Abstract
Granulocyte colony-stimulating factor (G-CSF) is the principal growth factor regulating the production of neutrophils, yet its role in lineage commitment and terminal differentiation of hematopoietic progenitor cells is controversial. In this study, we describe a system to study the role of G-CSF receptor (G-CSFR) signals in granulocytic differentiation using retroviral transduction of G-CSFR–deficient, primary hematopoietic progenitor cells. We show that ectopic expression of wild-type G-CSFR in hematopoietic progenitor cells supports G-CSF–dependent differentiation of these cells into mature granulocytes, macrophages, megakaryocytes, and erythroid cells. Furthermore, we show that two mutant G-CSFR proteins, a truncation mutant that deletes the carboxy-terminal 96 amino acids and a chimeric receptor containing the extracellular and transmembrane domains of the G-CSFR fused to the cytoplasmic domain of the erythropoietin receptor, are able to support the production of morphologically mature, chloroacetate esterase-positive, Gr-1/Mac-1–positive neutrophils in response to G-CSF. These results demonstrate that ectopic expression of the G-CSFR in hematopoietic progenitor cells allows for multilineage differentiation and suggest that unique signals generated by the cytoplasmic domain of the G-CSFR are not required for G-CSF–dependent granulocytic differentiation.
GRANULOCYTE colony-stimulating factor (G-CSF) is the principal growth factor regulating the production of mature neutrophils. In fact, G-CSF is widely used to ameliorate neutropenia in a variety of clinical settings.1 Multiple actions of G-CSF have been described that may contribute to the neutrophilic response. First, G-CSF stimulates the proliferation of granulocytic precursors.2,3 Second, it reduces the average transit time through the granulocytic compartment.3-5Although the effect of G-CSF on neutrophil production is well established, its role in the commitment of multipotent hematopoietic progenitors to the myeloid lineage and their subsequent terminal differentiation into mature neutrophils is controversial.
The biological effects of G-CSF are mediated through its interaction with the G-CSF receptor (G-CSFR), a member of the cytokine receptor superfamily.6 To define further the role of G-CSF in the control of granulopoiesis, we recently generated G-CSFR–deficient mice.7 Similar to G-CSF (cytokine)–deficient mice,8 G-CSFR–deficient mice have chronic neutropenia with a uniform decrease in myeloid cells in the bone marrow.7,9No accumulation of immature granulocytic cells in the bone marrow was observed, suggesting that the residual granulocytic precursors present in these mice were able to differentiate normally into mature neutrophils. In agreement with this conclusion, G-CSFR–deficient myeloid progenitors demonstrated normal granulocytic differentiation in vitro in response to interleukin-3 (IL-3) or granulocyte-macrophage colony-stimulating factor (GM-CSF).7 Surprisingly, near normal numbers of myeloid-committed progenitors were observed in the bone marrow of these animals. These data demonstrated that G-CSFR signals are not required for lineage commitment or terminal differentiation.
On the other hand, several studies have shown that the addition of G-CSF to cultures of certain multipotential hematopoietic cell lines results in their granulocytic differentiation.10-14Furthermore, a carboxy-terminal region of the cytoplasmic domain of the G-CSFR was identified that was required for granulocytic differentiation.11-13 These observations lead to the hypothesis that G-CSFR signals play an active (instructive) role in directing granulocytic differentiation. This conclusion is compatible with data from the G-CSFR–deficient mice, because alternative granulocytic differentiation signals (eg, from other hematopoietic cytokines) may be able to compensate for the loss of G-CSFR signals in vivo. In contrast, other studies have shown that granulocytic differentiation can occur in a cytokine-independent fashion. Suppression of apoptosis by ectopic expression of bcl-2 allowed for granulocytic differentiation of hematopoietic cell lines in the absence of added cytokines (although certain features of granulocytic differentiation seemed to require G-CSF).15 16 These conflicting data highlight the controversy as to the presence and contribution of specific G-CSFR signals to granulocytic differentiation.
In this study, we describe a system to study the role of G-CSFR signals in the granulocytic differentiation of primary hematopoietic progenitor cells that lack endogenous G-CSFR protein. In addition to wild-type G-CSFR, two mutant G-CSFR were studied: a truncation mutant that deletes the carboxy-terminal 96 amino acids (including the putative maturation domain) and a chimeric receptor containing the extracellular and transmembrane domains of the G-CSFR fused to the cytoplasmic domain of the erythropoietin receptor. The effect of ectopic expression of these receptors on G-CSF–dependent hematopoietic proliferation and differentiation was assessed.
MATERIALS AND METHODS
Cytokines, cell lines, and mice.
Human flt-3 ligand and murine kit ligand were generously provided by Immunex (Seattle, WA). Human thrombopoietin was a generous gift from Dr John DiPersio (Washington University, St Louis, MO). The amphotropic and ecotropic packaging cell lines GP+EnvAm12 and GP+E86,17respectively, were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (FCS), 15 μg/mL of hypoxanthine, 250 μg/mL of xanthine, and 25 μg/mL of mycophenolic acid (HXM media) at 37°C in a 5% CO2, humidified atmosphere. The generation of G-CSFR–deficient mice has been described previously.7 All mice were housed in a specific pathogen-free environment.
Construction of G-CSFR retroviral plasmids.
The wild-type and mutant G-CSFR cDNAs were subcloned into the retroviral vector pMPncrdlneo.18 The d716 G-CSFR mutation was generated using a polymerase chain reaction (PCR)-based method to introduce a C to T mutation at nucleotide 2403, as described.19 The murine G-CSFR cDNA was used as the template in PCR reactions with the following oligonucleotide primer pairs: exon 16 forward primer (5′-CCCACAGTAGCCTGAGCTCC-3′) and exon 17 reverse mutagenesis primer (5′-AGAGGAATTCTAGGACTGGTTGGA-3′); exon 17 forward mutagenesis primer (5′-CCAGTCCTAGAATTCCTCTCGCAC-3′) and exon 17 reverse primer (5′-CCCCAAAGTTCTAGAAACCC-3′). The primary PCR products were purified by gel electrophoresis, annealed in a 1:1 molar ratio, and amplified with the exon 16 forward and exon 17 reverse primers listed above. The resulting product was digested withSac I and Xba I and subcloned into a plasmid containing the murine G-CSF cDNA that had been digested with Sac I andXba I. The GEpoR construct was generated as follows. The murine erythropoietin cDNA was used as the template in a PCR reaction using a forward primer (5′-AAGAAAGACTTCCAAGATCTGGCCTGGCA-3′) containing a Xmn I site and a reverse primer (5′-AATCTAGACTAGGAGCAGGCCACATAG-3′) containing anXba I site. The resulting 690-bp amplicon was digested withXmn I and Xba I and subcloned into a plasmid containing the murine G-CSFR cDNA that had been digested with Xmn I andXba I. Note that this subcloning strategy retains the first four amino acids of the G-CSFR. Sequence analysis was performed to confirm the fidelity of each of these constructs.
Retroviral infection of bone marrow cells.
The retroviral constructs were linearized with Sac-2, transfected into GP+EnvAm12 cells using lipofectamine (GIBCO BRL, Gaithersburg, MD), per the manufacturer's recommendations, and selected in HXM media supplemented with 800 μg/mL geneticin (GIBCO BRL). The amphotropic retrovirus-rich supernatant from geneticin-resistant cells was used to infect GP+E86 cells. Individual geneticin-resistant GP+E86 clones were derived, and their supernatant was tested for viral production on NIH3T3 cells. Clones producing 0.5 to 1.0 × 106infectious particles/mL were obtained for each construct.
Hematopoietic cells were harvested from the femurs and tibiae of 5- to 8-week-old G-CSFR–deficient mice, and the light-density (progenitor-enriched) fraction was collected after centrifugation through a Histopaque 1077 density gradient (Sigma, St Louis, MO). Cells were cocultured on irradiated (1,500 cGy) GP+E86 viral producer cells in α-minimum essential medium (α-MEM) supplemented with 20% FCS, murine IL-3 (10 ng/mL; R&D systems, San Diego, CA), human flt-3 ligand (50 ng/mL), human thrombopoietin (50 ng/mL), murine kit ligand (50 ng/mL), and polybrene (6 μg/mL) for 4 days at 37°C in a 5% CO2, humidified atmosphere.
Flow cytometry.
To assess surface G-CSFR expression, nonadherent cells from the cultures described above were incubated at 4°C for 1 hour with biotinylated G-CSF (generated as described7; 5 ng per 106 cells) in the presence or absence of a 100-fold molar excess of nonlabeled G-CSF, followed by incubation with phycoerythrin (PE)-conjugated streptavidin (GIBCO BRL). Cells were coincubated with the following cocktail of lineage-restricted fluorescien isothiocyanate (FITC)-conjugated rat monoclonal antibodies: antimouse B220 (M1/70, IgG2b), antimouse CD3 (M1/70, IgG2b), and antimouse CD11b (M1/70, IgG2b). In other experiments, PE-conjugated rat antimouse CD11b (M1/70, IgG2b; PharMingen, San Diego, CA) and FITC-conjugated rat antimouse Gr-1 (RB6-8C5, IgG2b) were used. In all experiments, cells were incubated with actinomycin D, 7-amino (7AAD) to exclude nonviable (7AAD-positive) cells from analysis. All antibodies were purchased from PharMingen. All cells were analyzed using a FACScan flow cytometer and CellQuest version 1.2.2 software (Becton Dickinson, Mansfield, MA).
Cell sorting.
Nonadherent cells from the cultures described above were incubated with biotin-conjugated rat antimouse CD34 (RAM34, IgG2a) and the same cocktail of FITC-conjugated lineage-restricted antibodies as described above. After incubation with PE-conjugated streptavidin, CD34+ lineage− cells were sorted using a Coulter Elite ESP cytometer (Coulter, Hialeah, FL).
Progenitor assays.
Seven hundred fifty to 2,000 CD34+lineage− cells were plated in 2.5 mL of methylcellulose media (MethoCult 3230; Stem Cell Technologies, Vancouver, British Columbia, Canada) supplemented with G-CSF at 1, 10, or 100 ng/mL (Amgen, Thousand Oaks, CA) or with 2.5 mL of methylcellulose media supplemented with erythropoietin and pokeweed mitogen-stimulated murine spleen cell-conditioned medium (MethoCult 3430; Stem Cell Technologies) and placed at 37°C in a humidified chamber with 5% CO 2 for 10 days. Colonies containing at least 50 cells were scored on day 10.
Cytological analysis.
Entire methylcellulose cultures were harvested on day 10 and leukocyte differentials were performed on Wright-stained cytospin preparations. Acetylcholine esterase20 and 2,7-diaminofluorene (DAF)21 stains were performed as described. For flow cytometry, cells were washed extensively and incubated with Fc block (PharMingen) per the manufacturer's recommendations before incubating with the indicated antisera.
Statistical analysis.
Data represent the mean ± SD. Statistical significance was assessed by the Student's t-test.
RESULTS
High efficiency transduction of G-CSFR–deficient hematopoietic cells with G-CSFR–expressing retrovirus.
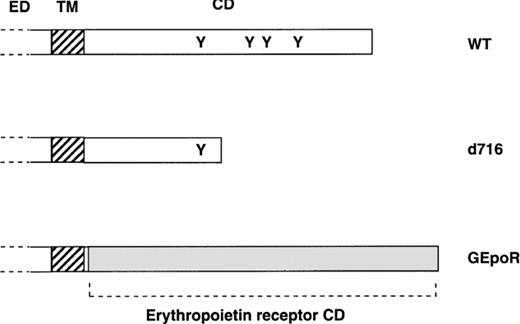
Most current models of granulocytic differentiation use immortalized cell lines; however, these models are often limited by incomplete differentiation and inappropriate gene expression. A system was developed to study the effect of wild-type (or mutant) G-CSFR expression on the granulocytic differentiation of primary hematopoietic cells using retroviral transduction. Hematopoietic cells isolated from G-CSFR–deficient mice were used in these studies because they lack endogenous G-CSFR. In initial experiments, the ability of three different G-CSFR proteins to support hematopoietic proliferation and differentiation were examined (Fig 1). In addition to wild-type murine G-CSFR (WT), two mutant receptors were studied. The d716 mutation truncates the distal 96 amino acids of the carboxy-terminal tail and reproduces a mutation of the G-CSFR found in a patient with severe congenital neutropenia.22 Expression of a similarly truncated G-CSFR in a myeloid cell line blocked G-CSF–dependent granulocytic differentiation.22 The GEpoR mutation produces a chimeric receptor with the extracellular (ligand-binding), transmembrane, and the first four amino-acids of the cytoplasmic domain derived from the G-CSFR and the remaining cytoplasmic domain derived from the murine erythropoietin receptor. The first four amino acids of the cytoplasmic domain of the G-CSFR were retained solely to facilitate the subcloning of the GEpoR construct. This chimeric receptor is predicted to transmit erythropoietin-specific signals in a G-CSF–dependent fashion.
Structure of mutant G-CSFR proteins. The extracellular (ED), transmembrane (TM), and cytoplasmic domain (CD) of the murine G-CSFR are shown. The position of tyrosine residues (Y) in the cytoplasmic domain also are shown. The d716 construct contains a point mutation at nucleotide 2403 of the murine cDNA that introduces a premature stop codon resulting in the truncation of the G-CSFR at amino-acid 716. The GEpoR mutation represents an in frame fusion of the extracellular, transmembrane, and first four amino acids of the cytoplasmic domain of the murine G-CSFR with the cytoplasmic domain of the murine erythropoietin receptor.
Structure of mutant G-CSFR proteins. The extracellular (ED), transmembrane (TM), and cytoplasmic domain (CD) of the murine G-CSFR are shown. The position of tyrosine residues (Y) in the cytoplasmic domain also are shown. The d716 construct contains a point mutation at nucleotide 2403 of the murine cDNA that introduces a premature stop codon resulting in the truncation of the G-CSFR at amino-acid 716. The GEpoR mutation represents an in frame fusion of the extracellular, transmembrane, and first four amino acids of the cytoplasmic domain of the murine G-CSFR with the cytoplasmic domain of the murine erythropoietin receptor.
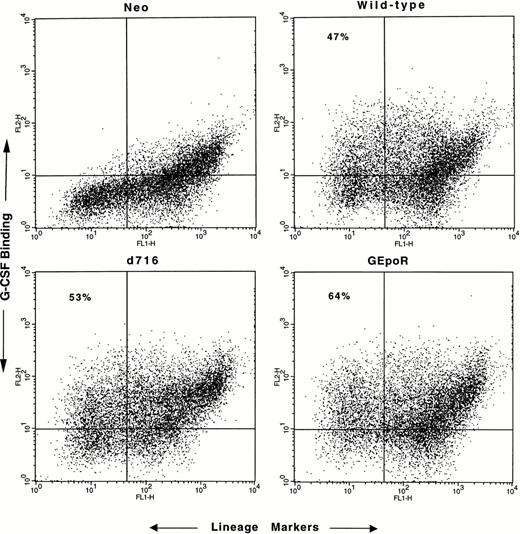
Hematopoietic cells isolated from the bone marrow of G-CSFR–deficient mice were cocultured with irradiated retroviral-producing cell lines for 4 days in the presence of hematopoietic growth factors (see the Materials and Methods). Transduction efficiency was assessed by measuring the percentage of cells that specifically bound G-CSF (Fig 2). In most experiments, approximately 50% of the immature (lineage−) cells expressed G-CSFR protein on their surface; no difference in transduction efficiency was observed with the different retroviruses. After transduction, CD34+ lineage− progenitor cells were isolated by flow cytometry (to eliminate mature cells) and cultured in methylcellulose media in the presence of G-CSF (or control cytokines) for 7 to 10 days. The number, size, and composition of the resulting colonies were evaluated.
Assessment of transduction efficiency. Nonadherent cells were harvested after 4 days of coculture on irradiated retroviral-producing fibroblasts, incubated with biotinylated G-CSF and a cocktail of lineage-restricted antisera, and analyzed by flow cytometry. The binding of biotinylated G-CSF by lineage+cells (upper-right quadrant) was seen even in the presence of a 100-fold molar excess of nonlabeled G-CSF (data not shown) and therefore represents nonspecific binding. Transduction efficiency was assessed by determining the percentage of lineage− cells that specifically bound G-CSF (upper left quadrant). No specific G-CSF binding was detected in cells transduced with the empty retroviral vector (neo). Nonviable cells were excluded from the analyses. Shown are representative results of one of five experiments.
Assessment of transduction efficiency. Nonadherent cells were harvested after 4 days of coculture on irradiated retroviral-producing fibroblasts, incubated with biotinylated G-CSF and a cocktail of lineage-restricted antisera, and analyzed by flow cytometry. The binding of biotinylated G-CSF by lineage+cells (upper-right quadrant) was seen even in the presence of a 100-fold molar excess of nonlabeled G-CSF (data not shown) and therefore represents nonspecific binding. Transduction efficiency was assessed by determining the percentage of lineage− cells that specifically bound G-CSF (upper left quadrant). No specific G-CSF binding was detected in cells transduced with the empty retroviral vector (neo). Nonviable cells were excluded from the analyses. Shown are representative results of one of five experiments.
All three G-CSFR proteins support G-CSF–dependent hematopoietic colony formation.
Hematopoietic cells transduced with WT, d716, GEpoR, or control (neo) retrovirus produced similar numbers of colonies in response to the cytokines present in pokeweed mitogen-stimulated spleen conditioned media (Fig 3A). No colonies were detected in any culture without added cytokine (Fig 3B). As expected, the neo-transduced cells did not produce any colonies in response to G-CSF even at the highest concentration (100 ng/mL). Cells transduced with the WT, d716, or GEpoR constructs produced similar number of colonies in response to G-CSF at concentrations of 10 or 100 ng/mL. Interestingly, at the lowest concentration of G-CSF (1 ng/mL), significant differences were detected in the frequency of colony formation. Compared with wild-type transduced cells, significantly fewer colonies were detected in cultures of GEpoR-transduced cells; in contrast, significantly greater numbers of colonies were detected in cultures of d716-transduced cells. A nonsignificant trend to increased colony size was observed with GEpoR-transduced cells compared with WT- or d716-transduced cells; on day 10 of G-CSF stimulation (100 ng/mL), the mean number of cells per colony ± SD was as follows: WT, 1,504 ± 322; d716, 2,068 ± 693; and GepoR, 3,098 ± 1,403.
Production of hematopoietic colonies. CD34+lineagelo cells transduced with the indicated retrovirus were cultured in the presence of pokeweed mitogen-stimulated spleen conditioned media (A) or the indicated amount of human G-CSF (B). The total number of hematopoietic colonies (CFU-C) observed after 10 days of culture are shown. *P value < .05 compared with cultures of WT-transduced cells. Data represent the mean ± SD.
Production of hematopoietic colonies. CD34+lineagelo cells transduced with the indicated retrovirus were cultured in the presence of pokeweed mitogen-stimulated spleen conditioned media (A) or the indicated amount of human G-CSF (B). The total number of hematopoietic colonies (CFU-C) observed after 10 days of culture are shown. *P value < .05 compared with cultures of WT-transduced cells. Data represent the mean ± SD.
All three G-CSFR proteins support a similar degree of granulocytic differentiation.
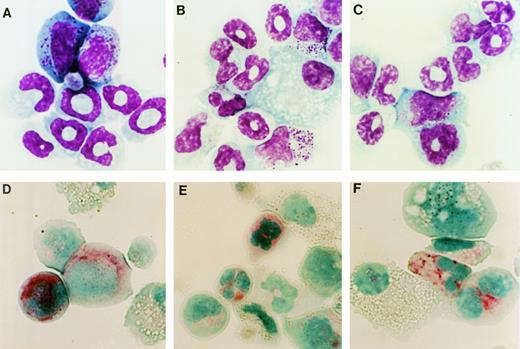
Hematopoietic colonies stimulated by G-CSF were harvested on day 10 of culture and their cellular composition was analyzed. In cultures of WT-, d716-, or GEpoR-transduced cells the predominant cell types observed in the G-CSF–stimulated colonies were macrophages and cells of the granulocytic lineage (Fig 4A through C and Table 1). In fact, a similar number of mature-appearing neutrophils were observed in each of these cultures. The only consistent difference observed in these experiments was a delay in granulocytic differentiation of GEpoR transduced cells; after 7 days of G-CSF stimulation, a greater fraction of cells were immature granulocytic precursors (data not shown). To confirm the presence of myeloid cells, the expression of Mac-1 and Gr-1 (proteins that are expressed predominantly on myeloid cells) was examined by flow cytometry (Fig 5). The majority of cells in cultures of WT-, d716-, or GEpoR-transduced cells stained brightly for Mac-1 and Gr-1, a pattern consistent with mature neutrophils and late granulocytic precursors.23 Another characteristic of mature neutrophils is the presence of chloroacetate esterase.24Although the intensity of staining was reduced relative to normal murine neutrophils, the majority of granulocytic cells in each of these cultures clearly stained positive for chloroacetate esterase (Fig 4D through F). Collectively, these data indicate that all three G-CSFR proteins are able to support a similar degree of granulocytic differentiation.
Cytological analysis of hematopoietic colonies. Cells were recovered from G-CSF–stimulated methylcellulose cultures of WT- (A and D), d716- (B and E), or GEpoR- (C and F) transduced cells. Cells were analyzed by Wright stain (A through C) or chloroacetate esterase stain (D through F). Shown are representative results of one of four experiments. Original magnification × 1,110.
Cytological analysis of hematopoietic colonies. Cells were recovered from G-CSF–stimulated methylcellulose cultures of WT- (A and D), d716- (B and E), or GEpoR- (C and F) transduced cells. Cells were analyzed by Wright stain (A through C) or chloroacetate esterase stain (D through F). Shown are representative results of one of four experiments. Original magnification × 1,110.
Cytological Analysis of Hematopoietic Colonies
| Cell Type . | WT (% ± SD) . | d716 (% ± SD . | GEpoR (% ± SD) . |
|---|---|---|---|
| Late myeloid | 29.3 ± 8.0 | 26.9 ± 8.5 | 36.5 ± 8.8 |
| Early myeloid | 29.1 ± 11.2 | 24.6 ± 4.5 | 30.8 ± 3.9 |
| Macrophage | 17.4 ± 8.0 | 31.9 ± 9.6 | 25.0 ± 10.0 |
| Erythroblast | 2.5 ± 1.8 | 1.9 ± 1.8 | 1.4 ± 1.1 |
| Basophil | 22.6 ± 8.3 | 14.9 ± 2.1 | 5.1 ± 3.0-150 |
| Cell Type . | WT (% ± SD) . | d716 (% ± SD . | GEpoR (% ± SD) . |
|---|---|---|---|
| Late myeloid | 29.3 ± 8.0 | 26.9 ± 8.5 | 36.5 ± 8.8 |
| Early myeloid | 29.1 ± 11.2 | 24.6 ± 4.5 | 30.8 ± 3.9 |
| Macrophage | 17.4 ± 8.0 | 31.9 ± 9.6 | 25.0 ± 10.0 |
| Erythroblast | 2.5 ± 1.8 | 1.9 ± 1.8 | 1.4 ± 1.1 |
| Basophil | 22.6 ± 8.3 | 14.9 ± 2.1 | 5.1 ± 3.0-150 |
Three hundred count manual leukocyte differentials were performed on cells isolated from methylcellulose cultures after 10 days of G-CSF stimulation. Late myeloid cells included polymorphonuclear, ring, and band neutrophils as well as neutrophilic metamyelocytes. Early myeloid cells included myelocytes, promyelocytes, and myeloblasts. Data represent the mean ± SD of four independent experiments.
P < .05 compared with WT.
Expression of Mac-1 and Gr-1. Cells were recovered from the indicated G-CSF–stimulated methylcellulose culture on day 10 and stained with FITC-conjugated Mac-1 (CD11b) and PE-conjugated Gr-1 or isotype controls. Distinct populations of Gr-1+Mac-1+ (neutrophil lineage) cells and Gr-1loMac-1+ (monocytic) cells were seen in each culture. Shown are representative results of one of four experiments.
Expression of Mac-1 and Gr-1. Cells were recovered from the indicated G-CSF–stimulated methylcellulose culture on day 10 and stained with FITC-conjugated Mac-1 (CD11b) and PE-conjugated Gr-1 or isotype controls. Distinct populations of Gr-1+Mac-1+ (neutrophil lineage) cells and Gr-1loMac-1+ (monocytic) cells were seen in each culture. Shown are representative results of one of four experiments.
Ectopic expression of the G-CSFR in hematopoietic progenitor cells allows for G-CSF–dependent multilineage differentiation.
In G-CSF–stimulated cultures of WT-, d716-, or GEpoR-transduced cells, a small number of granulocyte-erythrocyte-macrophage-megakaryocyte colony-forming unit (CFU-GEMM) and burst-forming unit-erythroid (BFU-E) colonies were observed (data not shown). In agreement with this observation, erythroid cells and megakaryocytes were consistently detected in these cultures (Table 1 and data not shown). The presence of megakaryocytes was confirmed by the detection of acetylcholine esterase-positive multinucleated cells (data not shown). Likewise, the presence of erythroid cells was confirmed by the demonstration of DAF-positive (hemoglobinized) cells (data not shown). Either the autocrine production of growth factors by the hematopoietic cells or the trace amounts of growth factors present in fetal calf could contribute to the multilineage differentiation observed. We therefore investigated whether neutralizing antibodies to erythropoietin and thrombopoietin could block erythroid or megakaryocytic differentiation in G-CSF–stimulated cultures. After 10 days of culture, a similar number of erythroid and megakaryocytic cells were observed (data not shown). Collectively, these data indicate that the G-CSFR is able to generate signals in progenitor cells that support the production of mature granulocytes, macrophages, megakaryocytes, and erythroid cells.
DISCUSSION
Hematopoietic cytokines clearly play an important role in the regulation of hematopoiesis, yet the mechanisms by which they exert their control are unclear. In this study, we describe a system to study the role of G-CSFR signals in granulocytic differentiation using retroviral transduction of G-CSFR–deficient, primary hematopoietic progenitor cells. This approach has several advantages over the use of immortalized myeloid cell lines to study granulocytic differentiation. First, the target cell population is comprised of primary progenitor cells (not cells that have been adapted to long-term culture). Second, the target cells completely lack endogenous G-CSFR. Using this system, we show that ectopic expression of wild-type G-CSFR in hematopoietic progenitor cells supports G-CSF–dependent differentiation of these cells into mature granulocytes and macrophages. Furthermore, we show that two mutant G-CSFR proteins, d716 (a truncation mutant that deletes the carboxy-terminal 96 amino acids) and GEpoR (a chimeric receptor containing the extracellular and transmembrane domains of the G-CSFR fused to the cytoplasmic domain of the erythropoietin receptor), also are able to support the production of morphologically mature, chloroacetate esterase-positive, Gr-1/Mac-1–positive neutrophils in response to G-CSF. Surprisingly, along with granulocytes and macrophages, these G-CSFR proteins also were able to support the production of mature megakaryocytes and erythroid cells.
The role of G-CSFR signals in the terminal differentiation of myeloid precursors to neutrophils is controversial. In the present study, we show that the truncated G-CSFR mutant (d716) is able to support G-CSF–dependent granulocytic differentiation of primary hematopoietic cells. The d716 mutation was derived from a patient with severe congenital neutropenia and acute myelogenous leukemia.22This mutation deletes the putative maturation domain of the G-CSFR and disrupts G-CSF–dependent activation of SHC and the JNK/SAPK pathway.22,25 26 Our results demonstrate that this region and these signal transduction pathways are not required for G-CSF–supported granulocytic differentiation. In agreement with this conclusion, we recently have shown that mice homozygous for a similar targeted knock-in mutation of their G-CSFR have normal resting granulopoiesis (manuscript submitted).
Recent studies showed that transduction of primary hematopoietic progenitor cells with a retrovirus encoding either for c-fms or for the nonhematopoietic prolactin receptor allowed for the generation of erythroid colonies in response to macrophage colony-stimulating factor or prolactin, respectively.27,28 These results suggested that signals uniquely generated by the erythropoietin receptor were not required for erythrocytic differentiation. Likewise, in the present study, we show that expression of the GEpoR chimera in hematopoietic progenitor cells allowed for G-CSF–dependent granulocytic differentiation. This result suggests that signals generated by the cytoplasmic domain of the erythropoietin are able to substitute for those generated by the G-CSFR. In contrast, recent studies have shown that ectopic expression of the full-length erythropoietin receptor,29 thrombopoietin receptor,30 or c-fms27 in primary murine hematopoietic cells does not support granulocytic differentiation. In addition to differences in the retroviral vectors and culture conditions, the major difference between these studies and the current study is the retention of the extracellular and transmembrane domains of the G-CSFR in the GEpoR chimera. It therefore is possible that these regions of the G-CSFR are sufficient to generate the signals required for granulocytic differentiation in this system (see discussion below). Studies are in progress to explore this hypothesis.
Smith et al31 recently showed that multiple regions of the β-common chain of the GM-CSF receptor may contribute to GM-CSF–dependent macrophage differentiation in a cell-specific manner. Their data suggested that both the strength of the differentiative signal and the pool of available signaling intermediates determined the differentiation response. It is therefore possible that the high level of receptor expression achieved with retroviral promoters may allow for an otherwise weak (and possibly nonphysiological) signal to initiate a differentiation program. For example, multiple regions of the G-CSFR may normally be required to induce granulocytic differentiation; the loss of one region (ie, membrane-distal region) may be compensated for by the overexpression of the remaining receptor regions. A confirmation of our findings in mice carrying a similar targeted G-CSFR mutation in which the level of GEpoR expression is physiological and lineage-restricted is therefore warranted.
Two general models for the role of cytokines in hematopoietic differentiation have been proposed.32 In the instructive model, cytokines transmit specific signals to multipotential hematopoietic cells directing lineage commitment and differentiation, whereas, in the stochastic model, lineage-commitment and terminal differentiation are intrinsically determined with cytokines providing only growth and survival signals. Studies of mice carrying targeted null mutations of the G-CSFR7 or erythropoietin receptor33,34 have shown that the production of the relevant lineage-committed progenitors is largely preserved. Likewise, megakaryocyte progenitors are present in thrombopoietin receptor-deficient mice, although at a much reduced level compared with wild-type mice.35,36 Collectively, these data indicate that the signals generated by these receptors are not required for lineage commitment. In the present study, we show that ectopic expression of the G-CSFR in hematopoietic progenitor cells supports multilineage differentiation with the production of mature granulocytes, macrophages, megakaryocytes, and erythroid cells. This result suggests that signals generated by the G-CSFR are able to substitute for those normally generated by the erythropoietin and thrombopoietin receptors to support erythrocytic and megakaryocytic differentiation, respectively. In agreement with this conclusion, ectopic expression of the receptors for erythropoietin,37-39thrombopoietin,30 or GM-CSF40,41 in primary hematopoietic progenitor cells does not result in preferential lineage commitment and allows for ligand-dependent multilineage differentiation (with the possible exception of granulocytes, see above).
Collectively, these data provide new and strong evidence in support of the stochastic model of hematopoietic differentiation in which cytokines provide important growth and survival signals but do not direct terminal differentiation. If specific signals directing terminal differentiation (or lineage commitment) are not being generated, then why do the cytoplasmic domains of members of the cytokine receptor family share so little homology? Two recent studies provide a potential explanation. Huffman et al42 recently showed that GM-CSF–deficient mice have a defect in macrophage function that leads to a clinical syndrome with features of pulmonary alveolar proteinosis.42-44 Likewise, we recently have discovered that neutrophils isolated from G-CSFR–deficient mice have significant but selective functional defects45 (and manuscript in preparation). These data suggest that the GM-CSF and G-CSF receptors are providing nonredundant signals essential for mature macrophage or neutrophil function, respectively. The need to selectively regulate the function of specific populations of mature hematopoietic cells may explain the diversity within the cytokine receptor family.
ACKNOWLEDGMENT
The authors thank Dr Mark Sands for his assistance in the generation of high-titer retrovirus. We also thank Jennifer Poursine-Laurent for her expert technical assistance.
Supported by a grant from Monsanto/Searle.
Address reprint requests to Daniel C. Link, MD, Washington University Medical School, Division of Bone Marrow Transplantation and Stem Cell Biology, Box 8007, 660 S Euclid Ave, St Louis, MO 63110-1093; e-mail:link@IM.wustl.edu.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal