Abstract
Fibrin is degraded by the fibrinolytic system in which a plasminogen activator converts plasminogen to plasmin, a serine protease that cleaves specific bonds in fibrin leading to solubilization. To elucidate further the biophysical processes involved in conversion of insoluble fibers to soluble fragments, fibrin was treated with either plasmin or the combination of plasminogen and plasminogen activator, and morphologic changes were observed using scanning electron microscopy. These changes were correlated with biochemical analysis and with characterization of released, soluble fragments by transmission electron microscopy. Initial changes in the fibrin matrix included creation of many free fiber ends and gaps in the continuity of fibers. With more extensive digestion, free fiber segments associated laterally, resulting in formation of thick fiber bundles. Supernatants of digesting clots, containing soluble derivatives, were negatively contrasted and examined by transmission electron microscopy. Large, complex fragments containing portions of multiple fibers were observed, as were pieces of individual fibers and smaller fragments previously identified. Some large fragments had sharply defined ends, indicating that they had been cleaved perpendicularly to the fiber direction. Other fibers showed splayed ends or a lacy meshwork of surrounding protofibrils. Longer times generated more small fragments whose molecular composition could be inferred from their appearance. These results indicate that fibrinolytic degradation results in larger pieces than previously identified and that plasmin digestion proceeds locally by transverse cutting across fibers rather than by progressive cleavage uniformly around the fiber.
FIBRIN PROVIDES a temporary hemostatic plug and physiologic mechanisms insure its efficient removal. Most important is the fibrinolytic system that generates the active protease, plasmin, through conversion of fibrin-bound plasminogen by plasminogen activators, including tissue-type plasminogen activator (t-PA) and urokinase-type plasminogen activator.1-4 Plasmin then cleaves fibrin at specific sites generating soluble fragments, many of which have been characterized.5-7 Both the rate of fibrinolysis and the structures of soluble derivatives are determined in part by the fibrin structure itself. Fibrin is produced from fibrinogen through the action of thrombin, which cleaves fibrinopeptides to produce fibrin monomers that then aggregate in a half-staggered manner to produce two-stranded protofibrils. These can aggregate laterally to yield small fibers that then associate and branch to form the typical fibrin matrix.
The activation of plasminogen by t-PA is accelerated by the conversion of fibrinogen to fibrin, and this property is determined in part by the structure of the fibrin formed. For example, clots made of thin fibers have a decreased rate of conversion of plasminogen to plasmin by t-PA, and they are lysed more slowly than fibrin composed of thick fibers.8-10 Under other conditions, however, thin fibers are lysed more rapidly.11 In addition, fibrin clots composed of abnormally thin fibers formed from certain dysfibrinogens display a lower rate of fibrinolysis and decreased plasminogen binding.12-14 The molecular basis for the slower rates of fibrinolysis within fibers remains unclear. The sequential polypeptide chain cleavages during plasmic degradation of fibrin have been characterized and have provided the basis for a molecular model of fibrin degradation,5,6,15 16 but less is known about the physical changes in the fibrin matrix that precede solubilization.
Although the molecular details of plasminogen and t-PA binding to fibrin remain unclear, some information provides an indication of the possible arrangement of the complex. Specific amino acid sequences that bind t-PA have been identified,17 and several fibrin sequences or fragments have been proposed as sites for plasminogen binding or enhancement of its activation.18-23 An electron microscope study identified the pocket at the end-to-end junction between two fibrin molecules in the protofibril as a plasminogen binding site.24 Soluble degradation products of fibrin have been characterized by both sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and transmission electron microscopy, providing the basis for a model of their structure.5,25 Changes in the polypeptide chain composition of the fibrin network occurring before solubilization have been characterized electrophoretically,6 but information is not available regarding the physical changes in fibrin during plasmin exposure. In this report, we characterize structural changes occurring in polymerized fibrin during fibrinolysis, combining visualization by transmission and scanning electron microscopy with electrophoretic characterization of the soluble and solid phases of fibrin clots during the course of plasmic degradation.
MATERIALS AND METHODS
Preparation of fibrin digests.
Fibrin clots were prepared in specially adapted tubes to facilitate removal of the clot after formation and digestion. Polyethylene tubes (9 × 32 mm) were obtained from Dynalab (Rochester, NY), and the bottom 2 mm was removed. The tops were covered with teflon tape and inverted over tight fitting caps. To prepare fibrin, 0.2 mL of fibrinogen (Kabi Pharmacia, Inc, Franklin, OH) at a concentration of 2 mg/mL and 0.05 mol/L Tris-HCl buffer, pH 7.4, containing 0.10 mol/L NaCl, 0.018 mol/L calcium chloride, and 0.01% sodium azide was pipetted into the inverted tubes, and human thrombin (Calbiochem, LaJolla, CA) was then added to a final concentration of 0.5 U/mL. As soon as clotting occurred (∼30 seconds) the clot was overlaid with 0.2 mL buffer and incubated at 37°C for 60 minutes. For enzymatic digestion, the buffer was removed, and the clot was rinsed 5 times with 0.2 mL of buffer to which was added t-PA (Genentech, South San Francisco, CA) or plasmin at 71 CTA units/mg (Bureau of Biologics Standards, Bethesda, MD) at varying concentrations. After incubation at 37°C, the soluble digestant was removed from the top of the clot and transferred to a separate tube containing 100 kallikrein inhibitory units (KIU)/mL of aprotinin (Bayer, Kankakee, IL). The remaining, partly digested clot was then overlaid with 0.2 mL of buffer containing 200 KIU/mL of aprotinin and allowed to incubate for 1 hour. The buffer was then removed and replaced with 0.1 mol/L phosphate buffer, pH 7.4, containing 200 KIU/mL of aprotinin and incubated for 16 hours at 25°C. The buffer was again removed and replaced with a new 0.2 mL aliquot of buffer containing 200 KIU/mL of aprotinin, and this step was repeated three times over 1 hour. Phosphate buffer was then removed, and the clot was fixed with 1 mL of 2% glutaraldehyde in 0.1 mol/L phosphate buffer, pH 7.4. Electrophoresis in 7% SDS polyacrylamide gels was performed as described.26 Gradient gels of 4% to 10% polyacrylamide were prepared as previously described27and run using a discontinuous buffer system.28
Scanning electron microscopy of digested clots.
Clots were prepared for scanning electron microscope experiments by fixation, dehydration, critical point drying, and sputter coating with gold-palladium as described previously.29 30Specimens were observed and photographed digitally using a Philips XL20 scanning electron microscope (Philips Electron Optics, Eindhoven, The Netherlands).
Transmission electron microscopy of negatively contrasted specimens.
A drop of the fiber suspension was transferred to a 500 mesh copper grid coated with a thin carbon film, rinsed with several drops of buffer, and negatively stained with 2% uranyl acetate.31 The specimens were observed in a Philips EM 400 electron microscope operating at 80 kV.
RESULTS
Clot digestion and biochemical characterization.
Fibrin was overlaid with plasmin at concentrations of 0.2, 0.5, and 2.0 U/mL or with buffer only for 0.5, 0.75, 1, 2, and 3.5 hours. Grossly, all control clots incubated with buffer for different times were similar in size with a cylindrical shape and an approximate diameter of 6 mm and height of 5 mm. The top surface had a slight concavity of approximately 0.5 to 0.7 mm. Fibrin clots treated with 0.2 U/mL of plasmin did not appear different for periods up to 1 hour, but developed deeper concavities at 2 and 3.5 hours. Clots overlaid with 2 U/mL of plasmin showed a clear decrease in clot thickness, declining to 4.0, 3.0, 2.5, and 0.5 mm at exposure times of 30 minutes, 1 hour, 2 hours, and 3.5 hours, respectively. Fibrin clots incubated with plasmin at the intermediate concentration of 0.5 U/mL had dimensions between those exposed to the high and lower concentrations.
Fibrin clots were also digested with plasmin produced by activation of endogenous plasminogen with t-PA. In these experiments, 0.75 μg/mL t-PA was layered on the clots and digestion was stopped after either 1 or 3 hours; the concentration of endogenous plasminogen in these preparations was measured to be 0.16 μmol/L. The clots showed similar changes in overall clot dimensions and electrophoretic appearance of residual clot and soluble products.
Analysis by SDS polyacrylamide gel electrophoresis showed that fibrin cross-linking was complete, with no residual monomeric γ or α chains remaining (Fig 1A), and the cross-linked fibrin showed characteristic β, γγ, and α polymer chains as prominent features. Degradation was stopped after 2 hours (lane 2) or 4 hours (lane 3), and the supernatant containing soluble derivatives was removed. Electrophoresis of the remaining, insoluble, partly degraded fibrin showed little change in polypeptide chain composition, with intact β and γγ chains and only some decrease in the amount of α polymer. This appearance was expected, considering that plasmin action was limited to the buffer-fibrin interface, so that a large amount of undegraded fibrin remained. Electrophoresis of the soluble products generated during a typical experiment showed little change in composition during plasmic degradation for up to 4 hours, at which time 26% of the original fibrin had been solubilized. The prominent derivatives present were fragments E, DD, DY, and YY (Fig1B).
Electrophoretic characterization of plasmic derivatives of fibrin. (A) SDS polyacrylamide gel electrophoresis of cross-linked fibrin (lane 1) and cross-linked fibrin after exposure to t-PA (1 μg/mL) for 1 hour (lane 2) or 4 hours (lane 3). After 2 and 4 hours, 9% and 26%, respectively, of the original fibrin clot had been solubilized. Electrophoresis of reduced protein was performed using 7% polyacrylamide gels. The locations of the polypeptide chains are indicated. (B) SDS polyacrylamide gel electrophoresis of digests of fibrin after incubation with plasmin for 45 minutes, 1 hour, 2 hours, and 3.5 hours in lanes 1 through 4, respectively. The percentage degradation of the original fibrin was 4% at 45 minutes, 5% at 1 hour, 11% at 2 hours, and 12% at 3.5 hours. The location of fragment E and the smallest cross-linked degradation products (DD, DY, and YY) are indicated. Electrophoresis of nonreduced protein was performed in 4% to 10% gradient gels.
Electrophoretic characterization of plasmic derivatives of fibrin. (A) SDS polyacrylamide gel electrophoresis of cross-linked fibrin (lane 1) and cross-linked fibrin after exposure to t-PA (1 μg/mL) for 1 hour (lane 2) or 4 hours (lane 3). After 2 and 4 hours, 9% and 26%, respectively, of the original fibrin clot had been solubilized. Electrophoresis of reduced protein was performed using 7% polyacrylamide gels. The locations of the polypeptide chains are indicated. (B) SDS polyacrylamide gel electrophoresis of digests of fibrin after incubation with plasmin for 45 minutes, 1 hour, 2 hours, and 3.5 hours in lanes 1 through 4, respectively. The percentage degradation of the original fibrin was 4% at 45 minutes, 5% at 1 hour, 11% at 2 hours, and 12% at 3.5 hours. The location of fragment E and the smallest cross-linked degradation products (DD, DY, and YY) are indicated. Electrophoresis of nonreduced protein was performed in 4% to 10% gradient gels.
Scanning electron microscopy of digested clot surfaces.
The appearance of fibrin clots before plasmin treatment or after incubation with buffer rather than plasmin was consistent with past observations demonstrating an extensively branched fiber network (Fig 2D). The mean diameter of fibers measured from micrographs of these control clots was 98 ± 19 nm. In some cases, the bottom surface of the digested clots was examined as an internal control and showed the same appearance as clots never exposed to plasmin.
Scanning electron micrographs of digested clot surfaces. Different concentrations of plasmin were applied to the clot surface and samples were prepared for microscopy at various times. (A) 0.2 U/mL plasmin, 30 minutes. (B) 0.2 U/mL plasmin, 1 hour. (C) 0.2 U/mL plasmin, 3.5 hours. (D) Control, no plasmin. (E) 0.5 U/mL plasmin, 30 minutes. (F) 2.0 U/mL plasmin, 3.5 h. Bar equals 2 μm.
Scanning electron micrographs of digested clot surfaces. Different concentrations of plasmin were applied to the clot surface and samples were prepared for microscopy at various times. (A) 0.2 U/mL plasmin, 30 minutes. (B) 0.2 U/mL plasmin, 1 hour. (C) 0.2 U/mL plasmin, 3.5 hours. (D) Control, no plasmin. (E) 0.5 U/mL plasmin, 30 minutes. (F) 2.0 U/mL plasmin, 3.5 h. Bar equals 2 μm.
After plasmin exposure, the most obvious feature observed by scanning electron microscopy was the presence of many fiber ends (Fig 2A), whereas fiber ends were observed rarely in control clots. Although the presence of many fiber ends in the early stages of digestion was greater than in control samples, there were no changes in pore sizes, fiber diameters, or other properties at the very earliest stages of digestion. After 30 or 45 minutes of digestion by plasmin at a concentration of 0.2 U/mL, the fiber bundles were larger in diameter (130 ± 49 nm). Both long and short cleaved fibers with sharply truncated ends were seen (Fig 2A).
In fibrin clots exposed for 1 hour to plasmin at a concentration of 0.2 U/mL, long individual fibers with cleaved ends were rare, but the ends of many shorter fibers were visible over the entire surface, as were fiber bundle aggregates composed of fibers cleaved at one end but remaining attached to the clot at the other (Fig 2B). The fiber bundles appeared to be covered with follicles of varying size representing the stubs of fibers that had been cleaved and removed. Although individual fibers making up the bundles could still be seen in some cases, these fiber bundles were different in appearance from the ones found in undegraded fibrin clots. The fiber bundles were larger in diameter (222 ± 89 nm) and they appeared flatter with rough and irregular surfaces that were often fragmented in one or more places. This fragmentation as well as the bundling of individual fibers into larger aggregates resulted in the formation of networks with larger pores than in control clots.
After 3.5 hours of plasmin treatment, fiber aggregates were larger and more numerous (Fig 2C) and individual cleaved fibers were difficult to identify. The fine structure of these aggregates also changed, with fiber bundles spread over a larger area, and their surfaces were more irregular. Many areas looked amorphous with poor definition of individual fibers, and there was extensive fragmentation with large pores evident. The average fiber diameter was 220 ± 78 nm.
Structural changes observed on the fibrin clot surface after treatment with 0.5, 1.0, or 2 U/mL of plasmin were similar but occurred earlier. For example, large fragmented fiber aggregates dominated the surface of clots treated with 0.2 U/mL plasmin after 2 or more hours of treatment, whereas at higher plasmin concentrations, these fragmented structures were prominent after only 30 minutes (compare Fig 2C and E). The fibers were also greater in diameter and had a flatter appearance than those seen with 0.2 U/mL of plasmin (compare Fig 2C and F). For example, with 1 U/mL plasmin at 30 minutes, the average diameter was 314 ± 106 nm. The individual fibers making up such aggregates were poorly defined and appeared fused along their lengths. Few individual fibers were identifiable.
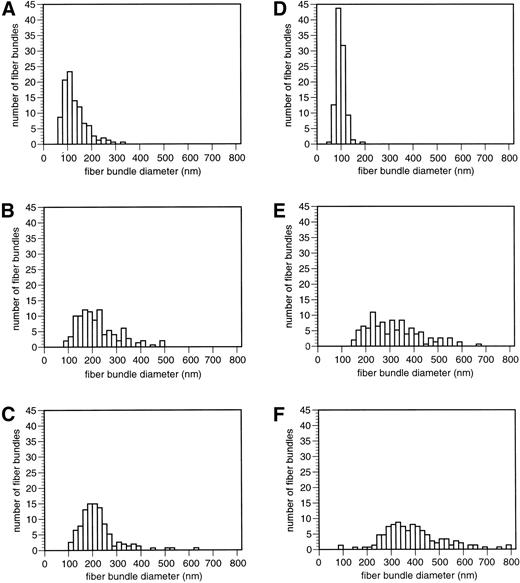
Histograms of fiber bundle diameters for different stages of digestion provide additional quantitative information about these changes (Fig 3). With increasing digestion, the histograms became broader, indicating greater heterogeneity in fiber diameter. The peaks also shifted to larger diameters as a result of the presence of thicker fiber bundles formed from the aggregation of fibers transected by plasmin. Clots digested with a combination of t-PA and plasminogen showed a similar sequence of changes and overall appearance.
Histograms of fiber bundle diameters from scanning electron micrographs of digested clot surfaces. Fiber bundle diameters were measured from micrographs similar to those shown in Fig 2. Fiber bundle diameters are in nanometers. The histograms for each digestion experiment are arranged in the same way as the corresponding representative micrographs in Fig 2, for ease of comparison. The same number of measurements was made for each digestion condition. (A) 0.2 U/mL plasmin, 30 minutes. (B) 0.2 U/mL plasmin, 1 hour. (C) 0.2 U/mL plasmin, 3.5 hours. (D) Control, no plasmin. (E) 0.5 U/mL plasmin, 30 minutes. (F) 2.0 U/mL plasmin, 3.5 hours.
Histograms of fiber bundle diameters from scanning electron micrographs of digested clot surfaces. Fiber bundle diameters were measured from micrographs similar to those shown in Fig 2. Fiber bundle diameters are in nanometers. The histograms for each digestion experiment are arranged in the same way as the corresponding representative micrographs in Fig 2, for ease of comparison. The same number of measurements was made for each digestion condition. (A) 0.2 U/mL plasmin, 30 minutes. (B) 0.2 U/mL plasmin, 1 hour. (C) 0.2 U/mL plasmin, 3.5 hours. (D) Control, no plasmin. (E) 0.5 U/mL plasmin, 30 minutes. (F) 2.0 U/mL plasmin, 3.5 hours.
Transmission electron microscopy of soluble derivatives.
To characterize the structures of soluble derivatives released from fibrin and to follow their digestion over time, the specimens were negatively contrasted and observed by transmission electron microscopy. A large variety of fragments with marked variability in size and appearance were observed, but the same structures were observed from digestion with plasmin or with t-PA and plasminogen. The largest products appeared to be composed of fragments of multiple fiber bundles (Fig 4A). The ends of these structures were often sharply delineated, showing that the fibers had been cut approximately perpendicular to the fiber axis (Figs 4A and D and 5D). In early digests at low plasmin concentration, these fiber bundles sometimes retained their overall shape so that individual fibers were still detectable and the 22.5 nm repeat was visible, although in most cases the normal fibrin band pattern was no longer clear. After more extensive digestions, there were loose amorphous aggregates made up of pieces of smaller size linked together to make complex lacy networks (Figs 4B and C and 5A through C). In such aggregates, few individual fibers could be recognized, although the size of these aggregates and their mesh-like appearance suggested that they consisted of several highly digested fibers or protofibrils fused together.
Transmission electron micrographs of negatively contrasted digestant removed from the clots. Large pieces of clot observed in the supernatants. (A) This chunk consisting of pieces of fibers and aggregated smaller structures has been cleaved so that it is nearly separated from a larger mass at the location indicated by the arrow. (B) Lacy meshwork of fibers, protofibrils, and other cleaved pieces. (C) Aggregate consisting of pieces of fibers and smaller structures. Bar for (A) through (C) equals 0.5 μm. (D) Fiber bundles with splayed ends. Bar for (D) equals 0.1 μm.
Transmission electron micrographs of negatively contrasted digestant removed from the clots. Large pieces of clot observed in the supernatants. (A) This chunk consisting of pieces of fibers and aggregated smaller structures has been cleaved so that it is nearly separated from a larger mass at the location indicated by the arrow. (B) Lacy meshwork of fibers, protofibrils, and other cleaved pieces. (C) Aggregate consisting of pieces of fibers and smaller structures. Bar for (A) through (C) equals 0.5 μm. (D) Fiber bundles with splayed ends. Bar for (D) equals 0.1 μm.
Transmission electron micrographs of negatively contrasted digestant removed from the clots. Pieces of fibers, protofibril aggregates, and smaller fragments observed in the supernatants. Arrows indicate areas that are darker as a result of removal of protein by digestion. Arrowheads point out brighter areas where stain was excluded because of plasmin binding. (A) Fiber surrounded by lacy meshwork of protofibril-like structures. (B) Fiber surrounded by meshwork made up of protofibrils and other smaller structures. (C) Fiber splayed into bundles of protofibrils. Bar for (A) through (C) equals 0.5 μm. (D) Fiber showing sharply cut end (at right) and another chunk that is largely separated from the rest of the fiber but still associated with it (asterisk). (E) Digested fiber with missing pieces and truncated end; note some smaller fragments in the background. (F) Piece of remaining fiber where individual protofibrils can be visualized. (G) Very thin fiber remnant with other smaller fragments. (H) Small fragments present at longer times of digestion or with higher plasmin concentrations. Some examples of DDE complexes are circled. Bar for (D) through (H) equals 0.1 μm.
Transmission electron micrographs of negatively contrasted digestant removed from the clots. Pieces of fibers, protofibril aggregates, and smaller fragments observed in the supernatants. Arrows indicate areas that are darker as a result of removal of protein by digestion. Arrowheads point out brighter areas where stain was excluded because of plasmin binding. (A) Fiber surrounded by lacy meshwork of protofibril-like structures. (B) Fiber surrounded by meshwork made up of protofibrils and other smaller structures. (C) Fiber splayed into bundles of protofibrils. Bar for (A) through (C) equals 0.5 μm. (D) Fiber showing sharply cut end (at right) and another chunk that is largely separated from the rest of the fiber but still associated with it (asterisk). (E) Digested fiber with missing pieces and truncated end; note some smaller fragments in the background. (F) Piece of remaining fiber where individual protofibrils can be visualized. (G) Very thin fiber remnant with other smaller fragments. (H) Small fragments present at longer times of digestion or with higher plasmin concentrations. Some examples of DDE complexes are circled. Bar for (D) through (H) equals 0.1 μm.
Individual digested fibrin fibers that were also found in all samples of soluble digestant displayed more structural detail (Fig 5A through G). Typically, these fibers had compact lateral packing in the center but splayed into thin strands laterally and at their ends. The darker areas corresponding to lower protein density in these regions arise from the presence of gaps as a consequence of protein removed by digestion (Fig 5A, D, and F, arrows). Some brighter areas of fibers were observed, indicating areas where stain was excluded because of a higher protein content, possibly representing sites of plasmin binding24 (Fig 5B and C, arrowheads). In some fibers, the lateral packing along their length was uniform, such that individual protofibrils with a lateral spacing of about 9 nm were visible (Fig 5B, E, and F). Areas with cleaved fiber ends were found in every sample of digested clot, indicating the cross-sectional direction of fibrin cleavage (Fig 5D, E, and F). In most preparations there were also small structures that could be identified as complexes arising at the later stages of plasmin digestion (Fig 5H). Globular regions corresponding to the nodules present in fibrin(ogen) could be visualized, permitting identification of complexes such as DD/E, DY/YD, and DXD/YY. These smaller fragments predominated at the longest digestion times.
DISCUSSION
Detailed information regarding the structures of fibrinogen and fibrin degradation products appearing during the course of plasmic degradation provided the basis for a model of their structure and origin from the fibrin fiber.5-7,15,16 The carboxyl terminal ends of the α chains and the amino terminal ends of the β chains are first removed from fibrinogen, yielding fragment X. Then all three polypeptide chains are cleaved at plasmin-sensitive sites in the coiled-coil region connecting the lateral and central domains. This digestion yields from each fibrin monomer a single fragment E consisting of the central domain and two D fragments, each consisting of lateral domains, as well as two αC fragments representing the carboxyl terminal α chains. If fibrin is cross-linked by factor XIIIa, the degradation products are different, with cross-linked D-dimers and αC multimers produced. Additional noncovalent interactions result in formation of a variety of complexes of which DD/E is the smallest.5
Much less is known about physical changes in the residual, insoluble fibrin matrix during plasmic degradation. One model proposes that a fibrin clot is digested from the outside-in with products of degradation released layer-by-layer.6,32 This analysis is based on characterization of fibrin degradation products released from clots that had been lyophilized and ground to small pieces before plasmin exposure. Recent investigations have also suggested that plasma clot degradation results in sequential, layer-by-layer reduction in size.33 34 These studies included dynamical changes during degradation monitored by confocal laser-scanning fluorescence microscopy, but there was not sufficient resolution to observe fine structural details at the clot surface.
At the level of individual fibers, Gabriel et al8 suggested an outside-in model in interpreting results of experiments with digestion of thick and thin fibrin fibers. In other words, it was assumed that plasmin will first digest the outer surface of the fibers, producing progressively thinner fibers as digestion proceeds. Similarly, a model of fibrinolysis developed to predict effects of clot transport also used an outside-in scheme to keep track of changes.35
This report presents direct observations of changes during fibrinolysis at the resolution of individual fibers. An important finding was that local plasmin action proceeds laterally, resulting in transection of fibers. With progressive degradation there was an increase in free fiber ends but little change in fiber diameter. We also observed an irregular or nodular appearance of fibers suggesting cleavage similar to the fragmented and flocculent appearance of fibrin during lysis of platelet-fibrin thrombi.36 At later stages of digestion, many cut fiber ends were observed, and mean fiber diameters increased rather than decreased, contrary to our expectations. We interpret this as lateral aggregation of the cut fibers to form thick bundles, which could have been expected, because lateral aggregation of fibrin fibers occurs during polymerization, yielding thick fiber bundles.37 The average size of the fiber bundles increased dramatically from 129 ± 49 to 220 ± 78 nm, and such fiber aggregates became the dominant feature of the surface of digesting fibrin. These changes were accompanied by a clear increase in the size of pores between bundles, providing further evidence of lateral cleavage and fiber aggregation. Fibers cleaved by plasmin have increased mobility, providing freedom for lateral polymerization.
Our observations are consistent with data obtained by other means. For example, during lysis of fibrin clots polymerized in the presence of plasminogen and t-PA, the turbidity increases during polymerization and then decreases as the clot is lysed. A spike of high turbidity can sometimes be observed at the initiation of clot degradation, consistent with the transient presence of very thick fibers at this time. Similar experiments have monitored clot stiffness during fibrinolysis, demonstrating a transient phase of hyper-rigidity before clot dissolution,38 which is also consistent with the transient generation of thicker fibers.
The results presented suggest an explanation for the observed resistance to fibrinolysis of fibrin composed of thin in comparison with thick fibers.8-10 12 If plasmin transects fibers at localized sites, fibrin composed of thick fibers will be degraded more rapidly, because there would be fewer fibers to transect. We also suggest that the progressive fiber aggregation during plasmic degradation may provide an additional mechanism for local enhancement and acceleration of the fibrinolytic process, because cleavage proceeds laterally.
Transmission electron microscopy of soluble products also provided new insight into the initial stages of fibrin solubilization in more detail. Larger and more heterogeneous soluble products were observed in this study than expected, reflecting the largest derivatives released from fibrin before complete solubilization of the matrix. Products that appeared to represent aggregates of several fibrin fibers were observed, as well as individual fibers. Smaller degradation products identical to those observed previously25 were also seen. Soluble products composed of sections of entire fibers or fibrin bundles could only arise from degradation due to localized proteolytic transection of fibers rather than an overall uniform action, which would result in a progressive decrease in fiber diameter.
The largest soluble derivatives, which had diameters up to 400 nm and displayed no visible internal structure, corresponded in appearance to large fiber aggregates observed by scanning electron microscopy of the clot surface before solubilization. The lack of structural order suggests extensive internal degradation before release from the fiber surface. In contrast, portions of individual fibrin fibers in solution demonstrated more structural detail and appeared similar to fibers seen in undigested clots. They exhibited sharp, transected ends, indicating that they were produced by cleavage across the fiber. The band pattern arising from the specific molecular packing was faint, suggesting some internal degradation or conformational change.
The most frequent structures observed in the digesting fibrin matrix appeared as fibers with a compact structure centrally but splayed into a lace-like mesh at the edges. The splaying of partially digested fibers may be explained from knowledge of fibrin structure and assembly. During clot formation, protofibrils of fibrin start aggregating laterally to form fibers after they reach a critical length.30,39,40 When this process is reversed by plasmic degradation, disaggregation of protofibrils may occur if transection generates a fragmented piece shorter than the critical length needed for lateral aggregation. This disaggregation may be modified by the extent of α chain cross-linking that stabilizes lateral aggregation.41
The pattern of degradation observed is consistent with evidence that plasminogen binds to a fiber in the pouch at the end-to-end junction of two fibrin molecules in a protofibril.24 In this location, plasmin is able to reach cleavage sites located on adjacent protofibrils, creating additional carboxyl-terminal lysines as new sites for plasmin binding as degradation proceeds.42 The results presented herein suggest that plasmin molecules then move across the fibers laterally, cleaving additional sites. In support of this concept, it should be noted that equivalent plasminogen binding sites along the length of a protofibril would be located 22.5 nm apart, whereas those on an adjacent protofibril are likely to be less than 5 to 10 nm away. Movement of plasmin across the fibrin may result from binding of a second kringle to the next fibrin binding site before release of the first kringle. Such a crawling of plasmin across the fiber is consistent with the presence of more than one binding kringle in each plasmin molecule43 and with its known conformational changes.44,45 Additional evidence for such a bridging mechanism is provided by the observation that plasminogen can precipitate fibrin degradation products, forming complexes with a ratio of one plasminogen to two fibrin degradation products.46Also, plasminogen added to polymerizing fibrin enhances lateral aggregation of protofibrils yielding clots made of thicker fibers.47 In addition, observations of plasminogen covalently bonded to fibrin with a photoaffinity activated cross-linker show examples of one plasminogen molecule linked to the ends of three or four fibrin molecules24 that must arise from adjacent protofibrils.
The results presented provide a new degree of detail regarding the physical process of fibrinolysis. At the level of individual fibers, the consequences of plasmin activity locally are to fully transect individual fibers and release a heterogeneous group of large, soluble products. However, from the perspective of a macroscopic clot, the process may still be viewed as digestion from the outside-in, as the action of plasmin is limited to the shell of fibers at the clot periphery. Its action proceeds centrally with progressive degradation. The observations also demonstrate that there are large-scale changes in the three-dimensional integrity and mechanical stability of fibrin coincident with release of the soluble degradation products. These changes in the fibrin matrix will enhance permeation of fluid into the clot, which is the most important mode of enzyme transport.35 48 Therefore, the cross-lateral cleavage of individual fibrin fibers by plasmin with generation of larger pores may result in progressive acceleration of fibrinolysis as resistance to permeability decreases. This will facilitate access of enzyme to deeper layers of clot and promote fiber degradation.
Supported in part by National Institutes of Health Grants No. HL30954 (to J.W.W.) and HL30616 (to C.W.F.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to John W. Weisel, PhD, Department of Cell and Developmental Biology, University of Pennsylvania School of Medicine, Philadelphia, PA 19104-6058.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal