the particulate to cytosolic fraction of TF-1 cells within 5 minutes of treatment with apoptosis-inducing levels of ionizing radiation. However, this postirradiation translocation did not occur when cells were rescued from apoptosis by GM-CSF. Furthermore, treatment of cells with Gö6976, an inhibitor of classical PKC isoforms, abrogated the rescue effect of GM-CSF. The calcium-independent novel PKC isoform, PKCδ appeared to be degraded in both the particulate and cytosolic fractions of TF-1 cells after treatment with apoptosis-inducing levels of ionizing radiation in either the presence or absence of GM-CSF rescue. Levels of ceramide, a lipid mediator of apoptosis, were measured at 2, 4, 8, 10, and 60 minutes after treatment with ionizing radiation and were substantially reduced in TF-1 cells rescued from apoptosis by GM-CSF compared with apoptotic TF-1 cells. The largest decrease in ceramide production seen was at 4 minutes postirradiation, with a 46% reduction in ceramide levels in TF-1 cells rescued from apoptosis by GM-CSF compared with those in apoptotic TF-1 cells. Because ceramide has been shown to affect PKCα subcellular distribution, these data implicate a role for ceramide in mediating the rapid postirradiation translocation and inhibition of PKCα in TF-1 cells not rescued from apoptosis by GM-CSF. Expression of the antiapoptotic protein Bcl-2 doubled in TF-1 cells rescued from apoptosis by GM-CSF, but did not increase in unrescued cells. Our findings suggest that activated PKCα and increased expression of Bcl-2 after γ irradiation determine survival in TF-1 cells rescued from apoptosis with GM-CSF and that PKCδ plays a role in mediating signals involved in sensing cellular damage and/or regulation of cell damage repair.
PROTEIN KINASE C (PKC) is a family of serine/threonine kinases with closely related structure and function. The 12 known members of the PKC family are divided into three categories on the basis of their activation requirements: the classical PKCs (cPKC), which are Ca2+-dependent and phorbol ester-requiring, (the α, β1, β2, and γ isoforms); the novel PKCs (nPKC), which are Ca2+-independent, but still require phorbol ester for optimal activity, (the δ, ε, η, and θ isoforms); and the atypical PKCs (aPKCs), which are Ca2+-independent and phorbol ester-independent, but are designated as PKC based on their kinase activity and sequence homology. PKC acts as an intracellular mediator in a wide variety of cellular processes including growth factor activation,1 hormonal response,2 neurotransmission,3 cell cycle regulation,4,5 proliferation,6-8differentiation,9-11 and tumor promotion.12Additionally, expression of PKC isoforms is highly dependent on cell type and differentiation state.13-16 For these reasons, it is hypothesized that individual PKC isoforms mediate a variety of biological effects.
PKC is known to activate c-Ras, c-Raf, and c-Mos, all of which are associated with radioresistance, thus implicating PKC signaling pathways in radiation-induced cellular responses and tumor promotion.17,18 Furthermore, PKC-mediated signaling pathways have been implicated during apoptosis induced by a wide variety of environmental stimuli including ionizing radiation.19-26 Additionally, the effect of activating or inhibiting PKC on cellular sensitivity to apoptosis is cell type and differentiation state-dependent.27-29 Because PKC isoform expression is also cell type and differentiation state-dependent,14-16 it is likely that the cell-specific nature of the apoptotic response to PKC activation and/or inhibition is the result of alterations in the expression and/or activity of individual PKC isoforms.
Recent studies suggest a role for individual PKC isoforms in mediating apoptosis-associated phenomena: growth factor rescue from apoptosis,26 ceramide-induced apoptosis,30 and alterations in Bcl-2 expression.31 To identify a role for individual PKC isoforms in mediating apoptosis, we investigated the subcellular distribution and protein levels of PKC isoforms during ionizing radiation-induced apoptosis and rescue from apoptosis by growth factor, granulocyte-macrophage colony-stimulating factor (GM-CSF). We demonstrated that PKCα was translocated from the particulate to cytosolic fraction during apoptosis in the human leukemia cell line TF-1, and that this translocation did not occur when cells were rescued from apoptosis by the addition of growth factor. These data identify a role for PKCα in control of TF-1 cell survival and/or entry into apoptosis. Our data also identify differences in ceramide levels between TF-1 cells dependent on whether cells were incubated in the absence or presence of GM-CSF after treatment with ionizing radiation. Because ceramide has been shown to affect PKCα activity and/or translocation and because the changes in ceramide were concomitant with changes in PKCα, these data support an interaction between ceramide and PKCα during the early stages of apoptosis. Furthermore, our data suggest that PKCα might regulate apoptosis by altering Bcl-2 levels, because increased Bcl-2 expression was seen with rescue from apoptosis by GM-CSF. Additionally, PKCδ appears to be degraded in both the particulate and cytosolic fractions of TF-1 cells after exposure to ionizing radiation in either the presence or absence of GM-CSF rescue at 1 and 6 hours postirradiation. Because PKCδ was degraded in both apoptotic and GM-CSF rescued TF-1 cells, this suggests a role for PKCδ in the cellular response to radiation-induced damage, rather than in direct control of the apoptotic process.
MATERIALS AND METHODS
Cell Culture
TF-1 cells were cultured in RPMI 1640 with 20% fetal bovine serum and 50 U/mL GM-CSF (Sandoz, Houston, TX). Cells were grown at 37°C in a humidified 5% CO2 incubator.
Protein Determination
Protein of all samples was determined using the Bicinchoninic protein assay kit from Pierce (Rockford, IL) with bovine serum albumin as the protein standard.
Preparation of Whole Cell Lysates
A total of 10 × 106 cells were washed once in cold phosphate-buffered saline (PBS). Cell pellets were suspended in sample loading buffer (62.5 mol/L Tris-HCl, pH 6.8, 2% [wt/vol] sodium dodecyl sulfate [SDS], 10% [vol/vol] glycerol, 5% [vol/vol] β-mercaptoethanol, 0.01% [wt/vol] bromophenol blue), and sonicated for 1 minute on ice.
Preparation of Cellular Extracts
Preparation of cytosolic and particulate fractions for immunoblotting.
Cytosolic and membrane fractions were prepared as described32,33 with modifications. A total of 15 × 106 cells were washed in cold PBS to remove growth factor and serum. Cells were resuspended in a hypotonic lysis buffer (20 mmol/L Tris-HCl, pH 7.4, 2 mmol/L EGTA, 1 mmol/L EDTA, 10 mg/mL leupeptin, 10 mg/mL aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF]) and passed 10 times through a 25-gauge needle on ice. The resultant cell lysate underwent two rounds of low speed centrifugation, first at 1,000g and then at 2,000g, at 4°C for 10 minutes each to remove the nuclear fraction. The supernatant was centrifuged at 60,000g for 1 hour at 4°C, the pellet of which comprises the particulate fraction and the supernatant of which contains the cytosolic fraction. Proteins were extracted from the particulate fraction by the addition of hypotonic lysis buffer with 1% Triton X-100 and gentle agitation.
Preparation of nuclear proteins for immunoblotting.
Nuclei were isolated by an adapted method of Neumann et al.34 A total of 10 × 106 cells were washed once in cold sucrose-TKM buffer (0.25 mol/L sucrose, 0.05 mol/L Tris-HCl, pH 7.5, 0.025 mol/L KCl, 0.005 mol/L MgCl2) and resuspended in sucrose-TKM buffer containing 0.1% (wt/vol) Triton X-100 and 1.0 mmol/L PMSF. Cells were disrupted by Dounce homogenization on ice and nuclei harvested at 1,000g at 4°C. Nuclei were washed in cold sucrose-TKM buffer and stored at −70°C. To extract nuclear proteins, nuclei were resuspended at 2 × 107 nuclei/mL high salt buffer (0.5 mol/L NaCl, 0.05 mol/L MgCl2, 0.1 mol/L Tris-HCl, pH 7.5, 1.0 mmol/L PMSF, 25 mg/mL DNase, 12.5 mg/mL RNase, 1 mg/mL aprotinin, 2 mg/mL leupeptin), incubated at 37°C for 20 minutes, and chilled on ice. Extracts were centrifuged at 12,000g at 4°C to remove nuclear debris and supernatants were stored at −70°C.
Immunoblotting
The samples (50 μg protein) were denatured by boiling in Laemmli sample buffer for 3 minutes and separated by electrophoresis on a 7.5% polyacrylamide gel and transferred electrophoretically to a nitrocellulose membrane. The membrane was blocked with a PBS-Tween (0.05%) solution with 5% (wt/vol) low fat dry milk at 4°C overnight. The membrane was incubated with a solution of recombinant rabbit anti-PKC isoform-specific antibody (1:100 dilution; Oxford, Oxford, MI) or goat antihuman Bcl-2 antibody (1:2,000 dilution; generous gift of Dr John C. Reed, Burnham Institute, La Jolla, CA) and incubated for 2 hours at room temperature (RT). Blots were washed in PBS-Tween solution and incubated with goat antirabbit antibodies conjugated to horseradish peroxidase (1:30,000 dilution; Oxford) for 30 minutes at RT. Following four washes with PBS-Tween solution, immunoreactive proteins were detected using the ECL chemiluminescence system (Amersham, Arlington Heights, IL) and recorded by fluorography on Hyperfilm (Amersham), according to the manufacturer's instructions. In some cases, the fluorograms were quantitated using a BioRAD (Hercules, CA) GS-670 laser densitometer and the Molecular Analyst software program (BioRAD).
Cell Irradiation
Cells were irradiated at a rate of 6.4 Gy/minute in a GammaCell127Ce irradiator. Just before irradiation, TF-1 cells in mid- to late-logarithmic growth were washed once in PBS and resuspended in normal growth media lacking GM-CSF or containing GM-CSF (100 U/mL) as indicated. Following irradiation, cells were incubated at 37°C at a cell density of 2.5 × 105 cells/mL for 6 hours (for DNA fragmentation analysis) or 48 hours (for cytochemical detection of apoptosis) unless otherwise indicated.
Electrophoretic Examination of DNA Fragmentation
DNA fragmentation analysis was performed as described35with modifications. A total of 4 × 106 TF-1 cells were resuspended in Nicolletti lysis buffer (10 mmol/L Tris-HCl, 100 mmol/L NaCl, 1 mmol/L EDTA, 1% [wt/vol] SDS) to which fresh proteinase K (1 mg/mL) had been added and incubated overnight at 45°C. The digest was heated to 55°C for 1 hour and nucleic acids phenol/chloroform extracted. Nucleic acids were subjected to RNase A (0.15 mg/mL) digestion for 1 to 2 hours at 37°C. DNA was phenol/chloroform extracted, concentrated with ethanol and separated on a 2% agarose-TBE gel at 80 V for 2 hours. DNA was stained with ethidium bromide (1 mg/mL) and visualized on a UV transilluminator.
Cytochemical Detection of Apoptotic Cells
Cells undergoing apoptosis were detected based on the morphologic changes seen in the nuclear chromatin associated with the apoptotic process. Nuclei were visualized using the DNA-binding fluorochrome bisbenzimide (Hoechst 33258; Sigma, St Louis, MO). Cells were stained as described36 with modifications. A total of 3.0 × 106 cells were harvested at 300g for 10 minutes and washed one time in PBS. The cell pellet was resuspended in 50 mL 10% formalin in PBS and incubated for 10 minutes at RT. Fixed cells were then washed one time in PBS, resuspended in 15 mL of the fluorescent dye bisbenzimide (50 mg/mL in PBS), and incubated for 15 minutes at RT. Cells were stored at 4°C in the dark until use. Cells were examined for apoptotic morphology using an Olympus BH-2 (Melville, NY) fluorescent microscope fitted with appropriate filters using Oncor (Gaithersburg, MD) visual systems software. Cells displaying nuclear fragmentation and chromatin condensation with an intact plasma membrane were considered apoptotic. Significance of differences in the numbers of apoptotic versus nonapoptotic cells between groups were tested by χ2 analysis.
Measurement of Ceramide 1-Phosphate Levels
Extraction and measurement of ceramide were performed as described37 by assay of 32P incorporated upon phosphorylation of ceramide to ceramide 1-P by diacylglycerol (DAG) kinase. Lipids were extracted by the method of Bligh and Dyer.38 Lipids were dissolved in chloroform, and samples corresponding to 200 mg were stored under N2 until the time of assay. Ceramide stored within the organic phase of the extract was resuspended in 20 mL of 7.5% α-octyl-β-D-glucopyranoside; 5 mmol/L cardiolipin; 1 mmol/L diethylenetriamine pentacetic acid (Sigma). Thereafter, 40 μL of purified DAG kinase in enzyme buffer (dithiothreitol; 1.5 mol/L NaCl, 250 mmol/L sucrose, and 15% glycerol, pH 7.4) was added to the organic phase extract. [γ32P]adenosine triphosphate (ATP) (20 μL 10 mmol/L; 1,000 dpm/pmol), in buffer, was added to start the reaction. After 30 minutes at 22°C, the reaction was stopped by extraction of lipids with 1 mL of chloroform:methanol:hydrochloric acid (100:100:1; vol/vol). Buffered saline solution (170 mL; 135 mmol/L NaCl; 1.5 mmol/L CaCl2, 0.5 mmol/L MgCl2, 5.6 mmol/L glucose, and 10 mmol/L HEPES, pH 7.2) and 30 mL of 100 mmol/L EDTA were added. The lower organic phase was dried under N2. Ceramide 1-P was resolved by TLC using CHCl3:CH3OH:acetic acid (65:15:5, vol/vol) as solvent, detected by autoradiography, and the incorporated 32P was quantified by phosphoimaging (Fugi BAS1000; Fugi Medical Systems, New Castle, DE). The level of ceramide 1-phosphate was determined by comparison to a concomitantly run standard composed of known amounts of ceramide.
RESULTS
GM-CSF Suppresses Ionizing Radiation-Induced Apoptosis in TF-1 Cells
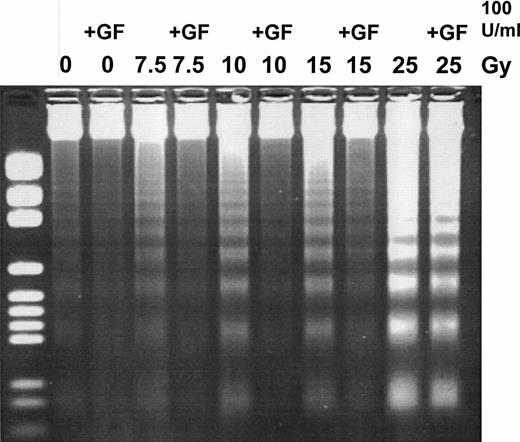
Because TF-1 cells can be rescued from growth factor deprivation-induced apoptosis by the addition of interleukin-3 (IL-3),39 we initially investigated whether TF-1 cells could be rescued from ionizing radiation-induced apoptosis by the addition of GM-CSF, another growth factor on which TF-1 cells are dependent for proliferation. Ionizing radiation induced apoptosis in TF-1 cells in a dose-dependent manner, as demonstrated by DNA fragmentation and cytochemical analysis (Fig 1 and Table 1, respectively). The addition of GM-CSF (100 U/mL) to culture medium at the time of irradiation led to significant rescue of TF-1 cells from radiation-induced apoptosis, as demonstrated by χ2 analysis of apoptosis induction between cells treated with the same dose of radiation in either the presence or absence of GM-CSF (Table 1). GM-CSF produced significant rescue of cells from apoptosis even at doses of radiation up to 25 Gy (P < .0001; Table 1). The concentration of GM-CSF necessary to rescue cells from apoptosis was twice that used for routine cell culture. GM-CSF deprivation alone did not produce significant increases in apoptosis between nonirradiated cells cultured in either the presence or absence of GM-CSF (P = .1269; Table 1).
Rescue of TF-1 cells from apoptosis with GM-CSF. TF-1 cells were treated with γ radiation (0, 7.5, 10, 15, or 25 Gy) at a rate of 6.41 Gy/minute in either the presence or absence of GM-CSF (100 U/mL) in complete RPMI supplemented with 20% FBS. Cells were subsequently incubated for 6 hours in complete RPMI supplemented with 20% FBS in either the presence or absence of growth factor. Postincubation, DNA was harvested for DNA fragmentation analysis. DNA was separated at 80 V for 2 hours on a 2% agarose-TBE gel, stained with EtBr, and visualized using UV transmission. This is a representative gel of three experiments.
Rescue of TF-1 cells from apoptosis with GM-CSF. TF-1 cells were treated with γ radiation (0, 7.5, 10, 15, or 25 Gy) at a rate of 6.41 Gy/minute in either the presence or absence of GM-CSF (100 U/mL) in complete RPMI supplemented with 20% FBS. Cells were subsequently incubated for 6 hours in complete RPMI supplemented with 20% FBS in either the presence or absence of growth factor. Postincubation, DNA was harvested for DNA fragmentation analysis. DNA was separated at 80 V for 2 hours on a 2% agarose-TBE gel, stained with EtBr, and visualized using UV transmission. This is a representative gel of three experiments.
Cytochemical Analysis of Rescue of Cells From Apoptosis With GM-CSF
| Treatment of Cells . | No. of Nonapoptotic Cells . | No. of Apoptotic Cells . |
|---|---|---|
| Without GM-CSF | ||
| 0 Gy | 949 | 551 |
| 5 Gy | 783 | 717* |
| 10 Gy | 695 | 805* |
| 15 Gy | 579 | 921* |
| 25 Gy | 506 | 994* |
| With GM-CSF | ||
| 0 Gy | 989 | 511-151P = .1269 |
| 5 Gy | 855 | 645*-151P = .0083 |
| 10 Gy | 858 | 642*-151P < .0001 |
| 15 Gy | 706 | 794*-151P = .0068 |
| 25 Gy | 718 | 782*-151P < .0001 |
| Treatment of Cells . | No. of Nonapoptotic Cells . | No. of Apoptotic Cells . |
|---|---|---|
| Without GM-CSF | ||
| 0 Gy | 949 | 551 |
| 5 Gy | 783 | 717* |
| 10 Gy | 695 | 805* |
| 15 Gy | 579 | 921* |
| 25 Gy | 506 | 994* |
| With GM-CSF | ||
| 0 Gy | 989 | 511-151P = .1269 |
| 5 Gy | 855 | 645*-151P = .0083 |
| 10 Gy | 858 | 642*-151P < .0001 |
| 15 Gy | 706 | 794*-151P = .0068 |
| 25 Gy | 718 | 782*-151P < .0001 |
*Based on χ2 values, represents a P < .0001 when compared with unirradiated cells treated with or without GM-CSF (100 U/mL).
Represents P values as shown when compared with samples irradiated with the same dose of radiation in the absence of GM-CSF rescue.
Effects of Ionizing Radiation-Induced Apoptosis and Rescue From Apoptosis on PKC Isoform Expression and Subcellular Distribution
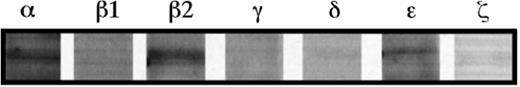
To assess which PKC isoforms might be associated with ionizing radiation-induced apoptosis and rescue from apoptosis by GM-CSF in TF-1 cells, PKC isoform-specific activity was measured indirectly by monitoring PKC isoform translocation between cytosolic and particulate fractions. TF-1 cells predominantly express the α, β2, δ, ε, and ζ isoforms of PKC, but do not express the β1 or γ isoforms of PKC, as determined by immunoblot (Fig 2). Therefore, subcellular distribution and levels of PKCα, β2, δ, ε, and ζ were determined by immunoblot at 5 minutes, and 1 and 6 hours postirradiation in either the presence or absence of rescuing levels of GM-CSF. Cells were irradiated with 5 Gy γ radiation, which was the lowest dose of radiation tested that resulted in apoptotic induction and from which TF-1 cells could be rescued from apoptosis by GM-CSF (Fig 1 and Table 1). While there were no consistent observable changes in the subcellular distribution or levels of PKCβ2, ε, or ζ with irradiation or rescue from radiation-induced apoptosis by GM-CSF (data not shown), there were reproducible changes in the subcellular distribution of PKCα and in cytosolic and particulate PKCδ levels.
PKC isoform expression in TF-1 cells. TF-1 whole cell lysates were analyzed for expression of PKCα, β1, β2, γ, δ, , and ζ isoforms by immunoblotting. This is a representative blot of two experiments.
PKC isoform expression in TF-1 cells. TF-1 whole cell lysates were analyzed for expression of PKCα, β1, β2, γ, δ, , and ζ isoforms by immunoblotting. This is a representative blot of two experiments.
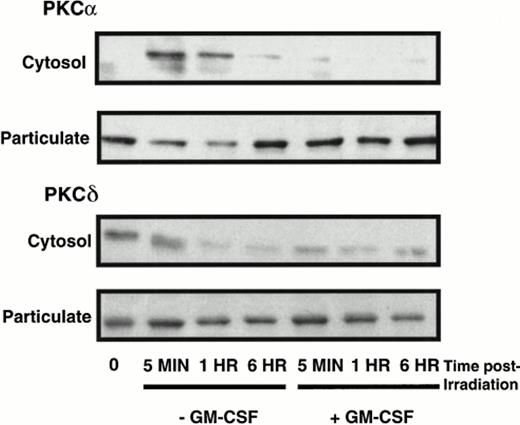
Under normal culture conditions, ie, in the presence of GM-CSF, PKCα resided predominantly in the particulate fraction (Fig 3). After treatment of TF-1 cells with γ radiation in the absence of GM-CSF rescue, PKCα was translocated from the particulate to cytosolic cell fraction at 5 minutes and was gradually translocated back to the particulate fraction by 6 hours. The level of PKCα in the cytosolic fraction increased at 5 minutes by 2.05- ± 0.88-fold when compared with unirradiated control cells incubated in the absence of GM-CSF, as determined by densitometric analysis. This change was concomitant with a decrease in PKCα levels in the particulate fraction to 78.0% ± 6.1% of control levels at 5 minutes postirradiation. By 6 hours, PKCα levels in the cytosolic and particulate fractions of cells irradiated in the absence of growth factor returned to levels comparable to those of unirradiated control cells incubated in the absence of growth factor and harvested at the same time. The immediate translocation of PKCα to the cytosolic fraction seen in irradiated TF-1 cells incubated in the absence of GM-CSF was not seen in cells that were irradiated in the presence of GM-CSF (Fig 3). PKCα levels remained relatively unchanged in the particulate fractions of TF-1 cells irradiated in the presence of GM-CSF when compared with control cells, which had not been irradiated, but were harvested at the same times. These results were consistent for three immunoblots.
The effect of apoptosis on PKCα and δ distribution in cytosolic and particulate fractions. TF-1 cells were treated with γ radiation (5 Gy), incubated in either the presence or absence of GM-CSF (100 U/mL) in complete RPMI supplemented with 20% FBS, and harvested at 5 minutes and 1 and 6 hours postirradiation. Nonnuclear cytosolic and particulate fractions were analyzed for PKCδ isoform expression by immunoblotting. This is an autoradiograph of a representative blot of three showing PKC expression.
The effect of apoptosis on PKCα and δ distribution in cytosolic and particulate fractions. TF-1 cells were treated with γ radiation (5 Gy), incubated in either the presence or absence of GM-CSF (100 U/mL) in complete RPMI supplemented with 20% FBS, and harvested at 5 minutes and 1 and 6 hours postirradiation. Nonnuclear cytosolic and particulate fractions were analyzed for PKCδ isoform expression by immunoblotting. This is an autoradiograph of a representative blot of three showing PKC expression.
PKCδ levels decreased in both the cytosolic and particulate fractions of TF-1 cells irradiated in either the presence or absence of GM-CSF rescue (Fig 3). By 1 hour postirradiation, PKCδ levels in the cytosolic and particulate fractions of TF-1 cells irradiated in the absence of GM-CSF decreased to 61% ± 2.7% and 71% ± 9.7%, respectively, of those in the same fractions of unirradiated control cells treated otherwise in the same manner, as determined by densitometric analysis of immunoblots (n = 3). Similar decreases were seen in the cytosolic and particulate fractions of TF-1 cells irradiated in the presence of GM-CSF. At 1 hour postirradiation, cytosolic levels of PKCδ of TF-1 cells irradiated in the presence of GM-CSF decreased to 69.0% ± 3.2% of those of control cells and those in the particulate fraction dropped to 49.0% ± 6.5% of PKCδ levels in control cells. PKCδ levels continued to decrease up to 6 hours posttreatment in both cellular fractions of cells irradiated either in the presence or absence of GM-CSF rescue. These changes were consistent for three immunoblots. Similar decreases in PKCδ levels were seen in nuclear fractions and whole cell lysates of PKCδ at 1 and 6 hours postirradiation (data not shown).
Effect of Ionizing Radiation Induced-Apoptosis on Ceramide Production
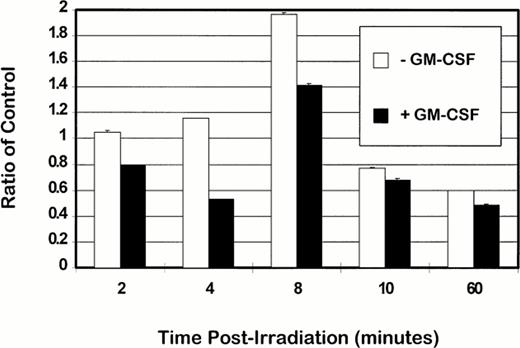
Because ceramide has been shown to inactivate cellular PKCα,40 ceramide production was examined during ionizing radiation-induced apoptosis and rescue from apoptosis by GM-CSF. Ceramide levels in apoptotic TF-1 cells and TF-1 cells rescued from apoptosis by GM-CSF were expressed as a ratio of control unirradiated cells incubated in the presence or absence of GM-CSF, respectively (Fig 4). For all times observed, ceramide production was reduced in TF-1 cells rescued from apoptosis by GM-CSF compared with that in apoptotic cells. The largest differences were seen at 4 minutes postirradiation, with a 46% reduction in ceramide levels of rescued cells compared with apoptotic cells. At all other time points observed, the levels of ceramide produced in TF-1 cells rescued from apoptosis by GM-CSF were 20.8% ± 6.0% lower than those in apoptotic TF-1 cells. The differences in ceramide production between cells irradiated in the presence or absence of GM-CSF are noteworthy because it is thought that ceramide is produced as a direct product of ionizing radiation-induced membrane lipid peroxidation. These data imply that ceramide production after irradiation is not a sole consequence of membrane damage and that ceramide production is to some extent regulated by cellular mechanisms, which may be influenced by other external factors.
The effect of GM-CSF rescue from apoptosis on ceramide levels in TF-1 cells. TF-1 cells were treated with γ radiation (5 Gy) in either the presence or absence of GM-CSF (100 U/mL) in complete RPMI supplemented with 20% FBS, and harvested at 2, 4, 8, 10, and 60 minutes postirradiation. Extraction and measurement of ceramide was performed as described37 by assay of 32P incorporated upon phosphorylation of ceramide to ceramide 1-P by DAG kinase. Ceramide 1-P was resolved by TLC using CHCl3:CH3OH:acetic acid (65:15:5, vol/vol) as solvent, detected by autoradiography and the incorporated32P was quantified by phosphoimaging (Fugi BAS1000, Fugi Medical Systems). The level of ceramide was determined by comparison to a concomitantly run standard composed of known amounts of ceramide. Ratios were derived by comparison to ceramide levels of unirradiated TF-1 cells grown in either the presence or absence of GM-CSF. The values were derived from duplicate experiments and error bars represent standard deviations into which mean values were incorporated.
The effect of GM-CSF rescue from apoptosis on ceramide levels in TF-1 cells. TF-1 cells were treated with γ radiation (5 Gy) in either the presence or absence of GM-CSF (100 U/mL) in complete RPMI supplemented with 20% FBS, and harvested at 2, 4, 8, 10, and 60 minutes postirradiation. Extraction and measurement of ceramide was performed as described37 by assay of 32P incorporated upon phosphorylation of ceramide to ceramide 1-P by DAG kinase. Ceramide 1-P was resolved by TLC using CHCl3:CH3OH:acetic acid (65:15:5, vol/vol) as solvent, detected by autoradiography and the incorporated32P was quantified by phosphoimaging (Fugi BAS1000, Fugi Medical Systems). The level of ceramide was determined by comparison to a concomitantly run standard composed of known amounts of ceramide. Ratios were derived by comparison to ceramide levels of unirradiated TF-1 cells grown in either the presence or absence of GM-CSF. The values were derived from duplicate experiments and error bars represent standard deviations into which mean values were incorporated.
Effect of the Calcium-Dependent PKC Isoform Inhibitor, Gö6976, on Apoptosis
Because different effects were seen in PKCα translocation in both the presence and absence of GM-CSF rescue from apoptosis (Fig 3), this suggested that PKCα is involved in mediating early apoptotic or cell survival signaling pathways in TF-1 cells. Because attempts to genetically alter PKCα levels in TF-1 cells were unsuccessful, the cPKC inhibitor, Gö 6976, was used to further investigate the role of PKCα in mediating ionizing radiation-induced apoptosis and GM-CSF rescue from apoptosis in TF-1 cells. Gö 6976 is a bisindolemaleimide compound, which has been shown to inhibit PKCα and β at nanomolar concentrations.41 In TF-1 cells, Gö6976 (5 mmol/L) reduced total 12-0-tetradecanoylphorbol-13-acetate (TPA)-induced PKC activity in TF-1 cells by 55.1% as examined by the method of Mirallia et al42 (data not shown). The remaining PKC activity seen was most probably due to the activity of other PKC isoforms expressed by TF-1 cells that were unaffected by Gö 6976.
TF-1 cells were treated with 5 mmol/L Gö 6976 for 20 minutes before irradiation and treated subsequently as described in Table 1. In the absence of Gö 6976 pretreatment, TF-1 cells irradiated at a dose of 10 Gy were rescued from apoptosis by the addition of GM-CSF to culture medium at the time of irradiation as demonstrated by significant differences (P < .0001) in the number of TF-1 cells undergoing apoptosis between cells receiving the same dose of radiation, but cultured postirradiation in the presence and absence of GM-CSF (Table 2). Pretreatment of TF-1 cells with Gö 6976 abrogated the GM-CSF rescue phenomenon as indicated by the lack of significant differences in the ratio of nonapoptotic to apoptotic cells between cells receiving the same dose of radiation, but cultured postirradiation in the presence and absence of GM-CSF (P = .6144). Additionally Gö 6976 pretreatment did not significantly alter apoptotic induction in unirradiated TF-1 cells incubated in either the presence or absence of GM-CSF (P= .4543).
Cytochemical Analysis of the Abrogation of Rescue of TF-1 Cells From Apoptosis by Treatment With Gö 6976
| Treatment of Cells . | No. of Nonapoptotic Cells . | No. of Apoptotic Cells . |
|---|---|---|
| Without GM-CSF | ||
| 0 Gy− Gö | 927 | 573 |
| 0 Gy+ Gö | 913 | 587 |
| 10 Gy− Gö | 696 | 804 |
| 10 Gy+ Gö | 675 | 825 |
| With GM-CSF | ||
| 0 Gy− Gö | 907 | 593*P = .4543 |
| 0 Gy+ Gö | 897 | 603*P = .5485 |
| 10 Gy− Gö | 833 | 667*P < .0001 |
| 10 Gy+ Gö | 715 | 785*P = .6144 |
| Treatment of Cells . | No. of Nonapoptotic Cells . | No. of Apoptotic Cells . |
|---|---|---|
| Without GM-CSF | ||
| 0 Gy− Gö | 927 | 573 |
| 0 Gy+ Gö | 913 | 587 |
| 10 Gy− Gö | 696 | 804 |
| 10 Gy+ Gö | 675 | 825 |
| With GM-CSF | ||
| 0 Gy− Gö | 907 | 593*P = .4543 |
| 0 Gy+ Gö | 897 | 603*P = .5485 |
| 10 Gy− Gö | 833 | 667*P < .0001 |
| 10 Gy+ Gö | 715 | 785*P = .6144 |
*Based on χ2 values, represents P values as shown when compared with samples irradiated with the same dose of radiation in the absence of GM-CSF rescue.
Rescue of TF-1 Cells From Apoptosis by GM-CSF Correlates With Increased Levels of the Antiapoptotic Protein Bcl-2
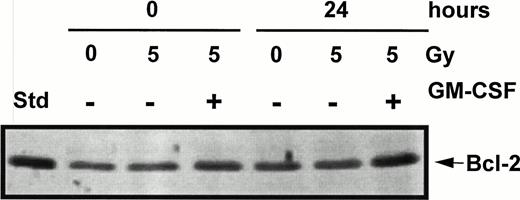
Because it has been suggested that IL-3 suppression of apoptosis in the myeloid cell line NFS/N1. H-7 is a result of cPKC-mediated alterations in Bcl-2 levels31 and because IL-3 suppression of TF-1 cell apoptosis is mediated through alterations in Bcl-2 levels,39 it is likely that GM-CSF rescue from apoptosis in TF-1 cells is also modulated by Bcl-2 levels. For that reason, TF-1 cells were examined to see if Bcl-2 levels were altered by GM-CSF rescue from ionizing radiation-induced apoptosis. GM-CSF–mediated rescue from apoptosis produced a 1.97 ± 0.34-fold increase in Bcl-2 levels by 24 hours when compared with Bcl-2 levels of unirradiated control cells, while radiation-induced apoptosis in the absence of growth factor rescue did not significantly alter Bcl-2 levels (95% ± 6.0% of control levels), as determined by densitometric analysis (Fig 5).
The effect of GM-CSF rescue from apoptosis on Bcl-2 levels in TF-1 cells. TF-1 cells were treated with γ radiation (5 Gy), incubated in either the presence or absence of GM-CSF (100 U/mL) in complete RPMI supplemented with 20% FBS, and harvested at 24 hours postirradiation. Whole cell lysates were analyzed for Bcl-2 expression by immunoblotting. The autoradiograph shows one of two blots assaying Bcl-2 expression.
The effect of GM-CSF rescue from apoptosis on Bcl-2 levels in TF-1 cells. TF-1 cells were treated with γ radiation (5 Gy), incubated in either the presence or absence of GM-CSF (100 U/mL) in complete RPMI supplemented with 20% FBS, and harvested at 24 hours postirradiation. Whole cell lysates were analyzed for Bcl-2 expression by immunoblotting. The autoradiograph shows one of two blots assaying Bcl-2 expression.
DISCUSSION
PKC is an important mediator in the control of apoptotic signaling pathways. These studies show that individual PKC isoforms play an integral role in apoptosis and rescue from apoptosis by GM-CSF in the human leukemia cell line, TF-1. Treatment of these cells with apoptosis-inducing levels of ionizing radiation resulted in alterations in the subcellular distribution of PKCα that were not seen when cells were rescued from apoptosis by GM-CSF. In particular, PKCα resided predominantly in the particulate fraction of TF-1 cells in both normal culture conditions and in irradiated TF-1 cells rescued from apoptosis by the addition of GM-CSF. However, PKCα was translocated from the particulate to the cytosolic fraction of irradiated TF-1 cells in the absence of GM-CSF rescue (Fig 3). Additionally, PKCδ levels decreased in both the particulate and cytosolic fractions of TF-1 cells irradiated in either the presence or absence of rescuing levels of GM-CSF (Fig 3). These results are similar to observations in the IL-6–dependent plasmacytoma cell line, PCT, in which suppression of IL-6 deprivation-induced apoptosis is mediated through PKCα and δ.43 PKCα was predominantly located in the cytosolic fraction of apoptotic PCT cells and was translocated to the particulate fractions when apoptotic PCT cells were treated with rescuing levels of IL-6. Furthermore, PKCδ levels decreased in both the cytosolic and particulate fractions of apoptotic PCT cells. Similarly, confocal microscopy studies of normal human tonsilar epithelium showed that cytosolic PKCα levels are greater in the apoptotic superficial layer of epithelium when compared with the nonapoptotic basal and suprabasal layers of epithelium.44 Others have reported comparable alterations in the levels and subcellular distribution of PKCα and/or PKCδ in other cell types.25,45 46
Because it is the current model that PKC is translocated upon activation from the cytosolic to the particulate fraction and because PKCα was rapidly translocated to the cytosolic fraction after irradiation in TF-1 cells not rescued from apoptosis by GM-CSF, these data suggest that PKCα is inactivated after irradiation in unrescued cells and that this inactivation is associated with apoptosis induction in TF-1 cells. These data further implicate a role for ceramide in mediating these alterations in PKCα distribution during apoptosis and GM-CSF rescue. Ionizing radiation acts on cell membranes to generate ceramide and commence apoptosis.35 Several studies show that ceramide affects PKCα activity and/or subcellular distribution. C2-ceramide blocks bradykinin-induced translocation of PKCα to the particulate fraction in the murine epidermal (HEL-37) and human skin fibroblast (SF 3155) cell lines.47 C2-ceramide treatment causes a decrease in PKCα levels in the particulate fraction in the human promyelocytic leukemia cell line HL-60.48 Additionally, Lee et al40 have shown that both C2 and C6-ceramide inhibit PKCα activity in Molt-4 human leukemia cells by inhibiting basal and induced phosphorylation (although these studies did not find C6-ceramide-induced changes in PKCα translocation). Therefore, it is likely that the translocation of PKCα to the particulate fraction of TF-1 cells during the early stages of apoptosis is the result of ceramide-induced changes in PKCα location. It is also likely that this translocation corresponds to PKCα inactivation and that this inactivation is necessary for apoptosis induction in TF-1 cells. It is therefore our hypothesis that PKCα mediates a long-term survival or proliferative signal. This hypothesis is supported by the fact that PKCα inhibition by pretreatment with Gö 6976 abrogated the GM-CSF rescue phenomenon (Table 2).
It should be noted that the proposed PKCα-mediated survival signal is probably a function of a signaling pathway distinct from that of the GM-CSF proliferative pathway. PKCε has been shown to mediate the IL-3/GM-CSF proliferative signal in TF-1 cells49 and in NIH 3T3 cells.50 However, PKCε levels and subcellular distribution did not change in apoptotic TF-1 cells or in GM-CSF rescued TF-1 cells (data not shown). That the proposed survival signal is mediated by PKCα, and not PKCε, is further supported by our finding that GM-CSF rescue was blocked by the cPKC isoform inhibitor, Gö 6976 (Table 2). While others have reported a role for PKCε during apoptosis in other cells types,30,51 our data do not support such a role in TF-1 cells. Because TF-1 cells express both PKCα and β2 and because Gö 6976 inhibits the activity of both isoforms, the possibility that PKCβ2 also plays a role in mediating GM-CSF rescue cannot be completely ruled out, however, a significant role for PKCβ2 in mediating long-term survival appears unlikely. We did not find alterations in PKCβ2 subcellular location or levels in TF-1 cells treated with apoptosis-inducing levels of ionizing radiation either in the presence or absence of GM-CSF rescue (data not shown). Additionally, overexpression of PKCβ did not lead to increased clonogenic survival or radioresistance in C3H 10 T1/2 cells, despite the fact that this cell line showed increased PKCβ expression after exposure to ionizing radiation.29 Further exclusion of a role for PKCβ, or other PKC isoforms, in mediating a cell survival signal in TF-1 cells awaits successful genetic manipulation of PKCβ expression and/or development of a method to monitor or manipulate PKC isoform-specific activity at the cellular level.
A mechanism by which PKCα might mediate long-term survival/growth factor rescue from apoptosis in TF-1 cells is through alterations in the levels of the antiapoptosis protein Bcl-2 (and possibly other members of the Bcl-2 family). This hypothesis is supported by the observation that Bcl-2 levels decrease in TF-1 cells undergoing apoptosis and in TF-1 cells treated with the PKC inhibitors calphostin and H-7.39 It has been suggested that IL-3 suppression of apoptosis in NFS/N1 H-7 cells is a result of cPKC-mediated alterations in Bcl-2 levels.31 Furthermore, the effect of PKC on Bcl-2 levels determines p21ras-mediated entry into pathways for cell growth or apoptosis in Jurkat cells.52 We have shown that rescue from apoptosis by GM-CSF in TF-1 cells corresponded with increased Bcl-2 expression (Fig 4).
Because PKCδ was degraded in irradiated TF-1 cells in either the presence or absence of growth factor rescue, our data tend to support a hypothesis that the changes in PKCδ levels are a response to γ irradiation. Immediately after cellular insult and genotoxic stress, cells must sense the insult, affect cell cycle arrest, and repair DNA and cellular damage, if possible; or enter apoptosis, if not. PKCδ may play a role in one or several of these events. A role for PKCδ in cell cycle control has been implicated in proliferation and differentiation control in the absence of cellular insult in other types of cells.8,53-56 Additionally, PKC has been shown to activate the growth inhibitory protein p53.57 However, the exact role of PKCδ in cell cycle control is unclear at present.
Sawai et al30 have reported that PKCδ translocation from the particulate to cytosolic fraction is essential to ceramide-induced apoptosis in three leukemia cell lines: the HL-60 promyelocyte, U937 monoblast, and HPB-A11 T cell lines. That finding is in contrast to our data in that, in those studies, PKCδ translocation was blocked during rescue by TPA, and we did not see significant differences in PKCδ translocation between apoptotic TF-1 cells and TF-1 cells rescued from apoptosis by GM-CSF addition. However, the feature that is common between both sets of data is that the removal of PKCδ from the particulate fraction is associated with the induction of apoptosis. Sawai et al30 also reported that PKCδ and ε were degraded in cytosolic fractions of leukemia cells when apoptosis was induced by tumor necrosis factor (TNF)α or anti-Fas antibody. Others have reported PKCδ proteolysis by an interleukin-β1 converting enzyme (ICE)-like protease to an active 40 kD cytosolic fragment during apoptosis induced by radiation, TNFα, or anti-Fas antibody stimulation in U937 cells.58 Those studies suggest that PKCδ may be translocated from the particulate fraction to the cytosolic fraction, where it is subsequently degraded during apoptosis by certain types of stimuli. Such an event would appear as a decrease in full size (78 kD) PKCδ in immunoblots of particulate and cytosolic fractions. Degradation in the cytosol of PKCδ by an ICE-like protease might explain the our data, however, a lower molecular weight immunoreactive protein was not detected by the methods used here in either whole cell lysates or the particulate, cytosolic, or nuclear fractions of apoptotic TF-1 cells.
The results we report offer a possible explanation for the functional diversity of two PKC isoforms in mediating cellular responses to ionizing radiation and in apoptosis. These data implicate a possible role for PKCδ in cell cycle regulation and/or sensing genotoxic stress. Furthermore, these data imply that PKCα mediates a long-term cell survival signal in TF-1 cells and that this survival signal may counteract the sphingomyelin pathway. Because it has been suggested that PKC may modulate Bcl-2 expression31,39 52and because Bcl-2 levels increased in TF-1 cells rescued from apoptosis with GM-CSF, it is likely that PKCα regulates its proposed cell survival signal through alterations in Bcl-2 expression. While additional studies are required to confirm this hypothesis, it does allow us to speculate that therapeutic blockade of this function may induce apoptosis and/or enhance cell death by radiotherapy.
ACKNOWLEDGMENT
The authors thank Dr Alan Miller (Tulane University, New Orleans, LA) for his generous gift of TF-1 cells and Dr John C. Reed (Burnham Institute, La Jolla, CA) for his generous gift of Bcl-2 antibodies.
Supported by grants from the Cancer Association of Greater New Orleans to M.L.K. and Department of Defense, Tulane/Xavier Center for Bioenvironmental Research Grant to S.C. and B.S.B.
Address reprint requests to Barbara S. Beckman, PhD, Department of Pharmacology, Tulane University School of Medicine, 1430 Tulane Ave, New Orleans, LA 70112.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal