Abstract
In sickle cell anemia (SS), some red blood cells dehydrate, forming a hyperdense (HD) cell fraction (>1.114 g/mL; mean corpuscular hemoglobin concentration [MCHC], >46 g/dL) that contains many irreversibly sickled cells (ISCs), whereas other SS red blood cells dehydrate to an intermediate density (ID; 1.090 to 1.114 g/mL; MCHC, 36 to 46 g/dL). This study asks if the potassium-chloride cotransporter (K:Cl) and the calcium-dependent potassium channel [K(Ca2+)] are participants in the formation of one or both types of dense SS red blood cells. We induced sickling by exposing normal density (ND; 1.080 to 1.090 g/mL; MCHC, 32 to 36 g/dL) SS discocytes to repetitive oxygenation-deoxygenation (O-D) cycles in vitro. At physiologic Na+, K+, and Cl−, and 0.5 to 2 mmol/L Ca2+, the appearance of dense cells was time- and pH-dependent. O-D cycling at pH 7.4 in 5% CO2-equilibrated buffer generated only ID cells, whereas O-D cycling at pH 6.8 in 5% CO2-equilibrated buffer generated both ID and HD cells, the latter taking more than 8 hours to form. At 22 hours, 35% ± 17% of the parent ND cells were recovered in the ID fraction and 18% ± 11% in the HD fraction. Continuous deoxygenation (N2/5% CO2) at pH 6.8 generated both ID and HD cells, but many of these cells had multiple projections, clearly different from the morphology of endogenous dense cells and ISCs. Continuous oxygenation (air/5% CO2) at pH 6.8 resulted in less than 10% dense cell (ID + HD) formation. ATP depletion substantially increased HD cell formation and moderately decreased ID cell formation. HD cells formed after 22 hours of O-D cycling at pH 6.8 contained fewer F cells than did ID cells, suggesting that HD cell formation is particularly dependent on HbS polymerization. EGTA chelation of buffer Ca2+ inhibited HD but not ID cell formation, and increasing buffer Ca2+ from 0.5 to 2 mmol/L promoted HD but not ID cell formation in some SS patients. Substitution of nitrate for Cl− inhibited ID cell formation, as did inhibitors of the K:Cl cotransporter, okadaic acid, and [(dihydroindenyl) oxy]alkanoic acid (DIOA). Conversely, inhibitors of K(Ca2+), charybdotoxin and clotrimazole, inhibited HD cell formation. The combined use of K(Ca2+) and K:Cl inhibitors nearly eliminated dense cell (ID + HD cell) formation. In summary, dense cells formed by O-D cycling for 22 hours at pH 7.4 cycling are predominately the ID type, whereas dense cells formed by O-D cycling for 22 hours at pH 6.8 are both the ID and HD type, with the latter low in HbF, suggesting that HD cell formation has a greater dependency on HbS polymerization. A combination of K:Cl cotransport and the K(Ca2+) activities account for the majority of dense cells formed, and these pathways can be driven independently. We propose a model in which reversible sickling-induced K+ loss by K:Cl primarily generates ID cells and K+ loss by the K(Ca2+) channel primarily generates HD cells. These results imply that both pathways must be inhibited to completely prevent dense SS cell formation and have potential therapeutic implications.
THE ORIGIN OF dehydrated sickle cells, either dense discocytes or irreversibly sickled cells (ISCs), found in sickle cell disease (SS) blood has been a challenge since first observed by Herrick nearly 90 years ago.1 Dehydration of SS red blood cells has pathophysiological consequences, including increased red blood cell rigidity2 and reduced life span.3 Moreover, dehydration of SS cells implies an increased mean corpuscular hemoglobin concentration (MCHC) that decreases the delay time for deoxygenation-induced polymerization of hemoglobin S (HbS) by a factor that is proportional to the 30th power of the Hb concentration.4 Dense SS cells are also rheologically incompetent5,6 and contribute directly to vaso-occlusion.7
SS cells are heterogeneous with respect to degree of dehydration, age, HbF levels, and ion transport properties.8,9 Some intermediate density (ID) cells (similar to the SS3 fraction previously defined by us as all red blood cells with MCHC of 37 to 42 g/dL) are dense discocytes, whereas a hyperdense (HD) fraction (similar to the SS4 fraction previously defined by us as all red blood cells with MCHC >42 g/dL) is the class of SS cells that contains the majority of ISCs as well as very dense discocytes.5
There are three operational ways to define dense cells in sickle cell anemia. The first is to define them as all red blood cells more dense than most normal (AA) red blood cells, which results in a density cut between 1.091 and 1.096 g/mL. The second is to define a more dehydrated group of SS cells that are rich in ISCs and depleted of HbF that are the result of a density cut between 1.103 and 1.118 g/mL. In this report, we have opted for a third approach. We have defined two classes of dense SS cells: ID cells (1.090 to 1.114 g/mL) and HD cells (>1.114 g/mL, which includes the ISC-rich cell fraction).
This approach is not new, because Clark et al10 proposed similar criteria for cutting discontinuous Stractan gradients; red blood cells of middle density began at 1.096 g/mL and hyperdense cells began at 1.115 g/mL. Other workers have also defined two classes of dense SS cells with cuts at 1.097 and 1.104 g/mL,11 1.087 and 1.118 g/mL,12 and 1.098 and 1.112 g/mL.13Densities exceeding 1.105,14 1.110,15 and 1.118 g/mL16 have been used to define a single class of dense SS cells. Others have made a broader definition of dense SS cells that included all cells greater than 1.092 g/mL17 or greater than 1.096 g/mL.18
In our previous work with continuous Percoll-Larex (Stractan) gradients,19,20 we defined SS3 cells (similar to ID cells) as having densities between 1.091 and 1.105 g/mL, whereas SS4 cells (similar to the most dense ID cells and all of the HD cells) have densities greater than 1.105 g/mL. SS3 cells and SS4 cells differ in a number of properties that support the contention that they should be studied separately and may have different origins: SS3 cells have a smaller percentage of ISCs (22.5% ± 12.8% v 70.2% ± 7.4%),5 a higher percentage of reticulocytes (6.3% ± 7.2% v 3.2% ± 5.8%),5 and a higher percentage of HbF (8% ± 2% v 4% ± 2%).9Moreover, Kaul et al5-7 have demonstrated that, whereas both SS3 and SS4 cells are rheologically incompetent, SS3 cells show the greatest change in rheological properties when oxygenated and deoxygenated conditions are compared. That is, SS4 cells are constitutively rigid, whereas SS3 cells rapidly become rigid due to HbS polymer formation.
The dehydration of SS cells is thought to involve a deoxygenation-induced increase in membrane permeability to monovalent alkali metals (Na+, K+) and divalent cations (Ca2+, Mg2+) that is unique to SS cells (reviewed in Canessa,8 Joiner,21 and Brugnara and Tosteson22). The existence of a Ca2+-dependent K+ channel [K(Ca2+)]23-26 and a K:Cl cotransport (K:Cl) pathway in red blood cells27-32 and particularly in SS red blood cells33,34 is well established, but their relative contributions to the formation of dense cells has remained controversial.8,21,22 It has been suggested that deoxygenation-induced stimulation of the K(Ca2+) results secondarily and persistently in the activation of K:Cl cotransport by virtue of the cellular acidification that accompanies cell dehydration.26 35 Nevertheless, the relative importance and individual effects of these pathways on dense SS cell formation are not clearly understood.
Other lines of evidence suggest that the formation of dense dehydrated SS cells may proceed by multiple pathways, some of which may be dependent on cell age and HbF content. For example, the dense cell population derived from whole SS blood contains both reticulocytes and more mature, although still young, cells,26 indicating that some SS cells become dense while very young, whereas other cells take considerably longer to become dense. The presence of a rapid dehydration pathway was first proposed by Bertles and Milner36 and later described in more detail by Bookchin et al.26 Moreover, cells in the high density fraction (SS4) contain a lower percentage of HbF than cells in the intermediate density fraction (SS3).9 This difference is compatible with a model in which SS4 cells are generated directly by a polymerization-related mechanism and SS3 cells by a polymerization-independent mechanism.
Fabry et al9 also demonstrated that only a fraction of SS2 cells have K:Cl volume regulation. More recently, Franco et al17 reported that, if SS cells are separated into very young (transferrin-receptor positive [TfR+]) SS reticulocytes and older cells (TfR−), the TfR+ cells have a lower HbF than TfR−cells, which they and others have attributed to a longer circulating lifespan of HbF-containing cells and the resultant enrichment in older, high HbF cells. They also found TfR+ cells in the density fraction defined as greater than 1.092 g/mL. That very young reticulocytes can become dense implies a rapid path for dehydration and their low level of HbF suggests that either their dehydration is polymer-driven, because of the inhibitory effect of HbF on polymerization, or that the older cells are HbF-rich, because these cells are protected from polymer-driven destruction.17Recently, Franco et al37 reported that, when unfractionated SS cells were subjected to cyclic oxygenation-deoxygenation (O-D) and then subjected to density fractionation, the TfR+ cells had undergone a larger density increase than the TfR−cells. They also demonstrated that, under conditions of cyclic O-D, both K:Cl and K(Ca2+) pathways play a role in SS red blood cell dehydration, which is in agreement with our previous report.38 However, they did not analyze their data in terms of different density classes of high-density cells.
Recent in vitro studies by others using repetitive O-D cycling to generate dehydrated SS cells used conditions that favored ID cell formation: short O-D cycling times (<6 hours) and buffer pH 7.4.25,37 Formation of HD cells in these studies was either negligible or not separately studied; thus, mechanisms by which HD cells are formed in vivo could not be evaluated. This is important, because dense SS cells are heterogeneous in terms of their functional abnormalities and contribution to disease pathophysiology. Cells in the ID cell fraction are more likely to be vaso-adhesive than HD cells, which are more likely to be mechanically rigid and participate in vaso-occlusive events.5 6
To test the hypothesis that different mechanisms may be involved in the sickling-induced formation of HD and ID SS cells, we used a technique, previously used by others,39-41 that simulates in vivo sickling by exposing SS cells to repetitive in vitro O-D cycles. Unlike some earlier studies, experimental conditions were selected to allow for in vitro generation of both ID and HD cells from a density-defined discoidal subpopulation (ND) of SS cells.
MATERIALS AND METHODS
Blood collection.
Blood was drawn from SS patients followed in the Day Hospital (Dr L. Benjamin, Director) or Hereditary Clinic (Dr H. Lachman, Director) of the Bronx Comprehensive Sickle Cell Center (Bronx, NY) after informed consent had been obtained. Diagnosis of homozygous βSgenotype was based on two types of electrophoresis (cellulose acetate, borate buffer, pH 8.6, and agar, citrate buffer, pH 6.4), anMst II restriction digest gene analysis, and a solubility test. Patients with concomitant α-thalassemia and those transfused within 3 months were excluded.
Primary density fractionation.
To avoid the complication of following new dense cell formation in the presence of pre-existing dense cells, all experiments were performed on an ND cell fraction, prepared as described. SS blood was washed 3 times in 10 mmol/L sodium phosphate, 140 mmol/L NaCl, pH 7.4 (PBS), and 0.5 mL of the washed cells were applied to a total volume of 6 mL of a continuous isotonic density gradient, composed of Percoll-Larex UF (Larex arabinogalactan; Larex International, St Paul, MN), and the cells were separated by centrifugation as previously described.19 The ND discocyte fraction (1.080 to 1.090 g/mL, similar to SS2) was isolated, as previously described,19 and used as starting material for all of the following experiments. Color instant Polaroid photographs and color transparencies (Polaroid Corp, Cambridge, MA) of the density gradients were taken. To quantitate cells in the different density fractions, analytical gradients were run simultaneously using 0.05 mL packed red blood cells/6 mL gradient, and the color transparencies were scanned by laser densitometry.
In vitro O-D cycling.
One milliliter of approximately 40% hematocrit ND SS cells was added to 14 mL of O-D cycling buffer in 50-mL Erlenmeyer flasks. O-D cycling buffer typically contained (as final concentrations) 20 mmol/L sodium HEPES, pH 7.4, 120 mmol/L sodium chloride, 0.9 mmol/L sodium phosphate, 4.5 mmol/L potassium chloride, 0.5 to 2 mmol/L calcium chloride (as indicated), 0.9 mmol/L magnesium chloride, 10 mmol/L glucose, 0.2% bovine serum albumin (BSA), 0.1 mg/mL streptomycin, and 100 U/mL penicillin at 290 mOsm/kg. For experiments in which Cl− was excluded, all salts in the O-D cycling buffer had nitrate (NO3−) substituted for Cl−.
The flasks were sealed with rubber stoppers and continuously flushed with alternating cycles of 5% CO2/balance air and 5% CO2/balance N2. Where buffer pH was 7.4, it was adjusted after equilibration with 5% CO2. Where buffer pH was 6.8, no adjustments were made and, in the absence of bicarbonate to buffer the CO2, the resulting pH after equilibration with CO2 was 6.8. Gas flow was set to approximately 30 mL/min. O-D cycling was accomplished using a three-way valve and intervolameter timer. A manifold was used to allow control and experimental conditions to be tested simultaneously on the same sample. During the course of our experiments, various times for the duration of the O and D component of the O-D cycle were tested; they ranged from 5 minutes (pO2 84 ± 4 mm Hg) to 15 minutes (pO2 118 ± 11 mm Hg) for the O cycle; for the D cycle, 10 and 15 minutes were tested and found to yield the same value (pO2 22 ± 13 mm Hg). For some experiments, buffer pH was continuously monitored using a needle pH electrode inserted into the sealed flask. The flasks were shaken at 80 to 90 rpm at 37°C. After O-D cycling (or exposure to continuous air or N2 equilibrated with 5% CO2), 100% humidified oxygen was introduced into the sealed flasks for an additional 30 minutes. Buffer pH at the end of the oxygenation period was 7.2 to 7.3 and was similar for Cl−- and NO3-containing buffers. After 22 hours, buffer osmolality increased from 294 ± 3 to 310 ± 3 mOsm/kg. Hemolysis after 22 hours, measured as Hb released into the buffer, was 2.3% ± 0.6% when the incubations took place at pH 7.4 and 5.6% ± 0.9% when the incubations took place at pH 6.8 (see below for method). Hemolysis was not affected by increasing buffer Ca2+ from 0.5 to 2 mmol/L or by using continuous gas exposure versus O-D cycling.
Secondary density fractionation.
After oxygenation, the flasks were opened, and the cells collected by centrifugation and washed three times in isotonic saline. The washed cells were then separated a second time by analytical and preparative density fractionation, using continuous Percoll-Larex gradients, as described above.19 Photographs and transparencies of the analytical gradients were taken. Density fractions were collected from the preparative gradients by cutting at defined density locations to yield low density (LD; <1.080 g/mL; MCHC, <32 g/dL), ND (1.080 to 1.090 g/mL; MCHC, 32 to 36 g/dL), ID (1.090 to 1.114 g/mL; MCHC, 36 to 46 g/dL), and HD (>1.114 g/mL; MCHC, >46 g/dL) cell fractions. This separation is very similar, but not identical to, our previously defined density populations SS1, SS2, SS3, and SS4.19 By analogy to this earlier separation scheme, the majority of reticulocytes are found in the LD and ND cell fractions.19In some experiments, a single density cut was made at 1.10 g/mL (MCHC, 40 g/dL), resulting in two cell populations: a top fraction containing lighter cells with densities less than 1.10 g/mL (MCHC, <40 g/dL) and a bottom fraction containing the most severely dehydrated cells with densities greater than 1.10 g/mL (MCHC, >40 g/dL). The reason for this density cut was to increase the number of dense cells available for this and other studies. Isolated density fractions were washed three times in isotonic saline to remove the gradient mixture. Aliquots of washed cells were fixed in glutaraldehyde for light and electron microscopy, as described below.
ATP, Hb, and F-cell determinations.
For ATP, 0.8 mL of the O-D cycling mixture (at 0 hours and after 22 hours of O-D cycling) was added to 0.8 mL ice-cold 12% trichloroacetic acid and vortexed to lyse the cells. The lysates were incubated on ice for 5 minutes, quick frozen using a dry ice-ethanol bath, and stored for less than 5 days at −80°C. Immediately before analysis (all of the samples were analyzed on the same day), the lysates were thawed and the supernatants were collected by microfuge. ATP was assayed using Sigma kit 366-UV (Sigma, St Louis, MO), according to the manufacturer and expressed as micromoles of ATP per gram of Hb. Hb was determined in 0.05 mL of the O-D cycling mixture using Drabkins reagent and compared with an Hb standard, according to Sigma kit 525 (Sigma).
For hemolysis determinations, Hb was determined in 0.5 mL of the O-D cycling buffer (after centrifuging out the intact red blood cells) using Drabkins reagent, as described above, and compared with a 100% hemolysis standard.
F cells were measured by fluorescence-activated cell sorting (FACS) analysis using PBS-washed red blood cells derived from whole blood or Percoll-Larex density fractions before and after O-D cycling using a protocol performed and developed by Wallac/Isolab, Inc (Akron, OH). Briefly, after washing red blood cells from blood or Percoll-Larex density fractions at least four times with PBS, the cell pellet was resuspended in an approximately equal volume of PBS. For fixation, 10 μL of the cell suspension was added to 1 mL of PBS/4% formaldehyde (Fisher Scientific, Pittsburgh, PA) for 1 hour at room temperature. PBS (0.25 mL)/0.05% glutaraldehyde (Fisher Scientific, Pittsburgh, PA) was added, and the cells were mixed for an additional 30 seconds and then immediately pelleted by centrifugation for 5 minutes at 150g. A brief exposure to glutaraldehyde was found to aid in preserving cell morphology; however, care must be taken to limit exposure to the glutaraldehyde to 30 seconds plus the 5 minutes of centrifugation time. The fixed cells were then washed in PBS, centrifuged, resuspended in 0.25 mL of PBS/5% nonfat dry milk, mixed gently for 10 minutes at room temperature, and centrifuged. For permeabilization, the cell pellet was resuspended in 0.5 mL of 0.01% Triton X-100 (Sigma/Aldrich, Milwaukee, WI) in PBS/0.1% BSA and aliquoted into two 0.1-mL fractions. One aliquot received fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody to HbF (Wallac/Isolab, Inc; affinity purified, 1 μg/0.1 mL cell suspension), whereas the other aliquot received an isotypic control. The cells were mixed gently for 30 minutes at room temperature, centrifuged, washed in PBS, pelleted, and resuspended in PBS. A minimum of 10,000 cells were analyzed using an Epics XL flow cytometer (Coulter, Hialeah, FL). For some experiments, red blood cell HbF was determined in hemolysates by electrophoresis on citrate agar.9
Ion transport inhibitors.
In some experiments, pharmacologic inhibitors of the K:Cl cotransporter or K(Ca2+) were added. To inhibit K:Cl cotransport, final concentrations of 0.5 μmol/L okadaic acid (ammonium salt; Research Biochemical International, Natick, MA; stock solution at 0.1 mmol/L in dimethyl sulfoxide [DMSO]) and 0.1 mmol/L [(dihydroindenyl)oxy]alkanoic acid (DIOA; Research Biochemical International, Natick, MA; stock solution at 50 mmol/L in DMSO) were used. To inhibit K(Ca2+), final concentrations of 10 μmol/L clotrimazole (Sigma; 10 mmol/L stock solution in DMSO) and 0.1 μmol/L charybdotoxin (Bachem Bioscience, Philadelphia, PA; stock solution at 0.15 mmol/L in water) were used. These experiments also contained 0.1 mmol/L ouabain to inhibit the Na+-K+-ATPase (Sigma; 10 mmol/L stock in water) and 10 μmol/L bumetanide to inhibit the Na+-K+-2Cl− cotransporter (Sigma; 10 mmol/L stock in DMSO).
Microscopy.
Cells (0.05 mL packed cells) were fixed for light and electron microscopy in 2 mL ice-cold 2% glutaraldehyde in PBS, pH 7.4. After incubation for 1 hour on ice, the cells were collected by centrifugation and washed three times in PBS. Fixed cells were used to make smears on slides that were stained with Giemsa stain in an automated slide stainer. Cells were examined under 1,000× light microscopy and photographs were taken of several fields. ISC counts were made from the photographs. Morphologically, ISCs were strictly defined as cells whose length was ≥2× width and having only two points at opposite ends of the cell after oxygenation for 30 minutes in 100% oxygen. The same investigator performed all of the ISC counts.
Statistical analysis.
Values are presented as the mean ± 1 SD. Statistical analysis was performed using SigmaStat for Windows (ver 1.0; Jandel Scientific, San Rafael, CA). The probability of the null hypothesis was calculated using the paired or unpaired t-test (two-tailed), as appropriate.
RESULTS
Effect of buffer pH and number of O-D cycles on dense cells formed from ND SS cells by O-D cycling.
O-D cycled cells were fixed in glutaraldehyde in both the oxygenated (O) and deoxygenated (D) stages of the O-D cycle. Cells fixed in the D stage were greater than 90% sickle shaped, with many cells exhibiting multiple projections, whereas cells fixed in the O stage were predominately biconcave discs, demonstrating that our O-D cycling protocol resulted in near complete cell sickling-unsickling during each O-D cycle (data not shown). This was true for O stages that ranged from 3 to 15 minutes and for D stages that ranged from 8 to 15 minutes.
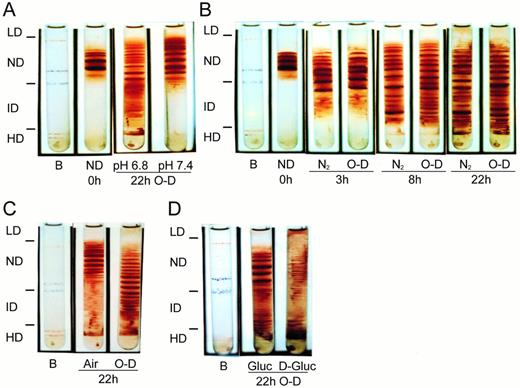
O-D cycling SS ND cells for 22 hours (88 cycles of 5 minutes O and 10 minutes D) in buffer containing physiologic levels of sodium, K+, and Cl− and 0.5 mmol/L Ca2+ in 5% CO2-equilibrated buffer at pH 6.8 resulted in both ID cell (density, 1.090 to 1.114 g/mL) and HD cell (density, >1.114 g/mL) formation (Fig1A). In contrast, O-D cycling at pH 7.4 in 5% CO2-equilibrated buffer did not result in generation of HD cells and only small numbers of cells that were more dense than the parent ND cells were formed (Fig 1A).
Formation of dense SS cells by in vitro O-D cycling. SS ND (1.080 to 1.090 g/mL) cells were isolated from SS whole blood by density gradient centrifugation using continuous density isotonic Percoll-Larex (Stractan) gradients with physiologic concentrations of Na+, K+, Mg2+, and Cl−. The ND cells were subject to O-D cycling in sealed flasks containing a bicarbonate-free HEPES buffer at pH 7.4 using 5% CO2/balance air (duration, 5 minutes) and 5% CO2/balance N2 (duration, 10 minutes) for the oxy (O) and deoxy (D) stages, respectively. Buffer pH was adjusted to 7.4 after equilibration with 5% CO2. Without adjustment, in the absence of bicarbonate and after equilibration with 5% CO2, the resulting buffer pH was 6.8. Experiments using continuous deoxygenation or continuous oxygenation also used 5% CO2-containing gasses. After O-D cycling (or continuous air or N2 exposure) the flasks were flushed under sealed conditions with 100% oxygen for 30 minutes, followed by collection of the cells and a second density separation, as described above. Color transparencies of analytical gradients were taken and scanned by laser densitometry to quantitate the percentage of cells in each density fraction. Cell density fractions were defined as follows: LD (<1.080 g/mL), ND (1.080 to 1.090 g/mL), ID (1.090 to 1.114 g/mL), and HD (>1.114 g/mL). Results shown are representative of from 2 to 15 experiments and are paired results using a single SS patient in all arms of the experiment. (A) Effect of pH on dense cells formed after 22 hours of O-D cycling. (B) Time course of dense cells formed by continuous deoxygenation (5% CO2/balance N2) or O-D cycling at pH 6.8. (C) Dense cells formed after 22 hours of continuous oxygenation (5% CO2/balance air) or O-D cycling at pH 6.8. (D) Effect of ATP depletion on dense cell formation by O-D cycling. The O-D cycling buffer contained either 10 mmol/L glucose (Gluc) or 10 mmol/L 2-deoxyglucose (D-Gluc). The final concentration of Ca2+ in the buffer was 1.5 mmol/L (for A and D) or 0.5 mmol/L (for B and C). B, density marker beads (Amersham Pharmacia Biotech, Piscataway, NJ).
Formation of dense SS cells by in vitro O-D cycling. SS ND (1.080 to 1.090 g/mL) cells were isolated from SS whole blood by density gradient centrifugation using continuous density isotonic Percoll-Larex (Stractan) gradients with physiologic concentrations of Na+, K+, Mg2+, and Cl−. The ND cells were subject to O-D cycling in sealed flasks containing a bicarbonate-free HEPES buffer at pH 7.4 using 5% CO2/balance air (duration, 5 minutes) and 5% CO2/balance N2 (duration, 10 minutes) for the oxy (O) and deoxy (D) stages, respectively. Buffer pH was adjusted to 7.4 after equilibration with 5% CO2. Without adjustment, in the absence of bicarbonate and after equilibration with 5% CO2, the resulting buffer pH was 6.8. Experiments using continuous deoxygenation or continuous oxygenation also used 5% CO2-containing gasses. After O-D cycling (or continuous air or N2 exposure) the flasks were flushed under sealed conditions with 100% oxygen for 30 minutes, followed by collection of the cells and a second density separation, as described above. Color transparencies of analytical gradients were taken and scanned by laser densitometry to quantitate the percentage of cells in each density fraction. Cell density fractions were defined as follows: LD (<1.080 g/mL), ND (1.080 to 1.090 g/mL), ID (1.090 to 1.114 g/mL), and HD (>1.114 g/mL). Results shown are representative of from 2 to 15 experiments and are paired results using a single SS patient in all arms of the experiment. (A) Effect of pH on dense cells formed after 22 hours of O-D cycling. (B) Time course of dense cells formed by continuous deoxygenation (5% CO2/balance N2) or O-D cycling at pH 6.8. (C) Dense cells formed after 22 hours of continuous oxygenation (5% CO2/balance air) or O-D cycling at pH 6.8. (D) Effect of ATP depletion on dense cell formation by O-D cycling. The O-D cycling buffer contained either 10 mmol/L glucose (Gluc) or 10 mmol/L 2-deoxyglucose (D-Gluc). The final concentration of Ca2+ in the buffer was 1.5 mmol/L (for A and D) or 0.5 mmol/L (for B and C). B, density marker beads (Amersham Pharmacia Biotech, Piscataway, NJ).
At pH 6.8, ID cells first became apparent after 3 hours of continuous deoxygenation or O-D cycling (Fig 1B). At 8 hours, there was considerable ID cell formation that was increased in continuously deoxygenated cells compared with O-D cycled cells. HD cell formation was not significant until 8 hours for both continuously deoxygenated and O-D cycled cells. After 22 hours, greater than 50% of the parent SS ND cells were found in the dense cell fractions (ID + HD; Fig 1A and B are representative, and data from 15 other SS patients are summarized in Fig 5, 22-hour chloride data).
SS patients were heterogeneous in the number of ID and HD cells formed by continuous deoxygenation or O-D cycling (data not shown). We noted that ID cell formation increased substantially when CO2-free gasses were used (data not shown). This may be due to the recently described inhibition of K:Cl activity by HCO3− at plasma levels (∼24 mmol/L)42; our buffer level of HCO3− after CO2-equilibration is approximately 24 mmol/L at pH 7.4, but only approximately 6 mmol/L at pH 6.8 (see Discussion).
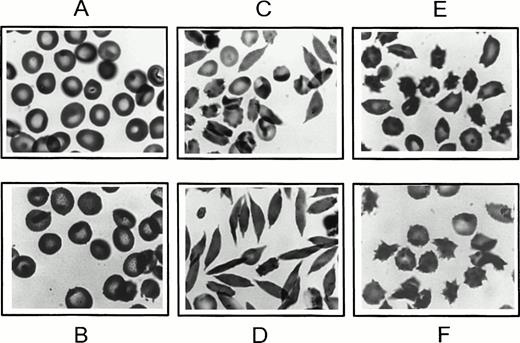
Five percent to 15% of the cells that had been O-D cycled at pH 6.8 for 22 hours in 5% CO2-equilibrated buffer (containing 0.5 mmol/L Ca2+) and then oxygenated with 100% oxygen for 30 minutes had a morphology closely resembling endogenous ISCs, ie, elongated cells with single projections at each end. Increasing oxygenation time to 1 hour or exposing the oxygenated cells to carbon monoxide (1 hour at 4°C) did not reduce ISC formation or change ISC morphology (data not shown). Moreover, electron micrographs of thin sections confirmed the absence of HbS polymer, establishing their classification as ISCs (data not shown). ISCs were more numerous in the most dehydrated bottom density cell fraction (density, >1.10 g/mL; MCHC, >40 g/dL) compared with lighter cells (density, <1.10 g/mL; Fig 2D and C, respectively).
Morphology of newly formed dense cells. After 22 hours at pH 6.8 in 5% CO2-equilibrated buffer, as in Fig 1 (for B-F), cells were oxygenated for 30 minutes with 100% oxygen, fixed in glutaraldehyde, and examined by light microscopy. (A) Uncycled parent SS ND cells; (B) SS ND cells exposed to continuous oxygenation for 22 hours; (C) top density cell fraction (<1.10 g/mL) formed after 22 hours of O-D cycling; (D) bottom density cell fraction (>1.10 g/mL) formed after 22 hours of O-D cycling; (E) top density cell fraction (<1.10 g/mL) formed after 22 hours of continuous deoxygenation; and (F) bottom density cell fraction (>1.10 g/mL) formed after 22 hours of continuous deoxygenation.
Morphology of newly formed dense cells. After 22 hours at pH 6.8 in 5% CO2-equilibrated buffer, as in Fig 1 (for B-F), cells were oxygenated for 30 minutes with 100% oxygen, fixed in glutaraldehyde, and examined by light microscopy. (A) Uncycled parent SS ND cells; (B) SS ND cells exposed to continuous oxygenation for 22 hours; (C) top density cell fraction (<1.10 g/mL) formed after 22 hours of O-D cycling; (D) bottom density cell fraction (>1.10 g/mL) formed after 22 hours of O-D cycling; (E) top density cell fraction (<1.10 g/mL) formed after 22 hours of continuous deoxygenation; and (F) bottom density cell fraction (>1.10 g/mL) formed after 22 hours of continuous deoxygenation.
Exposure of ND SS cells to continuous oxygenation with air for 22 hours at pH 6.8 with 5% CO2 equilibrated buffer resulted in less than 10% dense cell formation (ID + HD), much less than that formed by O-D cycling (Fig 1C), and most of the continuously oxygenated cells had a morphology similar to uncycled parent ND cells (Fig 2B and A, respectively). Many of the continuously deoxygenated cells had multiple projections, clearly different from the morphology of endogenous ISCs. These abnormally shaped cells were more common in the dehydrated cell fraction (density, >1.10 g/mL) compared with the lighter cells (density, <1.10 g/mL; Fig 2F and E, respectively). Hematologically normal AA cells exposed to either 22 hours of O-D cycling or continuous deoxygenation at pH 6.8 resulted in less than 1% dense cell formation, and cell morphology was normal (data not shown).
Effect of ATP depletion on dense SS cells formed by O-D cycling.
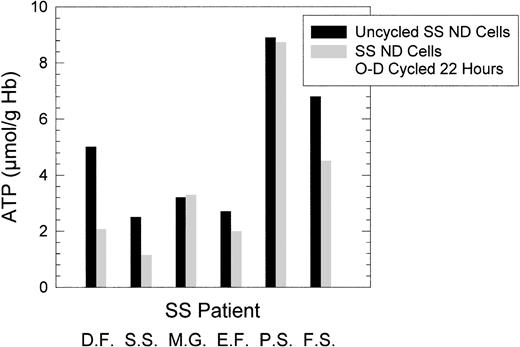
To evaluate the role of ATP depletion on O-D cycling-induced dense cell formation, SS ND cells were O-D cycled for 22 hours at pH 6.8 in 5% CO2-equilibrated buffers, containing either 10 mmol/L glucose or 10 mmol/L 2-deoxyglucose, a nonmetabolizable carbohydrate used by others to ATP-deplete red blood cells.43 As shown in Fig 1D, O-D cycling for 22 hours in the presence of 2-deoxyglucose resulted in a large increase in the formation of HD cells and a decrease in the formation of ID cells compared with O-D cycling in the presence of glucose. In six SS patients in whom ATP levels before and after 22 hours of O-D cycling at pH 6.8 in the typical O-D cycling buffer (containing 10 mmol/L glucose) were measured, ATP levels after O-D cycling were heterogeneous, with no appreciable ATP loss in three SS patients and decreases of between 26% and 58% in three SS patients (Fig 3). However, in all six SS patients, and in every SS patient we have studied, there was formation of both ID and HD cells under these conditions.
ATP levels after 22 hours of O-D cycling SS ND cells at pH 6.8. Six paired SS patient samples were tested before and after O-D cycling as described in Fig 1. The cells collected after O-D cycling represented all cells, that is, before density separation. ATP levels are expressed as micromoles per gram of Hb.
ATP levels after 22 hours of O-D cycling SS ND cells at pH 6.8. Six paired SS patient samples were tested before and after O-D cycling as described in Fig 1. The cells collected after O-D cycling represented all cells, that is, before density separation. ATP levels are expressed as micromoles per gram of Hb.
F-cell content of ID and HD cells formed by O-D cycling.
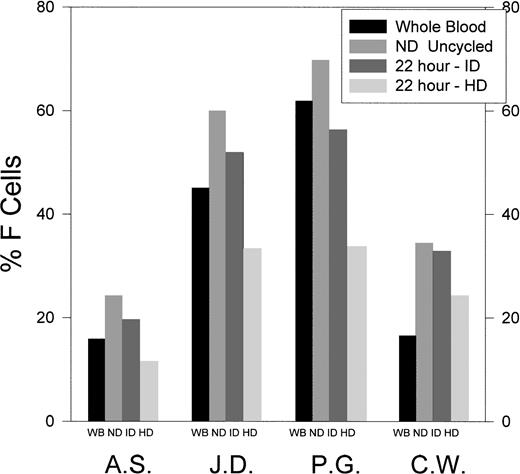
The F-cell content of unfractionated red blood cells, ND cells derived from whole blood, and ID and HD cells formed from the ND cells after 22 hours of O-D cycling at pH 6.8 in 5% CO2-containing gasses was determined by FACS analysis in four SS patients (Fig 4). ID cells formed after 22 hours of O-D cycling at pH 6.8 contained a percentage of F cells that was only slightly lower than the ND cells from which they were derived (ID/ND = 0.76 to 0.95). In contrast, HD cells formed after 22 hours of O-D cycling at pH 6.8 contained a much lower percentage of F cells compared with the ND cells from which they were derived (HD/ND = 0.42 to 0.74).
The percentage of F cells in unfractionated, ND uncycled, and ID and HD cells formed after 22 hours of O-D cycling at pH 6.8. SS ND cells derived from SS whole blood (WB) were O-D cycled for 22 hours at pH 6.8, and the newly formed dense cells were separated into ID and HD cells using continuous density gradients, as in Fig 1. Results are shown for four SS patients.
The percentage of F cells in unfractionated, ND uncycled, and ID and HD cells formed after 22 hours of O-D cycling at pH 6.8. SS ND cells derived from SS whole blood (WB) were O-D cycled for 22 hours at pH 6.8, and the newly formed dense cells were separated into ID and HD cells using continuous density gradients, as in Fig 1. Results are shown for four SS patients.
Role of the K:Cl cotransport in dense SS cell formation.
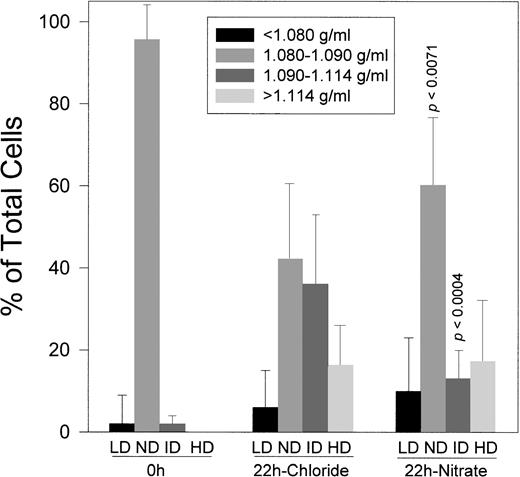
To evaluate the role of K:Cl cotransport in O-D cycling-induced dense SS cell formation, Cl− in the O-D cycling buffer was replaced by NO3− to inhibit this pathway. Experiments were conducted using 15 paired samples of ND SS cells O-D cycled for 22 hours at pH 6.8 in 5% CO2-containing gasses. As shown in Fig 5, O-D cycling in buffer containing Cl− resulted in the formation of 35% ± 17% ID cells and 18% ± 11% HD cells. Substitution of NO3− for Cl− reduced ID cell formation to 15% ± 9% (P < .0004) without significantly affecting HD cell formation, along with a concomitant increase in parent ND cells compared with O-D cycling in Cl− (P < .0071).
Effect of chloride or nitrate on dense cells formed by O-D cycling. SS ND cells were O-D cycled for 22 hours at pH 6.8 in buffer containing chloride or nitrate, each with 0.5 mmol/L Ca2+. After O-D cycling, the cells were reapplied to density gradients and LD, ND, ID, and HD fractions were quantitated as described and defined in Fig 1. Results are presented for 15 SS patients where paired data were available. p calculated using the paired t-test (two-tailed).
Effect of chloride or nitrate on dense cells formed by O-D cycling. SS ND cells were O-D cycled for 22 hours at pH 6.8 in buffer containing chloride or nitrate, each with 0.5 mmol/L Ca2+. After O-D cycling, the cells were reapplied to density gradients and LD, ND, ID, and HD fractions were quantitated as described and defined in Fig 1. Results are presented for 15 SS patients where paired data were available. p calculated using the paired t-test (two-tailed).
Effect of calcium on dense SS cell formation.
HD cell formation at pH 6.8 in 5% CO2-containing gasses was enhanced by the presence of Ca2+ in the cycling buffer, but the requirement for Ca2+ was not absolute, because substitution of Ca2+ with EGTA reduced, but did not completely prevent, HD cell formation (Table 1). Consistent with this finding, increasing the concentration of Ca2+ in the O-D cycling buffer from 0.5 to 2 mmol/L increased approximately twofold HD cell formation in two SS patients (Table 2).
Effect of Replacing Extracellular Ca2+With EGTA on Dense Cell Formation
| Condition (n = 4) . | % Cells After 22 Hours of O-D Cycling . | |||
|---|---|---|---|---|
| LD . | ND . | ID . | HD . | |
| 0.5 mmol/L Ca2+ | 6 ± 2 | 32 ± 24 | 38 ± 26 | 24 ± 8 |
| 0.5 mmol/L EGTA | 7 ± 3 | 33 ± 20 | 51 ± 13 | 9 ± 8 |
| p < .043v Ca2+ | ||||
| Condition (n = 4) . | % Cells After 22 Hours of O-D Cycling . | |||
|---|---|---|---|---|
| LD . | ND . | ID . | HD . | |
| 0.5 mmol/L Ca2+ | 6 ± 2 | 32 ± 24 | 38 ± 26 | 24 ± 8 |
| 0.5 mmol/L EGTA | 7 ± 3 | 33 ± 20 | 51 ± 13 | 9 ± 8 |
| p < .043v Ca2+ | ||||
SS-ND cells were O-D cycled for 22 hours at pH 6.8 in buffer containing physiologic levels of Cl−, after which the cells were separated using continuous density gradients and quantitated, as in Fig 1. LD, <1.080 g/mL; ND, 1.080 to 1.090 g/mL; ID, 1.090 to 1.114 g/mL; HD, >1.114 g/mL.
Effect of Increasing Extracellular Ca2+ on Dense Cell Formation by O-D Cycling
| SS Patient . | Ca2+ (mmol/L) . | % Cells After 22 Hours of O-D Cycling . | |||
|---|---|---|---|---|---|
| LD . | ND . | ID . | HD . | ||
| N.M. | 0.5 | 7 | 16 | 46 | 31 |
| 2.0 | 3 | 7 | 38 | 52 | |
| A.O. | 0.5 | 2 | 39 | 43 | 16 |
| 2.0 | <1 | 15 | 48 | 37 | |
| SS Patient . | Ca2+ (mmol/L) . | % Cells After 22 Hours of O-D Cycling . | |||
|---|---|---|---|---|---|
| LD . | ND . | ID . | HD . | ||
| N.M. | 0.5 | 7 | 16 | 46 | 31 |
| 2.0 | 3 | 7 | 38 | 52 | |
| A.O. | 0.5 | 2 | 39 | 43 | 16 |
| 2.0 | <1 | 15 | 48 | 37 | |
SS-ND cells were O-D cycled for 22 hours at pH 6.8 in buffer containing physiologic levels of Cl−, after which the cells were separated using continuous density gradients and quantitated, as in Fig 1. LD, <1.080 g/mL; ND, 1.080 to 1.090 g/mL; ID, 1.090 to 1.114 g/mL; HD, >1.114 g/mL.
Effect of ion transport inhibitors on dense SS cell formation.
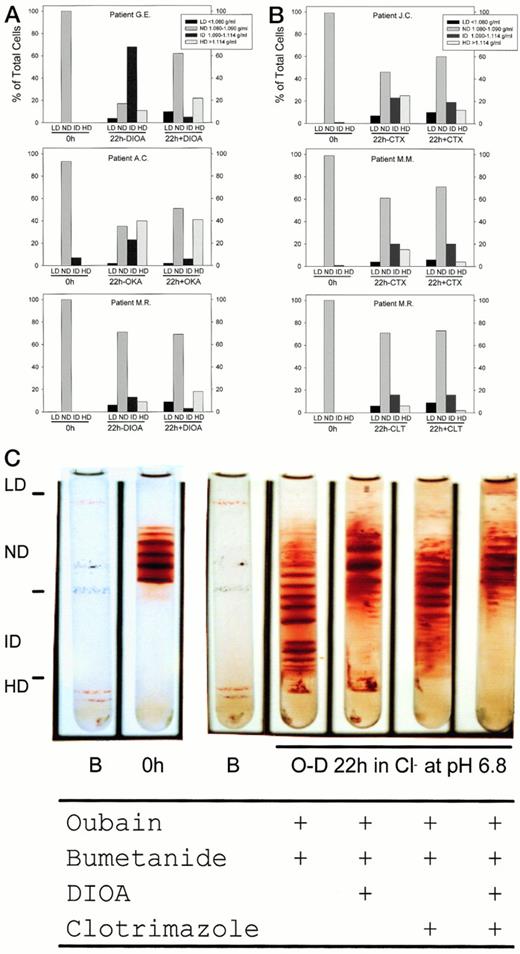
Ouabain (100 μmol/L) and bumetanide (10 μmol/L), inhibitors of the Na+-K+-ATPase and Na+-K+-2Cl− cotransporter, respectively, had no detectable effect on 22-hour O-D cycling-induced dense cell formation at pH 6.8 in 5% CO2-containing gasses in the absence of other inhibitors (data not shown). Inhibitors of K:Cl cotransport, DIOA (100 μmol/L) and okadaic acid (0.5 μmol/L), inhibited by 84% ± 8% (n = 4) the formation of ID cells but did not appreciably inhibit the formation of HD cells. This was observed in SS patients who produced large numbers of ID cells (G.E. in Fig 6A is representative), intermediate numbers of ID cells (A.C. in Fig 6A is representative), and low numbers of ID cells (M.R. in Fig 6A is representative). In some SS patients we noted a small increase in the number of HD cells formed in the presence of DIOA, but not okadaic acid. In contrast, inhibitors of K(Ca2+), charybdotoxin (0.1 μmol/L), and clotrimazole (10 μmol/L) inhibited by 42% ± 28% (n = 5) the formation of HD cells without appreciably inhibiting ID cell formation. This was observed in SS patients who produced large numbers of HD cells (J.C. in Fig 6B is representative), intermediate numbers of HD cells (M.M. in Fig 6B is representative), and low numbers of HD cells (M.R. in Fig 6B is representative). Figure 6C shows the results of an experiment in which both K:Cl and K(Ca2+) inhibitors were tested alone or together on the same SS patient. Consistent with results shown in Fig6A and B, DIOA primarily inhibits ID cell formation and clotrimazole primarily inhibits HD cell formation. Combined use of both DIOA and clotrimazole nearly abolished ID and HD cell formation. The small number of dense cells that were formed in the presence of these inhibitors were irregularly shaped, suggesting that cell damage had occurred (data not shown).
Effect of ion transport inhibitors on dense cell formation by O-D cycling. SS ND cells were O-D cycled for 22 hours at pH 6.8 and density separated into LD, ND, ID, and HD fractions, and the percentage of cells in each fraction was quantitated as described and defined in Fig 1. All experiments contained ouabain (100 μmol/L final concentration) and bumetanide (10 μmol/L final concentration) to inhibit the Na+-K+-ATPase and Na+-K+-2Cl− cotransporter, respectively. (A) Inhibitors of K:Cl cotransport, DIOA (100 μmol/L final concentration), and okadaic acid (OKA; 0.5 μmol/L final concentration) primarily inhibited ID cell formation (1.090 to 1.114 g/mL). (B) Inhibitors of K(Ca2+), charybdotoxin (CTX; 0.1 μmol/L final concentration), and clotrimazole (CLT; 10 μmol/L final concentration) inhibited primarily HD cell formation (>1.114 g/mL). (C) Combined use of K:Cl cotransport inhibitor (DIOA) and K(Ca2+) inhibitor (CLT) in the same SS patient on ID and HD cell formation. Inhibitor concentrations are as described above. A manifold was used to allow for the simultaneous O-D cycling of all four test conditions.
Effect of ion transport inhibitors on dense cell formation by O-D cycling. SS ND cells were O-D cycled for 22 hours at pH 6.8 and density separated into LD, ND, ID, and HD fractions, and the percentage of cells in each fraction was quantitated as described and defined in Fig 1. All experiments contained ouabain (100 μmol/L final concentration) and bumetanide (10 μmol/L final concentration) to inhibit the Na+-K+-ATPase and Na+-K+-2Cl− cotransporter, respectively. (A) Inhibitors of K:Cl cotransport, DIOA (100 μmol/L final concentration), and okadaic acid (OKA; 0.5 μmol/L final concentration) primarily inhibited ID cell formation (1.090 to 1.114 g/mL). (B) Inhibitors of K(Ca2+), charybdotoxin (CTX; 0.1 μmol/L final concentration), and clotrimazole (CLT; 10 μmol/L final concentration) inhibited primarily HD cell formation (>1.114 g/mL). (C) Combined use of K:Cl cotransport inhibitor (DIOA) and K(Ca2+) inhibitor (CLT) in the same SS patient on ID and HD cell formation. Inhibitor concentrations are as described above. A manifold was used to allow for the simultaneous O-D cycling of all four test conditions.
DISCUSSION
SS red blood cells in sickle cell blood are heterogeneously dehydrated, with some cells becoming HD (defined as >1.114 g/mL; MCHC, >46 g/dL; similar to SS4) and others dehydrating to ID (defined as 1.090 to 1.114 g/mL; MCHC, 36 to 46 g/dL; similar to SS3; reviewed in Nagel and Fabry44). Although it is clear that both the K(Ca2+) and the K:Cl cotransport pathways contribute to SS red blood cell dehydration, it is not known to what extent each pathway is involved in the generation of different classes of dense SS cells.
To test the hypothesis that ID and HD cells are generated by separate pathways, we adapted an in vitro O-D cycling protocol to generate ID and HD cells from ND SS discocytes. The O-D cycling protocol was adapted from earlier protocols originally described by Ohnishi et al39 and modified by others40,41 and has been used to define mechanisms involved in sickling-induced ISC and dense cell formation and SS red blood cell rheologic abnormalities. Our adaptation was to perform the O-D cycling at pH 6.8 (in buffer equilibrated with 5% CO2) at which activity of K:Cl cotransport is maximal33 and sickling is favored. In addition, we started with a relatively homogeneous ND cell population that precludes the problems of trying to measure newly formed dense cells in a mixed population containing preformed dense cells that may also change their distribution during O-D cycling. Because the ND cell fraction (similar to SS2) is enriched in young cells and reticulocytes (reviewed in Nagel and Fabry44), it is likely that this fraction is physiologically relevant for the study of dense SS cell formation in vivo. However, we recognize that our ND fraction is a selected cell fraction and that it does not contain cells that became dense in vivo. Thus, it is possible that we may not be studying cells that have the greatest tendency to become dense. Unfortunately, it is impossible to test this experimentally, because one cannot study dense cell formation in vitro using preformed dense cells.
With our O-D cycling protocol (physiologic levels of Na+, K+, Mg2+, and Cl−; 0.5 mmol/L to 2 mmol/L Ca2+; and equilibration with 5%CO2, at pH 6.8), greater than 50% of the parent ND SS cells dehydrated (they are recovered in the ID and HD cell fractions) after O-D cycling for 22 hours, and some cells were morphologically similar to endogenous ISCs. These conditions were selected because, when shorter O-D cycling times (<8 hours) or buffer pH 7.4, in the absence of CO2, were used by others,25,37 the yield of newly formed high-density cells (>1.103 g/mL, as defined in Brugnara et al25 and Franco et al37) was low.
O-D cycling ND SS cells for 22 hours at pH 7.4 (in 5% CO2-equilibrated buffer) resulted in a small amount of ID cell formation that was seen in some but not all SS patients, and there was no appreciable HD cell formation in any SS patient. In contrast, O-D cycling at pH 6.8 (in 5% CO2-equilibrated buffer) for 22 hours resulted in approximately 20% of the parent ND cells dehydrating to the HD cell fraction. Exposure of ND SS cells to continuous air for 22 hours at pH 6.8 (in 5% CO2-equilibrated buffer) resulted in less than 10% dense cell (ID + HD) formation, substantially less than that formed by O-D cycling (>50%). This supports the contention that the formation of HbS polymer is essential for the generation of at least some types of dense cells in vitro. HD cells were also formed by exposure of ND cells to continuous deoxygenation (N2/5% CO2) for 22 hours at pH 6.8; indeed, continuous deoxygenation at pH 6.8 promoted HD cell formation to an even greater extent than did O-D cycling. This suggests that continuous deoxygenation at pH 6.8 stimulates K+ loss by the K(Ca2+) pathway. Our results are consistent with those of Franco et al,37 who reported that continuous deoxygenation of TfR+ SS cells resulted in density increases that were inhibited by removal of Ca2+but not by replacement of Cl− with NO3−, suggesting that dense cells formed by continuous deoxygenation (at pH 7.4) proceeded by a Ca2+-dependent, Cl−-independent pathway, presumably the K(Ca2+) pathway, and did not require the K:Cl cotransport pathway. Alternatively, because ATP depletion accelerates HD cell formation, it is possible that ATP levels are affected differently depending on whether the cells are repetitively sickled or continuously deoxygenated. Another possibility is that continuous deoxygenation inhibits K:Cl cotransport activity. This possibility is consistent with the results of Franco et al,37 who reported that cyclic O-D but not continuous deoxygenation results in an increase in SS red blood cell density mediated by a Cl−-dependent pathway, presumably the K:Cl cotransporter.
Consistent with the hypothesis that the K(Ca2+) pathway is principally involved in HD cell formation (by O-D cycling at pH 6.8), addition of the Ca2+ chelator EGTA to the O-D cycling buffer inhibited, but did not prevent, HD cell formation, and increasing Ca2+ in the O-D cycling buffer from 0.5 to 2 mmol/L increased HD cell formation without affecting ID cell formation.
ID cells formed after 22 hours of O-D cycling at pH 6.8 contained a percentage of F cells that was only slightly smaller than the ND cells from which they were derived (ID/ND = 0.76 to 0.95). In contrast, HD cells formed after 22 hours of O-D cycling at pH 6.8 contained a much smaller percentage of F cells compared with the ND cells from which they were derived (HD/ND = 0.42 to 0.74). This result is consistent with earlier findings that the most dense circulating SS cells had the lowest HbF content9 36 and further suggests that the formation of the most severely dehydrated cells (HD cells) is more dependent on HbS polymerization than is the formation of ID cells. These results are also consistent with our contention that ID and HD cells can be formed by independent mechanisms.
Franco et al17 reported that the HbF content of very young transferrin-receptor positive (TfR+) circulating SS cells was negligible (≤0.6%) in dense cells (>1.092 g/mL, equivalent to the densest 50% of our ID fraction and all of our HD cell fraction) compared with TfR+ light cells (<1.092 g/mL), implying that HbF protects against cell dehydration in this very young TfR+ population. Thus, it is likely that HbF is protective against cell dehydration both in the youngest and in the more mature, albeit still young SS cells that are destined to become the most severely dehydrated cells. Our results are also consistent with recent data of Franco et al,45 who found that HbF is an important determinant in the formation of high-density (density, >1.094 g/mL) TfR+ SS reticulocytes, but does not mediate the formation of TfR+ moderate density (density, 1.083 to 1.094 g/mL) SS reticulocytes. This is also in agreement with our previous conclusion that both F reticulocytes and non-F reticulocytes have a similar K:Cl driven response to hypotonic stimulation.9 Together, these results suggest that sickling is not required to activate the K:Cl cotransporter or form moderate density cells, but does not rule out the possibility that sickling may enhance K:Cl cotransport activity and increase ID cell formation. Regardless, the conclusions of Franco et al45 and our conclusions from the results presented here are in agreement that different mechanisms are likely to be involved in the formation of different classes of dense young SS cells.
There are several arguments to support O-D cycling at pH 6.8 for 22 hours as pathophysiologically relevant:
(1) Although blood pH approaching 6.8 is not encountered in the general circulation, acid pH is found in regions of the spleen,46kidney,47 and in situations in which blood stasis occurs, such as vessels which are partially vaso-occluded for short periods of time.48 The final acidity depends in part on the length of stasis and the extent of deoxygenation. In this regard, SS patients are in particular danger of long periods of stasis in organs with sinusoids and in localized regions of the general circulation in which frequent transient vaso-occlusions are likely.49
(2) This protocol generates both ID and HD cells (Fig 1) and better represents than other methods the actual pattern of dense cells found endogenously in SS whole blood.19
(3) Some of the O-D cycling-induced dense cells are morphologically similar to endogenous ISCs (Fig 2), whereas dense cells resulting from continuous deoxygenation contain multiple projections clearly different from endogenous ISCs.
(4) HbF protects against the formation of the most severely dehydrated HD cells generated by O-D cycling (Fig 4), consistent with the known protective effect of HbF on in vivo dense cell formation (reviewed in Nagel and Fabry44).
Hence, it is reasonable to contend that O-D cycling for 22 hours at pH 6.8 simulates dense cell formation in vivo, validating this approach.
ATP depletion is a potential complication in our studies using 22-hour incubations, because, in the presence of Ca2+, ATP depletion is associated with cell K+ loss and dehydration.24 We find that, in the presence of 10 mmol/L glucose, ATP loss was heterogeneous, with no significant loss for some samples after 22 hours and decreases of between 26% and 58% in other samples. That dense ID and HD cells were formed in all cases suggests that ATP depletion is not required for ID and HD cell formation. Substitution of 2-deoxyglucose, a nonmetabolizable carbohydrate43, for glucose in the O-D cycling buffer greatly stimulated HD cell formation and slightly inhibited ID cell formation after 22 hours. These data are consistent with multiple pathways being involved in the formation of dense SS cells and are consistent with the known metabolic dependence of K:Cl cotransport activity (reviewed in Lauf et al50). This conclusion is also in accordance with the observation that ISCs are ATP depleted in vivo51 and with the known stimulation of K(Ca2+) by ATP-depletion in the presence of Ca2+.52
It is unlikely that K:Cl cotransport is involved in generating the HD cells in the ATP-depleted population, because increased free Mg2+ resulting from decreased ATP:Hb ratios would be expected to result in K:Cl cotransport inhibition,53 thus supporting our argument that the most severely dehydrated HD cells are more likely formed by the K(Ca2+) pathway (also see below).
Our experiments were performed using gasses containing 5% CO2. It can be calculated that the HCO3− concentration at equilibration with dissolved CO2 at pH 6.8 is approximately 6 mmol/L and at pH 7.4 is approximately 24 mmol/L [log10(HCO3−) = pH + log10(pCO2) − 7.604 at 37°C, where pCO2 at 5% CO2 = 38mm Hg]. It was recently shown that SS red blood cell K:Cl cotransport activity is strongly inhibited by physiologic levels of HCO3−found in plasma at pH 7.4 (∼24 mmol/L),42 and this may explain why we found little formation of ID cells at pH 7.4 using 5% CO2-equilibrated buffer, whereas we found significantly more ID cell formation at pH 7.4 using CO2-free buffer (data not shown).
To study the contribution of the different transport pathways involved in dense cell formation, we first defined the requirement for Cl− (indispensable for K:Cl cotransport in human red blood cells) in O-D cycling-induced dense cell formation. Whereas NO3− has been reported to bind Hb and alter red blood cell volume,54 the effect is too small (<10%) in human red blood cells to be of major concern, and the use of other anions would prevent us from comparing results with a large body of data previously collected using NO3− to inhibit human red blood cell K:Cl cotransport.
SS ND cells were O-D cycled in the presence of Cl− or NO3− (and 0.5 mmol/L Ca2+) at pH 6.8. In Cl−, both ID and HD cells were generated; with NO3−, there was a 43% reduction of ID cells without significantly affecting HD cell formation. This demonstrates that both a Cl−-dependent and a Cl−-independent pathway are present for sickling-induced SS cell dehydration from ND cells. We also noted considerable individual to individual variability in the extent of ID + HD cell formation, both in the Cl− and NO3−-O-D cycled experiments, suggesting that the generation of ID + HD cells is subject to genetic or epigenetic regulation and is consistent with previous observations of variability in ion transport activity between SS patients.8,9,53,55,56 It is likely that most of the K:Cl cotransport activation occurs during the oxy stage of the O-D cycle, because deoxygenation partially inhibits K:Cl cotransport activity.53
The effect of ion transport inhibitors further addresses the issue of the potential differences in the mechanisms of ID versus HD cell formation. Both ID and HD cells are formed by O-D cycling in the presence of Cl−, in contrast, O-D cycling in the absence of Cl− (substitution of NO3− for Cl−) results in the formation of HD cells. That K:Cl cotransport is involved in ID cell formation is further suggested by the strong inhibition of ID cell formation by DIOA18 and okadaic acid,57inhibitors of K:Cl cotransport. In contrast, charybdotoxin23 and clotrimazole,25 inhibitors of K(Ca2+), primarily inhibit HD cell formation. Combined use of both K:Cl cotransporter and K(Ca2+) inhibitors (DIOA and clotrimazole, respectively) almost completely abolished ID and HD cell formation, suggesting that these two pathways are the major contributors to in vitro dense cell formation by repetitive episodic sickling.
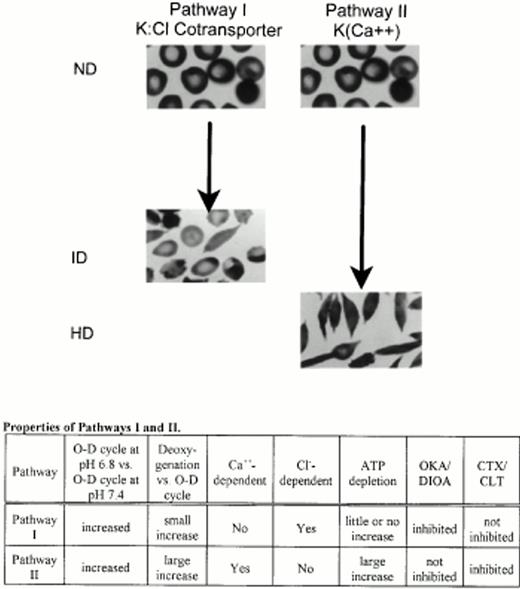
In conclusion, our results demonstrate that 22 hours of repetitive in vitro sickling at pH 6.8 in 5% CO2-containing gasses generates both ID and HD cells from ND SS discocytes. The ID and HD cells most likely arise by separate pathways; ID cells are primarily formed by a Ca2+-independent, Cl−-dependent pathway that is inhibited by pharmacologic inhibitors of K:Cl cotransport and by HCO3− and is less dependent on HbS polymerization. HD cells are primarily formed by a Ca2+-dependent, Cl−-independent pathway that is inhibited by pharmacologic inhibitors of K(Ca2+) and is more dependent on HbS polymerization.
These results support a model for repetitive sickling-induced SS normal density discocyte dehydration in which K+ loss mediated by the K:Cl cotransporter primarily generates ID cells, and K+loss mediated by K(Ca2+) primarily generates HD cells (Fig 7). This model predicts that cells with relatively high K(Ca2+) and low K:Cl (due to either increased transporter number, increased activation of regulatory intermediates, or altered set-points) will dehydrate quickly, ie, that these cells will not spend much time as ID intermediates. Cells with relatively low K(Ca2+) and high K:Cl will dehydrate more slowly, spending more time as ID intermediates. Thus, some ID cells are likely to be precursors of HD cells. This model also predicts, importantly, that inhibiting only K(Ca2+) or only K:Cl cotransport will not completely prevent sickling-induced dense cell formation.
Proposed model for the formation of ID and HD cells from young ND SS red blood cells by two independent pathways. Pathway I primarily mediates formation of ID cells and its properties are consistent with K:Cl cotransport. HbS polymerization plays a smaller role in dense cells formed by this pathway. Pathway II primarily mediates formation of HD cells and its properties are consistent with the K(Ca2+) channel. HbS polymerization plays a larger role in dense cells formed by this pathway. ND, ID, and HD cells are defined in Fig 1. O-D, O-D cycling; deoxygenation, continuous deoxygenation with 5% CO2/balance N2; Cl−-dependency was determined as the activity remaining after substitution of Cl− with NO3−; ATP depletion was accomplished by substitution of 2-deoxyglucose for glucose; OKA, DIOA, CTX, and CLT are defined in Fig 6.
Proposed model for the formation of ID and HD cells from young ND SS red blood cells by two independent pathways. Pathway I primarily mediates formation of ID cells and its properties are consistent with K:Cl cotransport. HbS polymerization plays a smaller role in dense cells formed by this pathway. Pathway II primarily mediates formation of HD cells and its properties are consistent with the K(Ca2+) channel. HbS polymerization plays a larger role in dense cells formed by this pathway. ND, ID, and HD cells are defined in Fig 1. O-D, O-D cycling; deoxygenation, continuous deoxygenation with 5% CO2/balance N2; Cl−-dependency was determined as the activity remaining after substitution of Cl− with NO3−; ATP depletion was accomplished by substitution of 2-deoxyglucose for glucose; OKA, DIOA, CTX, and CLT are defined in Fig 6.
ACKNOWLEDGMENT
The authors thank Dr Mitzy Canessa (who unfortunately passed away in 1997) for the critical review of early editions of this manuscript and for helpful suggestions. We dedicate this report to her, in the memory of her scientific career, enthusiasm, and creativity. We are grateful to Wallac/Isolab, Inc (Dr Tom Campbell and Marsha Mason) for performing F-cell FACS analysis. The technical assistance of Sandra Suzuka is gratefully acknowledged.
Supported by National Institutes of Health Grant No. HL38655.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Robert S. Schwartz, PhD, Division of Hematology, Albert Einstein College of Medicine-Montefiore Medical Center, 111 E 210th St, Bronx, NY 10467; e-mail: rschwart@worldnet.att.net.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal