Abstract
Stem cell localization, conservation, and differentiation is believed to occur in niches in the marrow stromal microenvironment. Our recent observation that long-term in vitro human hematopoiesis requires a stromal heparan sulfate proteoglycan (HSPG) led us to hypothesize that such HSPG may orchestrate the formation of the stem cell niche. We compared the structure and function of HS from M2-10B4, a hematopoiesis-supportive cell line, with HS from a nonsupportive cell line, FHS-173-We. Long-term culture-initiating cell (LTC-IC) maintenance was enhanced by PG from supportive cells but not by PG from nonsupportive cells (P < .005). The supportive HS were significantly larger and more highly sulfated than the nonsupportive HS. Specifically, supportive HS contained higher 6-O-sulfation on the glucosamine residues. In agreement with these observations, purified 6-O-sulfated heparin and highly 6-O-sulfated bovine kidney HS similarly maintained LTC-IC. In contrast, completely desulfated heparin, N-sulfated heparin, and unmodified heparin did not support LTC-IC maintenance. Moreover, the supportive HS promoted LTC-IC maintenance but not differentiation of CD34+/HLA-DR−cells into colony-forming cells (CFCs) and mature blood cells. The supportive HS but not the nonsupportive HS bound both cytokines and matrix components critical for hematopoiesis, including interleukin-3 (IL-3), macrophage inflammatory protein-1 (MIP-1), and thrombospondin (TSP). Significantly more CD34+ cells adhered directly to immobilized O-sulfated heparin than to N-sulfated or desulfated heparin. Thus, hematopoiesis-supportive stromal HSPG possessing large, highly 6-O-sulfated HS mediate the juxtaposition of hematopoietic progenitors with stromal cells, specific growth-promoting (IL-3) and growth-inhibitory (MIP-1 and platelet factor 4 [PF4]) cytokines, and extracellular matrix (ECM) proteins such as TSP. We conclude that the structural specificity of stromal HSPG that determines the selective colocalization of cytokines and ECM components leads to the formation of discrete niches, thereby orchestrating the controlled growth and differentiation of stem cells. These findings may have important implications for ex vivo expansion of and gene transfer into primitive hematopoietic progenitors.
THE REGULATED proliferation, commitment, and terminal differentiation of undifferentiated hematopoietic stem cells (HSCs) occurs in intimate contact with the bone marrow (BM) microenvironment. This results in preservation of the stem cell pool while permitting controlled cell proliferation and differentiation.1-5 Stem cells are thought to be localized in stem cell niches or local area networks in the microenvironment, where they interact with the components of their niche-including stromal cells, extracellular matrix (ECM) proteins, and cytokines that are present on stromal cells or bound to ECM macromolecules. Regional variation in these components within the hematopoietic microenvironment may create niches that are specific for cells at a given stage of differentiation.6 7 Specific niches might exist that induce conservation and maintenance of primitive progenitors and other niches that promote proliferation and differentiation, depending on the specific cytokines and matrix components present within it. At present, the identity and structural characteristics of the macromolecules that mediate the formation of such niches remain unknown.
Stromal cell lines that differ in their hematopoietic supportive capability (reviewed in Deryugina and Müller-Sieburg6) may represent niches containing specific components critical for either survival, proliferation, or differentiation of stem cells.8 To define components of niches that support maintenance of primitive human hematopoietic progenitors, we initiated studies to identify and compare the factors produced by hematopoiesis-supportive and nonsupportive cells. We have previously demonstrated that 50% of human long-term culture-initiating cells (LTC-ICs) can be maintained for 5 to 8 weeks in vitro when cultured in medium conditioned by human mixed cell type marrow stromal feeders or by the murine BM stroma-derived fibroblast cell line M2-10B4 (the supportive cells).9-11 Furthermore, 100% of LTC-ICs are maintained for 8 weeks in stroma-conditioned medium supplemented with the heparin-binding cytokines interleukin-3 (IL-3) + macrophage inflammatory protein-1α (MIP-1α)11 or IL-3 + platelet factor 4 (PF4).12 Interestingly, even though cytokine levels in medium conditioned by the human embryonic cell line FHS-173-We (the nonsupportive cells) are similar to marrow stromal supernatant (SN),13-16 conditioned medium from this cell line maintains only 10% of LTC-ICs for 5 weeks.13 Thus, microenvironmental factors other than cytokines appear to be responsible for differences in LTC-IC supportive capability of these two cell lines.
We have shown that one such microenvironmental factor essential for maintenance of human LTC-ICs is a heparan sulfate proteoglycan (HSPG) secreted into the SN of the supportive cells.17 HSPGs are a ubiquitous component of all tissue microenvironments, including the BM microenvironment,18-21 and are present both on cell surfaces and in the ECM. They can mediate cell adhesion, bind and modulate the activity of cytokines, and also bind diverse ECM proteins.22 HSPGs at least partly mediate adhesion of hematopoietic progenitors to stromal cells.7,23-25Furthermore, a number of hematopoietic cytokines, such as IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), basic fibroblast growth factor (bFGF), and hepatocyte growth factor (HGF), bind to HS.4,5,26,27 They may therefore form the backbone of macromolecular complexes in the microenvironment that regulate cell growth and differentiation.18,22 28-31
Structurally, HSPGs are highly heterogeneous macromolecules composed of a core protein and covalently linked, sulfated HS side chains. These HS glycosaminoglycans (GAGs) consist of repeating disaccharide units of D-glucosamine (GlcN) and a hexuronic acid (UA) that is either D-glucuronic acid (GlcA) or L-iduronic acid (IdoA). HSPGs from different tissues of origin have unique structural characteristics due to differences in their core proteins as well as in the nature, number, and pattern of sulfation of the disaccharides, including variability in N-, 6-O-, and, rarely, 3-O-sulfation of GlcN, 2-O-sulfation of IdoA, and sometimes 2-O-sulfation of GlcA.32 It is believed that these differences are responsible for highly specific interactions of GAGs with diverse macromolecules.32 33
Specificity in the pattern of O-sulfation of HS is one of the important determinants of its functional specificity and its ability to bind cytokines and to modulate cell proliferation and differentiation.26,27,34-38 Bovine kidney HS has a high degree of 6-O-sulfation of the first N-acetylated glucosamine adjacent to the terminal N-sulfated glucosamine at the interfaces between sulfated and nonsulfated blocks.39 We have recently shown that this HS supports LTC-IC maintenance.17
We hypothesized that (1) differences in HS secreted by the hematopoiesis-supportive and nonsupportive cells may be responsible for the observed differences in LTC-IC maintenance by these two cell lines and (2) the hematopoiesis-supportive activity of these HS may depend on specific patterns of sulfation that allow colocalization of HSC with cytokines and ECM components that support the conservation and proliferation of HSC. Such HSPGs would thus form the backbone of a functional stem cell niche. In this report, we describe the structural and functional characteristics of heparan sulfate required to form a functional microenvironment that supports long-term in vitro human hematopoiesis.
MATERIALS AND METHODS
Stromal Feeders and Cell Line Cultures
Long-term bone marrow culture (LTBMC) medium consisted of Iscove’s modified Dulbecco’s medium (IMDM) with 12.5% fetal calf serum (FCS), 12.5% horse serum, 2 mmol/L L-glutamine, 1,000 U/mL penicillin, 100 U/mL streptomycin, and 10−6 mol/L hydrocortisone.40 The M2-10B4 cell line (a kind gift from Dr C.J. Eaves, Terry Fox Laboratories, Vancouver, British Columbia, Canada) was maintained by passage in RPMI-1640 medium + 10% FCS and the FHS-173-We cell line (from ATCC, Rockville, MD) in Dulbecco’s modified Eagle’s medium (DMEM) + 10% FCS. When confluent, cells were irradiated at 6,000 rads and subsequently maintained in LTBMC medium. Human stromal feeders were established from human BM mononuclear cells as previously described, irradiated at 1,250 rads when confluent, and maintained in LTBMC medium.40Supernatants from irradiated cultures of human stroma or cell lines were harvested 2 to 3 days after a half-medium change, centrifuged to remove cell debris, and frozen at −70°C until use.
Long-Term Cultures
Cell separation.
BM was aspirated in preservative-free heparin from the posterior iliac crest of healthy young volunteers after obtaining informed consent. BM mononuclear cells were separated by Ficoll-Hypaque centrifugation (specific gravity, 1.077) and enriched for CD34+ cells using Ceprate LC CD34-avidin immunoadsorption columns (CellPro Inc, Bothell, WA).41 The CD34+enriched cell population was labeled with anti-CD34-phycoerythrin (PE) and anti-HLA-DR-fluorescein isothiocyanate (FITC) antibodies (Becton Dickinson, San Jose, CA) and CD34+/HLA-DR− cells selected on a FACStar Plus flow cytometry system equipped with a Consort 32 computer.9 40
Cytokines and glycosaminoglycans.
Recombinant human cytokines used in long-term cultures included granulocyte colony-stimulating factor (G-CSF; Neupogen; Amgen, Thousand Oaks, CA), GM-CSF (Immunex Corp, Seattle, WA), stem cell factor (SCF; a kind gift from Amgen), leukemia inhibitory factor (LIF; R & D Systems Inc, Minneapolis, MN), MIP-1α (R&D Systems), and IL-6 (a kind gift from Dr G. Wong, Genetics Institute, Cambridge, MA). Bovine kidney HS and heparin were from Sigma (St Louis, MO), and the differentially chemically desulfated heparins were from Seikagaku America (Rockville, MD).
Culture system.
DR− cells were suspended in a Transwell insert with a microporous 0.4-μm membrane placed in stroma-free wells of 24-well plates. Media in the lower well were replaced 5 times weekly with 0.8 mL fresh unconditioned LTBMC medium, LTBMC medium supplemented with cytokines alone or in combination with cell line-derived PGs (see below for method of purification), bovine kidney HS, and differentially sulfated heparin GAGs or with SN from stromal feeders. Cultures were maintained for 5 weeks at 37°C in humidified 5% CO2atmosphere. The progeny of DR− cells recovered by vigorous washing were plated at limiting dilutions (22 replicates per concentration) onto irradiated M2-10B4 feeders in 96-well plates to enumerate LTC-ICs as described.9 The absolute number of LTC-IC was calculated using Poisson statistics.9 42
Structural Analysis of Proteoglycans
Purification of PGs from SN of supportive and nonsupportive cells.
For use in long-term cultures, PGs in the SN of irradiated supportive and nonsupportive cells were radiolabeled (1 of 10 flasks were labeled) on the sulfate groups by addition of 50 μCi/mL Na235SO4 (ICN Biomedicals Inc, Irvine, CA) in sulfate-replete (sulfate content of IMDM, 0.8 mmol/L) LTBMC medium for 24 hours. PGs were purified by diethyl aminoethyl (DEAE)-Sephacel anion exchange high-performance liquid chromatography (HPLC; Beckman, Fullerton, CA), as previously described.17 For structural analysis, PGs were labeled with 20 μCi/mL3H-glucosamine (to label the GAG backbone; DuPont NEN, Boston, MA) and 50 μCi/mL Na235SO4 in LTBMC medium for 18 to 24 hours, before purification by anion exchange HPLC.
Preparation of HSPGs.
Purified, 3H- and 35S-labeled PGs were digested by chondroitinase ABC (cABC; Seikagaku) and chromatographed on a Sephadex G-50 (Sigma) column, and undigested HSPGs eluting at V0 were collected. Free HS chains were released from the core protein by treatment of the HSPG with sodium hydroxide in the presence of sodium borohydride (NaBH4). Between 96% and 99% of the purified HS from the various peaks was digestible by nitrous acid,43,44 indicating that this material was highly purified for HS. The size of the HSPG and HS was estimated by gel filtration chromatography on a 0.75 × 95 cm Sepharose CL-6B column equilibrated in 4 mol/L guanidine hydrochloride and 0.05 mol/L sodium acetate, pH 5.8. The approximate sizes of PG and GAG were estimated by the methods of Heinegard and Hascall45 and Wasteson,46 respectively.
Distribution of N-sulfation and O-sulfation in HS.
35S-and 3H-labeled HS from both cells were subjected to low pH nitrous acid (pH 1.5) deaminative cleavage of N-sulfated regions as described.44 The digested oligosaccharides were resolved by gel filtration chromatography on a 0.75 × 110 cm Sephadex G-25 column equilibrated and eluted at a rate of 6 mL/h with 0.2 mol/L ammonium acetate, pH 7.0; 0.3-mL fractions were collected. The resulting oligosaccharides were analyzed as described previously.47
CarboPac PA1 chromatography.
3H- and 35S-labeled CS/DS PGs in the SN of the two cells were recovered from DEAE-Sephacel anion exchange HPLC, dialyzed against water, and lyophilized. Analysis of sulfated disaccharides was performed as described.48 Briefly, CS/DS were digested using cABC, the resulting disaccharides were reduced by a modified borohydride reduction reaction to stabilize unsaturated disaccharides to alkali, and the reduced alditols were desalted using Dowex 50 hydrogen. The disaccharides were resolved by HPLC using a CarboPac PA1 column eluted at 1 mL/min with a sodium trifluoroacetic acid gradient in 0.1 mol/L NaOH. Fractions of 1 mL were collected. Disaccharides were detected by integrated pulse amperometry on a pulsed electrochemical detector module and identified by comparison to known disaccharide standards. The 3H and 35S radioactivity incorporated in the disaccharides was measured, and the relative metabolism of 3H-glucosamine by the 2 cells was estimated by the ratio of 3H:35S in the sulfated disaccharide peaks.48
2-O-Desulfation of GAGs
Unmodified heparin or O-sulfated heparin was dissolved in dH2O (4 mg/mL) and the pH was adjusted to 12.5 using 0.1 N NaOH.49 The solution was frozen, lyophilized, and then rehydrated in the original volume of dH2O and dialyzed against water.
Iodination of HS
Purified HS from the two cells was labeled with tyramine50,51 and iodinated using carrier-free Na2125I (Amersham, Arlington Heights, IL) and the Iodo-gen iodination reagent (Pierce, Rockford, IL).51Free 125I was removed from the labeled HS by separation on a Sephadex G-25 column, followed by exhaustive dialysis. The specific activity was 3.4 μCi/μg for the iodinated supportive HS and 7.2 μCi/μg for the nonsupportive HS.
Affinity Coelectrophoresis (ACE)
Binding of 35S-labeled HS from the supportive and nonsupportive cells to cytokines including IL-3 (R & D Systems), PF4, MIP-1α, and bFGF (R & D Systems) and to ECM molecules including thrombospondin (TSP; a kind gift from Dr Robert Hebbel, University of Minnesota, Minneapolis, MN) and fibronectin (FN; Sigma) was examined using ACE. To digest residual core proteins of the HSPGs and any other remaining proteins before ACE, 3H- and35S-labeled HS preparations obtained from the two cells as described above were further incubated with proteinase K (Sigma) followed by heating at 100°C for 1 minute to inactivate the enzyme. ACE was performed as described.52 Briefly, a 1% agarose gel (low melting point Seaplaque; FMC, Rockland, MD) in 50 mmol/L sodium MOPSO (Sigma), pH 7.0, 125 mmol/L NaCl, 0.5% CHAPS buffer was cast with a strip well (for HS) and a perpendicular 8-lane comb (for proteins). Protein samples prepared at twice the desired concentration in MOPSO/NaCl/CHAPS electrophoresis buffer were mixed with an equal volume of 2% agarose and allowed to gel in appropriate wells created by the 8-lane comb. The HS preparation in MOPSO/NaCl/CHAPS electrophoresis buffer containing bromophenol blue and sucrose was added to the strip well. Electrophoresis was performed in a Hoefer electrophoresis apparatus in MOPSO/NaCl running buffer prepared without CHAPS at 330 mA, 45 to 60 V for 2.5 to 2.75 hours at 20°C to 25°C. Gels were air-dried, submerged in Amplify solution (Amersham, Arlington Heights, IL), redried, and autoradiographed at −80°C on Kodak Biomax MS film using a Kodak Biomax TranScreen LE intensifying screen (Eastman Kodak, Rochester, NY). In other experiments, the binding of 125I-labeled cell line HS to TSP and MIP-1α was examined by ACE.
Surface Plasmon Resonance (SPR)
The binding of GAGs to PF4 was examined on BIAcore biosensor equipment (Pharmacia, Uppsala, Sweden) using surface plasmon resonance, which is highly sensitive and monitors real-time binding of an analyte (GAG) to a ligand (PF4) immobilized on a sensor chip.53 54 A change in mass at the surface of the chip upon binding of the analyte causes a change in refractive index, changing the angle at which plasmon resonance occurs. This is measured in resonance units (RU), which correlate with the amount of analyte bound (1 RU = 1 pg/mm2). For assessment of binding of GAG in solution to immobilized PF4, 1 μg/mL biotinylated PF4 in HEPES buffer containing 150 mmol/L NaCl, 1 mmol/L CaCl2, and 1 mmol/L MgCl2 was perfused over an SA5 (with immobilized streptavidin) sensor chip. GAGs in equilibration buffer (HEPES buffer as above with 0.005% surfactant p20) in a range of concentrations between 10−4 and 10−7 mol/L were perfused over the chip at 20 μL/min. The KD was calculated using BIAevaluation 2.1 software (Pharmacia Biosensor AB, Uppsala, Sweden) from the average of ka (association or run-on phase) and kd (dissociation or wash-out phase) kinetics: KD = kd/ka (where ka is derived from the ks plot).
Adhesion of CD34+ Cells
Unmodified or differentially sulfated heparins were conjugated to ovalbumin by amide linkage, using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC).55Briefly, GAG and ovalbumin were dissolved (2 mg each) in 1 mL distilled water and 20 mg of EDC in 50 μL water was added (all chemicals from Sigma). The mixture was shaken at 4°C for 12 hours to couple GAGs to ovalbumin. EDC was removed by dialysis against phosphate-buffered saline (PBS). For adhesion experiments, 48-well plates were coated by overnight incubation at 37°C with 150 μL/well of 5% fatty acid free bovine serum albumin (BSA; Sigma) or 100 μg/mL ovalbumin-conjugated GAGs in Voller’s bicarbonate buffer. For some experiments, plates were incubated for an additional 24 hours at 4°C. Wells were washed and blocked with 1% BSA in PBS before the adhesion assay. Fluorescence-activated cell sorting (FACS)-sorted CD34+ cells were labeled with51Cr and resuspended in IMDM + 0.3% BSA, and 20,000 cells in 250 μL volume/well were allowed to adhere to the coated wells for 4 hours at 37°C. The total radioactivity present in an equivalent number of cells (20,000 cells in 250 μL volume) was measured separately. Nonadherent cells were removed by four to six gentle washings with PBS, and their removal was confirmed by visual inspection. Adherent cells were lysed by addition of 500 μL/well of 1% Triton X-100 in PBS and 51Cr radioactivity was measured. The percentage of adhesion was calculated as: % adhesion = cpm in adherent cell lysate × 100/total cpm in 20,000 cells plated.
Binding of 125I-HS to CD34+ Cells
CD34+ cells were suspended in 0.5 mL cold IMDM + 0.3% BSA, and 125I-HS from the supportive or nonsupportive cells was added. After incubation on ice with gentle mixing for 90 minutes (maximal binding was seen at 60 to 90 minutes in preliminary experiments; data not shown), the cells were washed with cold IMDM + 0.3% BSA and the bound radioactivity in the cell pellet counted using a gamma counter.
Statistics
Results of data are reported as the mean ± SEM. Levels of significance were determined by the two-sided Student’st-test.
RESULTS
PGs from Hematopoiesis-Supportive Cells But Not From Nonsupportive Cells Support LTC-IC Maintenance
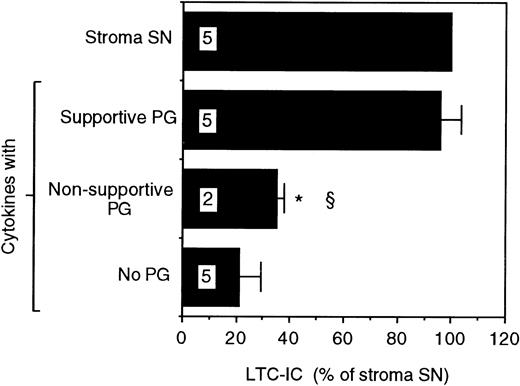
Proteoglycans purified from the SN of the two cells were evaluated for their ability to support LTC-IC maintenance. A combination of supportive cell PGs and the picogram concentrations of cytokines found in stromal SN supported maintenance of the same number of LTC-IC as cultures fed with unfractionated stromal SN. However, supportive cell PG alone or cytokines alone were unable to maintain LTC-IC (Fig 1). In contrast, when purified PGs from the SN of the nonsupportive cells were combined with the same cytokines, no increase in LTC-IC maintenance was seen over that with cytokines alone. Maintenance of LTC-IC in cultures supplemented with nonsupportive cell PGs + cytokines or with supportive cell PGs + cytokines in the present study was comparable to the LTC-IC maintenance observed in the presence of unfractionated nonsupportive cell SN and supportive cell SN previously described by our group.13This indicates that a combination of cytokines at concentrations found in stromal SN + purified PGs recreates the hematopoiesis-supportive capabilities of unfractionated SN from the two cell lines.
LTC-IC supportive capability of proteoglycans from the SN of the supportive and nonsupportive cells. A total of 14,000 to 17,000 DR− cells were plated in 0.4-μm transwell inserts in 24-well tissue culture clusters. Cultures were maintained by daily replacement of the medium in the lower chambers of the wells by either stroma supernatant (Stroma SN) or by LTBMC medium supplemented with a combination of cytokines (500 pg/mL G-CSF, 50 pg/mL GM-CSF, 200 pg/mL SCF, 50 pg/mL LIF, 2 ng/mL IL-6, and 200 pg/mL MIP-1) with or without proteoglycans from the SN of the supportive or nonsupportive cells. Cultures were harvested after 5 weeks and cells were replated at limiting dilutions for estimation of LTC-IC frequency. The absolute number of LTC-IC in the starting cell population at day 0 was 0.67 ± 0.01 per 100 DR− cells plated. The absolute number of LTC-IC after 5 weeks of culture in stroma SN was 0.32 ± 0.03 per 100 DR− cells initially plated at day 0 (48% of day 0 LTC-IC). Numbers within bars indicate number of experiments. Comparison between Stroma SN and other conditions: *P < .001. Comparison between the supportive cells and other conditions: §P < .005.
LTC-IC supportive capability of proteoglycans from the SN of the supportive and nonsupportive cells. A total of 14,000 to 17,000 DR− cells were plated in 0.4-μm transwell inserts in 24-well tissue culture clusters. Cultures were maintained by daily replacement of the medium in the lower chambers of the wells by either stroma supernatant (Stroma SN) or by LTBMC medium supplemented with a combination of cytokines (500 pg/mL G-CSF, 50 pg/mL GM-CSF, 200 pg/mL SCF, 50 pg/mL LIF, 2 ng/mL IL-6, and 200 pg/mL MIP-1) with or without proteoglycans from the SN of the supportive or nonsupportive cells. Cultures were harvested after 5 weeks and cells were replated at limiting dilutions for estimation of LTC-IC frequency. The absolute number of LTC-IC in the starting cell population at day 0 was 0.67 ± 0.01 per 100 DR− cells plated. The absolute number of LTC-IC after 5 weeks of culture in stroma SN was 0.32 ± 0.03 per 100 DR− cells initially plated at day 0 (48% of day 0 LTC-IC). Numbers within bars indicate number of experiments. Comparison between Stroma SN and other conditions: *P < .001. Comparison between the supportive cells and other conditions: §P < .005.
Supportive HS Are Larger and More 6-O-Sulfated Than Nonsupportive HS
Because the LTC-IC maintaining activity of the supportive PGs is due to their HS,17 we examined the differences in HS from the supportive and the nonsupportive cells. Upon anion exchange HPLC,35SO4- and 3H-glucosamine-labeled PG from SN of the supportive cells eluted as a single major peak, whereas PGs from the nonsupportive cells eluted in three peaks (labeled A, B, and C; data not shown). The three peaks in PGs from the nonsupportive cells and comparable regions from the supportive cells’ PGs were analyzed separately.
HS from both cells eluted largely in peak A, with a small proportion of HS in peak B from the nonsupportive cells. In three independent experiments, the percentage of HS in peak A of PGs from the nonsupportive cells was 89% ± 3%, in peak B was 19% ± 11%, and in the supportive cell PG peak A was 33% ± 8%. The rest of the GAGs in these peaks, and almost all the GAGs in peak C, were CS/DS. The overall proportion of HSPG in nonsupportive cell PGs (41%) was higher than in supportive cell PGs (13%), and total35SO4 and 3H-glucosamine in HSPG from the nonsupportive cells was greater than in HSPG from the supportive cells. Therefore, structural rather than the quantitative differences in HS from the two cells must be responsible for the observed difference in LTC-IC maintenance.
Size of HPSGs and GAGs.
The size of HSPGs from the two cells was similar (average molecular weight [Mr], 160 to 210 kD). However, the average size of supportive HS (45 kD) was larger than nonsupportive HS (both peak A [22 kD], which contains the majority of its HS, and peak B [33 kD], which constitutes a smaller proportion of its HS).
Degree of sulfation of HS.
Sulfation, determined as the ratio of dpm of 35S-sulfate to3H-glucosamine, was higher in PGs from the supportive cells than from the nonsupportive cells in three independent experiments. Because different cell types can have different glucosamine pool sizes and fluxes, the specific activity of the sulfated disaccharides generated by cABC was used to correct the measured incorporation of3H-glucosamine in GAGs by the supportive cells and the nonsupportive cells, as outlined by Midura et al.48 The average ratio of 3H:35S in the sulfated disaccharides (ΔDi-4S and ΔDi-6S) was 1.60 for the nonsupportive cells and 0.66 for the supportive cells. This indicates that the nonsupportive cells incorporate more radiolabeled glucosamine into identical sulfated disaccharides than the supportive cells. However, even after correction for this difference in 3H-glucosamine incorporation, the 35SO4 to3H-glucosamine ratio was higher in the supportive cell’s proteoglycans, HSPGs, and HS than in the nonsupportive cells (the 35S:3H ratio was 1.11 in supportive HS and 0.43 in nonsupportive HS).
These results were consistent with elution from DEAE-Sephacel. Nonsupportive HS eluted at lower NaCl concentrations than supportive HS. This indicates that differences in elution of HSPGs are due to differences in sulfation of their HS rather than to differences in the number of HS chains per molecule of core protein. Nonsupportive HS eluted at 0.39 mol/L NaCl (peak A) and at 0.42 mol/L NaCl (peak B). The supportive HS eluted as a major peak at 0.48 mol/L, with minor peaks at 0.37 and 0.43 mol/L. Thus, a second difference in HS from the two cells is the higher proportion of more highly sulfated HS secreted by the supportive cells compared with HS from the nonsupportive cells.
Sulfation pattern of HS.
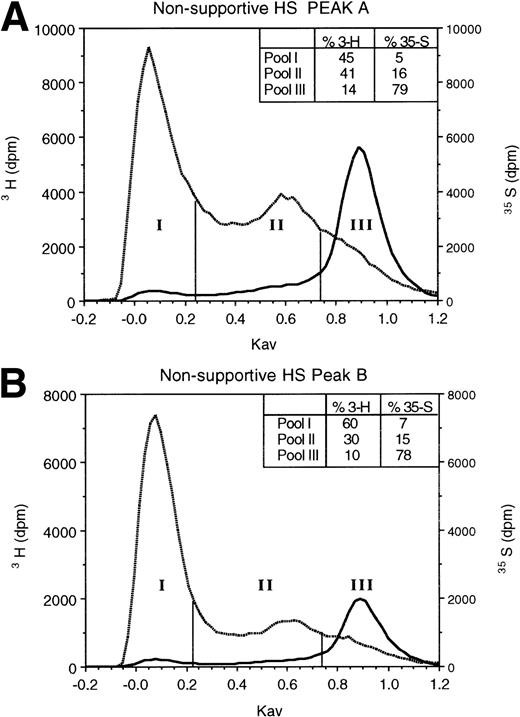
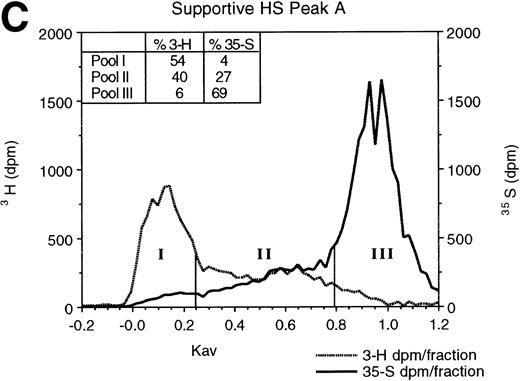
To determine if supportive and nonsupportive HS differ in sulfation pattern, we digested 3H-glucosamine- and35SO4-labeled HS with low pH nitrous acid. The resulting oligosaccharides were resolved by Sephadex G-25 column chromatography. At low pH, nitrous acid cleaves HS at GlcNSO3 residues, leaving regions with GlcNAc as intact oligosaccharides.44 When the resulting oligosaccharides and disaccharides are resolved by G-25 gel filtration chromatography,47 large undigested oligosaccharides (>8 monosaccharide residues) containing contiguous GlcNAc residues elute in the excluded volume (pool I in Fig 2), disaccharides and free sulfate derived from regions containing contiguous GlcNSO3 residues elute in the total volume (pool III), and oligosaccharides of 3-8 monosaccharides derived from cleavage of alternating [-(UA-GlcNSO3-UA-GlcNAc)n-] or variably spaced regions containing both GlcNAc and GlcNSO3residues elute in the included volume (pool II; fractions between vertical bars). The 3H-glucosamine in pool II is indicative of the proportion of the total carbohydrate backbone contained in such oligosaccharides. The 35S in pool II oligosaccharides is largely as 6-O-sulfated GlcN, rather than as 2-O-sulfated UA,39,47 and is therefore representative of the extent of 6-O-sulfation on GlcNAc residues adjacent to the terminal GlcNSO3 residue.39 56
Gel filtration chromatography of HS oligosaccharides following nitrous acid digestion. HS in HPLC peaks A and B from the nonsupportive cell PG (A and B) and in peak A from the supportive cell peak A PG (C) were subjected to low pH nitrous acid digestion and separated on a Sephadex G-25 column, as described in Materials and Methods. Fractions of 0.3 mL were collected and incorporated3H and 35S radioactivity was monitored.
Gel filtration chromatography of HS oligosaccharides following nitrous acid digestion. HS in HPLC peaks A and B from the nonsupportive cell PG (A and B) and in peak A from the supportive cell peak A PG (C) were subjected to low pH nitrous acid digestion and separated on a Sephadex G-25 column, as described in Materials and Methods. Fractions of 0.3 mL were collected and incorporated3H and 35S radioactivity was monitored.
As shown in Fig 2, the proportion of the total carbohydrate in these oligosaccharides was equivalent in nonsupportive HS (41%3H in peak A) and the supportive HS (40% 3H in peak A). However, in two independent experiments, the proportion of35S in pool II from supportive HS (27%) was 1.7- to 1.8-fold greater than in nonsupportive HS (15% to 16%). Moreover, even after correction for differential 3H-glucosamine incorporation by the two cells (see above), the sulfate:carbohydrate backbone ratio (35S:3H) in the supportive cell oligosaccharides (0.63:1) was higher than that in the nonsupportive cell peak A (0.26:1) or in the nonsupportive cell peak B oligosaccharides (0.43:1). Our results therefore support the conclusion that the observed differences in percent sulfation in pool II in the two HS are largely a result of a higher degree of 6-O-sulfation of the terminal GlcN residues in supportive HS than in nonsupportive HS. Thus, supportive HS are more 6-O-sulfated than nonsupportive HS.
6-O-Sulfation Is Essential for the LTC-IC Maintaining Capability of GAGs
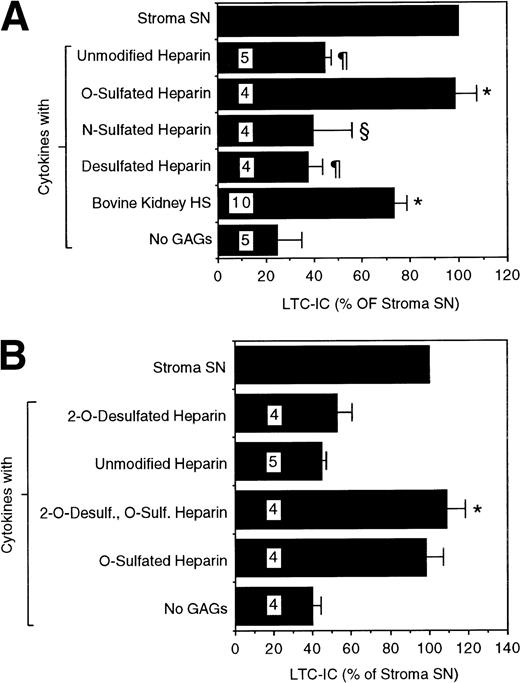
We next examined if these differences in sulfation patterns have functional implications for the ability of GAGs to support LTC-IC. We evaluated the ability of chemically modified heparins, which are selectively desulfated at one or more positions, to maintain LTC-IC. A combination of unmodified heparin and cytokines did not significantly increase LTC-IC maintenance over that seen with cytokines alone (Fig 3A). This suggested that the common, highly N- and O-sulfated heparin motif [-IdoA(2-OSO3)-GlcNSO3(6-OSO3)-] may not be optimal for LTC-IC maintenance. A completely N- and O-desulfated, N-reacetylated heparin (desulfated heparin) of comparable chain length also did not have any LTC-IC maintaining activity. Completely N- and O-desulfated, N-resulfated heparin, which is largely depleted of 2-and 6-O-sulfate groups but retains N-sulfation (N-sulfated heparin), had only partial activity. In contrast, a combination of cytokines with N-desulfated, N-reacetylated heparin, which retains 2- and 6-O-sulfate but not N-sulfate groups (O-sulfated heparin), maintained LTC-IC to the same extent as unfractionated stromal SN or HS from the supportive cells. Highly 6-O-sulfated bovine kidney HS maintained 70% to 80% LTC-IC. The optimal concentration of bovine kidney HS for support of LTC-IC maintenance was in the range of 5 μg/mL, because higher (20 μg/mL) or lower (1 μg/mL) concentrations were less effective (data not shown). In contrast to their effects on LTC-IC maintenance, the addition of PGs from the two cells, chemically desulfated heparins, or bovine kidney HS to cytokines did not change the number of colony-forming cells (CFCs) or mature cells generated over 5 weeks compared with cultures using cytokines alone (data not shown).
LTC-IC supportive activity of differentially sulfated glycosaminoglycans. A total of 10,000 to 17,000 DR− cells were plated in 0.4-μm transwell inserts in 24-well tissue culture clusters. Cultures were maintained by daily replacement of the medium in the lower chambers of the wells by either stroma supernatant (Stroma SN) or by LTBMC medium supplemented with a combination of cytokines (500 pg/mL G-CSF, 50 pg/mL GM-CSF, 200 pg/mL SCF, 50 pg/mL LIF, 2 ng/mL IL-6, and 200 pg/mL MIP-1) with or without 5 μg/mL of the indicated GAGs. Cultures were harvested after 5 weeks and cells were replated at limiting dilutions for estimation of LTC-IC frequency. The absolute number of LTC-IC in the starting cell population at day 0 was 0.40 ± 0.07 per 100 DR− cells plated. The absolute number of LTC-IC after 5 weeks of culture in stroma SN was 0.21 ± 0.04 per 100 DR− cells initially plated at day 0 (52% of day 0 LTC-IC). Numbers within bars indicate number of experiments. (A) Comparison between cytokines only (No GAGs) and other conditions: *P < .002. Comparison between O-sulfated heparin and other conditions: §P < .02, ¶P < .002. (B) LTC-IC supportive activity of 2-O-desulfated glycosaminoglycans, prepared from unmodified heparin or from O-sulfated heparin as described in Materials and Methods. Comparison between cytokines only (No GAGs) and 2-O-desulfated O-sulfated heparin: *P < .002.
LTC-IC supportive activity of differentially sulfated glycosaminoglycans. A total of 10,000 to 17,000 DR− cells were plated in 0.4-μm transwell inserts in 24-well tissue culture clusters. Cultures were maintained by daily replacement of the medium in the lower chambers of the wells by either stroma supernatant (Stroma SN) or by LTBMC medium supplemented with a combination of cytokines (500 pg/mL G-CSF, 50 pg/mL GM-CSF, 200 pg/mL SCF, 50 pg/mL LIF, 2 ng/mL IL-6, and 200 pg/mL MIP-1) with or without 5 μg/mL of the indicated GAGs. Cultures were harvested after 5 weeks and cells were replated at limiting dilutions for estimation of LTC-IC frequency. The absolute number of LTC-IC in the starting cell population at day 0 was 0.40 ± 0.07 per 100 DR− cells plated. The absolute number of LTC-IC after 5 weeks of culture in stroma SN was 0.21 ± 0.04 per 100 DR− cells initially plated at day 0 (52% of day 0 LTC-IC). Numbers within bars indicate number of experiments. (A) Comparison between cytokines only (No GAGs) and other conditions: *P < .002. Comparison between O-sulfated heparin and other conditions: §P < .02, ¶P < .002. (B) LTC-IC supportive activity of 2-O-desulfated glycosaminoglycans, prepared from unmodified heparin or from O-sulfated heparin as described in Materials and Methods. Comparison between cytokines only (No GAGs) and 2-O-desulfated O-sulfated heparin: *P < .002.
To examine if 2-O-sulfation was required for the LTC-IC maintaining activity of the GAGs, unmodified heparin and the O-sulfated heparin were subjected to 2-O-desulfation.49 LTC-IC maintaining capability of the O-sulfated heparin was not reduced by removal of the 2-O-sulfate groups (Fig 3B). These results suggest that 6-O-sulfation of GAGs is essential for supporting the maintenance of primitive hematopoietic progenitors and suggest further that N-sulfation may be detrimental to this activity.
O-Sulfated HS Selectively Bind Early-Acting Cytokines and ECM Components and Directly Mediate Adhesion of CD34+Cells
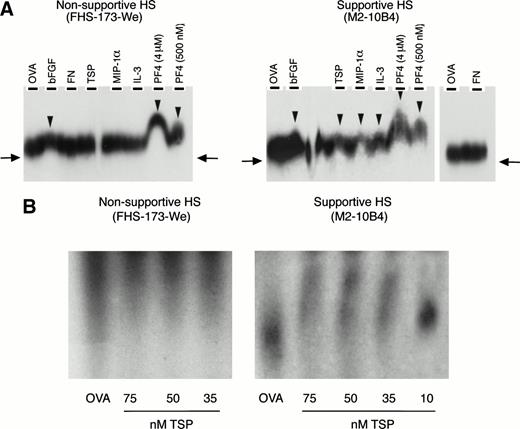
We then examined if these GAGs might contribute to the colocalization of primitive progenitors with early-acting cytokines and matrix components, thereby facilitating the formation of an appropriate microenvironmental niche. Affinity coelectrophoresis experiments demonstrated that supportive 35S-labeled HS bound to IL-3, MIP-1α, PF4, TSP, and bFGF. In contrast, nonsupportive35S-labeled HS bound only PF4 and bFGF but not IL-3, MIP-1α, or TSP (Fig 4A). Neither HS bound to FN.
(A) Binding of 35S-labeled HS to cytokines and matrix components. Binding was examined by affinity coelectrophoresis, as described in Materials and Methods. Proteins cast in the lanes were 500 nmol/L ovalbumin (OVA), 25 nmol/L bFGF, 275 nmol/L FN, 100 nmol/L TSP, 500 nmol/L MIP-1, 275 nmol/L IL-3, and PF4 (at the indicated concentrations). HS (40,000 to 50,000 dpm35S per gel) from supportive and nonsupportive cells were electrophoresed into the protein-containing gels. The right-side panel in the autoradiograph for the supportive HS shows lack of binding to OVA and FN. 35S radioactivity was detected by autoradiography. The arrows indicate the extent of migration of unbound HS. The arrowheads indicate retardation of migration of HS due to binding to proteins in the gel. (B) Binding of 125I-HS to TSP. 125I-labeled HS (10,000 cpm 125I per lane) from the two cells was electrophoresed through ovalbumin (OVA; 500 nmol/L) or the indicated concentrations of TSP. The migration of a component of the supportive HS was retarded by binding to TSP in a dose-dependent manner, compared with migration through ovalbumin. No binding was seen for the nonsupportive HS.
(A) Binding of 35S-labeled HS to cytokines and matrix components. Binding was examined by affinity coelectrophoresis, as described in Materials and Methods. Proteins cast in the lanes were 500 nmol/L ovalbumin (OVA), 25 nmol/L bFGF, 275 nmol/L FN, 100 nmol/L TSP, 500 nmol/L MIP-1, 275 nmol/L IL-3, and PF4 (at the indicated concentrations). HS (40,000 to 50,000 dpm35S per gel) from supportive and nonsupportive cells were electrophoresed into the protein-containing gels. The right-side panel in the autoradiograph for the supportive HS shows lack of binding to OVA and FN. 35S radioactivity was detected by autoradiography. The arrows indicate the extent of migration of unbound HS. The arrowheads indicate retardation of migration of HS due to binding to proteins in the gel. (B) Binding of 125I-HS to TSP. 125I-labeled HS (10,000 cpm 125I per lane) from the two cells was electrophoresed through ovalbumin (OVA; 500 nmol/L) or the indicated concentrations of TSP. The migration of a component of the supportive HS was retarded by binding to TSP in a dose-dependent manner, compared with migration through ovalbumin. No binding was seen for the nonsupportive HS.
We calculated the maximal retardation of HS migration through PF4 as the retardation of the most avidly binding component of HS (which is represented by the uppermost end of the peak/smear). The retardation coefficient52 was calculated as follows: R = (M0 − M)/M0, where M0 was the mobility of free heparin (taken as mobility in presence of ovalbumin [OVA]) and M was the mobility of heparin through a protein containing lane (PF4). Mobility was measured as the distance from the loading well at the top of the gel to the upper end of the HS smear. Thus, in presence of 4 μmol/L PF4: maximal R = 0.58 for the supportive HS, and maximal R = 0.36 for the nonsupportive HS. Because retardation is a function of the binding affinity of the HS to the protein, these results indicate that a subgroup of the supportive HS binds with greater affinity to PF4 than the nonsupportive HS. In contrast, the nonsupportive HS were more homogenous in regard to PF4 binding and did not possess a comparable subset of HS that bound PF4 with high affinity.
To further confirm this binding, additional experiments were performed using the 125I-labeled HS from the two cells. As shown in Fig 4B, a component of the supportive HS, but not the nonsupportive HS, bound to TSP in a dose-dependent manner. Similarly, the125I-labeled supportive HS but not the nonsupportive HS bound the cytokine MIP-1α in a dose-dependent manner, between 10 and 250 nmol/L MIP-1α (not shown). Thus, differences in the ability of HS from the two cell lines to support LTC-IC correlate with the ability of the HS to bind cytokines and ECM components that are important for support of primitive hematopoietic progenitors.
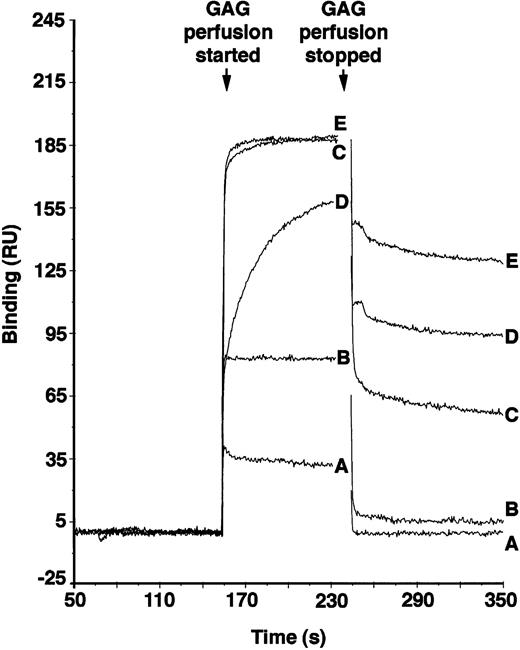
PF4 is active in maintaining LTC-IC,12 and its localization in the microenvironment is dependent on avid binding to GAGs. To examine if a high degree of O-sulfation, as is present in supportive HS but not in nonsupportive HS, may be responsible for localization of PF4, we tested the PF4 binding affinities of chemically desulfated heparins. Because the differentially sulfated heparins were not radiolabeled, binding was examined using surface plasmon resonance. As shown in Fig 5, the O-sulfated heparin and bovine kidney HS (which has a high degree of 6-O-sulfation)39 bound to PF4 with high affinity (KD 4.26 × 10−8 mol/L and 4.0 × 10−7 mol/L, respectively), N-sulfated heparin bound with significantly lower affinity (KD 1.11 × 10−6 mol/L), and completely desulfated heparin failed to demonstrate any detectable binding. The affinity of O-sulfated heparin was comparable to that of unmodified heparin (KD 4.16 × 10−8 M; see also Maccarana and Lindahl57), which contains a high degree of both O- and N-sulfation of the GAG chains. These results suggest that O-sulfation of heparin is necessary and sufficient for binding of PF4 and that N-sulfation of GlcN may not significantly affect PF4 binding under these conditions. Although binding affinity of heparin to PF4 increases with increasing size of the heparin,57differences in size are not likely to explain differences in binding of the modified heparins, because all preparations have similar average Mr.
Binding of differentially sulfated GAGs to PF4. GAGs were perfused over biotinylated PF4 immobilized on a streptavidin chip in BIAcore biosensor equipment. Binding was measured in resonance units (1 RU = 1 pg/mm2) using surface plasmon resonance. Binding of each GAG was measured in triplicate at each concentration for five different concentrations. Representative binding curves are shown for the following concentrations of GAGs: (A) desulfated heparin, 3.4 × 10−6 mol/L; (B) N-sulfated heparin, 3.4 × 10−6 mol/L; (C) O-sulfated heparin, 1.7 × 10−6 mol/L; (D) bovine kidney heparan sulfate, 8.33 × 10−7 mol/L; and (E) unmodified heparin, 8.33 × 10−7 mol/L.
Binding of differentially sulfated GAGs to PF4. GAGs were perfused over biotinylated PF4 immobilized on a streptavidin chip in BIAcore biosensor equipment. Binding was measured in resonance units (1 RU = 1 pg/mm2) using surface plasmon resonance. Binding of each GAG was measured in triplicate at each concentration for five different concentrations. Representative binding curves are shown for the following concentrations of GAGs: (A) desulfated heparin, 3.4 × 10−6 mol/L; (B) N-sulfated heparin, 3.4 × 10−6 mol/L; (C) O-sulfated heparin, 1.7 × 10−6 mol/L; (D) bovine kidney heparan sulfate, 8.33 × 10−7 mol/L; and (E) unmodified heparin, 8.33 × 10−7 mol/L.
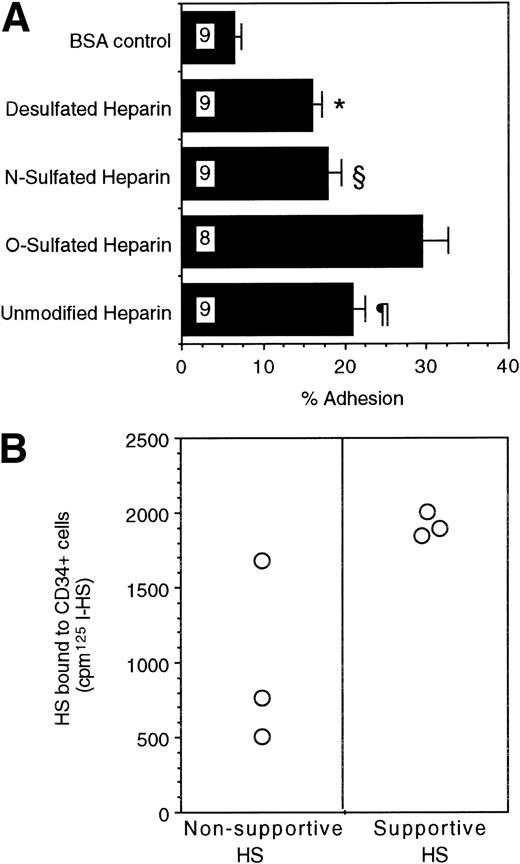
Lastly, we examined if progenitors adhere directly to these GAGs. Direct adhesion of FACS-purified human BM CD34+ cells to ovalbumin-conjugated, differentially sulfated purified heparins was evaluated in the complete absence of stromal cells or other adhesive ligands. Sixteen percent to 18% of CD34+ cells adhered to completely desulfated heparin or to N-sulfated heparin and 21% ± 2% to unmodified heparin (Fig 6A). In contrast, a significantly higher proportion (29% ± 3%; n = 8) of CD34+ cells adhered to O-sulfated heparin (P < .002 v desulfated heparin, P < .005 vN-sulfated heparin, and P < .05 vunmodified heparin; n = 9 for each type of heparin). These results indicate that immobilized O-sulfated GAGs directly mediate the adhesion of human CD34+ cells. Using 125I-labeled HS from the two cells, we also observed that the supportive HS bound consistently to CD34+ cells in suspension (Fig 6B). The binding of the nonsupportive HS was variable. These binding studies with cell line-derived HS complement the adhesion data with differentially sulfated heparins.
(A) Adhesion of CD34+ cells to differentially sulfated GAGs. 51Cr-labeled CD34+ cells (20,000 cells/well) were incubated for 4 hours at 37°C in 48-well plates previously coated with 5% BSA or with 100 μg/mL ovalbumin-conjugated GAGs. The percentage of adhesion was calculated from the proportion of input cpm present in the adherent cells. Numbers within bars indicate the number of experiments. Comparison between O-sulfated heparin and other conditions: *P< .002; §P < .005; ¶P < .05. (B) Binding of 125I-HS to CD34+ cells. The binding of 106 cpm of 125I-HS from the two cells to 0.75 × 106 CD34+ cells suspended in 0.5 mL medium was estimated, as described in Materials and Methods. Radioactivity bound to the cells was counted using a gamma counter.
(A) Adhesion of CD34+ cells to differentially sulfated GAGs. 51Cr-labeled CD34+ cells (20,000 cells/well) were incubated for 4 hours at 37°C in 48-well plates previously coated with 5% BSA or with 100 μg/mL ovalbumin-conjugated GAGs. The percentage of adhesion was calculated from the proportion of input cpm present in the adherent cells. Numbers within bars indicate the number of experiments. Comparison between O-sulfated heparin and other conditions: *P< .002; §P < .005; ¶P < .05. (B) Binding of 125I-HS to CD34+ cells. The binding of 106 cpm of 125I-HS from the two cells to 0.75 × 106 CD34+ cells suspended in 0.5 mL medium was estimated, as described in Materials and Methods. Radioactivity bound to the cells was counted using a gamma counter.
DISCUSSION
We demonstrate that the addition of the hematopoiesis-supportive cell-derived PGs but not the nonsupportive cell derived PGs increases LTC-IC maintenance significantly. Compared with nonsupportive HS, the supportive HS that are responsible for this effect are larger and are more highly sulfated, specifically on the 6-O-position of GlcN located adjacent to modified regions having GlcNSO3. HS possessing these characteristics bind both ECM components and cytokines important for growth of primitive hematopoietic progenitors and mediate the direct adhesion of CD34+ cells to HS. Therefore, HSPGs with large, highly 6-O-sulfated HS side chains may be central components of the human hematopoietic stem cell niche in which conservation, proliferation, and differentiation of primitive progenitors is regulated. This conclusion is supported by a number of observations.
Firstly, the requirement of 6-O-sulfation of GAG for LTC-IC maintenance was demonstrated directly by studies examining the ability of differentially sulfated, purified heparins to support LTC-IC. Heparin and HS share important structural attributes, including the composition of disaccharide units and a high degree of sulfation. However, compared with HS, heparin contains a significantly higher proportion of the trisulfated disaccharide [-IdoA(2-OSO3)-GlcNSO3(6-OSO3)-] sequences and consequently is more N-sulfated.33 After chemical N-desulfation and N-re-acetylation (O-sulfated heparin), these disaccharides retain 2-O-and 6-O-sulfation and are therefore highly enriched in repeating disaccharide units of [-IdoA (2-OSO3)-GlcNAc(6-OSO3)-]. After 2-O-desulfation of the O-sulfated heparin, the major sulfate residues remaining on the heparin are as 6-O-sulfate. LTC-IC maintenance in the presence of this heparin possessing only 6-O-sulfate (2-O-desulfated, O-sulfated heparin) was equivalent to that seen with unfractionated stromal SN or in the presence of the supportive cell PG.
Secondly, like the supportive HS, bovine kidney HS maintained a large proportion of LTC-IC. Bovine kidney HS contains a high proportion of 6-O-sulfated GlcN but an extremely small proportion (0.3%) of 2-O-sulfated GlcA.39
Thirdly, the capability of unmodified heparin, which has a high degree of both N- and O-sulfation, to support LTC-IC maintenance was significantly lower than heparin from which N-sulfate groups had been removed (O-sulfated heparin). The LTC-IC supportive capability of N-sulfated heparin that lacks O-sulfated groups was no better than that of completely N- and O-desulfated heparin or cytokines alone. This suggests that the presence of a high degree of N-sulfation may interfere with the capability of O-sulfated GAGs to support LTC-IC maintenance. In agreement with this observation, the nonsupportive HS that are unable to support LTC-IC had less O-sulfation than supportive HS.
Fourthly, the ability of O-sulfated GAG or the supportive HS to enhance primitive progenitor maintenance correlates with their ability to colocalize CD34+ progenitors with cytokines and ECM components with known activity in early hematopoiesis. Previous studies indicate that GAGs contribute to cell-stroma adhesive interactions22-25 by showing that target cell adhesion to multimolecular ECM or to stromal cells can be inhibited by soluble heparin or HS or by removal of HS from the stromal layer. However, it is possible that additional ECM or cell surface adhesive ligands or cytokines58-60 contribute to the observed adhesion. To demonstrate a direct role of structurally defined GAGs in progenitor adhesion, we determined adhesion of CD34+ cells to chemically desulfated heparins conjugated to ovalbumin and coated on cell culture plates in the absence of stromal cells or other ECM components. Adhesion of CD34+ cells was highest to the O-sulfated GAG and significantly less to desulfated or N-sulfated heparins. Furthermore, HS from the supportive cells directly bound to CD34+ cells in suspension. We also show that supportive HS, but not the nonsupportive HS, directly bound to a number of cytokines and ECM components important in early hematopoiesis, including IL-3, MIP-1α, and TSP. Whether specific GAG sequences are required for binding IL-3, MIP-1α, or TSP is not known. The supportive HS, which are larger and possess a high content of 6-O-GlcN, bind all three of these proteins with significantly greater avidity than the smaller, less 6-O-sulfated nonsupportive HS.
IL-3 and MIP-1α are particularly critical for regulation of hematopoiesis,61 because the ex vivo survival of primitive human hematopoietic progenitors with myeloid and lymphoid differentiation potential depends on the presence of IL-3 and MIP-1α, but not on other early-acting cytokines that do not bind GAGs.11,62,63 We have previously shown that IL-3 and MIP-1α require O-sulfated GAGs for their LTC-IC maintaining activity (LTC-IC maintenance 32% ± 2% with IL-3 + MIP-1α and 95% ± 7% with O-sulfated heparin + IL-3 + MIP-1α).64 Also, we have demonstrated that PGs and MIP-1α secreted by stromal cells are both sequestered on the microporous membranes of Transwells, on which primitive progenitors can be maintained in the absence of contact with stromal cells.17 Likewise, previous studies have shown that stromal HSPGs not only bind and present IL-3 in a biologically active form to hematopoietic cells, but may increase its mitogenic activity.4,5 65 These studies, together with our observation that purified supportive HS directly bind IL-3 and MIP-1α, support the hypothesis that these HS may mediate colocalization of progenitors with functionally active cytokines.
The supportive HS also bound the ECM macromolecule TSP in a dose-dependent manner. Specific HS are essential for the localization of TSP in the ECM in vitro66 and in vivo,67 for the interaction of TSP with cell surfaces,68 and for TSP + growth factor-mediated modulation of proliferation of diverse cells, including hematopoietic progenitors.69 Additionally, HS increases the capacity of cytokines and TSP to localize hematopoietic progenitors by adhesion.70 Therefore, the capability of the supportive HS but not the nonsupportive HS to bind TSP along with cytokines may be an important characteristic of the activity of HS on primitive hematopoietic progenitors.
The sulfation pattern described here for supportive HS is not unique, but may be present in variable proportions in HS from different sources.39 Several cell lines from human and nonhuman sources support the growth of human hematopoietic progenitors.6,8 HS from one additional cell line that actively supports human LTC-IC have sulfation patterns and relatively longer HS chains, similar to HS from the supportive cells (P.G., unpublished observations). Thus, HS with relatively long (but not unusually so) chains and common short-block motifs, but with a higher extent of 6-O-sulfation, may be part of the minimal structural requirements for supporting primitive hematopoietic progenitors. Aside from the presence of specific sulfate groups, the organization of domains within the supportive cell HS may be an additional important determinant of its biologic activity.33 71 Current studies are further examining the structural characteristics of HS from the two cells.
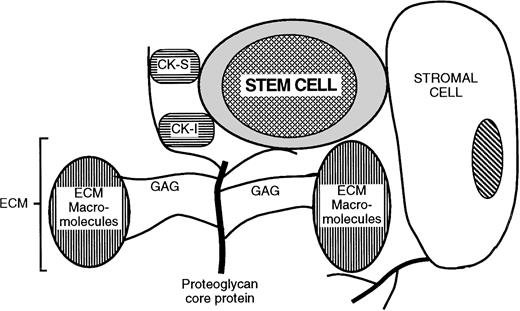
These results in the hematopoietic system lead us to propose a role for specific HSPGs in the orchestration of local microenvironmental niches. In this model (Fig 7), HSPGs are responsible for the juxtaposition of target cells (hematopoietic progenitors in the present study) with microenvironmental cells, cytokines, and ECM proteins. These HSPGs may be expressed on stromal cells (eg, glypican, syndecans) or deposited as ECM (eg, perlecan). The PGs bind, sequester and present to the target cell growth-promoting (eg, IL-3 and bFGF) and growth-inhibitory (eg, MIP-1α and PF4) cytokines produced locally or by distant accessory cells. In addition, the same PGs bind ECM components (eg, TSP). To successfully organize a target cell niche, HS may require distinct structural characteristics that allow concomitant binding of the specific cytokines and ECM components in multimolecular cytokine/matrix/PG complexes. Thus, the structural diversity of HSPGs that results in the selective colocalization of cytokines and ECM components may lead to formation of discrete niches that induce target cell quiescence and survival and other niches that promote target cell proliferation or terminal differentiation. Our observation that the supportive cell HS is required for LTC-IC maintenance but not for differentiation of CD34+ cells into CFCs and mature blood cells further supports this notion.
Model for proteoglycan-mediated juxtaposition of stem cells, cytokines, and ECM components. CK-S, growth stimulatory cytokines; CK-I, growth/cell cycle inhibitory cytokines and chemokines; ECM, extracellular matrix; GAG, glycosaminoglycan. See detailed discussion in the text.
Model for proteoglycan-mediated juxtaposition of stem cells, cytokines, and ECM components. CK-S, growth stimulatory cytokines; CK-I, growth/cell cycle inhibitory cytokines and chemokines; ECM, extracellular matrix; GAG, glycosaminoglycan. See detailed discussion in the text.
ACKNOWLEDGMENT
The authors acknowledge the excellent technical assistance of Laurel Deloria and Brad Anderson and thank Dr James D. San Antonio (Jefferson Medical College, Philadelphia, PA) for helpful discussions.
Supported by the US Department of Veterans Affairs; National Institutes of Health Grants No. R01-HL-48738, P01-CA-65493, and AR-32372; the Minnesota Medical Foundation; the University of Minnesota Bone Marrow Transplant Research Fund; American Heart Association Grant-in-Aid No. 94012990; and the University of Minnesota Hospital and Clinic. C.M.V. is a Scholar of the Leukemia Society of America.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Pankaj Gupta, MD, Hematology/Oncology Section, VA Medical Center, One Veterans Drive, Minneapolis, MN 55417, e-mail: gupta013@gold.tc.umn.edu; or Catherine M. Verfaillie, MD, Department of Medicine, Box 806 UMHC, 420 Delaware St SE, Minneapolis, MN 55455, e-mail: verfa001@maroon.tc.umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal