Abstract
Central nervous system (CNS) relapse has been an obstacle to uniformly successful treatment of childhood acute lymphoblastic leukemia (ALL) for many years. We therefore intensified intrathecal chemotherapy (simultaneously administered methotrexate, hydrocortisone, and cytarabine) for 165 consecutive children with newly diagnosed ALL enrolled in Total Therapy Study XIIIA from December 1991 to August 1994. The 64 patients (39%) who had 1 or more blast cells in cytocentrifuged preparations of cerebrospinal fluid at diagnosis, with or without associated higher-risk features, received additional doses of intrathecal chemotherapy during remission induction and the first year of continuation treatment. Patients with higher-risk leukemia, regardless of cerebrospinal fluid findings, also received additional doses of intrathecal chemotherapy during the first year of continuation treatment. Cranial irradiation was reserved for patients with higher-risk leukemia (22% of the total). The 5-year cumulative risk of an isolated CNS relapse among all 165 patients was 1.2% (95% confidence interval, 0% to 2.9%), whereas that of any CNS relapse was 3.2% (0.4% to 6.0%). The probability of surviving for 5 years without an adverse event of any type was 80.2% ± 9.2% (SE). Our results suggest that early intensification of intrathecal chemotherapy will reduce the risk of CNS relapse to a very low level in children with ALL, securing a higher event-free survival rate overall.
APPROXIMATELY 70% of children with acute lymphoblastic leukemia (ALL) can be cured with contemporary forms of chemotherapy.1 One approach to improving this result would be to lower the incidence of central nervous system (CNS) relapse, which in most studies ranges from 5% to 11%.2-12 Growing appreciation that CNS irradiation can cause potentially serious neurotoxicity, including brain tumors, has increased the emphasis on stringent risk assessment to ensure that patients are neither undertreated nor overtreated within this site.13 Thus, we have reported that fewer than 5 leukocytes/μL with definable blast cells in the cerebrospinal fluid (CSF) increases the CNS relapse hazard in children with ALL.5 This suggests that any number of leukemic cells in the CSF identifies patients who may benefit from intensified intrathecal chemotherapy, which effectively prevents CNS relapse in cases of intermediate- or high-risk ALL.10 14-16In the study reported here, early intensification of triple intrathecal chemotherapy (methotrexate, hydrocortisone, and cytarabine), especially for patients with blast cells in the CSF and those with standard higher-risk features, reduced the incidence of CNS relapse to a very low level, improving clinical outcome overall.
MATERIALS AND METHODS
Patients.
From December 1991 to August 1994, 165 consecutive children and adolescents, 18 years of age or younger, with newly diagnosed ALL were enrolled in Total Therapy Study XIIIA at St Jude Children's Research Hospital.17 The treatment protocol was approved by the institutional review board, and signed informed consent was obtained from the patients' parents or guardians.
The diagnosis of ALL was based on morphologic and cytochemical evaluation of bone marrow smears as well as immunophenotyping and cytogenetic analysis of lymphoid blast cells. Depending on the pattern of blast cell reactivity to a panel of monoclonal antibodies, cases were classified as T-cell or B-cell precursor, as previously described.18
Samples of CSF (1.0 mL from cases with fewer than 500 cells/μL or a smaller volume from cases with higher counts, increased to 1.0 mL with normal saline) were mixed with one drop of 22% bovine albumin (Organon Teknika, Durham, NC), placed in a cytospin sample chamber, and centrifuged at 1,000 revolutions per minute for 5 minutes (Shandon centrifuge; Shandon, Cheshire, UK). All slides were reviewed by two examiners (a hematopathologist and a certified medical technician) who classified cases as CNS-1, no definable blast cells;CNS-2, fewer than 5 leukocytes/μL with definable blast cells;CNS-3, 5 or more leukocytes/μL with definable blast cells or the presence of cranial-nerve palsies; and contaminated, more than 10 erythrocytes/μL with detectable blast cells. Cell counts in the CSF were performed with a hemocytometer.
Treatment.
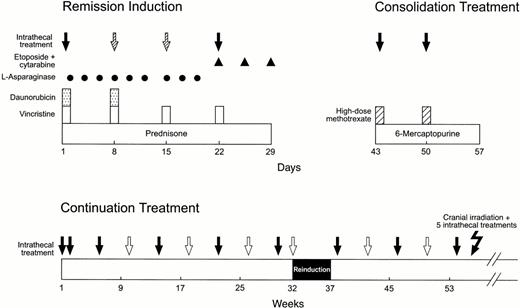
Initial treatment consisted of methotrexate alone19followed 4 days later by remission induction therapy with prednisone, vincristine, daunorubicin, asparaginase, and etoposide plus cytarabine (Fig 1).17 All patients received 2 weeks of consolidation therapy with high-dose methotrexate and mercaptopurine upon attaining complete remission. Continuation therapy for higher-risk cases consisted of drug pairs administered in weekly rotation: etoposide plus cyclophosphamide, mercaptopurine plus methotrexate, methotrexate plus cytarabine, prednisone plus vincristine plus asparaginase, etoposide plus cyclophosphamide, mercaptopurine plus high-dose methotrexate (replaced by low-dose methotrexate after 1 year of therapy), etoposide plus cytarabine, and prednisone plus vincristine plus asparaginase. Reinduction therapy (similar to that used initially) was administered from weeks 32 to 37. Postremission treatment for lower-risk cases consisted of daily mercaptopurine and weekly methotrexate reinforced by high-dose methotrexate every 8 weeks (for the first year) and prednisone plus vincristine pulse every 4 weeks.
Schema of remission induction, consolidation treatment, and continuation therapy for the first year. Solid arrows indicate triple intrathecal treatment that was administered to all patients; hatched arrows, additional doses administered only to patients with a CNS-2, CNS-3, or contaminated status; open arrows, additional doses administered during the continuation phase of therapy to patients with a higher risk of CNS relapse, as defined by CSF findings or other features (Table 1). Cranial irradiation plus 5 triple intrathecal treatments was administered only to patients with high-risk leukemia. See the Materials and Methods for other details.
Schema of remission induction, consolidation treatment, and continuation therapy for the first year. Solid arrows indicate triple intrathecal treatment that was administered to all patients; hatched arrows, additional doses administered only to patients with a CNS-2, CNS-3, or contaminated status; open arrows, additional doses administered during the continuation phase of therapy to patients with a higher risk of CNS relapse, as defined by CSF findings or other features (Table 1). Cranial irradiation plus 5 triple intrathecal treatments was administered only to patients with high-risk leukemia. See the Materials and Methods for other details.
The schedule of triple intrathecal chemotherapy (simultaneously administered methotrexate, hydrocortisone, and cytarabine) is shown in Fig 1. Briefly, the intensification plan specified additional intrathecal treatments, in age-appropriate doses,20 on days 8 and 15 of remission induction for all patients whose CSF sample had a CNS-2, CNS-3, or contaminated status. These subgroups then received additional intrathecal doses every 4 weeks during the first 56 weeks of continuation therapy, as did patients with a CNS-1 status who were judged to have an increased risk of CNS relapse based on other features (Table 1). Cranial irradiation was reserved for patients in the higher-risk category (1,800 cGy or 2,400 cGy plus 5 intrathecal treatments from weeks 56 to 69). Intrathecal chemotherapy was not administered after the first 59 weeks of continuation treatment.
Subgroups Receiving Intensified Therapy Directed to the CNS
| CSF Finding* . | No. of Patients . | No. of Intrathecal Treatments . | Dose of Cranial Irradiation (cGy) . | |
|---|---|---|---|---|
| First 12 wks . | Total . | |||
| Lower risk | ||||
| CNS-1 | 89 | 6 | 13 | 0 |
| CNS-2 | 27 | 8 | 21 | 0 |
| Contaminated | 12 | 8 | 21 | 0 |
| Higher risk-151 | ||||
| CNS-1 | 12 | 6 | 24 | 1,800 |
| CNS-2 | 15 | 8 | 26 | 1,800 |
| Contaminated | 4 | 8 | 26 | 1,800 |
| CNS-3 | 6 | 8 | 26 | 2,400 |
| CSF Finding* . | No. of Patients . | No. of Intrathecal Treatments . | Dose of Cranial Irradiation (cGy) . | |
|---|---|---|---|---|
| First 12 wks . | Total . | |||
| Lower risk | ||||
| CNS-1 | 89 | 6 | 13 | 0 |
| CNS-2 | 27 | 8 | 21 | 0 |
| Contaminated | 12 | 8 | 21 | 0 |
| Higher risk-151 | ||||
| CNS-1 | 12 | 6 | 24 | 1,800 |
| CNS-2 | 15 | 8 | 26 | 1,800 |
| Contaminated | 4 | 8 | 26 | 1,800 |
| CNS-3 | 6 | 8 | 26 | 2,400 |
*CNS-1 status is defined by no identifiable blast cells in CSF; CNS-2, fewer than 5 leukocytes/μL with definable blast cells in CSF; CNS-3, 5 or more leukocytes/μL with definable blast cells in CSF or the presence of cranial nerve palsy; andcontaminated, more than 10 erythrocytes/μL with detectable blast cells in CSF.
B-cell precursor phenotype with a leukocyte count of at least 100 × 109/L, a T-cell phenotype with a leukocyte count of at least 50 × 109/L, or a karyotype with the Philadelphia chromosome. All other cases were considered lower risk.
Statistical analysis.
Differences in the distribution of base-line characteristics among subgroups defined by CNS status were assessed with Fisher's exact test. To account for the competing effects of failures other than CNS relapse, we estimated the cumulative incidence of CNS relapse (either isolated or combined with relapse in any other site) as an initial adverse event, using the method of Kalbfleisch and Prentice21 as implemented by Gray.22 Survival free of CNS relapse and event-free survival were estimated by the Kaplan-Meier method. Follow-up times on the date of analysis ranged from 3 to 5.7 years (median, 4.3 years).
RESULTS
Sixty-four patients (39%) had unequivocal lymphoblasts in their CSF samples: 42 had CNS-2 status; 6 had CNS-3 status; and 16 had contaminated status (Table 1). Twenty-five were considered to have a higher risk of CNS relapse by standard criteria (B-cell precursor immunophenotype with a leukocyte count of at least 100 × 109/L, a T-cell immunophenotype with a leukocyte count of at least 50 × 109/L, or a karyotype with the Philadelphia chromosome), whereas 39 were in the lower-risk category (absence of higher-risk features). By comparison with the CNS-1 subgroup, patients with a CNS-2 status were significantly more likely to have several adverse presenting features: leukocyte counts greater than 100 × 109/L (33% v 5%, P < .001), a germline TEL status (76% v 58%, P< .01), or an MLL rearrangement (17% v 1%,P < .001). There were too few patients with a CNS-3 status to permit meaningful statistical testing.
One hundred sixty-three of the 165 patients entered complete remission. Of the 28 adverse events that have occurred, 13 were hematologic relapses, 2 were isolated CNS relapses, 3 were combined CNS and hematologic relapses, and 2 were deaths in remission (1 accidental and 1 due to presumed sepsis). There have been 8 cases of therapy-induced leukemia. The cumulative risk of an isolated CNS relapse at 5 years postremission was 1.2% (95% confidence interval, 0% to 2.9%) and of any CNS relapse was 3.2% (0.4% to 6.0%; Fig 2A). The 5-year event-free survival estimate for all 165 patients was 80.2% ± 9.2% (SE) (Fig 2B).
(A) Cumulative risk of CNS relapse, either isolated or combined with relapse in other sites. Numbers in parentheses are the 95% confidence intervals. (B) Event-free survival and survival free of CNS relapse. Five-year estimates are the means ± SE. Three patients did not achieve complete remission and therefore were not at risk for CNS relapse during the immediate postinduction period.
(A) Cumulative risk of CNS relapse, either isolated or combined with relapse in other sites. Numbers in parentheses are the 95% confidence intervals. (B) Event-free survival and survival free of CNS relapse. Five-year estimates are the means ± SE. Three patients did not achieve complete remission and therefore were not at risk for CNS relapse during the immediate postinduction period.
Two adolescent boys (with B-cell precursor leukemia and a CNS-2 or contaminated CSF status) had isolated CNS relapses at 16 and 19 months of continuation treatment. Both patients had relatively low presenting leukocyte counts (17 and 18.9 × 109/L, respectively), and neither had recognized high-risk genetic features. Three other boys, 3 to 6 years of age with B-cell precursor leukemia and a CNS-1 status, had combined CNS and bone marrow relapses at 13, 19, and 37 months. Both cases of early relapse had high-risk genetic features, either the Philadelphia chromosome with a leukocyte count of 271 × 109/L or a near-haploid karyotype with a leukocyte count of 2.7 × 109/L. The 1 late relapse occurred in a patient with hyperdiploid leukemia (>50 chromosomes per leukemic cell). The patient with Philadelphia chromosome-positive ALL relapsed just before scheduled cranial irradiation, and in this study near-haploidy was not used as a criterion for cranial irradiation. Hence, none of these 5 patients had received cranial irradiation before relapse.
DISCUSSION
We attribute the very low incidence of CNS relapse in this study to early intensification of intrathecal treatment in the context of effective systemic chemotherapy. Although the need for intensive CNS-directed therapy is well recognized in patients with a CNS-3 status,9 we are the first to have extended this requirement to patients with a CNS-2 status. Our use of cranial irradiation was limited to a subset of patients (22% of the total group) who were at higher risk of CNS relapse, with or without positive CSF findings. The efficacy of this strategy is supported by results from an interim analysis of our ongoing Total Therapy trial: no CNS relapses among 200 patients observed for a median of 20 months (C.-H.P., unpublished observation). Others have reported similarly low risks of CNS relapse in recent years, but in each instance, a majority of the patients received cranial irradiation.23-26 The high event-free survival estimate in our study (80.2% ± 9.2% [SE] at 5 years) reflects at least in part the near elimination of CNS relapse as a major adverse event in the clinical course of ALL patients and represents improvement over previous results from this center.2 5
In therapeutic trials that did not include early intensification of intrathecal therapy, the isolated CNS relapse rates have ranged from 5% to 11%.2-12 Addition of cranial irradiation did not appear useful in lowering the hazard of CNS relapse in several of these studies.6,10,11 In the trial preceding Study XIIIA, we did not administer intrathecal treatment during consolidation therapy or early in the continuation phase, and neither did we intensify CNS-directed therapy for patients in whom a CNS-2 status was the only feature predicting relapse.5 Virtually all of the CNS relapses in that study occurred during the first year of continuation treatment, before scheduled administration of cranial irradiation.
In the future, it may be possible to avoid cranial irradiation in some patients with high-risk leukemia, eg, those with a rapid early response to induction chemotherapy.14,16 The use of dexamethasone, which may penetrate into the CSF better than prednisolone,27 could further reduce the proportion of patients requiring cranial irradiation.28 However, the requirement for radiation in other subgroups remains controversial. Among children with T-cell leukemia and an initial leukocyte count of at least 100 × 109/L, those treated with intensive intrathecal therapy alone had an inferior outcome compared with those receiving cranial irradiation,15 although this comparison was not based on patients treated with the same systemic regimen. Nonetheless, it may be possible to reduce the dose of prophylactic cranial irradiation to as low as 1,200 cGy without loss of therapeutic efficacy.15 Some investigators use craniospinal irradiation to treat patients with a CNS-3 status.11,12,23 29 We suggest that cranial irradiation plus intensified intrathecal chemotherapy is sufficient therapy for this subgroup, because none of the 13 patients with a CNS-3 status in the present study or our ongoing trial has relapsed in the CNS.
The frequency of detection of a CNS-2 status has increased significantly at this center (P < .01), from 17.6% (Mahmoud et al5) to 25.5% in the present study. We would emphasize that the distribution of other presenting features (eg, leukocyte count, age, leukemic cell lineage or ploidy, and the presence of the Philadelphia chromosome) did not differ between study patients and the historical comparison group (data not shown). However, even though the median leukocyte count in the CSF (n = 1) was identical between these two cohorts, the median percentage of blasts in cytospin preparations was higher in the current study (6% v 3%, P = .03). We attribute both the higher incidence of CNS-2 findings and the increased percentage of blasts in the CSF to improved preparation of cytospin samples (with an upgraded cytocentrifuge), more rapid delivery of CSF samples to the laboratory (thus avoiding excessive degradation of blast cells), and greater vigilance for leukemic blasts in CSF samples. With immunologic assays, the proportion of children with leukemic cells in their CSF may increase still further, to as high as 45%.30 31
Early intensification of systemic chemotherapy to prevent the emergence of drug-resistant blast cells is the cornerstone of successful treatment of ALL.1 13 As demonstrated in this report, the same approach to intrathecal chemotherapy can reduce the CNS relapse hazard to near zero, boosting the overall effectiveness of ALL treatment programs. Additional study is needed to determine if patients with a CNS-2 status require more intensive early intrathecal treatment than do those with a CNS-1 status.
ACKNOWLEDGMENT
The authors thank John Gilbert for critical comments and editing assistance and Virginia Norris for preparing the manuscript.
Supported by Grants No. CA-20180 and CA-21765 (CORE) from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC).
Address reprint requests to Ching-Hon Pui, MD, St Jude Children's Research Hospital, 332 N Lauderdale, Memphis, TN 38105-0318.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal