Abstract
Reduction of mitochondrial membrane potential (Ψm) and release of cytochrome c from mitochondria appear to be key events during apoptosis. Apoptosis was induced in IC.DP premast cells by the withdrawal of interleukin-3 (IL-3). Ψm decreased by 12 hours and cytochrome c was detected in the cytosol at 18 hours. Despite these changes in the mitochondria after 18 hours of IL-3 deprivation, clonogenicity was unaffected when IL-3 was replenished at 18 hours. Activation of v-Abl tyrosine kinase (v-Abl TK) in IC.DP cells before IL-3 depletion led to increased levels of Bcl-XL, prevented reduction of Ψm and the release of mitochondrial cytochrome c, and suppressed apoptosis. Activation of v-Abl TK 18 hours after withdrawal of IL-3 when ≤10% of the cells had died restored Ψm in the remaining cells. More than 40% of cells thus rescued by v-Abl TK between 18 and 42 hours could subsequently form colonies in the presence of IL-3. These data suggest that reduction in Ψm precedes loss of mitochondrial cytochrome c in IC.DP cells; that v-Abl TK activation, probably via upregulation of Bcl-XL, prevents loss of Ψm and blocks the release of cytochrome c from mitochondria; and that neither of these mitochondrial events is sufficient for commitment to apoptosis.
APOPTOSIS IS A GENETICALLY regulated cell death process, initially defined by distinctive morphological criteria1 and subsequently characterized by the involvement of a number of cell death-associated genes.2 Although induced by a wide range of disparate stimuli, the final apoptotic response of the cell is relatively stereotypic involving cell shrinkage, chromatin condensation, nonrandom DNA fragmentation, and selective proteolysis by a series of cysteine proteases collectively named caspases. This strongly implies that a common biochemical effector mechanism mediates the latter stages of apoptosis regardless of type of stimulus. An important question that we address here is the identity of the commitment event(s) that engages the execution machinery. Because mitochondria are believed to play an important role in the induction of apoptosis,3 we wish to examine whether changes in mitochondria exemplified by the release of cytochrome c from mitochondria and a reduction in Ψm are sufficient for commitment to death.
After exposure to a variety of stimuli that induce apoptosis, the electron transport protein cytochrome c is released from the mitochondria into the cytosol,4,5 where it binds apoptosis protease activating factor-1 (apaf-1), a newly characterized 130-kD protein that shares homology with CED-4, theCaenorhabditis elegans death gene.6,7As well as acting as an adaptor protein for cytochrome c (also termed apaf-2), it is suggested that apaf-1 may also bind to dATP and a so-far uncharacterized protein called apaf-3 (reviewed in Vaux8). This putative molecular complex is thought to trigger the cleavage of the inactive precursor of caspase 3 yielding active caspase 3, which in turn activates a cascade of other caspases and other molecules, including the DNA fragmentation factor DFF,9 10 the executionary machinery of apoptotic cell death.
Bcl-2 suppresses apoptosis and can block the release of cytochrome c from mitochondria and prevent the activation of caspase-3.5,11 The Bcl-2 homolog Bcl-XL also prevents the accumulation of cytosolic cytochrome c, possibly by binding directly to it.12 Bcl-2 and certain other Bcl-2 family members, including Bcl-XL, are tethered to the outer mitochondrial membrane, as well as to the endoplasmic reticulum (ER) and nuclear membranes.2 The efflux pathway of cytochrome c from its loose connection to the inner mitochondrial membrane through the mitochondrial intermembrane space remains undefined. A proposed common mechanism for Bcl-2 and other antiapoptotic family members is that they either block the efflux pathway of cytochrome c from mitochondria or its binding to apaf-1 or both.8 In this context, it is worthwhile noting that, under special conditions, Bcl-XL can form pores in artificial lipid bilayers13 14; however, the relevance of this to its antiapoptotic function remains to be formally demonstrated.
Another mitochondrial event that has been demonstrated to be an early event in apoptosis in a large number of cell systems is loss of electrical potential across the inner mitochondrial membrane (Ψm).3,15 The reduction in Ψmis thought to be due to the opening of a Ca 2+-activated, ADP-inhibited, and voltage-dependent megachannel in mitochondria giving rise to the mitochondrial permeability transition (PT) that facilitates mitochondrial swelling. The functional relevance of the induction of PT to apoptosis is implied by observations that apoptosis can be inhibited by cyclosporin A or bongrekic acid, compounds that inhibit PT. The relationship between loss of mitochondrial cytochrome c and loss of Ψm is currently unclear. In HL60 myeloid leukemia cells, cytochrome c is released before a reduction in Ψm,5 whereas the reverse kinetics have been observed in the liver.16 Moreover, during Fas-driven apoptosis of Jurkat T cells, cytochrome c appears to be inactivated but not lost from mitochondria.17 Furthermore, Brunet et al18 showed in a human lymphoblastic cell line (CEM C7A) that, during dexamethasone-induced apoptosis, a reduction in Ψm is downstream of commitment to apoptosis. Taken together, these data suggest that the precise order of a reduction in Ψm, the release of cytochrome c from mitochondria, and commitment to apoptosis can vary with cell type and apoptotic stimulus.
We address here the question of whether loss of Ψm and release of cytochrome c from mitochondria are reversible or irreversible events in terms of commitment to apoptosis in IC.DP pre-mast cells deprived of interleukin-3 (IL-3). In particular, we sought to determine whether cells receiving an apoptotic stimulus and that had reduced their Ψm and contained cytosolic cytochrome c could subsequently clone given appropriate survival stimuli. To approach these questions, we have exploited the IL-3–dependent murine pre-mast cell line IC.DP that contains a temperature-sensitive mutant of v-Abl tyrosine kinase (v-Abl TK).19 Activation of v-Abl TK does not promote cell proliferation, but results in the upregulation of Bcl-XL20 and suppresses apoptosis induced by IL-3 withdrawal.21
MATERIALS AND METHODS
Unless stated, all materials were from Sigma (Poole, UK). Sucrose, KCl, MgCl2, and HEPES were from Boehringer Mannheim BDH Laboratories (Mannheim, Germany). Murine cytochrome c monoclonal antibody was from Pharmingen (San Diego, CA). Cytochrome a monoclonal antibody, DiOC(6)3, nonyl acridine orange (NAO), and carbonyl cyanide m-chlorophenyl-hydrazone (mCCCP) were from Molecular Probes, Inc (Eugene, OR). Annexin V apoptosis detection kit is from R&D Systems (Oxford, UK). Murine IgG2anti-Bcl-XL antibody was from Transduction Laboratories (Lexington, KY).
Cell Culture
The hematopoietic cell line IC2.9 is an IL-3–dependent murine pre-mast cell line that has been stably transfected with a temperature-sensitive mutant of v-Abl TK to generate the IC.DP subclone.19 v-Abl TK is active at 32°C but inactive at 39°C. IC.DP and IC2.9 cells were cultured in Fischer’s medium (Life Technology, Paisley, Scotland) supplemented with 10% horse serum and 3% X60-Ag-653 cell-conditioned medium containing IL-3.22 IL-3 withdrawal-mediated induction of apoptosis was performed as described previously.21 Briefly, cells were incubated for 18 hours at 39°C to ensure that v-Abl TK was inactivated. Cells were then incubated for 2 hours at 32°C to activate v-Abl TK or were maintained at 39°C for 2 hours with inactive v-Abl TK. After extensive washing in Fischer’s medium containing only glutamine and antibiotics, cells were resuspended at 1 × 106/mL in this medium and reincubated at 32°C or 39°C. This time point, after IL-3 withdrawal, is referred to as 0 hours in the text and figures. IC2.9 cells were treated using an identical protocol and were included to control for temperature effects. None of the results presented was due to temperature, but rather due to the activation status of v-Abl TK.
Measurement of Cell Death
The mode of cell death upon depletion of IL-3 has been extensively studied in IC.DP cells and confirmed as being apoptosis using various techniques.21,23 24 In this study, we routinely examined cell viability by trypan blue exclusion. In selected studies, flow cytometric analysis of Annexin V staining simultaneously with uptake of propidium to detect increased plasma membrane permeability was used (see flow cytometry section below).
Flow Cytometry
All studies were performed using a Becton Dickinson FacsVantage with a Enterprise laser (Becton Dickinson [BD], Palo Alto, CA). Excitation was at 250 mW using the 488 nm laser line. Cells were examined at a flow rate of 200 to 300 events per second, and 10,000 events were analyzed per sample. Cellular debris and, in certain instances, cells already undergoing apoptosis (with reduced forward and increased orthogonal light scatter) were excluded from the analysis. All data were analyzed using Lysis II software (BD).
Apoptosis.
Apoptotic cells expose phosphatidyl serine at their plasma membrane and this can be detected using fluorescein isothiocyanate (FITC)-conjugated antibodies to annexin V. Apoptosis was measured using the annexin V-based R&D Systems detection kit as directed by the manufacturer. Green fluorescence of annexin V staining was collected at 530 ± 30 nm and red fluorescence due to DNA bound propidium was collected at 630 ± 22 nm.
Mitochondrial membrane potential.
The Ψm indicator DiOC(6)3 (2 μL of 2 μmol/L stock solution in dimethyl sulfoxide [DMSO]) was added by hamilton syringe to 0.4 mL IC.DP cell suspension (4 × 105 cells/mL) in fresh Fischer’s medium (pH 7.2) and incubated at room temperature for 5 minutes. A change in DiOC(6)3 fluorescence indicates a change that could represent a change in Ψm and/or a change in mitochondrial mass. Therefore, parallel experiments were performed using mitochondrial-specific dye NAO to determine mitochondrial mass. NAO (100 nmol/L, final concentration) was added to cell samples as described above. In both types of assay, propidium iodide (PI; 4 μL of 1 mg/mL stock) was added 30 seconds before analysis. DiOC(6)3 fluorescence or NAO fluorescence was collected at 530 ± 30 nm. DiOC(6)3 data were validated by addition of 20 μmol/L mCCCP after 5 minutes of DiOC(6)3 loading. Median values of green fluorescence from the subpopulation of cells that were negative for red fluorescence were determined. Comparative experiments were performed on the same day and the data were normalized against the 0-hour time point.
Clonogenic Assay
Clonogenic assays were performed to determine whether v-Abl TK activity could protect cells from apoptosis and that cells rescued in this way could subsequently proliferate if a mitogenic stimulus were subsequently applied. Cellular clonogenicity was measured as previously described.25 Briefly, cells were incubated for various periods of time in the absence of IL-3 and serum with v-Abl TK either active or inactive. Subsequently, single-cell suspensions were serially diluted to 1 or 2 cells per well in fresh Fischer’s medium supplemented with 10% horse serum and 3% IL-3 containing medium plus glutamine and antibiotics. This cell suspension was then aliquotted into a U-shaped 96-well plate (Costar, Cambridge, UK), with each well containing 100 μL of medium. Cell colonies were counted 14 days after initial plating and the percentage of cells that were clonogenic was calculated by ratioing the theoretical Poisson density for the number of negative wells observed, against the initial cell plating density, that is, % Clonogenicity = (ln × [96/NegWells])/(Plate Density) × 100, where NegWells is the number of wells that have failed to grow colonies and Plate Density is the original cell plating density per well. IL-3–replete IC.DP cells have a absolute cloning efficiency of 40%.
Cell Fractionation
Cells (1 × 107) were washed once with Fischer’s Medium and then suspended in ice-cold mitochondria isolation buffer containing 20 mmol/L HEPES-KOH, 100 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 250 mmol/L sucrose plus protease inhibitors phenylmethyl sulfonyl fluoride (PMSF; 1 mmol/L), aprotinin (10 μg/mL), leupeptin (10 μg/mL), pepstatin (10 μg/mL), and dithiothreitol (1 mmol/L). Five volumes of this buffer was added to the cell pellet and left on ice for 20 minutes. The cell suspension was then homogenized with a dounce homogenizer (15 to 25 strokes) and the homogenate was centrifuged at 750g for 5 minutes. The pellet containing any remaining intact cells and nuclei was washed once with the isolation buffer and then discarded. The supernatant was pooled and then centrifuged at 10,000g for 15 minutes. The resultant pellet containing mitochondria (designated as P10) was used for further experiments. The supernatant (termed S10) was subjected to further ultracentrifugation at 100,000g. The resultant supernatant was the cytosolic fraction (designated as S100) and the pellet contained cytoplasmic membranes (termed P100). Cytochrome oxidase assays were routinely performed (as described in Storrie and Madden26), and these assays demonstrated that P10 contained mitochondria and that mitochondria were not detectable in the S100 fraction containing cytosol. Cell fractions were also examined by Western blotting (see below) for the presence of cytochrome a and cytochrome c, which confirmed the cytochrome oxidase assay results.
Protein Analysis by Western Blotting
At specific time points after temperature switch to activate or inactivate v-Abl TK, 1 × 107 cells were harvested, washed in phosphate-buffered saline (PBS; pH 7.4), and lysed in buffer containing Tris-HCl (50 mmol/L, pH 7.4), NP-40 (1% vol/vol), sodium deoxycholate (0.25% wt/vol), NaCl (150 mmol/L), EGTA (1 mmol/L), EDTA (1 mmol/L), PMSF (1 mmol/L), aprotinin (10 μg/mL), leupeptin (10 μg/mL), pepstatin (10 μg/mL), and NaF (50 mmol/L). Protein content was determined using the standard Bio-Rad reagents (Bio-Rad, Hemel Hempstead, UK). Forty micrograms of S100 cytosolic or P10 mitochondria enriched fraction was loaded per sample and cellular proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 15% wt/vol gel before transfer onto a nitrocellulous membrane. The membranes were then probed using mouse, anti–Bcl-XL, anti-cytochrome c, or anti-cytochrome a antibody overnight at 4°C, follwed by antimouse secondary antibody for 2 hours at room temperature. Equal protein loading was confirmed by staining the filters with Ponceau S and/or reprobing for actin using mouse IgG2a anti-actin monoclonal antibody (Sigma). Immunreactive bands were detected using the ECL system (Amersham Life Science, Amersham, UK).
RESULTS
Kinetics of Apoptosis After Withdrawal of IL-3 in the Presence or Absence of v-Abl TK Activity
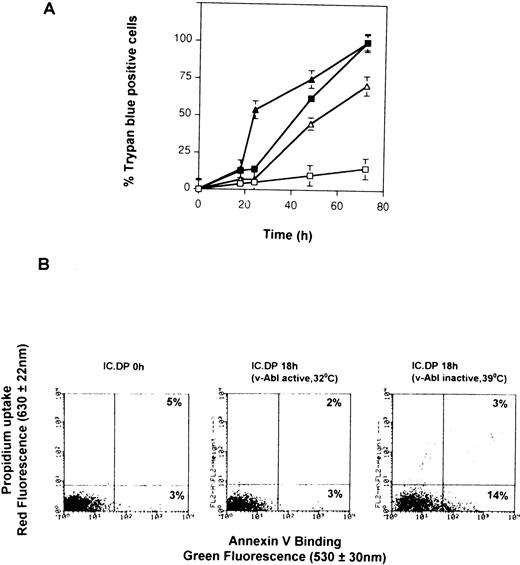
Figure 1A shows the rate of cell death in IC.DP (expressing ts v-Abl) and IC2.9 (no v-Abl) cells cultured at 32°C or 39°C, confirming the results of our previous studies.21 23 Note that there is approximately 10% cell death in each cell sample as determined by trypan blue uptake at the 18-hour time point. Figure 1B demonstrates that the mode of cell death in IL-3–depleted IC.DP and IC2.9 cells is apoptosis, identified by positive staining for annexin V, and shows that, at 18 hours, less than 10% cells take up propidium. In the absence of v-Abl TK activity, the percentage of cells staining positive for annexin V at 18 hours was always slightly greater than the percentage of cells staining positive for trypan blue or the percentage that take up propidium (Fig 1B, right-hand panel).
Induction of apoptosis by withdrawal of IL-3 from IC.DP and IC2.9 cells. (A) Kinetics of cell death of IC2.9 cells (triangles) and IC.DP cells (squares) after withdrawal of IL-3 measured by trypan blue exclusion. Cells were maintained at either 32°C (open symbols) or 39°C (solid symbols). Data points are the mean value ± SEM of three repeated experiments. (B) Flow cytometric analysis of apoptosis in IC.DP and IC2.9 cells maintained at either 32°C or 39°C for 18 hours after withdrawal of IL-3 determined by binding of annexin V and uptake of propidium iodide. Results are representative of three repeated experiments.
Induction of apoptosis by withdrawal of IL-3 from IC.DP and IC2.9 cells. (A) Kinetics of cell death of IC2.9 cells (triangles) and IC.DP cells (squares) after withdrawal of IL-3 measured by trypan blue exclusion. Cells were maintained at either 32°C (open symbols) or 39°C (solid symbols). Data points are the mean value ± SEM of three repeated experiments. (B) Flow cytometric analysis of apoptosis in IC.DP and IC2.9 cells maintained at either 32°C or 39°C for 18 hours after withdrawal of IL-3 determined by binding of annexin V and uptake of propidium iodide. Results are representative of three repeated experiments.
Withdrawal of IL-3 From IC.DP Cells Results in a Reduction in Ψm That Is Prevented and Reversed by Activated v-Abl TK
A reduction in Ψm is believed to be an early step during the induction of apoptosis in several cell types (eg, Decaudin et al27). We investigated whether this was also true in IL-3–depleted IC.DP cells. Figure 2A shows the measurement of Ψm using DiOC(6)3 and propidium iodide. Cells excluding propidium were gated (panel i) and the green DiO(6)3 fluorescence histogram was generated. A collapse of Ψm was observed after treatment with the proton ionophore mCCCP that corresponded on average to a 2.3-fold decrease in DiOC(6)3 staining (panel ii). Removal of IL-3 from IC.DP cells with v-Abl TK inactive resulted in a reduction of Ψm that was first detected at 12 hours (Fig 2B) and at 18 hours (the nadir of Ψm) corresponded to a 1.4-fold reduction in DiOC(6)3. Activation of v-Abl TK from 0 to 18 hours completely prevented this loss in Ψm (Fig 2B). These effects in IC.DP cells were not merely due to temperature changes, because IC2.9 cells exhibited a similar reduction in Ψm at 32°C or 39°C as that observed for IC.DP cells at 39°C (Fig 2B). Parallel experiments performed using the mitochondrial-selective probe NAO demonstrated that the observed changes in Ψm measured by DiOC(6)3 in IC.DP cells were not attributable to changes in mitochondrial mass (data not shown).
Activation of v-Abl TK prevents reduction in Ψm after withdrawal of IL-3. (A [i]) Viable cells were defined in region R1 by exclusion of propidium iodide. (A [ii]) Example of the measurement of Ψm in IC.DP cells (0 hours) using DiOC(6)3 (solid histogram) and collapse of Ψm with 10 μmol/L mCCCP (open histogram). (B) Kinetics of changes in Ψm in IC.DP (squares) and IC2.9 (triangles) cells after withdrawal of IL-3. Cells were maintained at either 32°C (open symbols) or 39°C (solid symbols). Data were normalized against the mean DiOC(6)3 fluorescence intensity for each sample at 0 hours. Data points are the mean ± SEM of three duplicate experiments.
Activation of v-Abl TK prevents reduction in Ψm after withdrawal of IL-3. (A [i]) Viable cells were defined in region R1 by exclusion of propidium iodide. (A [ii]) Example of the measurement of Ψm in IC.DP cells (0 hours) using DiOC(6)3 (solid histogram) and collapse of Ψm with 10 μmol/L mCCCP (open histogram). (B) Kinetics of changes in Ψm in IC.DP (squares) and IC2.9 (triangles) cells after withdrawal of IL-3. Cells were maintained at either 32°C (open symbols) or 39°C (solid symbols). Data were normalized against the mean DiOC(6)3 fluorescence intensity for each sample at 0 hours. Data points are the mean ± SEM of three duplicate experiments.
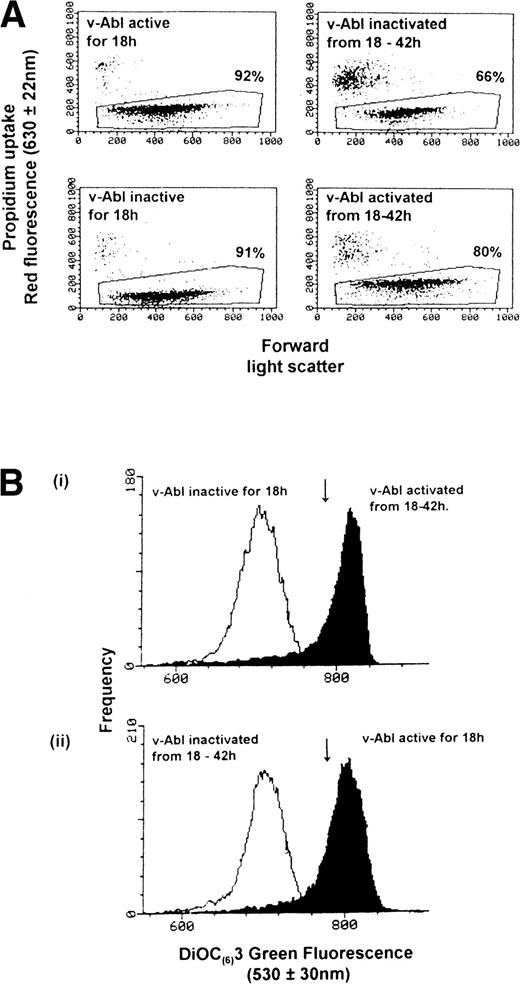
We asked whether this reduction of Ψm that occurred in the absence of IL-3 and v-Abl TK activity could be reversed if the survival stimulus provided by v-Abl TK were applied at 18 hours. IC.DP cells (106/mL) were deprived of IL-3 with v-Abl active (32°C) or inactive (39°C) for 18 hours. Cells were then either maintained at these temperatures for a further 24 hours or had their culture temperature switched from either from 32°C to 39°C (to inactivate v-Abl TK) or from 39°C to 32°C (to activate v-Abl TK) for the next 24 hours (see Fig 3, a schematic of the experimental protocol). Total cell number, Ψm, and the percentage of dead cells (uptake of propidium) were examined at 0, 18, and 42 hours for each of these conditions. Figure 4 shows typical results from such an experiment. Figure 4A shows the percentage of viable cells in each sample from which the mean values of Ψm were generated. Activation of v-Abl TK at 18 hours rescued all but 9% cells when cell viability was assessed at 42 hours (compare the bottom two dot plots of Fig 4A). Conversely, inactivation of v-Abl TK at 18 hours resulted in the further loss of 26% of cells between 18 and 42 hours (compare the top two dot plots of Fig 4A). The total cell number (including the trypan blue-positive corpses) remained constant throughout the 42-hour period congruent with the lack of proliferation that occurs in the absence of IL-3 with v-Abl active or inactive.21 The data for cell viability and Ψm from three repeat experiments are summarized in Table 1. Figure 4B shows the changes in Ψm that occurred at 18 and 42 hours after the v-Abl TK activating and inactivating temperature switches. The downward arrows indicate the cell population mean value for Ψm at 0 hours. Figure 4B (i) shows that rescue of IC.DP cells by v-Abl TK activation at 18 hours resulted in an elevation of Ψmfrom the point of rescue by temperature switch (open histogram) to that observed at 42 hours (solid histogram). Conversely, inactivation of v-Abl TK at 18 hours resulted in a lowering of Ψm (from the solid histogram to the open histogram), as expected for cells that are destined to die by apoptosis (Figure 4B [ii]). Figure 4B (i) shows that there is minimal overlap (5% cells) between the solid and open histograms, implying that 95% of the propidium negative cells in the population increase their Ψm after the applied temperature switch to activate v-Abl TK (Fig 4A). Taken together, these data show that the reduction in Ψm elicited by IL-3 withdrawal for 18 hours can be reversed by a survival stimulus such as activation of v-Abl TK.
Activation of v-Abl TK reverses the loss of Ψm. IL-3 was withdrawn from IC.DP cells, which were then maintained at 32°C or 39°C for 18 hours and then switched to the other temperature, ie, from 39°C to 32°C or from 32°C to 39°C for a further 24 hours. (A) The percentage of cells that exclude PI at each time point. The upper two dot plots show the increase in cell death after inactivation of v-Abl TK at 18 hours compared with that observed when v-Abl TK was activated at 18 hours (lower two dot plots). (B) The changes in Ψm occurring before and after activation (Bi) and inactivation (Bii) of v-Abl TK. The arrow indicates the Ψm at 0 hours. Results are representative of three independent experiments.
Activation of v-Abl TK reverses the loss of Ψm. IL-3 was withdrawn from IC.DP cells, which were then maintained at 32°C or 39°C for 18 hours and then switched to the other temperature, ie, from 39°C to 32°C or from 32°C to 39°C for a further 24 hours. (A) The percentage of cells that exclude PI at each time point. The upper two dot plots show the increase in cell death after inactivation of v-Abl TK at 18 hours compared with that observed when v-Abl TK was activated at 18 hours (lower two dot plots). (B) The changes in Ψm occurring before and after activation (Bi) and inactivation (Bii) of v-Abl TK. The arrow indicates the Ψm at 0 hours. Results are representative of three independent experiments.
Summary of Results
| Time After IL-3 Withdrawal (h) . | v-ABL TK Status . | % Dead (trypan blue positive) . | Ψm(fluo- rescence units normalized to 0 h) . | Cytosolic Cyto C . | Clonogenicity (% normalized to 0 h) . |
|---|---|---|---|---|---|
| 0 | 2 | 100 | −− | 100 | |
| 18 | Active | 5 | 148 | −− | 163 |
| 18 | Inactive | 8 | 64 | ++ | 100 |
| 18-42 | Inactivated at 18 h | 33 | 88 | ND | 10 |
| 18-42 | Activated at 18 h | 17 | 148 | + | 40 |
| 42 | Active | 10 | ND | 100 | |
| 42 | Inactive | 49 | ++ | 0 |
| Time After IL-3 Withdrawal (h) . | v-ABL TK Status . | % Dead (trypan blue positive) . | Ψm(fluo- rescence units normalized to 0 h) . | Cytosolic Cyto C . | Clonogenicity (% normalized to 0 h) . |
|---|---|---|---|---|---|
| 0 | 2 | 100 | −− | 100 | |
| 18 | Active | 5 | 148 | −− | 163 |
| 18 | Inactive | 8 | 64 | ++ | 100 |
| 18-42 | Inactivated at 18 h | 33 | 88 | ND | 10 |
| 18-42 | Activated at 18 h | 17 | 148 | + | 40 |
| 42 | Active | 10 | ND | 100 | |
| 42 | Inactive | 49 | ++ | 0 |
Results are the average of at least three independent experiments.
Abbreviation: ND, not determined.
Release of Cytochrome c From Mitochondria Elicited by IL-3 Withdrawal Is Prevented by v-Abl TK Activation
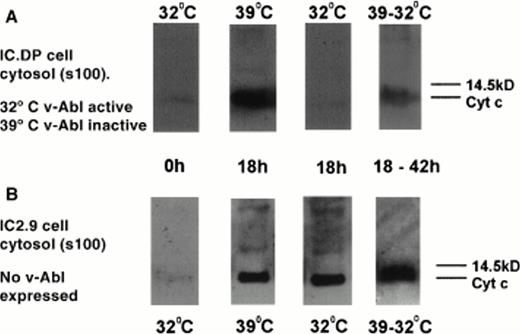
Figure 5 shows Western blots of a cytosolic extract of IC.DP (Fig 5A) and IC2.9 cells (Fig 5B) probed for cytochrome c. No cytosolic cytochrome c is detectable in IC.DP or IC2.9 cells at the start of the experiment (0 hours). At 39°C in the absence of v-Abl TK activity, the cytosol of IC.DP cells deprived of IL-3 for 18 hours contained cytochrome c. In contrast, at 32°C in the presence of v-Abl TK activity, cytosolic cytochrome c was not detectable in IC.DP cells deprived of IL-3 for 18 hours. This effect was not a merely a reflection of cell culture temperature, because cytosolic cytochrome c was detected at 18 hours after IL-3 withdrawal in the cytosol of IC2.9 cells (which do not contain v-Abl TK) at both temperatures. Maximal levels of detectable cytosolic cytochrome c were seen at 18 hours. At later time points (up to 42 hours) with v-Abl TK inactive, the percentage of cells undergoing apoptosis increased and cytosolic cytochrome c remained detectable but did not increase in level (data not shown). Cytosolic cytochrome a was undetectable in IL-3–deprived IC.DP and IC2.9 cells throughout the experimental time course (data not shown), ruling out the possibility that the fractionation procedure itself disrupted mitochondria and caused general spillage of mitochondrial components into the cytosolic fraction. When v-Abl TK was activated 18 hours after IL-3 withdrawal and the cytosolic cytochrome c level was examined 24 hours later (at 42 hours), it was detectable but reduced compared with that observed at the time of v-Abl TK activation (Fig 5).
Protein levels of cytochrome c in the cytosolic fraction (s100) of IC.DP and IC2.9 cells after IL-3 deprivation in the presence and absence of v-Abl TK activation. (A) IC.DP cells. (B) IC2.9 cells. Results shown are representative of three repeated experiments.
Protein levels of cytochrome c in the cytosolic fraction (s100) of IC.DP and IC2.9 cells after IL-3 deprivation in the presence and absence of v-Abl TK activation. (A) IC.DP cells. (B) IC2.9 cells. Results shown are representative of three repeated experiments.
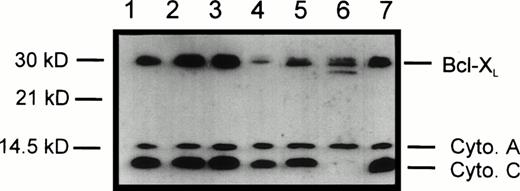
Figure 6 shows the levels of cytochrome c, cytochrome a, and Bcl-XL in the p10 intracellular membrane fraction of IL-3–deprived IC.DP cells. Using a previously reported procedure,28 P10 fractions from IC.DP were assessed for cytochrome c and cytochrome a levels at 0, 18, and 42 hours after the removal of IL-3 in the presence or absence of v-Abl TK activity and in cell populations in which v-Abl TK had been activated or inactivated at 18 hours to 42 hours. Cytochrome a remained constant in all samples. Cytochrome c remained at a constant level from 0 to 42 hours with v-Abl TK active (lanes 1, 2, and 3). In contrast, when v-Abl TK was inactive, the levels of cytochrome c in the p10 were decreased at 18 hours (lane 5) and barely detectable at 42 hours (lane 6). At 42 hours, despite a massive loss of cytochrome c from the p10 fraction, we did not detect a corresponding increase in the cytosolic s100 fraction, perhaps suggesting degradation of cytosolic cytochrome c during the apoptotic process. Inactivation of v-Abl TK at 18 hours resulted in a decrease in mitochondrial cytochrome c (lane 4) and, conversely, rescue of IL-3–deprived cells by activation of v-Abl TK between 18 and 42 hours resulted in cytochrome c levels equivalent to that seen at the start of the experiment (lane 7). We have previously shown that v-Abl TK activity results in the upregulation of Bcl-XL protein levels in whole cell lysates by 18 hours.22 Western blots of the p10 fraction were reprobed for Bcl-XL to correlate the levels of this suppressor of apoptosis with changes in the mitochondrial content of cytochrome c. As predicted, Bcl-XLlevels were elevated 18 and 42 hours after v-Abl TK activation (lanes 2 and 3) and were dramatically reduced if v-ABL TK was inactivated between 18 and 42 hours (lane 4). Conversely, when IL-3–deprived cells were not protected by v-Abl TK activity, Bcl-XL levels decreased (lanes 5 and 6), but were reestablished in cells that were rescued by v-Abl TK activation between 18 and 42 hours (lane 7). Thus, the levels of Bcl-xL and cytochrome c in the p10 fraction were positively correlated.
Protein levels of cytochrome c, cytochrome a, and Bcl-XL in the mitochondria-enriched subcellular fraction (p10) of IC.DP cells after IL-3 deprivation in the presence and absence of v-Abl TK activation. Lane 1, 0 hours; lane 2, 18 hours, v-Abl TK active; lane 3, 42 hours, v-Abl TK active; lane 4, 18 hours, v-Abl TK active, and then 24 hours, v-Abl TK inactive; lane 5, 18 hours, v-Abl TK inactive; lane 6, 42 hours, v-Abl TK inactive; lane 7, 18 hours, v-Abl TK inactive, and then 24 hours, v-Abl TK active. Data are representative of three separate experiments.
Protein levels of cytochrome c, cytochrome a, and Bcl-XL in the mitochondria-enriched subcellular fraction (p10) of IC.DP cells after IL-3 deprivation in the presence and absence of v-Abl TK activation. Lane 1, 0 hours; lane 2, 18 hours, v-Abl TK active; lane 3, 42 hours, v-Abl TK active; lane 4, 18 hours, v-Abl TK active, and then 24 hours, v-Abl TK inactive; lane 5, 18 hours, v-Abl TK inactive; lane 6, 42 hours, v-Abl TK inactive; lane 7, 18 hours, v-Abl TK inactive, and then 24 hours, v-Abl TK active. Data are representative of three separate experiments.
IC.DP Cell Populations That Have Reduced Their Ψm and Contain Cytosolic Cytochrome c Retain Clonogenicity When Provided With IL-3
Clonogenic assays were performed to determine whether cells that appeared viable at the conclusion of the reversal procedure described above (and shown in Fig 3) would survive in the long term and were capable of proliferation upon readdition of IL-3. The absolute cloning efficiency of IC.DP cells at 0 hours was 40% ± 8%, and the data described below and in Table 1 have been normalized, taking the 0-hour value as 100%. The most striking results was observed when cells were deprived of IL-3 for 18 hours with inactive v-Abl TK and then assessed for their ability to form colonies when IL-3 was readded; the clonogenicity remained 100%. Thus, long-term survival as assessed by clonogenicity was completely unaffected in a cell population in which 95% cells had reduced Ψm (Fig 4B) and that contained cells that we demonstrated to have cytosolic cytochrome c. There were no colonies formed when cells were left without IL-3 or v-Abl TK activity for 42 hours. When IL-3 was removed for 42 hours but cells were protected for the last 24 hours of this period by v-Abl TK (Fig3), at least 40% of cells were viable as judged by their ability to form colonies. In this cell population, Ψm had decreased in the first 18 hours and had then been restored by v-Abl TK action in the subsequent 24-hour rescue period. Moreover, cells rescued by v-Abl TK activity from 18 to 42 hours had a reduced amount of cytosolic cytochrome c (Fig 5). When v-Abl TK was switched off between 18 and 42 hours after IL-3 removal and the decrease in Ψm was not restored, only 10% cells subsequently formed colonies.
DISCUSSION
Many studies of the induction of apoptosis demonstrate that there are several phases of the process, namely initiation, commitment, and execution.18 If the promise of therapeutic intervention along the pathway(s) leading to apoptosis in pathological conditions is to be fulfilled, the molecular events defining these phases need to be established. Notably, those events that commit a cell to irreversibly engage the execution machinery might provide useful drug targets.
Recent interest in the regulation of apoptosis has been focussed on events occurring within mitochondria.3 In particular, the release of apoptogenic substances from mitochondria such as cytochrome c and apoptosis-inducing factor (AIF) is linked to the activation of caspases and thus may be an irreversible commitment event. We asked whether a reduction in Ψm and the release of cytochrome c were irreversible events in a well-characterized model of IL-3 withdrawal-mediated induction of apoptosis.
In contrast to other reports in different cell types,11,29IL-3 withdrawal from IC.DP cells resulted in a reduction in Ψm 6 hours before detectable cytochrome c in cytosolic fractions and the appearance of cells with apoptotic morphology (Figs 1through 5).20 21
Activation of v-Abl TK both at the point of IL-3 depletion (from 0 hours) and, more importantly, after a reduction in Ψm and appearance of cytosolic cytochrome c had occurred (after 18 hours in the absence of IL-3 and v-Abl TK activity), resulted in a cell population 24 hours later in which the vast majority of the cells had intact plasma membranes and appeared viable (Table 1). When Ψm was reexamined 24 hours after v-Abl TK activation, it was restored. Intriguingly, we noted that v-Abl TK activity for more than 12 hours resulted in an increase in Ψm above that observed at 0 hours (Fig 2B), and this is not a reflection of increased mitochondrial mass. We have not investigated this phenomenon further but speculate that it may be due to the increased availability of metabolic substrates, because v-Abl TK upregulates glucose uptake.30 When put into the clonogenic assay with IL-3, 40% of these cells that had been rescued (by v-Abl TK activation for 24 hours) formed colonies (Table 1). Notably, readdition of IL-3 at 18 hours to IL-3–deprived cells that had not been protected by v-Abl TK and that contained cytosolic cytochrome c resulted in 100% clonogenicity (Table 1). We argue that, taken together, these experiments suggest that both mitochondrial events are reversible. There are several pieces of evidence to support our argument. First, there is only a small fraction of cells dying during the v-Abl TK–mediated rescue period (typically <10%; compare bottom panels of Fig 4A), and in any case, measurements of Ψm are made after the cells already dead are excluded. Second, there has been minimal if any cell division during the course of the experiment, so we are not comparing different populations of cells. Thirdly, the histograms for Ψm shown in Fig 4B(i) show single populations before and after rescue, and there is minimal overlap in the solid and open histograms. Fourth, and shown for the first time, cells that had reduced Ψm and had released cytochrome c from their mitochondria that were subsequently prevented from undergoing apoptosis by v-Abl TK or readdition of IL-3 at 18 hours retain the capacity for proliferation in a clonogenic assay upon readdition of IL-3 (Table 1).
Enforced overexpression of Bcl-XL in U937 cells prevented the accumulation of cytochrome c in the cytosol and suppressed apoptosis induced by DNA damage.12 Our data also demonstrate that v-Abl TK, which upregulates the level of Bcl-XL and potently suppresses apoptosis induced by cytokine depletion or DNA damage (Fig 1),31 also prevents the release of cytochrome c from mitochondria. Our preliminary data suggest that the upregulation of Bcl-XL by v-Abl TK is of functional significance with respect to the suppression of apoptosis. Application of antisense Bcl-XL oligonucleotide but not sense oligonucleotide reduces the level of Bcl-XL with v-Abl TK active and restored an apoptotic response to the withdrawal of IL-3 (data not shown). A putative protein protein interaction pertinent to events occurring in IL-3–deprived IC.DP cells is that between Bcl-XL and cytochrome c. This protein partnership was demonstrated by immunoprecipitation of whole U937 cell lysates.12 The mechanism by which Bcl-xL prevents release of cytochrome c remains unresolved. Cytochrome c is located in the intermitochondrial membrane space loosely associated with the inner mitochondrial membrane, whereas Bcl-XL is thought to be tethered to the outer mitochondrial membrane, with the majority of the molecule facing outwards into the cytoplasm.3 Studies of apoptosis in Jurkat cells suggest that physical disruption of the outer mitochondrial membrane early in apoptosis provides an efflux pathway for cytochrome C.28 However, in contrast to these studies, the proapoptotic protein Bax can release cytochrome c from the mitochondria without inducing a PT or physical disruption to the outer mitochondrial membrane.32 Another potential efflux pathway might be the PT pore complex, which forms in the outer mitochondrial membrane early in apoptosis. However, reconstitution experiments of the PT in vesicles containing cytochrome c suggest that PT pore opening does not permit passage of cytochrome c.33
The data presented here suggest that the release of cytochrome c from mitochondria by whatever mechanism, observed after IL-3 depletion from IC.DP cells at least, is a recoverable position. This invokes a model whereby an important level of regulation of apoptosis occurs within the cytosol, preventing immediate irreversible engagement of apoptosis as soon as cytochrome c leaves the mitochondria. Other studies also suggest that release of cytochrome c does not inevitably lead to caspase activation and cell death, at least in short-term assays.34,35 Bcl-2 can prevent Bax-induced release of cytochrome c but can also prevent activation of caspases in cells in cells containing cytosolic cytochrome c.34 Overexpressed Bcl-2 delayed cell death induced by microinjection of cytochrome c (20 μmol/L).35 However, neither study examined the survival of cell in the long term with analysis of clonogenicity. Given our data that does show that cells with cytosolic cytochrome c can clone, either the death promoting cytosolic partner(s) for cytochrome c are not immediately available or active or cytochrome c has to be modified to fulfil a lethal function. The recent identification of apaf-1,6 which in the presence of dATP is a death-promoting partner for cytochrome c coupling its release into the cytosol to the activation of caspases, invites the speculation that the availability of the apaf-1 binding site for cytosolic cytochrome c might be the regulationary step leading to an elusive commitment point for the engagement of apoptosis.
We acknowledge that these findings are based on a single cell line and it should be noted that IL-3–dependent cell lines tend to use anerobic glycolysis for ATP generation.36 This has not been assessed in our experiments but could explain, at least in part, why IL-3–deprived IC.DP cells can tolerate the presence of cytosolic cytochrome c for a period of time and still be rescued by v-Abl TK or readdition of IL-3. Moreover, we do not yet know whether IC.DP cells harbor a defect in the cell death pathway downstream of cytochrome c release. If they do, such a defect might serve to delay the onset of apoptosis after IL-3 withdrawal (or exposure to chemotherapy) after cytochrome c release from mitochondria, but cannot prevent its occurrence. Studies are underway to examine the expression of Apaf-1 and cellular inhibitor of apoptosis (c-IAP)37 and the activation of caspase 3 in IC.DP cells and to determine whether these molecules are regulated by v-Abl TK and/or IL-3. Despite these caveats, our conclusions drawn from studies of IC.DP premast cells are that release of cytochrome c per se does not commit a cell to death. These conclusions are consistent with those obtained for fibroblasts and melanoma cells in which overexpressed Bcl-2 can attenuate Bax killing downstream of cytochrome c release.34
Most recently, it was reported that constitutive Bcr-Abl TK activity prevented the accumulation of cytochrome c in the S100 cytosolic fraction of HL60 and K562 hematopoietic cells treated with cytotoxic drugs or sphingoid bases.38 Our data confirm that a related oncogenic Abl tyrosine kinase (v-Abl TK) can also prevent release of cytochrome c in another cellular context. In addition, we provide data to suggest that v-Abl TK or IL-3 can act downstream of a reduction in Ψm and cytochrome c release from mitochondria to promote long-term cell survival.
ACKNOWLEDGMENT
The authors thank Sukbinder Heer for excellent technical support of all the flow cytometry experiments. We thank John A. Hickman for his constructive critique of this manuscript.
Supported by a Medical Research Grant to C.D. and A.J.M.W. C.D. is a Lister Institute Research Fellow. N.T. is an Honorary Visiting Research Fellow from Kansai Medical University (Osaka, Japan).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Caroline Dive, PhD, School of Biological Sciences, Stopford Building G38, Victoria University of Manchester, Oxford Road, Manchester M13 9PT, UK; e-mail: cdive@man.ac.uk.

![Fig. 2. Activation of v-Abl TK prevents reduction in Ψm after withdrawal of IL-3. (A [i]) Viable cells were defined in region R1 by exclusion of propidium iodide. (A [ii]) Example of the measurement of Ψm in IC.DP cells (0 hours) using DiOC(6)3 (solid histogram) and collapse of Ψm with 10 μmol/L mCCCP (open histogram). (B) Kinetics of changes in Ψm in IC.DP (squares) and IC2.9 (triangles) cells after withdrawal of IL-3. Cells were maintained at either 32°C (open symbols) or 39°C (solid symbols). Data were normalized against the mean DiOC(6)3 fluorescence intensity for each sample at 0 hours. Data points are the mean ± SEM of three duplicate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/12/10.1182_blood.v92.12.4545/5/m_blod42441002x.jpeg?Expires=1769094402&Signature=Q3eZ-JLkhS5ssLdljctElXN1qj74H54I6lEZQynBp3KOIf82ofO9N7SOku7oW-0GOGEYCWPYQStH77BXBHgAD67igwGgXQRIG8CQJk5i~DOu3XdpVeUPtR4twQq0qrXWJcPKz-PIwxO2NuIBePtNRfM0wMwDi7x~iGdcuUCG2lBpTwE9EGPR6KY~PFidTwfUJA0ByDA8dZ6UfhjFdvCSnN4wlU3F5pCeEFw5xMuHHHjPoMn9mL0QBEYtCa8NpB8YsqzpEd1MZsKUBA4ft8v8C-HwrohlUg1~~w0gtnVs0MOekRg58MKeStcYV-UGljBOGAKqA2ESj9G2QddMPJsLRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal