Abstract
Bispecific antibodies (CD3x19) against the CD3ɛ-chain of the T-cell–receptor/CD3 complex and the CD19 antigen on B cells can target polyclonal, nontumor-specific T cells to B lymphoma cells. This induces T-cell activation, and generation of cytotoxic T cells (CTLs). These polyclonal CTLs, targeted by the CD3x19 bispecific antibodies, can lyse CD19+ B-lymphoma cells. In a xenotransplant model in severe combined immunodeficiency deficient (SCID) mice, we and others observed that CD28 triggering is required for efficient elimination of B-lymphoma cells and cure from the tumor in addition to CD3x19 administration. We also showed that the activation and targeting of CTLs to the target cell by signal one alone, ie, the CD3x19 mab, induces T-cell death by apoptosis. In blocking experiments we showed that this “veto” apoptosis is mediated by the CD95/Fas ligand. Addition of anti-CD28 (signal 2) renders the T cells resistant for veto apoptosis both in vitro and in vivo. We therefore conclude that the role of costimulation in immunotherapy with bispecific antibodies or other T-cell–based immune strategies is not only to facilitate T-cell activation but also to prevent T-cell deletion by apoptosis.
BISPECIFIC ANTIBODIES have been generated to circumvent the problem of antigen recognition in T-cell–based immunotherapy models. Such antibodies consisting of an anti–CD3ε-part (OKT3) and a tumor-cell recognizing moeity can build a bridge between a T cell and the tumor cell to induce T-cell activation by CD3 cross-linking. Previous reports have shown that bispecific antibodies against the CD3 ε-chain and the pan–B-cell antigen CD19 (CD3x19) efficiently induce cytotoxic T cells in a syngeneic situation that then can kill malignant B cells.1-3 In vivo studies using a CD3x30 bispecific antibody in a severe combined immunodeficiency disorder (SCID)-mouse model for CD30+ Hodgkin’s lymphoma showed that efficient elimination of established tumors was achieved only when a second bispecific antibody against CD30 and CD28 (CD28x30) was administered during T-cell targeting.4
It is generally accepted that T-cell activation requires two distinct signals. The first signal depends on the ligation of the T-cell receptor (TCR)/CD3 complex and the CD4 or CD8 coreceptors.5The second signal can be provided by cell surface molecules that mediate essential costimulatory signals, thereby complementing the TCR/CD3-mediated events.6,7 CD28 is such a potent costimulatory molecule, and ligation of CD28 with agonistic antibodies or its natural ligands (B7.1 (CD80) and B7.2 (CD86)) synergizes with TCR-mediated signaling to initiate and maintain T-cell responses.7,8 Recently, ligation of CD28 by agonistic antibodies has been shown to prevent activation-induced cell death (AICD) of naive T cells during primary stimulation.9 This was related to the upregulation of bcl-xL, a potent apoptosis-preventing member of the bcl-2 gene family.
We previously described that cytotoxic T cells undergo AICD on target-cell recognition.10 Thus, the cells hit and kill but then die by AICD. This may have importance in limiting potentially dangerous immune responses, eg, in the control of autoimmunity. We coined the term “veto” apoptosis to describe this phenomenon in analogy to the classical veto T-cell suppression.11 Veto apoptosis, like other forms of T-cell AICD,12,13 is mediated by the Fas ligand.10 Costimulation of the T cells by B7.1 during target-cell recognition could overcome the veto apoptosis.10 We therefore asked the question whether targeting a T cell to a B-lymphoma cell by bispecific antibodies as well induces veto apoptosis and if cytotoxic T-cell (CTL) apoptosis can be prevented by costimulation via agonistic antibodies against the CD28 antigen. This is of particular importance in view of the ongoing or planned clinical trials based on T-cell targeting, because such an event would preferentially lead to T-cell deletion in contrast to tumor-cell elimination.
MATERIALS AND METHODS
Cell culture and preparation of T cells for in vitro and in vivo assays.
All cells were maintained in 1640 RPMI (Seromed-Biochrom, Hamburg, FRG), 10% heat-inactivated fetal calf serum (FCS; GIBCO-BRL, Karlsruhe, FRG), 2 mmol/L L-Glutamine (GIBCO-BRL, Karlsruhe, FRG), and penicilline-streptomycin (Seromed-Biochrom, Hamburg, FRG). T cells were prepared from peripheral blood by means of Ficoll density-gradient centrifugation. B cells were removed by depletion with anti-CD19 magnetic activated cell sorting (MACS) magnetic beads (Miltenyi Biotec, Bergisch Gladbach, FRG). Adherent cells were removed by plastic adherence for 2 hours at 37°C. The purity of the T cells was controlled by staining with PE-labelled anti-CD3 (Becton Dickinson, Heidelberg, FRG) and analyzed on a FACSort (Becton Dickinson). The purity of the T cells was over 94%. T cells were activated in 75 cm2 culture flasks (Nunc, Copenhagen, Denmark) by immobilized OKT3 (flasks coated overnight at 10 μg/mL antibody in phosphate-buffered saline [PBS]) in the presence of the agonistic anti-CD28 mab 15E8 (1μg/mL). 30 U IL-2 (Chiron, Ratingen, FRG) were added after 24 hours to maintain and expand the T cells for a further 5 days. The cytotoxicity of T cells was then determined in a standard 4-hour 51Cr release assay against Raji or Nalm-6 targets.
Bispecific antibodies.
CD3x19 Quadroma cells were generated and cultured as described14 in a hollow-fiber bioreactor (Tecnomara, Fernwald, FRG). The derived antibody mixture was first purified by affinity-chromatography on a Protein-A Sepharose CL-4B column (Pharmacia, Uppsala, Sweden) to remove immunoglobulin G1 (IgG1) parental antibodies. Subsequently, the eluent was subjected to HPLC purification on a Bakerbond ABx column (JT Baker Inc, Philippsburg, NJ) that allowed a separation of the bispecific antibody fraction by means of a morpholinoethane sulfonic acid/sodium acetate gradient. Purity of the eluted material was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions as described.14
Immunofluorescence.
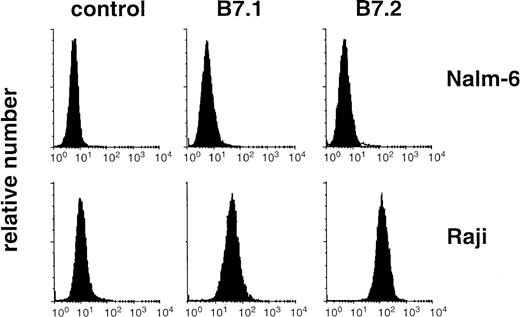
B7.1 or B7.2-expression on the B-cell lines Raji and Nalm-6 was determined by direct staining of 106 cells with phycoerythrin-labeled anti-CD80 or anti-CD86 mab (Becton-Dickinson). Surface fluorescence was determined using a FACSort (Becton-Dickinson). Dead cells were excluded by propidium iodide (PI) staining.
Measurement of apoptosis: Modified cell-cycle analysis.
Activated T cells (106) were exposed to irradiated target B cells at a 10:1 ratio. After 24 hours the malignant B cells were removed by depletion with anti-CD19 immunomagnetic beads (Dynal, Chantilly, VA). There was a slight reduction of T-cell numbers in the range of 5% to 10% after the immunomagnetic depletion, but no significant difference was observed in T-cell recovery as measured by absolute numbers of CD2+ cells in the cultures induced with or without the CD3x19 bs ab in the presence or absence of B-lymphoma cells. The purity of CD2+ cells was above 97% after MACS separation. These recovered T cells were pelleted, washed with ice-cold PBS in U-form 96-well plates, and gently resuspended in 300 μL hypotonic fluorochrome solution (propidium iodide 50 μg/mL, 0.1 mol/L sodium citrate plus 0.1% Triton X100) as previously described.15 After overnight incubation at 4°C in the dark, the propidium-iodide content of the individual nuclei was measured on a FACSort (Becton-Dickinson). Cell debris was excluded by raising the forward-scatter threshold adequately. Apoptotic nuclei displayed a decreased DNA content below the G1 peak, paralleled by an increase of the side scatter. Anti-CD95 (anti–APO-1 IgG3) or FII23c F(ab)2 fragments were produced as previously described.16Anti-B7.1 antibody BB-1 and control-immunoglobulinM(IgM) were purchased from Pharmingen (San Diego, CA). CD3x19 bs abs were prepared as described.1
Measurement of apoptosis: Detection of DNA single-strand breaks by in situ nick translation (ISNT).
To quantify apoptotic cells we used the method as previously described for detection of DNA single-strand breaks in apoptotic cells.17 Briefly, the cells were fixed with 1% formaldehyde followed by 70% ethanol. The fixed cells were washed with nick buffer (50 mmol/L Tris HCl pH 7.8, 5 mmol/L MgCl2, 0.1 mol/L β-mercaptoethanol, 10 μg/mL bovine serum albumin [BSA]) and incubated with a mixture of 1.3 μL dATP, dCTP, dGTP each 0.2 mmol/L (Perkin-Elmer-Cetus, Überlingen, FRG), 1.6 μL nick buffer, 0.3 μL 1mmol/L biotinylated-16-dUTP (Boehringer Mannheim, Mannheim, FRG) and 1 unit E.coli DNA polymerase I (AGS, Heidelberg, FRG) for 90 minutes at 15°C to complete DNA single-strand breaks. The biotin-labeled cells were stained with avidin-fluorescein isothiocyanate (FITC) (cell sorter grade; Camon, Wiesbaden, FRG). The DNA was stained with 1 μg/mL propidium iodide before analysis on a FACScan (Becton-Dickinson). Percentage of DNA fragmentation corresponds with percent green fluorescence in the propidium iodide (PI)+, eg, nuclei-containing population.
Animal experiments.
Animals (C.B-17 scid/scid mice [SCID]) from our own breeding colony) were kept and treated in accordance with local animal protection laws. The mice were kept in isolators under gnotobiotic conditions. Food and water were autoclaved. The mice were not subjected to antibiotic drug treatment. No mouse pathogens were detected. For serology, sterile sentinel mice were added to the colony. These mice were serologicaly negative for Sendai, PVM, MVM, Reo3, MHV (Corona), Theiler’s GD VII, Polyoma, K-, and m.-Adeno virus. Leakiness was determined by measuring mouse IgM and mouse IgG and mice expressing serum titers above 50 μg/ml IgM or IgG were excluded from the study. Tumor cells were injected subcutaneously in the inguinal region (Raji, 107 per animal) or intraperitoneally (Nalm-6, 107 per animal). Human T cells were prepared and preactivated in vitro by immobilized OKT3, soluble anti-CD28, and subsequent culture in low-dose IL-2 as described above. 107activated T cells were injected together with the Raji cells subcutaneously or, with a delay of 1 day, intraperitoneally in the Nalm-6 experiments. Antibodies were injected intraperitoneally together with the T cells at the following doses: CD3x19 bs ab at 200 μg/animal, monospecific anti-CD3 (OKT3) plus anti-CD19 (HD37) at 100 μg/animal, anti-CD28 (15E8) at 50 μg/animal. Serum half-life of the antibodies was determined as described18 19 and was 7.4 days for the CD3x19 and 8.1 days for the anti-CD28 mab. Peritoneal washout was performed by rigorously injecting 10 mL ice-cold PBS into the peritoneal cavity of sacrificed mice. Contaminating murine cells were removed via MACS with anti-H2D d mab and strepatavidin-magnetic beads (Miltenyi); Nalm-6 cells were depleted simultaneously as described above.
RESULTS
CD28 costimulation is required for tumor-cell elimination by bispecific antibodies in vivo.
To determine the in vivo antitumor activity of the CD3x19 bispecific antibodies, we established xenotransplant models of the Raji Burkitt lymphoma and Nalm-6 pre–B-ALL in SCID mice. Raji cells, when injected subcutaneously led to locally growing tumors that, at late stages, also showed lymphoid dissemination to the inguinal, axillar, thoracic, and abdominal lymph nodes as described.20 Nalm-6 cells were injected intraperitoneally and rapidly disseminated to liver, bone marrow and peripheral blood. The mice rapidly developed hind limb paralysis due to meningeal infiltration and had to be sacrificed. Human T cells were preactivated in vitro by immobilized OKT3, soluble anti-CD28 and low-dose IL-2. These T cells were applied together with the tumor cells s.c. (Raji) or injected intraperitoneally with a delay of 1 day (Nalm-6). Control mice received (a) in vitro activated T cells together with the tumor cells without antibodies or (b) T cells plus the monospecific anti-CD3 and anti-CD19 antibodies (Fig 1). These mice showed no increased survival as compared with mice injected with tumor cells alone. Coinjection (intraperitoneally) of CD3x19 bs ab together with the T cells led only to a slightly prolonged survival of mice transplanted with the Nalm-6 lymphoma cells. Nevertheless, 4 of 10 of the mice injected with Raji cells were cured by injection of the bispecific antibody plus T cells. In contrast to this limited survival improvement with the bs ab CD3x19 alone, the coinjection of anti-CD28 with the bispecific CD3x19 antibody protected all mice injected with Raji (Fig1a) and 9 of 10 mice that received Nalm-6 from tumor growth and dissemination (Fig 1b). The surviving mice were maintained for a further 6 months and remained disease free during this time. In the above experiments, the T cells were activated in vitro because SCID mice transplanted intraperitoneally or intravenously with resting human peripheral blood lymphocytes showed no antitumor response on anti-CD3x19 injection. This may be due to the limited recirculation and survival of human lymphocytes in SCID mice.17 In addition, there was no significant difference observed in survival (or kinetics of tumor outgrowth) in control mice injected with tumor cells, monospecific antibodies, and anti-CD28 as compared with those who received no anti-CD28 (data not shown). This rules out a nonspecific, CD3x19-independent enhancement of alloreactivity by the anti-CD28 mab as cause for the antitumor response mediated by CD3x19 plus anti-CD28 combination therapy.
Antitumor effect of bispecific CD3x19 in xenotransplanted SCID mice. (a) 107 Raji Burkitt lymphoma were injected subcutaneously together with 107 activated T cells. CD3x19 and anti-CD28 (clone 15E8) were injected intraperitoneally at the same time (b) 107 Nalm-6 cells were injected intraperitoneally. Activated T cells (107), CD3x19, and anti-CD28 were injected intraperitoneally 24-hours later. ▵, tumor cells alone; □, tumor cells plus monospecific control antibodies (anti-CD19 [HD37] plus anti-CD3 [OKT3], 100 μg each as single dose); ○, tumor cells plus CD3x19 (200 μg single dose); •, tumor cells plus CD3x19 (200 μg single dose) plus anti-CD28 (clone 15E8, 50 μg single dose).
Antitumor effect of bispecific CD3x19 in xenotransplanted SCID mice. (a) 107 Raji Burkitt lymphoma were injected subcutaneously together with 107 activated T cells. CD3x19 and anti-CD28 (clone 15E8) were injected intraperitoneally at the same time (b) 107 Nalm-6 cells were injected intraperitoneally. Activated T cells (107), CD3x19, and anti-CD28 were injected intraperitoneally 24-hours later. ▵, tumor cells alone; □, tumor cells plus monospecific control antibodies (anti-CD19 [HD37] plus anti-CD3 [OKT3], 100 μg each as single dose); ○, tumor cells plus CD3x19 (200 μg single dose); •, tumor cells plus CD3x19 (200 μg single dose) plus anti-CD28 (clone 15E8, 50 μg single dose).
CD3x19 bispecific antibodies induce apoptosis in the antibody-targeted cytotoxic T cells.
Because we observed in previous experiments that CTLs can undergo apoptosis on target-cell contact,10 we tested if this was also the case in T-cell targeting with CD3x19 bs ab. T cells were activated polyclonally with plastic-immobilized OKT3 mab plus anti-CD28 mab and maintained in IL-2–containing medium for 5 days. This stimulation induced optimal T-cell proliferation as measured by3H-thymidine uptake. The addition of anti-CD28 to the CD3-cross-linking also yields an improved cytotoxicity of the activated T cells.1 The activated T cells were then exposed to allogeneic Raji target cells and cytotoxicity against Raji was measured in a standard 51Cr-release assay. Bispecific CD3x19 ab was added in solution to the effector/target cell mixture and triggered specific lysis of the Raji Burkitt lymphoma target cells at concentrations above 0.05 μg/mL (data not shown). Addition of anti-CD28 ab to the CD3x19-targeted T-cell/Raji coculture did improve killing of the Raji target cells. Similar data were obtained with Nalm-6 as target cell (data not shown). To test if the T cells themselves underwent activation-triggered apoptosis in this system, we assessed T-cell apoptosis by measuring nuclear DNA content of the T cells. A large percentage of the T cells was induced to apoptosis by the T-cell targeting during coculture with the target cells (Fig 2). The addition of CD3x19 bispecific ab to the target cells induced apoptosis in 65% of the T cells when Nalm-6 was employed as target (Fig 2a) and 30% of the T cells in the case of Raji target cells (Fig 2b). The addition of anti-CD28 reduced the amount of T-cell apoptosis in both systems by about 50%.
Effect of anti-CD28 on CTL apoptosis during T-cell targeting. T cells were generated by activation of peripheral blood T cells by CD3 cross-linking and culture for 7 days in IL-2–supplemented medium (30 U/ml). T cells were then cultured for 24 hours at an E/T ratio of 10:1 in the absence or presence of CD3x19 bs ab (10 μg/mL). Control cultures were performed with medium alone (ie, only T cells plus [a] Nalm-6 or [b] Raji), monospecific control antibodies plus tumor cells (OKT3 and HD37 at 5 μg/mL), anti-CD28 (15E8, 1 μg/mL), IgM-control mab (G155-228, 10 μg/ml), or anti-B7.1 (BB-1, 10 μg/ml). After incubation, the remaining B lymphoma cells were removed from the coculture by immunomagnetic depletion with anti-CD19 and anti-CD20 as described.10 T-cell apoptosis was measured on the single cell level as described.15
Effect of anti-CD28 on CTL apoptosis during T-cell targeting. T cells were generated by activation of peripheral blood T cells by CD3 cross-linking and culture for 7 days in IL-2–supplemented medium (30 U/ml). T cells were then cultured for 24 hours at an E/T ratio of 10:1 in the absence or presence of CD3x19 bs ab (10 μg/mL). Control cultures were performed with medium alone (ie, only T cells plus [a] Nalm-6 or [b] Raji), monospecific control antibodies plus tumor cells (OKT3 and HD37 at 5 μg/mL), anti-CD28 (15E8, 1 μg/mL), IgM-control mab (G155-228, 10 μg/ml), or anti-B7.1 (BB-1, 10 μg/ml). After incubation, the remaining B lymphoma cells were removed from the coculture by immunomagnetic depletion with anti-CD19 and anti-CD20 as described.10 T-cell apoptosis was measured on the single cell level as described.15
Nalm-6 is essentially void of B7.1 (CD80) and B7.2 (CD86) expression (Fig 3). It was therefore tempting to speculate that the lower rate of T-cell AICD observed during targeting to Raji cells as compared with Nalm-6 could be attributed to the B7-expression of the Raji cells. Differences in CD3 crosslinking were unlikely to be involved in this effect because both lines express comparable amounts of CD19 and yield comparable amounts of51Cr release when tested in CD3x19-mediated T-cell targeting. Thus, we added a blocking anti-B7.1 (BB1) mab to the T-cell/target-cell coculture system. The anti-B7.1 mab and an isotype-matched IgM control mab had no intrinsic effect on T-cell death. Whereas blocking of B7.1 had no effect on the apoptosis of T cells targeted to (B7−) Nalm-6 cells, CD3x19-induced T-cell AICD in the presence of Raji cells was nevertheless increased suggesting that endogenous B7.1 can prevent target-induced T-cell AICD (Fig 2b) . None of the antibodies used had a stimulatory or inhibitory influence on T-cell apoptosis in the absence of B-lymphoma target cells (data not shown).
B7 expression on Raji and Nalm-6. Raji and Nalm-6 were stained for B7.1 (CD80) and B7.2 (CD86) expression by the use of phycoerythrin-labeled antibodies. Surface fluorescence was analyzed on FACSort cytometer. Dead cells were excluded by staining with propidium iodide.
B7 expression on Raji and Nalm-6. Raji and Nalm-6 were stained for B7.1 (CD80) and B7.2 (CD86) expression by the use of phycoerythrin-labeled antibodies. Surface fluorescence was analyzed on FACSort cytometer. Dead cells were excluded by staining with propidium iodide.
Veto apoptosis is mediated by the Fas ligand.
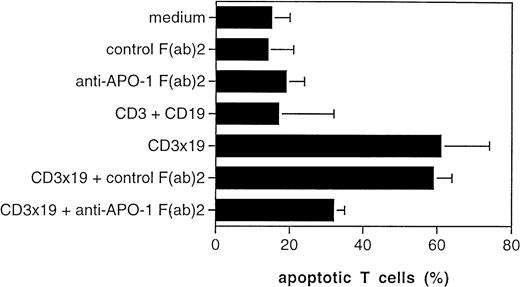
The CD95/Fas ligand (FasL) has been implicated in T-cell AICD.12 We previously observed that AICD of CTLs, which we termed veto apoptosis, is mediated in part by autocrine production of the FasL by the T cells on target-cell contact.13 The addition of F(ab)2 fragments of anti-CD95 (anti–APO-1 IgG3) ab could inhibit T-cell AICD by preventing binding of the FasL to the CD95 antigen.12 We therefore investigated if the target-induced veto apoptosis is, like other forms of AICD, as well mediated by the FasL. We used Nalm-6 as target cells, exposed them to activated T cells and added control F(ab)2 or anti-CD95 F(ab)2 to the CD3x19-triggered cultures. Although 61% of the T cells were induced to apoptotic death by the CD3x19 bs ab in the presence of Nalm-6 targets, the addition of anti-CD95 F(ab)2 fragments decreased T-cell AICD to 32%. No reduction was observed with the control F(ab)2 (Fig4).
Fas ligand expression and function. Peripheral T cells were activated by CD3-crosslinking and were maintained for 7 days in IL-2–containing medium (30 U/ml). T cells were then cultured for 24 hours at an E/T ratio of 10:1 in the absence or presence of CD3x19 bs ab (10 μg/mL). Control cultures were performed with medium alone (ie, only T cells plus Nalm-6), monospecific control antibodies (OKT3 and HD37 at 5 μg/mL), or control F(ab)′ fragments (FII23c IgG3, 5 μg/mL19). Anti-CD95 F(ab)′2 fragments (anti–APO-1 IgG3, 5 μg/mL) were used to block interaction between CD95/Fas and Fas ligand. After incubation, the remaining B lymphoma cells were removed from the coculture by immunomagnetic depletion with anti-CD19 and anti-CD20 as described.10 T-cell apoptosis was measured on the single-cell level as described.15
Fas ligand expression and function. Peripheral T cells were activated by CD3-crosslinking and were maintained for 7 days in IL-2–containing medium (30 U/ml). T cells were then cultured for 24 hours at an E/T ratio of 10:1 in the absence or presence of CD3x19 bs ab (10 μg/mL). Control cultures were performed with medium alone (ie, only T cells plus Nalm-6), monospecific control antibodies (OKT3 and HD37 at 5 μg/mL), or control F(ab)′ fragments (FII23c IgG3, 5 μg/mL19). Anti-CD95 F(ab)′2 fragments (anti–APO-1 IgG3, 5 μg/mL) were used to block interaction between CD95/Fas and Fas ligand. After incubation, the remaining B lymphoma cells were removed from the coculture by immunomagnetic depletion with anti-CD19 and anti-CD20 as described.10 T-cell apoptosis was measured on the single-cell level as described.15
CD28 costimulation prevents activation-induced cell death in vivo.
The previous experiments showed that T-cell activation by CD3x19 bs ab alone leads to AICD and that CD28-mediated costimulation protects from T-cell AICD in vitro during target-cell contact. In addition, we knew that costimulation through CD28 drastically improved the antitumor effect of the CD3x19 bispecific ab in the SCID xenotransplant models. We therefore assessed whether such a protection from AICD also occurs in vivo. Because the Nalm-6 model completely depends on anti-CD28 for tumor prevention we selected it for the in vivo apoptosis assays. Groups of three SCID mice were injected intraperitoneally with Nalm-6 cells alone or in combination with T cells, CD3x19 bs ab, with or without anti-CD28. After 16-hour incubation in vivo, the T cells were recovered by peritoneal washout and T-cell apoptosis was determined, after removal of the contaminating murine cells and Nalm-6 cells, by ISNT staining for DNA strand breaks. The average of background apoptosis of the T cells in the absence of Nalm-6 or antibodies was 9%. When the T cells were injected together with Nalm-6 and the monospecific control antibodies, the apoptosis rate of the T cells was 8%. In the presence of the CD3x19 antibody and Nalm-6, 30.7% of the T cells showed apoptotic DNA strand breaks that could be decreased to 14.4% (P < .01) in the presence of anti-CD28 mab (Table 1). The half life of the antibodies was 7.1 days for the CD3x19 bs ab and 8.2 days for 1.5 E8 anti CD28 mab (data not shown).
Effect of CD28 Costimulation on Activation-Induced Cell Death of CD3x19-Targeted T Cells in Vivo*
| . | Antibodies and Cells Injected . | |||
|---|---|---|---|---|
| T cells | + | + | + | + |
| Nalm-6 target cells | − | + | + | + |
| Anti-CD19 + anti-CD3 | − | + | − | − |
| CD3x19 bs ab | − | − | + | + |
| Anti-CD28 | − | − | − | + |
| . | Antibodies and Cells Injected . | |||
|---|---|---|---|---|
| T cells | + | + | + | + |
| Nalm-6 target cells | − | + | + | + |
| Anti-CD19 + anti-CD3 | − | + | − | − |
| CD3x19 bs ab | − | − | + | + |
| Anti-CD28 | − | − | − | + |
| . | Percentage of Apoptotic Cells . | |||
|---|---|---|---|---|
| Animal 1 | 6.1 | 5.1 | 21.0 | 11.1 |
| Animal 2 | 9.0 | 8.9 | 30.0 | 14.8 |
| Animal 3 | 12.5 | 11.3 | 36.0 | 17.4 |
| Mean | 9.2 | 8.4 | 30.7 | 14.4 |
| SD | 3.2 | 3.1 | 6.1 | 3.2 |
| . | Percentage of Apoptotic Cells . | |||
|---|---|---|---|---|
| Animal 1 | 6.1 | 5.1 | 21.0 | 11.1 |
| Animal 2 | 9.0 | 8.9 | 30.0 | 14.8 |
| Animal 3 | 12.5 | 11.3 | 36.0 | 17.4 |
| Mean | 9.2 | 8.4 | 30.7 | 14.4 |
| SD | 3.2 | 3.1 | 6.1 | 3.2 |
Groups of 3 SCID mice were injected intraperitoneally with Nalm-6 (107) and activated T cells (5 × 107). Control groups were mock-injected with PBS, or monospecific control antibodies (anti-CD19 (HD37) and anti-CD3 (OKT3), 100 μg each). The other groups were treated with CD3x19 bs ab (200 μg), or CD3x19 plus anti-CD28 (50 μg/mL). After 16 hours, the cells were washed out of the peritoneal cavity, B cells were depleted as described above, and cells were stained for DNA strand breaks using ISNT. Percentages of strand-break–positive cells as a marker for apoptosis were measured on the single level using a FACSort flow cytometer.
Although these data are consistent with the overall hypothesis that CD28 triggering prevents T-cell apoptosis during T-cell targeting, the effect also could have been the consequence of the upregulation of adhesion molecules on T-cell activation, which may impact on the ability to retrieve these cells. To exclude such an effect we counted the absolute numbers of cells recovered from the peritoneal cavity and stained with anti-CD2 mab to identify the human T cells. Absolute numbers of T cells recovered from the peritoneal cavity 16 hours after injection are shown in Table 2. The T-cell recovery ranged from 29% to 38% and there was no significant difference between animals injected with T cells alone, T cells plus tumor cells plus monospecific antibodies, or the bs ab in the presence or absence of CD28.
T-cell Recovery After T-cell Targeting With CD3x19 bs ab in Vivo
| Injection of: . | T Cells Alone . | T Cells, B Cells . | T Cells, B Cells Plus Anti-CD3 and anti-CD19 . | T Cells, B Cells Plus Anti-CD3x19 . |
|---|---|---|---|---|
| Without anti-CD28 | 3.7 × 106 ± 0.25 × 106 | 3.4 × 106 ± 0.41 × 106 | 3.3 × 106 ± 0.21 × 106 | 3.8 × 106 ± 0.34 × 106 |
| With anti-CD28 | 3.5 × 106 ± 0.21 × 106 | 2.9 × 106 ± 0.6 × 106 | 3.1 × 106 ± 0.45 × 106 | 3.4 × 106 ± 0.3 × 106 |
| Injection of: . | T Cells Alone . | T Cells, B Cells . | T Cells, B Cells Plus Anti-CD3 and anti-CD19 . | T Cells, B Cells Plus Anti-CD3x19 . |
|---|---|---|---|---|
| Without anti-CD28 | 3.7 × 106 ± 0.25 × 106 | 3.4 × 106 ± 0.41 × 106 | 3.3 × 106 ± 0.21 × 106 | 3.8 × 106 ± 0.34 × 106 |
| With anti-CD28 | 3.5 × 106 ± 0.21 × 106 | 2.9 × 106 ± 0.6 × 106 | 3.1 × 106 ± 0.45 × 106 | 3.4 × 106 ± 0.3 × 106 |
107 T cells were injected intraperitoneally in the presence or absence of 107 irradiated B cells (Nalm-6) plus/minus antibodies. After 16 hours the cells were washed out and stained with CD2-FITC conjugate. Absolute numbers of CD2+cells are given as calculated from total cell numbers and the percentage of CD2+ cells after the peritoneal washout and immunomagnetic separation procedure, ie, the removal of the B cells via immunomagnetic B-cell depletion. Mean ± SD from three animals per group are shown.
These data clearly show that the recovery rate of T-cell numbers, despite low, is not influenced by the activation procedure during the first 16 hours. Thus, this in vivo experiment excludes an effect of the activation procedure on the T-cell recovery and therefore strongly supports, together with the data shown in Table 1, our hypothesis that induction of T-cell apoptosis as well occurs in vivo after T-cell targeting by bispecific antibodies.
To exclude that the induction of apoptosis occurs only at high concentrations of bs ab, we performed titration experiments in which groups of three SCID mice were injected intraperitoneally with irradiated Nalm-6 B lymphoma cells and activated T cells (Fig 5). Control animals were injected with the monospecific control antibodies (anti-CD19 [HD37] and anti-CD3 [OKT3]) that were titrated from 100 μg down to 12.5 μg each. The other groups were treated with CD3x19 bs ab (200 μg, titrated down to 25 μg), or CD3x19 (200 μg, titrated down to 25 μg) plus anti-CD28 (50 μg/mL). An additional group of animals received anti-CD28 mab alone, ie, without anti-CDx19 or the CD3 and CD19 monospecific control mabs. In this group the anti-CD28 mab was titrated from 200 μg per animal down to 25 μg per animal. After 16 hours, the cells were washed out of the peritoneal cavity, murine peritoneal cells and B cells were depleted as described above and cells were stained for DNA strand breaks by means of ISNT. Apoptosis of the CD3x19-targeted T cells was detected down to a CD3x19 concentration of 25 μg per mouse. The anti-CD28 mab that was added at a fixed amount of 50 μg per mouse could suppress the induction of T cell apoptosis at all CD3x19 concentrations. No changes in T-cell background apoptosis were observed in the group treated with the monospecific CD3 and CD19. CD28 mab alone did neither enhance nor decrease T-cell apoptosis when applied in the absence of CD3x19 bs ab.
Titration of CD3x19 bs ab and apoptosis induction in vivo. In analogy to the experiment shown in Table 1, induction of T-cell apoptosis was measured on the single-cell level by ISNT after CD3x19 bs ab targeting in vivo. Groups of three SCID mice were injected intraperitoneally with irradiated Nalm-6 (107) and activated T cells (5 × 107 ). Control groups were mock-injected with PBS (not shown, values were in the range of the anti-CD3 + anti-CD19 group), anti-CD28 (white circles; mab titrated from 200 down to 25 μg per mouse), or monospecific control antibodies (white squares, anti-CD19 [HD37] plus anti-CD3 [OKT3], titrated from 100 μg down to 12.5 μg each). The other groups were treated with CD3x19 bs ab (black squares; titrated from 200 μg down to 25 μg), or CD3x19 plus anti-CD28 (black circles; 200 μg CD3x19 titrated down to 25 μg plus anti-CD28 at a fixed amount of 50 μg/animal). After 16 hours, the cells were washed out of the peritoneal cavity, B cells were depleted as described above, and cells were stained for DNA strand-breaks using ISNT. Percentages of strand-break–positive cells as a marker for apoptosis were measured on the single level using a FACSort flow cytometer.
Titration of CD3x19 bs ab and apoptosis induction in vivo. In analogy to the experiment shown in Table 1, induction of T-cell apoptosis was measured on the single-cell level by ISNT after CD3x19 bs ab targeting in vivo. Groups of three SCID mice were injected intraperitoneally with irradiated Nalm-6 (107) and activated T cells (5 × 107 ). Control groups were mock-injected with PBS (not shown, values were in the range of the anti-CD3 + anti-CD19 group), anti-CD28 (white circles; mab titrated from 200 down to 25 μg per mouse), or monospecific control antibodies (white squares, anti-CD19 [HD37] plus anti-CD3 [OKT3], titrated from 100 μg down to 12.5 μg each). The other groups were treated with CD3x19 bs ab (black squares; titrated from 200 μg down to 25 μg), or CD3x19 plus anti-CD28 (black circles; 200 μg CD3x19 titrated down to 25 μg plus anti-CD28 at a fixed amount of 50 μg/animal). After 16 hours, the cells were washed out of the peritoneal cavity, B cells were depleted as described above, and cells were stained for DNA strand-breaks using ISNT. Percentages of strand-break–positive cells as a marker for apoptosis were measured on the single level using a FACSort flow cytometer.
DISCUSSION
T-cell activation is initiated by signals transmitted through the T-cell receptor/signal transduction complex (signal 1). Other costimulatory receptor-ligand interactions between T-cells and antigen-presenting cells (APC) are needed, however, for complete activation (signal 2). Signaling by APC that provide the antigen signal in combination with costimulation, leads to activation, proliferation, and differentiation. Signaling through the T-cell receptor alone can lead to anergy5 or AICD.10 Previous in vivo studies showed that the efficacy of T cells against tumor cells in immunotherapy approaches can be improved by delivering costimulatory signals. Costimulation by CD28-mediated signals affects a wide variety of T-cell activation parameters (reviewed in7,8) and can protect T cells from AICD.9,10 In bispecific antibody-mediated T-cell targeting, immunodeficient mice were cured from established human tumors when the mice were treated with both the CD3xCD30 bs ab and received a costimulatory signal via a second CD28xCD30 bs ab.21 New strategies for costimulation of T cells to generate anticancer immunity focus on the generation of B7 constructs that may be delivered to the tumor-cell targets, eg, via (retro-) viral vectors,10 or by injection of chimeric fusion proteins in which the B7.1 or B7.2 is linked by genetical engineering to molecules such as single-chain antibodies22which then mediate targeting of the costimulatory construct. In this line, the costimulation via agonistic CD28 antibodies was shown to overcome anergy induced by signal 1 alone and to allow the activation of primed or preactivated T cells but not necessarily of the naive T cells in a syngeneic murine model.23 Such a requirement for costimulation was shown as well in other studies, in which costimulation via B7/CD28 facilitated the generation of CTLs during CD3-redirected cytotoxicity assays in vitro not only from the CD45RO+ memory population but also from small resting (CD45RO-) T cells.24
These observations prompted us to investigate the question of whether T cells targeted to tumor cells by bispecific antibodies, ie, the signal 1 alone, undergo apoptosis on target-cell contact and cross-linking of the CD3 complex.
We found that a substantial proportion of the activated and antibody-targeted CTLs dies by apoptosis on target-cell contact. Blocking experiments showed that this is mediated by production of the FasL on targeting of the T cells to the target cells by the CD3x19 bs ab. In previous reports, induction of the CD95/Fas ligand (FasL) has been shown to mediate activation-induced death of T cells.12 CD95/Fas is a surface receptor that is induced in lymphoid cells by activation.17,25 In recent studies, expression of the FasL has been reported at immunologically privileged sites and on tumor cell lines.26-29 We therefore investigated whether the target-cell and bispecific antibody-induced T-cell AICD in our systems could be attributed to the production of FasL. We previously showed, however, that the expression of FasL on a tumor cell line is not required to trigger CTLs to die by veto apoptosis. The T cells themselves produced the FasL on target-cell contact and this is sufficient to mediate T-cell death.10We also observed that normal, nonmalignant human B cells and a panel of B-cell lines do not express the FasL, no matter if resting or activated.13 Thus, we provided evidence that the FasL is derived from the T cells and not from the B lymphoma cells. Given the potential importance of this novel regulatory concept, we would suggest using the term veto apoptosis for T-cell AICD induced on target-cell contact.10 The veto apoptosis can be mediated by expression of the FasL or other molecules on the target cell26-30 or, as we describe here, by autocrine production of the FasL in the absence of the costimulatory signal 2. In analogy to the well-established veto phenomenon,11,31,32 the T-cell activation by signal 1 alone would lead to activation and induce sensitivity for AICD on target-cell contact. Apart from the case of T-cell targeting by bispecific antibodies, such an apoptosis-inducing signal can theoretically be delivered by each (MHC+) cell of the body that is capable of antigen presentation.11,31,32 Veto apoptosis appears to be mediated by additional mechanisms other than CD95/Fas because blocking with anti-CD95 F(ab)2 fragments did not completely prevent apoptosis in our systems. Possible additional mechanisms may involve TNF-receptors or other death receptors such as DR3, or DR4.33
Our data show that such a mechanism acts also in vivo because CD3x19 bs ab induced T-cell AICD in xenotransplanted SCID mice. In parallel to our in vitro data, the administration of anti-CD28 decreased T-cell AICD in vivo. Nevertheless, it was a possibility that such an induction of apoptosis on CD3x19-mediated T-cell targeting occurs in vivo only at high bs ab concentrations. We therefore performed a titration experiment in which we titrated the bs ab amount from 200 down to 25 μg CD3x19 per mouse. The rate of T-cell apoptosis induced by CD3x19 in the presence of Nalm-6 target cells is declining below 50 μg CD3x19 bs ab per mouse, but is still detectable at 25 μg bs ab. The additional application of anti-CD28 mab decreases the rate of T-cell apoptosis, in analogy to the in vitro experiments, by approximately 50% whereas the injection of anti-CD28 alone at different doses did not influence background T-cell apoptosis.
Such a protection from T-cell AICD through costimulation is, however, short-lived.13 Protection from AICD lasts at least 3-days after signal 1 (CD3/TCR crosslinking) and signal 2 (B7.1 recognition), whereas B7.1-mediated costimulation only marginally protected from AICD when the antigenic signal was delivered 7-days after the primary activation and costimulation.10
We believe that our observation is of potential importance for many immune-based therapeutic strategies. T-cell activation by antigen alone could preferentially lead to activation and priming for subsequent T-cell deletion (or anergy induction). Hence, the efficiency of costimulatory signals in experimental tumor therapy might be explained not only by a more efficient T-cell activation but also by the prevention of in vivo T-cell AICD. Interestingly, the endogenous expression of costimulatory B7 by Raji Burkitt lymphoma cells may be responsible for the higher efficiency of the CD3x19 bs ab to prevent tumor growth in the SCID mouse Raji xenotransplant model as compared with Nalm-6 cells. A blocking experiment showed that the endogenous B7 expression of Raji contributes to the lower T-cell AICD encountered during T-cell AICD in CD3x19 targeting to Raji. This is supported by the less pronounced T-cell apoptosis when T cells were targeted to Raji cells instead of Nalm-6 cells. Our experiments on retrovirally modified, B7.1-expressing MCF-7 breast cancer cells showed that B7.1 expression on the target cell protected alloreactive CTLs from veto apoptosis as compared with mock-transfected MCF-7.10Nevertheless, a significant number of T cells underwent apoptotic cell death on coculture with the Raji cells in the presence of CD3x19 bs ab. This might indicate that the B7 expressed by these malignant cells is not fully functional.
Altogether, our data strongly suggest that costimulation is essential for T-cell– based immunotherapy such as in the case of T-cell targeting by bispecific antibodies. Our results help to explain the requirement of CD28 costimulation for bispecific antibody in tumor therapy in animal models (present study and4) and lack of efficiency in CD3x19 alone in clinical studies.34 The costimulation appears to be required not only for T-cell activation but also to prevent deletion of the activated T cells by rendering them apoptosis-resistant. This extends the concept of immune therapy to the problem of T-cell depletion by the therapeutic stimulus itself and should motivate to further investigate not only how to activate T cells but also how to save them from deletion by AICD.
ACKNOWLEDGEMENT
We thank R.A.W. van Lier, CLB, Amsterdam, The Netherlands and G. Moldenhauer, German Cancer Research Center, Heidelberg, FRG for the most generous gift of purified 15E8 anti-CD28 antibody and P.H. Krammer for kindly providing the anti–APO-1 IgG3 antibody. We are grateful to C.H. Köhne, University of Rostock, FRG, for helpful discussions. This work was supported by the European Union in the Biomed2 and the TMR (training and mobility of researchers) program.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Antitumor effect of bispecific CD3x19 in xenotransplanted SCID mice. (a) 107 Raji Burkitt lymphoma were injected subcutaneously together with 107 activated T cells. CD3x19 and anti-CD28 (clone 15E8) were injected intraperitoneally at the same time (b) 107 Nalm-6 cells were injected intraperitoneally. Activated T cells (107), CD3x19, and anti-CD28 were injected intraperitoneally 24-hours later. ▵, tumor cells alone; □, tumor cells plus monospecific control antibodies (anti-CD19 [HD37] plus anti-CD3 [OKT3], 100 μg each as single dose); ○, tumor cells plus CD3x19 (200 μg single dose); •, tumor cells plus CD3x19 (200 μg single dose) plus anti-CD28 (clone 15E8, 50 μg single dose).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/12/10.1182_blood.v92.12.4750/5/m_blod42434001x.jpeg?Expires=1771325897&Signature=sKAph6GLmgjA6A-2kmeGv4TKooeh7DLeS1htGUdOSHHl6pQb4nNaNgNfAJ~m9~BD0a2X1e3K-ynmH5srxx7pafIvwR9e0imhXgq98ig135xZClvPEvh1j7fNQerQYG95~ubtQR9RG681893Om1hpbCo2W-VlYkB-eBStkgiWvK0JTnpBPsSx1jNDWd9Sy~m7cFaB5uUafMr3ae7mGV-STM4ZHsq2S9PqDolsSeMG7OC3sQxDackZvwPSR59wkxoyX8~cfH~yN7JhlQjKyRFm8PhnSQqXbFxakW-JbWOEjeNk90e-StY7bCnrtZr6ub-v-rY7kClJB7-rXPa83MqrEQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Effect of anti-CD28 on CTL apoptosis during T-cell targeting. T cells were generated by activation of peripheral blood T cells by CD3 cross-linking and culture for 7 days in IL-2–supplemented medium (30 U/ml). T cells were then cultured for 24 hours at an E/T ratio of 10:1 in the absence or presence of CD3x19 bs ab (10 μg/mL). Control cultures were performed with medium alone (ie, only T cells plus [a] Nalm-6 or [b] Raji), monospecific control antibodies plus tumor cells (OKT3 and HD37 at 5 μg/mL), anti-CD28 (15E8, 1 μg/mL), IgM-control mab (G155-228, 10 μg/ml), or anti-B7.1 (BB-1, 10 μg/ml). After incubation, the remaining B lymphoma cells were removed from the coculture by immunomagnetic depletion with anti-CD19 and anti-CD20 as described.10 T-cell apoptosis was measured on the single cell level as described.15](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/12/10.1182_blood.v92.12.4750/5/m_blod42434002x.jpeg?Expires=1771325897&Signature=WNHLtfCCA82XRKnfn8G3yHP~H-srj2cYD8lH8sWc3LJ4upvk2sqJV5ogKLZqzUfDoqFSftahl27oza~orPdL9cET6wrSoQBgUrWAFPtRg9GxiD7f6SrQ893F~7dEiHpm4CqIxcer9laRY83ueBIFfCaR25tFdhjwIoye0z2VG4MMWK8b3UXYLlMW1aDO5VpZOr6XloMkV8nhjoQ27FWYnvXvkwFstKyBJIvjEhEi1HmLp-bh0V620AwKpTAgk7lmXJUxomLfp2Olhnry~77pnqgx3FD3fel~C2w6xFVkEpXc6rZLi4xRKA3ojSwa5Cg5WKYtosbFGIEkRdbIGMCu1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Titration of CD3x19 bs ab and apoptosis induction in vivo. In analogy to the experiment shown in Table 1, induction of T-cell apoptosis was measured on the single-cell level by ISNT after CD3x19 bs ab targeting in vivo. Groups of three SCID mice were injected intraperitoneally with irradiated Nalm-6 (107) and activated T cells (5 × 107 ). Control groups were mock-injected with PBS (not shown, values were in the range of the anti-CD3 + anti-CD19 group), anti-CD28 (white circles; mab titrated from 200 down to 25 μg per mouse), or monospecific control antibodies (white squares, anti-CD19 [HD37] plus anti-CD3 [OKT3], titrated from 100 μg down to 12.5 μg each). The other groups were treated with CD3x19 bs ab (black squares; titrated from 200 μg down to 25 μg), or CD3x19 plus anti-CD28 (black circles; 200 μg CD3x19 titrated down to 25 μg plus anti-CD28 at a fixed amount of 50 μg/animal). After 16 hours, the cells were washed out of the peritoneal cavity, B cells were depleted as described above, and cells were stained for DNA strand-breaks using ISNT. Percentages of strand-break–positive cells as a marker for apoptosis were measured on the single level using a FACSort flow cytometer.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/12/10.1182_blood.v92.12.4750/5/m_blod42434005x.jpeg?Expires=1771325897&Signature=BWdtCWb5fqkDRcHziCC9uRPBa5emtKxjx4gHfv9DXJ1flUdckvdg2bUp66JskreMWrjSlYe-2pAPEq2XCPobeZxVhk7uhBN2AuIRyQLItP0MOLs-pC8wMwj6kY8ekkvxE0e44nGLtHbyjd8XbhzGTG5Ufuo5yTwfvaCWUmMva~YunXLBOZAg~OY3qsVd32aQPXj1eQMqYSHG7cbf6rZUrKxVlziziiHjDBf~P37qGuTV0G1XFI6IRTbqtHJ7HhgN6cTcKKiCqY~9GggaNzGRPj-N3P98KpuGT4D9a5E1ZKl9DaRoDByeN2Kl3ix1jH3viXBHoSFkYZbm-huLJHUOEg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal