Abstract
Crkl, a 39-kD SH2, SH3 domain-containing adapter protein, is constitutively tyrosine phosphorylated in hematopoietic cells from chronic myelogenous leukemia (CML) patients. We recently reported that thrombopoietin induces tyrosine phosphorylation of Crkl in normal platelets. In this study, we demonstrate that thrombopoietin induces association of Crkl with a tyrosine phosphorylated 95- to 100-kD protein in platelets and in UT7/TPO cells, a thrombopoietin-dependent megakaryocytic cell line. With specific antibodies against STAT5, we demonstrate that the 95- to 100-kD protein in Crkl immunoprecipitates is STAT5. This coimmunoprecipitation was specific in that Crkl immunoprecipitates do not contain STAT3, although STAT3 becomes tyrosine phosphorylated in thrombopoietin-stimulated platelets. The coimmunoprecipitaion of Crkl with STAT5 was inhibited by the immunizing peptide for Crkl antisera or phenyl phosphate (20 mmol/L). After denaturing of Crkl immunoprecipitates, Crkl was still immunoprecipitated by Crkl antisera. However, coimmunoprecipitation of STAT5 was not observed. Coincident with STAT5 tyrosine phosphorylation, thrombopoietin induces activation of STAT5 DNA-binding activity as demonstrated by electrophoretic mobility shift assays (EMSA). Using a β-casein promoter STAT5 binding site as a probe, we have also demonstrated that Crkl antisera supershift the STAT5-DNA complex, suggesting that Crkl is a component of the complex in the nucleus. Furthermore, interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and erythropoietin also induce Crkl-STAT5 complex formation in responding cells in a stimulation-dependent manner. In vitro, glutathione S-transferase (GST)-Crkl bound to STAT5 inducibly through its SH2 domain. These results indicate that thrombopoietin, IL-3, GM-CSF, and erythropoietin commonly induce association of STAT5 and Crkl and that the complex translocates to the nucleus and binds to DNA. Interestingly, such association between STAT5 and Crkl was not observed in cytokine-stimulated murine cells, suggesting an intriguing possibility that components of the human STAT5-DNA complex may be different from those of the murine counterpart.
THROMBOPOIETIN IS essential for megakaryocytopoiesis/thrombocytopoiesis (reviewed in Kaushansky1). One of the earliest biochemical events after treatment of cells with thrombopoietin is the induction of tyrosine phosphorylation. Using cell lines or platelets expressing thrombopoietin receptors (c-Mpl), several groups have shown that thrombopoietin treatment leads to tyrosine phosphorylation of Jak2, Tyk2, Shc, c-Cbl, Vav, STAT proteins, and c-Mpl.2-17
Crkl is an SH2/SH3 domain-containing adapter protein that was shown to be the major tyrosine-phosphorylated protein in chronic myelogenous leukemia (CML) neutrophils.18-22 We recently reported that thrombopoietin induces tyrosine phosphorylation of Crkl in normal platelets and FDCP-hMpl5 cells, a cell line genetically engineered to express human c-Mpl.17 Our data suggest that Crkl can be phosphorylated in the absence of p210Bcr-Abl and that Crkl may have a role in signaling by thrombopoietin. We have also shown that Crkl is constitutively tyrosine phosphorylated in platelets from CML patients.17
To further evaluate the role of Crkl in thrombopoietin signaling, we examined Crkl immunoprecipitates for the presence of tyrosine-phosphorylated proteins. In this report, we demonstrate the presence of a 95- to 100-kD tyrosine-phosphorylated protein in Crkl immunoprecipitates after thrombopoietin treatment of platelets and UT7/TPO cells, a thrombopoietin-dependent megakaryocytic cell line. This 95- to 100-kD protein was identified as STAT5. Interestingly, a Crkl-STAT5 complex is present in the nucleus, as demonstrated by the supershift of STAT-DNA complexes with Crkl antisera. Furthermore, we demonstrate that the Crkl-STAT5 complex formation also occurs in cells in response to interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), or erythropoietin, suggesting that the complex is commonly involved in signaling pathways elicited by various cytokines.
MATERIALS AND METHODS
Reagents.
Prostaglandin E1 (PGE1), aspirin, apyrase (type VIII), N-2 hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), sodium dodecyl sulfate (SDS), 2-mercaptoethanol, isopropyl β-D-thiogalactopyranoside (IPTG), sodium orthovanadate, chicken egg albumin, protein A-Sepharose, Triton X-100, bovine serum albumin (BSA), dithiothreitol (DTT), phenyl phosphate, and Tris (hydroxymethyl) aminomethane (Tris) were purchased from Sigma (St Louis, MO). Polyvinylidene difluoride (PVDF) membranes (pore size, 0.45 μm) were from Millipore Corp (Bedford, MA). SDS-polyacrylamide gel electrophoresis (SDS-PAGE) molecular standards and [γ-32P]ATP were from Amersham (Arlington Heights, IL) or Bio-Rad (Richmond, CA). Double-stranded Poly (dI-dC) was from Pharmacia Biotech (Milwaukee, WI). The Jak2 immunizing peptide (DSQRKLQFYEDKHQLPAPKC) was from Upstate Biotechnology Inc (Lake Placid, NY). Enhanced chemiluminesence (ECL) reagents, including secondary antibodies, were purchased from Amersham. The antiphosphotyrosine murine monoclonal antibody (MoAb; 4G10) was used as described.17 Crkl, STAT3, and c-Cbl antisera; an anti-GST MoAb; and the Crkl immunizing peptide (amino acids 283-302 mapping at the carboxy terminus of human Crkl) were from Santa Cruz (Santa Cruz, CA). Iscove’s modified Dulbeco’s medium (IMDM), nitroblue tetrazolium chloride (NBT), and 5-bromo-4 chloro-3-indolyl phosphate p-toluidine salt (BCIP) were from GIBCO BRL (Gaithersburg, MD). For electrophoretic mobility shift assay (EMSA), an anti-STAT5 antiserum, described previously, was used.23 An anti-STAT5 MoAb was from Transduction Laboratories (Lexington, KY). STAT5A and STAT5B antisera were from R&D Systems (Minneapolis, MN). Recombinant thrombopoietin, erythropoietin, IL-3, and GM-CSF were kindly provided by Kirin Brewery Co Ltd (Maebashi, Japan).
Platelet preparation.
Blood from healthy volunteers and three CML patients was drawn by venipuncture into 1/10 vol of 3.8% (wt/vol) trisodium citrate and gently mixed. Before drawing blood, informed consent was obtained. The three CML patients were BCR-ABL positive, as demonstrated by reverse transcriptase-polymerase chain reaction (RT-PCR) on peripheral blood samples. Platelet-rich plasma (PRP) was prepared by centrifuging the whole blood at 200g for 20 minutes and aspirating PRP. PRP was incubated with aspirin (2 mmol/L) for 30 minutes at room temperature. PGE1 (1 μmol/L) was added from a stock solution in absolute ethanol (1 mmol/L). The PRP was spun at 800g to form a soft platelet pellet. The pellet was resuspended in 1 mL of a modified HEPES-Tyrode buffer (129 mmol/L NaCl, 8.9 mmol/L NaHCO3, 0.8 mmol/L KH2PO4, 0.8 mmol/L MgCl2, 5.6 mmol/L dextrose, and 10 mmol/L HEPES, pH 7.4) containing apyrase (2 U/mL) and was washed twice with this buffer. Platelets were resuspended in the same buffer at a concentration of 3 × 108 cells/mL with apyrase (2 U/mL) and 1 mmol/L CaCl2 at 37°C. The contaminating white blood cells were counted and were regularly less than 0.01% of platelets. If the contaminating white blood cell count exceeded this value, the cell suspension was repeatedly centrifuged at 200g for 5 minutes until this value of the supernatant was less than 0.01%.
Gel electrophoresis and Western blotting.
Platelet stimulation was terminated by the addition of an equal volume of 2× concentrated Laemmli’s sample buffer (10% glycerol, 1% SDS, 5% 2-mercaptoethanol, 50 mmol/L Tris-HCl [pH 6.8], and 0.002% bromophenol blue)24 with 10 mmol/L EGTA and 1 mmol/L sodium orthovanadate. After boiling at 95°C for 5 minutes, one-dimensional electrophoresis was performed on SDS-7.5% to 15% polyacrylamide gels as described.24 Separated proteins were electrophoretically transferred from the gel onto PVDF membranes or nitrocellulose in a buffer containing Tris (25 mmol/L), glycine (192 mmol/L), and 20% methanol at 0.2 amps for 12 hours at room temperature. To block residual protein binding sites, membranes were incubated in TBST (Tris-buffered-saline [TBS], 10 mmol/L Tris, 150 mmol/L NaCl, pH 7.6, with 0.1% Tween 20) with 10% chicken egg albumin. The blots were washed with TBST and incubated overnight with primary antibodies at a final concentration of 1.0 μg/mL in TBST. The primary antibody was removed and the blots were washed four times in TBST and incubated with horseradish peroxidase-conjugated or alkaline phosphatase-conjugated second antibodies diluted 1:3,000 in TBST. Blots were then washed four times in TBST. Antibody reactions were detected with NBT/BCIP or chemiluminescence according to the manufacturer’s instructions.
Immunoprecipitation.
Cells in suspensions (0.5 mL) were lysed by the addition of an equal amount (0.5 mL) of lysis buffer (15 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF], 10 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.8 μg/mL leupeptin, 2% Triton X-100 [vol/wt], pH 7.4). After 20 minutes on ice, the lysates were centrifuged at 10,000g (at 4°C) for 20 minutes. The supernatant was removed and precleared with preimmune serum and protein A-Sepharose (40 μL of a 50% slurry) for 1 hour. Crkl antisera (2 μg/mL), STAT3 antisera (2 μg/mL), or the mixture of STAT5A and STAT5B antisera (3 μL each) were then added and incubated for 2 to 3 hours on ice. Protein A-Sepharose (40 μL of a 50% slurry) was added and incubated for 1 hour. The immune complexes were washed with 1 mL of cold washing buffer (15 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L PMSF, 10 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 0.8 μg/mL leupeptin, 1% Triton X-100 [vol/wt], pH 7.4) three times and then resuspended in Laemmli’s sample buffer.
Cell lines.
UT7/TPO and UT7/EPO, sublines of UT7, have been previously characterized.25 TF-1 cells were kindly provided by Dr Hisamaru Hirai (Tokyo University, Tokyo, Japan).26FDCP-hMpl5 cells were described previously.10 17 Cells were maintained in IMDM containing 10% fetal calf serum (FCS) in the presence of 10 ng/mL thrombopoietin (for UT7/TPO or FDCP-hMpl5), 10 U/mL erythropoietin (for UT7/TPO), or 1 ng/mL GM-CSF (for TF-1 and UT7). Before treatment of the cells with various cytokines, they were incubated in IMDM containing 10% FCS without cytokines for 18 hours. Before stimulation with thrombopoietin, cells were washed twice with phosphate-buffered saline (PBS; pH 7.4) and then resuspended in PBS at 2 × 107 cells/mL.
EMSA.
Nuclear extracts (40 μL, containing 10 to 20 μg protein) were prepared from UT-7/TPO cells (2 × 107 cells) by lysis followed by high salt extraction, as described previously.23 Two microliters of nuclear extracts was mixed with 20 μL of binding buffer [10 mmol/L Tris-HCl, 50 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L DTT, 0.1% NP-40, 5% glycerol, 1 mg/mL BSA, and 2 mg/mL poly(dI-dC), pH 7.5] containing 50,000 cpm of an end-labeled bovine β-casein promoter probe (5′-AGATTCTAGGAATTCAAATC-3′). The mixture was incubated for 30 minutes at room temperature. Complexes were separated on 5% nondenaturing polyacrylamide gels in 0.25× TBE and detected by autoradiography.
GST binding assays.
The GST-Crkl fusion proteins were generated as previously described.21 27 GST-fusion constructs were transformed intoEscherichia coli DH-5α and protein expression was induced by adding 0.5 mmol/L IPTG to exponentially growing cells. The GST-fusion proteins were isolated from sonicated bacterial lysates using glutathione sepharose beads. Coomassie-stained gels were used to normalize for the expression of the various GST-fusion proteins. Between 2.5 and 5 μg of GST-fusion proteins was incubated with 50 μL of glutathione sepharose beads in bacterial lysis buffer (150 mmol/L NaCl, 16 mmol/L Na2HPO4, 4 mmol/L NaH2PO4 [pH 7.3] containing 10 μg of aprotinin, 1 mmol/L orthovanadate, 1 mmol/L PMSF, and 0.1% 2-mercaptoethanol). The beads were washed four times in PBS and incubated for 4 hours with normalized cellular lysate of UT7 or TF-1 cells. The beads were washed three times with PBS and boiled in SDS-sample buffer. Proteins were separated by SDS-PAGE and transferred onto PVDF membranes for immunoblot analysis.
Isolation of platelet cytoskeleton.
The Triton X-100–insoluble cytoskeleton was isolated as described, with the following modification.17 An equal amount of lysis buffer was added to platelet suspensions to solubilize platelets. After 5 minutes on ice, the lysates were centrifuged at 10,000g. The resulting pellet was washed twice in washing buffer. For one-dimensional SDS electrophoresis, the Triton X-100–insoluble residue was solubilized in SDS sample buffer. The supernatant was diluted with an equal volume of 2× concentrated SDS sample buffer.
RESULTS
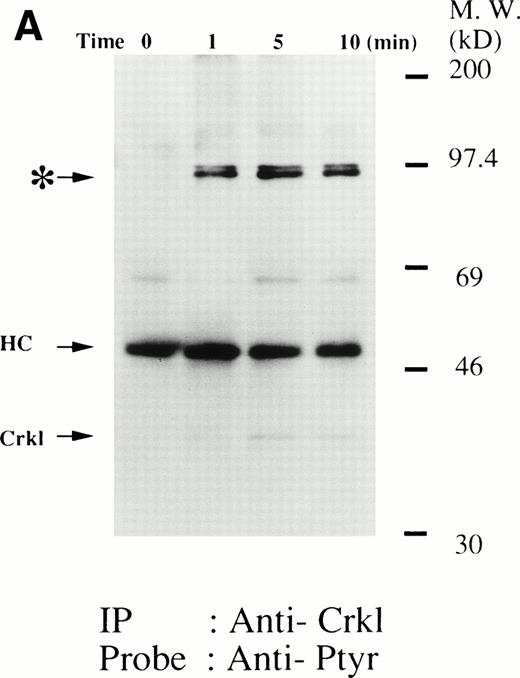
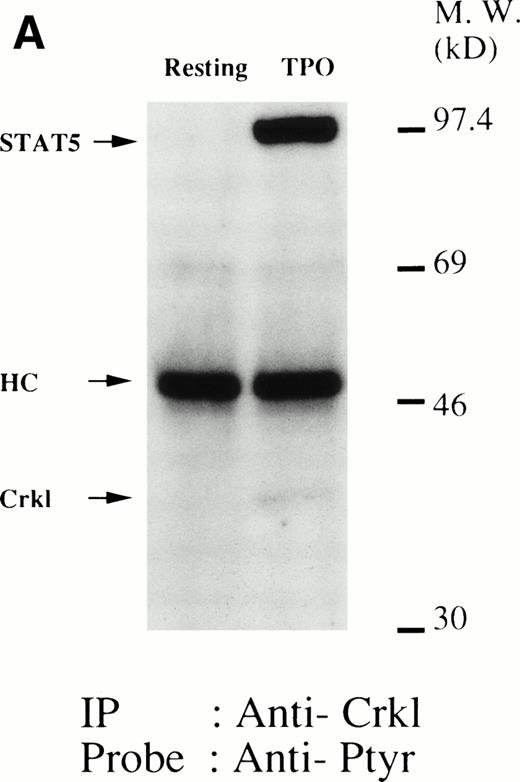
As we have previously reported, Crkl becomes tyrosine phosphorylated after thrombopoietin treatment of human platelets (Fig1A).17 Examination of phosphotyrosine immunoblots of the Crkl immunoprecipitates from thrombopoietin-treated platelets demonstrates the presence of a protein with a molecular weight of approximately 95 to 100 kD (often seen as a doublet). Whole cell lysate antiphosphotyrosine immunoblots show a protein of similar molecular weight that is inducibly tyrosine phosphorylated.9 As seen in Fig 1A, the association of this 95-to 100-kD tyrosine-phosphorylated protein with Crkl occurs rapidly and is maximal 5 minutes after treatment with thrombopoietin.
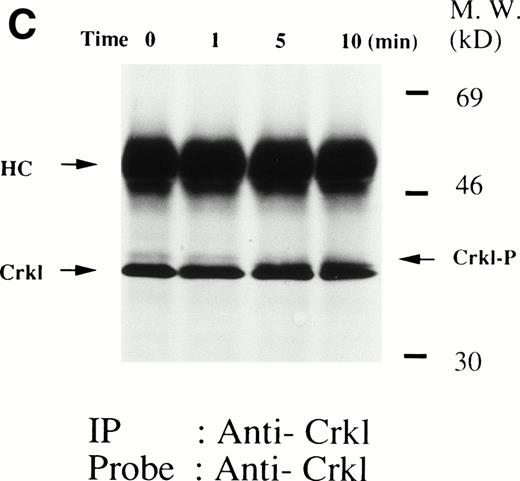
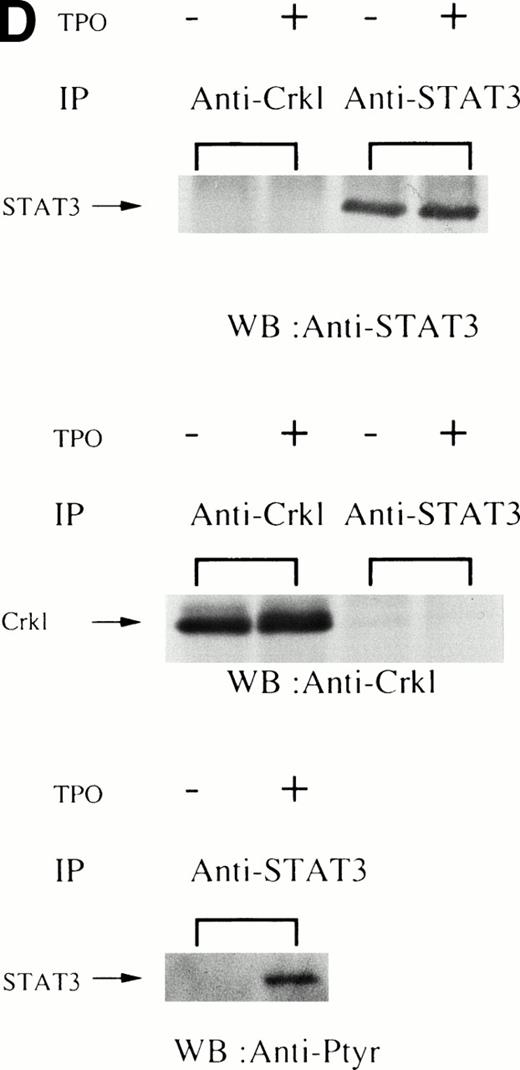
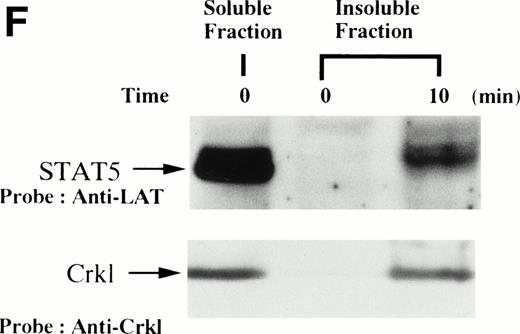
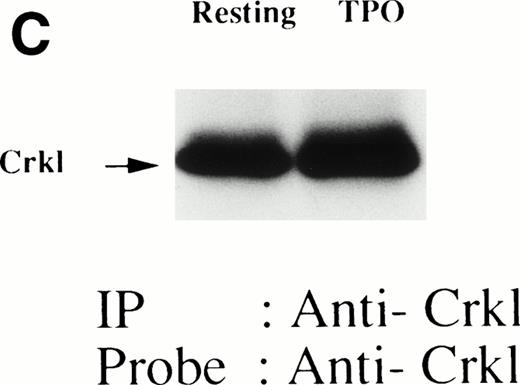
(A and B) Crkl immunoprecipitates from thrombopoietin-treated normal platelets contain a tyrosine-phosphorylated 95- to 100-kD protein that is also recognized by an anti-STAT5 MoAb. Platelets were lysed at the indicated times after thrombopoietin treatment by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to thrombopoietin (100 ng/mL). Crkl was immunoprecipitated with specific Crkl antisera. Immune complexes were resuspended in SDS-sample buffer. Proteins were separated by 7.5% to 15% SDS-PAGE and transferred onto PVDF membranes. Immunoblots were probed with phosphotyrosine antibodies (A) or a STAT5 MoAb (B) and bands were visualized by chemiluminescence. The asterik in (A) indicates the tyrosine-phosphorylated 95- to 100-kD protein. (C) The same PVDF membrane used in (B) was stripped of the antibody and reprobed with Crkl antisera. Bands were visualized using alkaline phosphatase-conjugated second antibody and NBT/BCIP. (D) Crkl immunoprecipitates do not contain STAT3. Platelets were lysed as in (A) before and after stimulation with thrombopoietin (100 ng/mL for 10 minutes). Crkl or STAT3 was immunoprecipitated with specific antisera as indicated. The membranes were probed with STAT3 antisera (top panel), Crkl antisera (middle panel), or 4G10 (bottom panel). Bands were visualized by ECL. (E) Thombopoietin-induced tyrosine phosphorylation of STAT5 in platelets. Platelets were lysed as in (A) before and after stimulation with thrombopoietin (100 ng/mL for 10 minutes). STAT5 was immunoprecipitated with specific antisera (3 μL of anti-STAT5A and STAT5B each). The membranes were probed with 4G10 (upper panel) or an anti-STAT5 MoAb (lower panel). Bands were visualized by ECL. (F) Association of STAT5 with the Triton X-100–insoluble residue. Platelets were lysed with Triton X-100–EGTA buffer before or after stimulation with thrombin (1 U/mL), with or without stirring. Lysates were separated by high-speed centrifugation into soluble and insoluble residues. Proteins from each fraction were separated by 10% SDS-PAGE and immunoblotted with an anti-STAT5 MoAb (upper panel) or Crkl antiserum (lower pane). (Left lane) Triton X-100–soluble residue of resting cells (1.5 × 107cells); (middle lane) Triton X-100–insoluble residue of resting cells (3.0 × 107cells); (right lane) Triton X-100–insoluble residue from 3.0 × 107 cells, prepared 10 minutes after exposure to thrombin (1 U/mL) with stirring.
(A and B) Crkl immunoprecipitates from thrombopoietin-treated normal platelets contain a tyrosine-phosphorylated 95- to 100-kD protein that is also recognized by an anti-STAT5 MoAb. Platelets were lysed at the indicated times after thrombopoietin treatment by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to thrombopoietin (100 ng/mL). Crkl was immunoprecipitated with specific Crkl antisera. Immune complexes were resuspended in SDS-sample buffer. Proteins were separated by 7.5% to 15% SDS-PAGE and transferred onto PVDF membranes. Immunoblots were probed with phosphotyrosine antibodies (A) or a STAT5 MoAb (B) and bands were visualized by chemiluminescence. The asterik in (A) indicates the tyrosine-phosphorylated 95- to 100-kD protein. (C) The same PVDF membrane used in (B) was stripped of the antibody and reprobed with Crkl antisera. Bands were visualized using alkaline phosphatase-conjugated second antibody and NBT/BCIP. (D) Crkl immunoprecipitates do not contain STAT3. Platelets were lysed as in (A) before and after stimulation with thrombopoietin (100 ng/mL for 10 minutes). Crkl or STAT3 was immunoprecipitated with specific antisera as indicated. The membranes were probed with STAT3 antisera (top panel), Crkl antisera (middle panel), or 4G10 (bottom panel). Bands were visualized by ECL. (E) Thombopoietin-induced tyrosine phosphorylation of STAT5 in platelets. Platelets were lysed as in (A) before and after stimulation with thrombopoietin (100 ng/mL for 10 minutes). STAT5 was immunoprecipitated with specific antisera (3 μL of anti-STAT5A and STAT5B each). The membranes were probed with 4G10 (upper panel) or an anti-STAT5 MoAb (lower panel). Bands were visualized by ECL. (F) Association of STAT5 with the Triton X-100–insoluble residue. Platelets were lysed with Triton X-100–EGTA buffer before or after stimulation with thrombin (1 U/mL), with or without stirring. Lysates were separated by high-speed centrifugation into soluble and insoluble residues. Proteins from each fraction were separated by 10% SDS-PAGE and immunoblotted with an anti-STAT5 MoAb (upper panel) or Crkl antiserum (lower pane). (Left lane) Triton X-100–soluble residue of resting cells (1.5 × 107cells); (middle lane) Triton X-100–insoluble residue of resting cells (3.0 × 107cells); (right lane) Triton X-100–insoluble residue from 3.0 × 107 cells, prepared 10 minutes after exposure to thrombin (1 U/mL) with stirring.
We and others have previously demonstrated that STAT3 and STAT5 become tyrosine phosphorylated after thrombopoietin treatment of platelets or various cell lines expressing c-Mpl.3,10,15,25 As expected, STAT3 and STAT5 were tyrosine phosphorylated in thrombopoietin-stimulated platelets (Fig 1D and E). Although STAT3 became tyrosine phosphorylated in thrombopoietin-stimulated platelets, Crkl immunoprecipitates did not contain STAT3 and vice versa (Fig 1D). However, Crkl immunoprecipitates from thrombopoietin-stimulated platelets did contain STAT5 as detected by either STAT5 MoAbs or polyclonal antibodies (Fig 1B and data not shown). The proteins detected by the STAT5 antibodies are often a doublet that may reflect STAT5A and STAT5B. No reactivity was seen with either STAT1 or Vav antisera (data not shown). We have previously shown that Crkl is incorporated into platelet cytoskleton during platelet aggregation (Oda et al17 and Fig 1F, lower panel). Accordingly, we postulated that STAT5, the function of which has been unknown in platelets, may be incorporated into the platelet cytoskeleton during platelet aggregation. STAT5 also became incorporated into the cytoskeletal pellet (Fig 1F, lower panel), suggesting that STAT5 may have a role in the reorganization of the cytoskeleton during platelet aggregation.
Because Crkl is constitutively tyrosine phosphorylated in platelets from CML patients, we next asked whether Crkl tyrosine phosphorylation is sufficient for an association with STAT5 (Fig 2). Before and after stimulation by thrombopoietin (100 ng/mL for 1 minute), Crkl was tyrosine phosphorylated. However, Crkl immunoprecipitates only contained STAT5 after stimulation with thrombopoietin (Fig 2), suggesting that Crkl tyrosine phosphorylation in CML platelets is not sufficient for association of Crkl with STAT5.
Crkl immunoprecipitates from thrombopoietin (100 ng/mL for 1 minute) -treated platelets of CML patients contain a tyrosine-phosphorylated 95- to 100-kD protein that is also recognized by an anti-STAT5 MoAb. The same methods as in Fig 1 were used, except that platelets from CML patients were analyzed. Results are representative of three experiments from three different donors. (−) Resting CML platelets; (+) thrombopoietin-treated CML platelets.
Crkl immunoprecipitates from thrombopoietin (100 ng/mL for 1 minute) -treated platelets of CML patients contain a tyrosine-phosphorylated 95- to 100-kD protein that is also recognized by an anti-STAT5 MoAb. The same methods as in Fig 1 were used, except that platelets from CML patients were analyzed. Results are representative of three experiments from three different donors. (−) Resting CML platelets; (+) thrombopoietin-treated CML platelets.
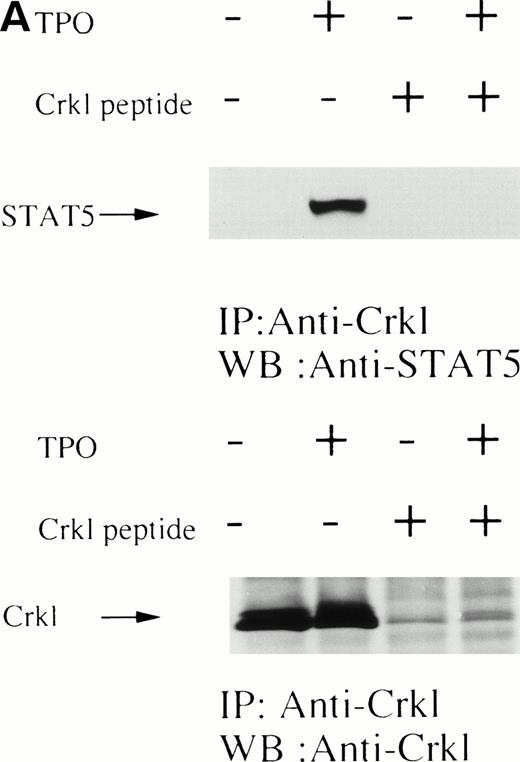
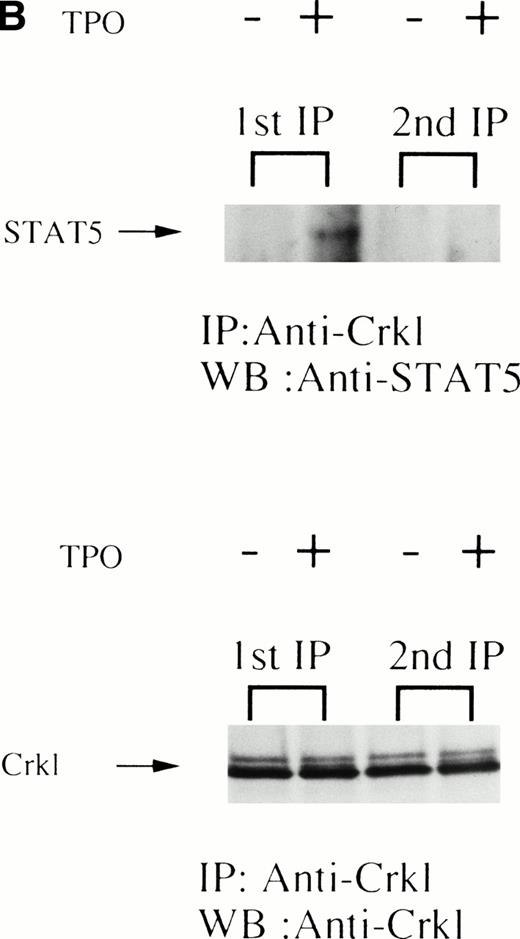
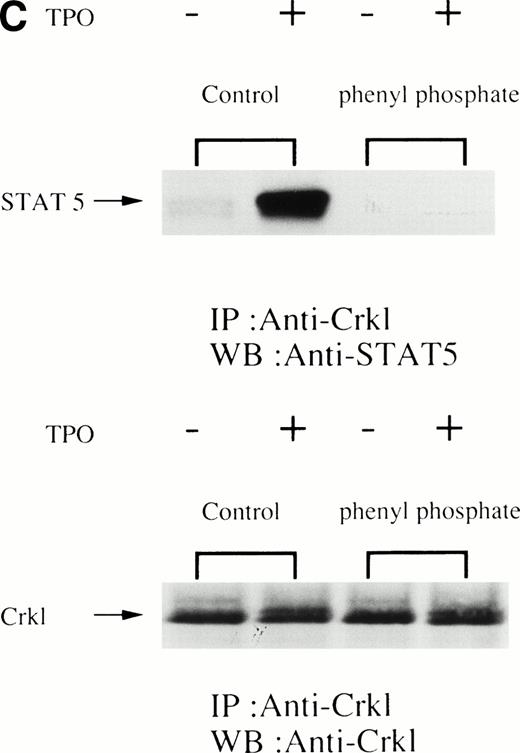
UT7/TPO cells are a recently characterized megakaryocytic cell line that is thrombopoietin-dependent.25 Treatment of UT7/TPO cells with thrombopoietin resulted in a similar induction of tyrosine phosphorylation of Crkl and association of Crkl with STAT5 as seen in platelets (Fig 3). To examine the specificity of the Crkl-STAT5 interaction, several experiments were performed. As seen in Fig 4A, the Crkl immunizing peptide but not a Jak2 peptide inhibited the Crkl immunoprecipitation and STAT5 coimmunoprecipitation. Crkl immunoprecipitates from UT7/TPO were next divided, and half of the beads were boiled in lysis buffer plus 2% SDS for 5 minutes. The denatured immunoprecipitates were diluted into lysis buffer containing 1% Triton X-100 to lower the concentration of SDS to 0.5%. Crkl was again immunoprecipitated (2nd IP). Before and after denaturing of Crkl immunoprecipitates, Crkl was immunoprecipitated by Crkl antisera (Fig4B, lower panel). However, no STAT5 was seen in the denatured Crkl immunoprecipitates (Fig 4B, upper panel). To see whether tyrosine phosphorylation is involved in Crkl and STAT5 association, Crkl was immunoprecipiated in the presence and absence of phenyl phosphate (Fig4C). Crkl was immunoprecipitated before and after stimulation of UT7/TPO cells, irrespective of the presence of phenyl phosphate (20 mmol/L; upper and lower panels). However, STAT5 coimmunoprecipitation after cell stimulation was inhibited by phenyl phosphate, suggesting that protein tyrosine phosphorylation is involved in the Crkl-STAT5 interaction.
(A, B, and C) Crkl immunoprecipitates from thrombopoietin-treated UT7/TPO cells contain a tyrosine-phosphorylated 95- to 100-kD protein that is also recognized by a STAT5 MoAb. UT7/TPO cells were lysed at the indicated times by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to thrombopoietin (100 ng/mL). Tyrosine-phosphorylated proteins (A), STAT5 (B), and Crkl (C) in the Crkl immunoprecipitates were detected as described in Fig 1.
(A, B, and C) Crkl immunoprecipitates from thrombopoietin-treated UT7/TPO cells contain a tyrosine-phosphorylated 95- to 100-kD protein that is also recognized by a STAT5 MoAb. UT7/TPO cells were lysed at the indicated times by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to thrombopoietin (100 ng/mL). Tyrosine-phosphorylated proteins (A), STAT5 (B), and Crkl (C) in the Crkl immunoprecipitates were detected as described in Fig 1.
(A) Crkl and STAT5 immunoprecipitation was inhibited by the Crkl immunizing peptide. (Upper and lower panels) Crkl immunoprecipitation was performed as in Fig 3A and C, except that immunoprecipitation was performed in the presence of the Jak2 (control; left 2 lanes) or the Crkl immunizing peptides (right 2 lanes). (B) STAT5 was not immunoprecipitated by Crkl antisera after denaturing of Crkl immunoprecipitates. The left two lanes are same as in (A; 1st IP). After denaturing of Crkl immunoprecipitates and dilution into lysis buffer containing 0.5% SDS and 1% Triton X-100, Crkl was again immunoprecipitated (2nd IP; the right 2 lanes). Crkl and STAT5 were detected as in Fig 2A and C. (C) Crkl and STAT5 coimmunoprecipitation was inhibited by phenyl phosphate (20 mmol/L). (Upper and lower panels) Crkl immunoprecipitation was performed as in Fig 3A and C, except that immunoprecipitation was performed in the presence (right 2 lanes) or absence (left 2 lanes) of phenyl phosphate (20 mmol/L).
(A) Crkl and STAT5 immunoprecipitation was inhibited by the Crkl immunizing peptide. (Upper and lower panels) Crkl immunoprecipitation was performed as in Fig 3A and C, except that immunoprecipitation was performed in the presence of the Jak2 (control; left 2 lanes) or the Crkl immunizing peptides (right 2 lanes). (B) STAT5 was not immunoprecipitated by Crkl antisera after denaturing of Crkl immunoprecipitates. The left two lanes are same as in (A; 1st IP). After denaturing of Crkl immunoprecipitates and dilution into lysis buffer containing 0.5% SDS and 1% Triton X-100, Crkl was again immunoprecipitated (2nd IP; the right 2 lanes). Crkl and STAT5 were detected as in Fig 2A and C. (C) Crkl and STAT5 coimmunoprecipitation was inhibited by phenyl phosphate (20 mmol/L). (Upper and lower panels) Crkl immunoprecipitation was performed as in Fig 3A and C, except that immunoprecipitation was performed in the presence (right 2 lanes) or absence (left 2 lanes) of phenyl phosphate (20 mmol/L).
STAT5 is thought to be tyrosine phosphorylated in the cytoplasm and translocates to the nucleus when cells are stimulated with various factors, including prolactin, GM-CSF, IL-3, epidermal growth factor (EGF), erythropoietin, and thrombopoietin25,28-31 (reviewed in Darnell32 and Ihle33). After tyrosine phosphorylation, STAT5 dimerizes and translocates to the nucleus, where it activates or represses transcription.
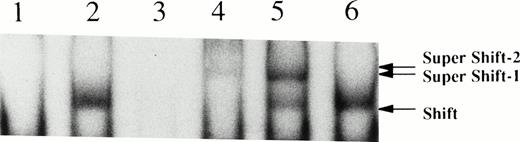
Binding of STAT5 to a radiolabeled probe from the β-casein promoter was analyzed by EMSA (Fig 5). Nuclear extracts were prepared from UT7/TPO cells before and after stimulation with thrombopoietin (100 ng/mL for 20 minutes). Thrombopoietin induced the electrophoretic shift of the end-labeled probe in UT7/TPO cells (Fig 5, Shifts). STAT5 but not STAT3 antisera supershifted the DNA-protein complex and bound to the labeled probe (Fig 5, Super Shift 2, lanes 4 and 6), indicating that a STAT5 or a STAT5-like factor was activated by thrombopoietin. Crkl antisera also supershifted a portion of the same DNA-protein complex induced by thrombopoietin, indicating that Crkl is a component of the DNA-STAT5 complex (Super Shift 1, lane 5). In contrast, STAT3 antiserum had no effect (lane 6), indicating that the effects of STAT5 and Crkl antisera are specific.
Thrombopoietin activates STAT5 in UT7/TPO cells. Growth factor-deprived UT-7/TPO cells were stimulated with 100 ng/mL thrombopoietin and nuclear extracts were prepared. Nuclear extracts were subject to EMSA using a β-casein probe. Lane 1, nuclear extracts from unstimulated cells; lane 2, nuclear extracts from thrombopoietin-stimulated cells; lane 3, the same as in lane 2 in the presence of 50-fold excess cold probe; lane 4, the same as in lane 2, except that the nuclear extracts were incubated with STAT5 antisera (2 μg/mL) before EMSA; lane 5, + Crkl antisera (2 μg/mL); lane 6, + STAT3 antisera (2 μg/mL). Shift, probe complex in the absence of the antibodies; Super Shift 1, the supershifted band in the presence of STAT5 antisera; Super Shift 2, the supershifted band in the presence of Crkl antisera.
Thrombopoietin activates STAT5 in UT7/TPO cells. Growth factor-deprived UT-7/TPO cells were stimulated with 100 ng/mL thrombopoietin and nuclear extracts were prepared. Nuclear extracts were subject to EMSA using a β-casein probe. Lane 1, nuclear extracts from unstimulated cells; lane 2, nuclear extracts from thrombopoietin-stimulated cells; lane 3, the same as in lane 2 in the presence of 50-fold excess cold probe; lane 4, the same as in lane 2, except that the nuclear extracts were incubated with STAT5 antisera (2 μg/mL) before EMSA; lane 5, + Crkl antisera (2 μg/mL); lane 6, + STAT3 antisera (2 μg/mL). Shift, probe complex in the absence of the antibodies; Super Shift 1, the supershifted band in the presence of STAT5 antisera; Super Shift 2, the supershifted band in the presence of Crkl antisera.
We also tested the possibility that IL-3, GM-CSF, and erythropoietin, which are known to induce activation STAT5 in various cells, may induce the Crkl-STAT5 complex formation. As shown in Fig 6, Crkl-STAT5 complex formation was also induced in IL-3– or GM-CSF–stimulated TF-1 cells (Fig 6A) or in erythropoietin-stimulated UT7/EPO cells (Fig 6B). Similar results were obtained in IL-3– or GM-CSF–stimulated UT7 cells (data not shown). In each set of experiments, the presence of Crkl-STAT5 complex formation was also confirmed by EMSA as in Fig 5 (data not shown).
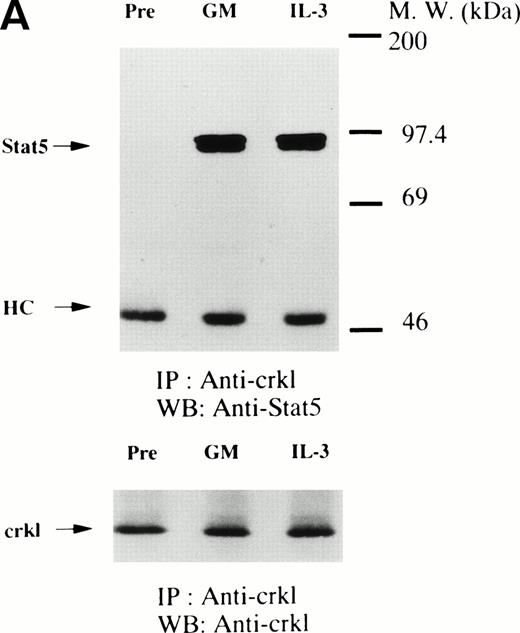
GM-CSF, IL-3, and erythropoietin induce the association of Crkl with STAT5. (A) (Upper panel) TF-1 cells were lysed before (pre) and after exposure to GM-CSF or IL-3 (100 ng/mL for 5 minutes). Crkl immunoprecipitates were resuspended in SDS-sample buffer. Proteins were separated by 10% SDS-PAGE and transferred onto PVDF membranes. Immunoblots were probed with an anti-STAT5 MoAb and bands were visualized by chemiluminescence. (Lower panel) The same PVDF membrane used in (A) was stripped of the antibody and reprobed with anti-Crkl antisera. The arrows indicated the relative position of STAT5, heavy chain of IgG, and Crkl. GM, GM-CSF. (B) Association of Crkl with STAT5 in UT7/EPO cells stimulated by erythropoietin. The same as in Fig 4A, except that UT7/EPO cells were stimulated by erythropoietin (10 U/mL for 10 minutes). EPO, erythropoietin.
GM-CSF, IL-3, and erythropoietin induce the association of Crkl with STAT5. (A) (Upper panel) TF-1 cells were lysed before (pre) and after exposure to GM-CSF or IL-3 (100 ng/mL for 5 minutes). Crkl immunoprecipitates were resuspended in SDS-sample buffer. Proteins were separated by 10% SDS-PAGE and transferred onto PVDF membranes. Immunoblots were probed with an anti-STAT5 MoAb and bands were visualized by chemiluminescence. (Lower panel) The same PVDF membrane used in (A) was stripped of the antibody and reprobed with anti-Crkl antisera. The arrows indicated the relative position of STAT5, heavy chain of IgG, and Crkl. GM, GM-CSF. (B) Association of Crkl with STAT5 in UT7/EPO cells stimulated by erythropoietin. The same as in Fig 4A, except that UT7/EPO cells were stimulated by erythropoietin (10 U/mL for 10 minutes). EPO, erythropoietin.
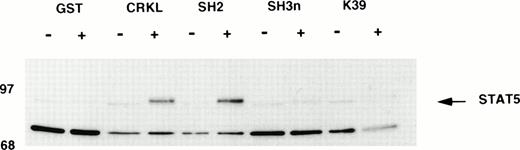
The interaction of Crkl and STAT5 was next examined using bacterially expressed Crkl (Fig 7). Lysates from starved (−) TF-1 cells or cells stimulated with 100 ng/mL of GM-CSF for 10 minutes (+) were incubated with various GST-Crkl constructs. Crkl constructs include full-length Crkl, full-length Crkl with a mutation of Arg to Lys in the SH2 domain FLVRES sequence (K39), the SH2 domain, and the N-terminal SH3 domain (SH3n) of Crkl. Bound proteins were boiled in 1% SDS and separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with STAT5 antisera. Full-length Crkl or the SH2 domain demonstrated inducible binding to STAT5 from starved cells, with a significant increase using lysates from GM-CSF–stimulated cells. No association with STAT5 was seen using the SH3n or K39 Crkl constructs. No binding was seen to GST or beads. Similar data were obtained using lysates from GM-CSF–treated UT7 cells or thrombopoietin-stimulated platelets (data not shown).
Binding of STAT5 to bacterially expressed Crkl. Lysates from starved (−) TF-1 cells or cells stimulated with 100 ng/mL of GM-CSF for 10 minutes (+) were analyzed for binding to GST or various GST-Crkl constructs. Crkl constructs include full-length Crkl, full-length Crkl with a mutation of Arg to Lys in the SH2 domain FLVRES sequence (K39), the SH2 domain, and the N-terminal SH3 domain (SH3n) of Crkl. Bound proteins were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with STAT5 antisera.
Binding of STAT5 to bacterially expressed Crkl. Lysates from starved (−) TF-1 cells or cells stimulated with 100 ng/mL of GM-CSF for 10 minutes (+) were analyzed for binding to GST or various GST-Crkl constructs. Crkl constructs include full-length Crkl, full-length Crkl with a mutation of Arg to Lys in the SH2 domain FLVRES sequence (K39), the SH2 domain, and the N-terminal SH3 domain (SH3n) of Crkl. Bound proteins were separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with STAT5 antisera.
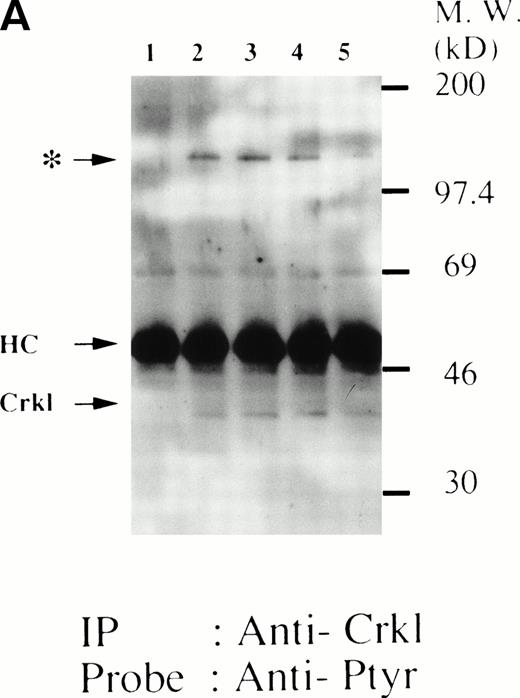
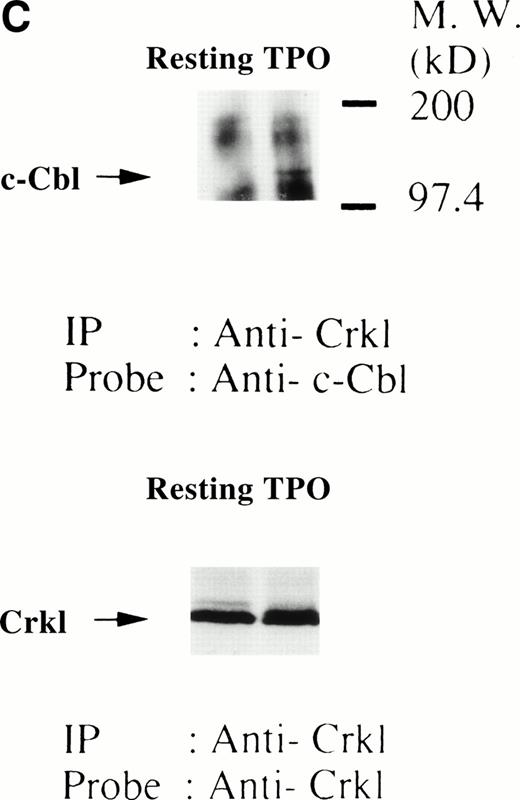
Finally, we also examined whether Crkl-STAT5 association may be observed in murine FDCP-hMpl5 cells in response to thrombopoietin. As we have reported, thrombopoietin (100 ng/mL) induced tyrosine phosphorylation of Crkl (Fig 8A). Thrombopoietin also induced coimmunoprecipitation with Crkl of 120-kD tyrosine-phosphorylated protein indicated by the asterik instead of 95- to 100-kD proteins. Crkl was immunoprecipitated similarly from each sample (Fig 8B). The Crkl immunoprecipitate contains a protein reactive with anti-cCbl (Fig 8C), suggesting that the 120-kD protein contains c-Cbl. Using the same samples, we have never seen any coimunoprecipitation of STAT5 using the same Crkl antiserum (data not shown), despite the fact that we regularly observe thrombopoietin-induced tyrosine phosphorylation of STAT5.10
Crkl and c-Cbl were coimmunoprecipitated from the lysates from FDCP-hMpl5 cells stimulated by thrombopoietin. (A and B) Crkl immunoprecipitates from thrombopoietin-treated FDCP-hMpl5 cells contain a tyrosine phosphorylated 120-kD protein (*). FDCP-hMpl5 cells were lysed at the indicated times by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to thrombopoietin (100 ng/mL). Tyrosine-phosphorylated proteins (A) and Crkl (B) in the Crkl immunoprecipitates were detected as described in Fig 1. Lane 1, resting FDCP-hMpl5 cells; lanes 2 through 5, the lysates from FDCP-hMpl5 cells 1, 5, 10, and 30 minutes after stimulation with thrombopoietin. (C) Crkl immunoprecipitate from thrombopoietin-stimulated FDCP-hMpl5 cells contains c-Cbl. FDCP-hMpl5 cells were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and 10 minutes after exposure to thrombopoietin (100 ng/mL). c-Cbl (upper panel) and Crkl (lower panel) in the Crkl immunoprecipitates were detected as described in Fig 1.
Crkl and c-Cbl were coimmunoprecipitated from the lysates from FDCP-hMpl5 cells stimulated by thrombopoietin. (A and B) Crkl immunoprecipitates from thrombopoietin-treated FDCP-hMpl5 cells contain a tyrosine phosphorylated 120-kD protein (*). FDCP-hMpl5 cells were lysed at the indicated times by the addition of an equal amount of a buffer containing 2% Triton X-100 before and after exposure to thrombopoietin (100 ng/mL). Tyrosine-phosphorylated proteins (A) and Crkl (B) in the Crkl immunoprecipitates were detected as described in Fig 1. Lane 1, resting FDCP-hMpl5 cells; lanes 2 through 5, the lysates from FDCP-hMpl5 cells 1, 5, 10, and 30 minutes after stimulation with thrombopoietin. (C) Crkl immunoprecipitate from thrombopoietin-stimulated FDCP-hMpl5 cells contains c-Cbl. FDCP-hMpl5 cells were lysed by the addition of an equal amount of a buffer containing 2% Triton X-100 before and 10 minutes after exposure to thrombopoietin (100 ng/mL). c-Cbl (upper panel) and Crkl (lower panel) in the Crkl immunoprecipitates were detected as described in Fig 1.
DISCUSSION
In this study, we have confirmed and extended our previous observations that Crkl becomes tyrosine phosphorylated upon thrombopoietin stimulation of cells (platelets and UT7/TPO cells). Crkl immunoprecipitates from platelets or UT7/TPO, treated with thrombopoietin, contained a prominent tyrosine-phosphorylated protein of 95 to 100 kD. We have identified this protein as STAT5. Furthermore, our results suggest that Crkl is also present in the nucleus with STAT 5 and is a component of a STAT5-DNA complex in cells stimulated with thrombopoietin (Fig 5).
The association of Crkl with STAT5 occurs within 1 minute after stimulation with thrombopoietin, when Crkl tyrosine phosphorylation in platelets is minimal (Fig 1). Thus, Crkl tyrosine phosphorylation may not be required for its association with STAT5. In support of this, constitutive tyrosine phosphorylation of Crkl in CML platelets was readily detected, and Crkl immunoprecipitates from lysates of resting CML platelets did not contain STAT5 (Fig 2). However, thrombopoietin (100 ng/mL) -induced treatment resulted in Crkl-STAT5 association without changing the degree of tyrosine phosphorylation of Crkl. In cells transformed by v-Crk, the v-Crk protein is not tyrosine phosphorylated, because it lacks the major site of Crk tyrosine phosphorylation.34 However, Crk immunoprecipitates from v-crk–transformed cells contain numerous tyrosine-phosphorylated proteins.35 Crk II has been shown to bind to a variety of tyrosine-phosphorylated proteins, such as c-Cbl, paxillin, and p130cas.34-36 In the case of c-Cbl, this association is dependent on the tyrosine phosphorylation of c-Cbl after EGF receptor activation or T-cell receptor engagement and does not require tyrosine phosphorylation of Crk II.34 36 Thus, Crkl may be capable of binding to STAT5 without becoming tyrosine phosphorylated.
Because phenyl phosphate inhibited Crkl-STAT5 association, protein tyrosine phosphorylation may be required for Crkl-STAT5 association. Because tyrosine phosphorylation of Crkl does not seem to be required for Crkl-STAT5 association as discussed above and the association occurs apparently after tyrosine phosphorylation of STAT5, it is likely that STAT5 tyrosine phosphorylation may be involved in the Crkl-STAT5 complex formation. Furthermore, the binding of Crkl to STAT5 occurs rapidly upon stimulation of the cells, suggesting that Crkl binds to STAT5 directly after tyrosine phosphorylation of STAT5. STAT5 could be binding to Crkl through a phosphotyrosine-dependent interaction of STAT5 with the SH2 domain of Crkl. The GST fusion protein data confirms such interaction of Crkl and STAT5. In these experiments, STAT5 binds to the SH2 domain of Crkl after stimulation of cells with cytokines. These data are consistent with the observed inducible coimmunoprecipitation of Crkl and STAT5 and suggest a phosphotyrosine-dependent interaction of STAT5 with the SH2 domain of Crkl. Such a possibility seemed to be unlikely, because STAT5 is thought to dimerize when tyrosine phosphorylated through its SH2 domain binding to the tyrosine phosphate of another STAT protein (reviewed in Darnell32 and Ihle33). This model suggests that, when STAT5 binds to DNA, its SH2 domain and tyrosine phosphate would not be available for binding to another SH2 domain-containing protein. Because STAT proteins are believed to be tyrosine phosphorylated at only one site, this suggests that Crkl must displace a STAT protein by binding through its SH2 domain to the tyrosine-phosphorylated residue of a STAT protein. A recent report suggests that STAT1 or STAT2 requires only a tyrosine-phosphorylated STAT protein, which binds to an SH2 domain of the other STAT protein for homodimerization or heterodimerization of the STAT complex and binding of the complex to DNA.37Thus, our data suggest that this may also be true for the STAT5 dimers and that one phosphotyrosine residue in a STAT5 dimer-DNA complex may be available for binding to SH2 domain of other molecules such as Crkl without disrupting the complex. Interestingly, this is not the case for murine STAT5, because as we did not see any association of Crkl with STAT5 in FDCP-hMpl5 cells stimulated by thrombopoietin (Fig 8). Because amino acid sequences of both STAT5 and Crkl are well conserved in both species, we initially suspected that the lack of coimmunoprecipitation was merely dilutional. However, even when we used large number of cells (108 cells/mL) for immunoprecipitation studies, we did not observe the coimmunoprecipitation (data not shown). Furthermore, we saw the coimmunoprecipitation of Crkl with c-Cbl (Fig 8), a process mediated by the binding of Crkl-SH2 domain to c-Cbl.38Furthermore, we did not detect any Crkl-STAT5 association in murine 32D cells stimulated by GM-CSF (data not shown). Thus, our data indicate that the lack of coimmunoprecipitation may not be a technical artifact, suggesting an intriguing possibility that human and murine STAT5-DNA complexes are different, because only the former contains Crkl.
STAT5 proteins are also known to be tyrosine phosphorylated in a variety of situations, including cellular stimulation with GM-CSF, IL-3, erythropoietin, and thrombopoietin.28-33 We have shown that Crkl is also associated with STAT5 under these conditions (Fig 6). These data suggest that the complex formation of STAT5 and Crkl may be solely dependent on activation of STAT5. Several reports have suggested that, in BCR/ABL-transformed cells, STAT5 is constitutively tyrosine phosphorylated and activated.39-42The molecular mechanisms of STAT5 activation are unknown, but one of the possible mechanisms is tyrosine phosphorylation of STAT5 directly by the BCR/ABL kinase.41 However, no direct association of STAT5 and the BCR/ABL kinase has been demonstrated.41 As expected, we also found that, in BCR/ABL-positive K562 cells, Crkl-STAT5 complex is constitutively formed (data not shown). As we have shown that Crkl-STAT5 formation is mediated by Crkl-SH2 domain, N-teminal SH3 domain of Crkl is available for the binding for BCR-ABL chimeric protein.38 Thus, it is possible that Crkl may bridge STAT5 and the BCR/ABL protein so that the kinase can phosphorylate STAT5.
In UT7 cell backgrounds, STAT5 seems to be dispensable for cell proliferation.43 Furthermore, Crkl-STAT5 complex formation is also observed in platelets that do not proliferate. As we have demonstrated that STAT5 become incorporated into platelet cytoskleton, it is possible that Crkl-STAT5 complex may have a role that is not directly related to STAT5 functions as a transcription factor. Such a view is not heretical, because inactive STAT1 and STAT2 may mediate interferon-induced apoptosis (reviewed in Scinder44).
In summary, we have found the novel physical interaction of Crkl-STAT5 in human but not in murine hematopoietic cells, suggesting that the components of the Crkl-STAT5 complex could be different in the cells from different species, and we have further clarified a part of the mechanisms of the Crkl-STAT5 association. Despite the extensive investigations, the pathophysiologic role of Crkl in blood cells is still obscure.38 Because Crkl is an adapter protein, it is critical to determine a potentially important ligand for Crkl. Our study indicates that STAT5 is a novel ligand for Crkl.
ACKNOWLEDGMENT
The authors thank Kirin Brewery Co Ltd for kindly providing recombinant cytokines. We also thank Dr Hisamaru Hirai for TF-1 cells.
K.O. and A.O. contributed equally to this work and should be regarded as cofirst authors.
Supported in part by Grants in Aid from The Ministry of Education, Science and Technology of Japan (A.O., A.M., and Y.I.), Torey Research Foundation (A.M.), New Energy and Industrial Technology Development Organization (A.M.), The Ryoichi Naito Foundation for Medical Research (1994 and 1996; A.O.), and Research Grants for Life Sciences and Medicine, Keio University Medical Science Fund (A.O.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Atsushi Oda, MD, PhD, Hokkaido Red Cross Blood Center, Yamanote 2-2, Nishi-ku, Sapporo 063-0002, Japan.






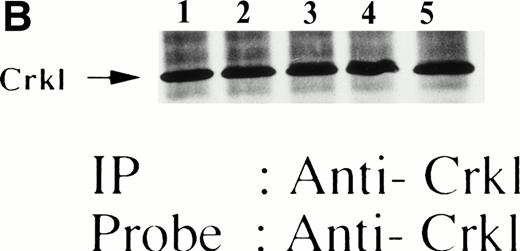
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal