Abstract
Hemolytic anemia is a major feature of paroxysmal nocturnal hemoglobinuria (PNH). Intravascular red blood cell (RBC) destruction is caused by increased sensitivity of the abnormal erythrocyte to complement-mediated lysis, due to the GPI absence of a membrane-bound glycosylphosphatidylinositol (GPI)-linked protein, which functions as an inhibitor of reactive lysis (CD59). Both in vivo and in vitro models have suggested the feasibility of cell-to-cell transfer of GPI proteins, and patients with hemolysis could potentially benefit from transfer of CD59 to their deficient erythrocytes. We studied the ability of RBC components prepared from outdated packed RBC collections, as well as high-density lipoprotein (HDL) preparations, rich in CD55 and CD59, to promote protein transfer, as assessed by flow cytometry, immunoblotting, and susceptibility to complement-mediated lysis. By flow cytometry, CD55 and CD59 were present on RBC-derived microvesicles that stained with an antiglycophorin antibody Ab; in addition, soluble CD59 and CD55 were detected by immunoblot in soluble fractions eluated from RBC units stored for more than 35 days, but not in fresh blood. Both commercial HDL preparations and those prepared in our laboratory contained CD55 and CD59, as assayed by immunoblot. When RBC that were deficient (GPI)-anchored protein, obtained from five patients, with PNH were incubated with HDL preparations for 2 to 4 hours, there was significant transfer of both proteins to the cell surface, as demonstrated by flow cytometry. Washed RBC microvesicles, prepared by ultrasonification, also mediated transfer of GPI-linked proteins to deficient RBC. Pretreatment of microvesicles, RBC eluate preparations, and HDL with phosphatidylinositol-specific, phospholipase C, abrogated protein transfer to deficient cells, indicating that increased cell-associated CD55 and CD59 levels were related to insertion of the intact GPI moiety, rather than to simple adhesion. PNH RBC that were exposed to HDL, RBC eluate preparations, or microvesicles demonstrated decreased in vitro complement-mediated hemolysis in the Ham test. Transfer of GPI-linked proteins from soluble preparations containing CD55 and CD59 to PNH erythrocytes is feasible and may have clinical utility.
PAROXYSMAL NOCTURNAL hemoglobinuria (PNH) is an acquired, clonal somatic stem cell disorder associated with hemolysis, thrombosis, and bone marrow failure.1 PNH is caused by somatic cell mutations in a gene called PIG-A that produces global deficiency of a class of proteins linked to the cell surface by a glycosylphosphatidylinositol (GPI) anchor.2,3 A large and diverse group of GPI-anchored proteins have been described, some of which have been clearly related to the pathophysiology of PNH.4 Absence of CD59, an important inactivator of complement present on the erythrocyte cell surface, results in the exaggerated sensitivity of erythrocytes to complement-mediated lysis (as measured in the Ham or sucrose hemolysis tests), intravascular hemolysis, and often severe anemia.5 6
Cell-to-cell transfer of GPI-linked proteins has been demonstrated in a mouse transgenic system,7 in “knock-out” embryonal bodies,8 and from trypanosomal membranes to red blood cells (RBC) of infected patients.9 High-density lipoproteins (HDL) act as carriers of CD59 and are capable of transferring this protein to erythrocytes.10 Data support the concept that GPI-linked proteins also can be transferred to RBC in their intact form (with retention of their ability to inactivate complement) by prostosomes obtained from seminal fluid11 and from RBC to endothelium in the transgenic mouse.7 Membrane-to-membrane transfer by prostosomes can occur without actual membrane fusion.11 In a different system, transfer of the GPI-linked protein, CD59, occurred after incubation of a nonmembrane-associated, protein-rich fraction with deficient cells.11 Retention of an intact phospholipid tail appears to be necessary for the retention of protein function, as soluble CD59, lacking its GPI anchor has only 1/200th the ability of GPI-linked CD59 to inactivate complement.12
In the current study, we examined the effect of transfer of two GPI-linked proteins, CD55 and CD59, on protection of deficient RBC from hemolysis, using readily available sources of GPI-linked proteins, HDL, and outdated RBC concentrates. Should function of transferred protein be maintained, either could provide potential therapeutic modalities for patients with PNH.
MATERIALS AND METHODS
Patient selection.
Informed consent was obtained according to a protocol approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute. Samples of venous blood were obtained and analyzed from 20 healthy volunteers, 10 patients with PNH, and 7 patients with aplastic anemia, not associated with PNH. PNH patients were identified based on the results of the Ham test and clinical findings of hemolysis. The diagnosis of aplastic anemia was established by bone marrow biopsy and pancytopenia in accordance with the criteria of the International Study of Aplastic Anemia and Agranulocytosis.
Flow cytometric analysis of RBC and RBC microvesicles.
RBC were incubated in the dark with monoclonal antibodies (MoAb) directed to CD55 and CD59 (Monosan, Caltag, San Francisco, CA) and then with fluorescein isothiocyanate (FITC)-conjugated or phycoerythrin (PE)-conjugated goat (Fab) antimouse IgG. For phenotypic identification, RBC were counterstained FITC-conjugated antiglycophorin MoAb (Becton Dickinson, Mountain View, CA). Phycoerythrin- or FITC-conjugated mouse isotype-matched MoAb served as controls (Becton Dickinson). Samples were analyzed by forward and wide-angled light scatter using a flow cytometer (FACScan; Becton Dickinson, San Jose, CA). Based on the light scatter properties of platelet and RBC, a region was drawn to include mostly erythrocytes and 1 × 104 events were collected. Binding of FITC-conjugated MoAb to RBC or RBC-derived microparticles was expressed as mean channel fluorescence (MCF) intensity or as a percentage of the particles binding MoAb. RBC-derived microvesicles reacting with antiglycophorin antibody were examined and distinguished from intact erythrocytes on the basis of size (light scatter) and from cellular debris. Microvesicles were washed with phosphate-buffered saline (PBS) and incubated with MoAb as described above.
Preparation of RBC eluate and microvesicles enriched for CD55 and CD59.
For preparation of RBC eluates, AB-negative units of packed RBC stored at 4°C for 35 days under standard transfusion practice conditions were washed once with 50 mL of PBS. The wash solution was filtered using a 100-kD filter to remove microvesicles and membrane fragments. The filtrate was concentrated using a 10-kD filter and complement inactivated by heating to 50°C for 30 minutes. Microvesicles were obtained from stored RBC by sonication for 10 seconds at 4°C and then separated from intact erythrocytes by differential centrifugation, followed by five cycles of washing in PBS.
Transfer of GPI-anchored proteins to deficient RBC.
Complement-inactivated RBC eluates prepared as detailed above, microvesicles, or HDL was added to GPI-anchored protein-deficient RBC from PNH patients or to erythrocytes from Rhesus monkeys (which do not cross-react with antihuman CD55 or CD59 MoAb). Samples were incubated with individual preparations for 2 hours, washed with PBS, and prepared for flow cytometry as described above.
Ham test.
A standard Ham test was performed, as previously described, using acidified AB negative sera as a source of activated complement.13 The increase in soluble hemoglobin after the reaction with activated complement was expressed as a percentage of the total hemoglobin content of the cell preparation (λ − 540 nm).
Western blot analysis.
RBC eluates, microvesicles, and HDL preparations were used for immunoblotting. Before immunoblotting, HDL preparations were filtered through a 100-kD filter. Protein preparations were electrophoresed in 14% of sodium dodecyl sulfate (SDS)-polyacrylamide gels, which were then equilibrated in transfer buffer (125 mmol/L TRIS [2-amino-2(hydroxymethyl-1,3 propanediol)]-base, 960 mmol/L glycine, 20% methanol), and separated proteins were electrophoretically transferred on polyvinylidene difluoride (PVDF) membranes (Immobilon-P Millipore, Bedford, MA). Membranes were blocked in TBST-milk (10 mmol/L TRIS-HCI [pH 8.0], 150 mmol/L NaCl, 0.5% Tween-20, 1% nonfat dry milk, 1% bovine serum albumin [Cohn fraction V; Miles, Kankakee, IL]) and treated with either CD55 or CD59 MoAb. Samples were incubated with goat anti-IgG after washing and then subsequently with goat antialkaline phosphatase MoAb (Dako, Carpinteria, CA). Specific bands were detected using nitroblue tetrazolium (NBT)/5-bromo-4 chloro-3-indolyl phosphate (BCIP) substrate (Pierce, Rockford, IL).
RESULTS
Production of cell-free CD55 and CD59.
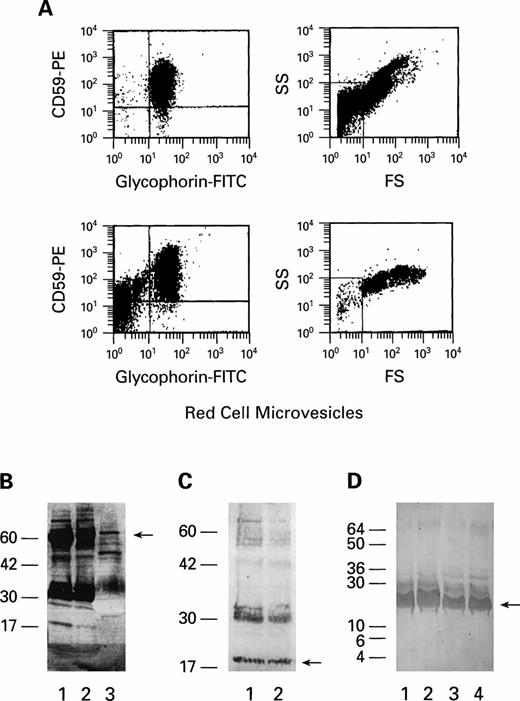
We first attempted to determine if storage of RBC under blood banking conditions resulted in the loss of GPI-linked proteins by shedding of either soluble CD55 and CD59 into solution or by formation of microvesicles containing CD55 and CD59. After the addition of PBS to stored RBC, intact RBC were removed by centrifugation at 1,000gand supernatant was analyzed for microvesicles. Microvesicles were gated by the flow cytometer to include particles that stained with antiglycophorin MoAb and were smaller than RBC; most such particles in the wash solution also stained with both CD55 and CD59 antibodies (Fig 1A) (n = 6). Few if any of these microvesicles were detected in fresh RBC preparations (data not shown). When the RBC eluate was filtered using a 100-kD filter to remove microvesicles and membrane fragments and further subjected to a 3-kD filter, soluble CD59 and CD55 antigens at 19 kD and 60 kD, respectively, were present on immunoblot, after staining with CD59 and CD55 MoAb (Fig 1B and C) (n = 10). Western blot of HDL concentrates (prepared in our laboratory or obtained commercially [Athens Research & Technology, Athens, GA; Biomedical Technologies, Stoughton, MA; or Fluka Chemical Corp, Ronkonkoma, NY]) also demonstrated bands of molecular weights corresponding to those of human CD55 (Fig 1B) and CD59 glycoproteins (Fig 1, D) (n = 10).
(A) Flow cytometric analysis of RBC microvesicles shed from stored RBC. Two units of packed RBC units, stored for 35 days at 4°C, were washed with PBS. RBC microvesicles were gated by forward scatter/side scatter to include microparticles, smaller than RBCs, staining with antiglycophorin; scattergrams with gates can be seen on the right. Gated microvesicles of the two units (above and below) stained with CD59 and antiglycophorin are demonstrated on the left. (B and C) Immunoblot of RBC eluate, microvesicles, and HDL. Samples of RBC eluates (1), microvesicles (2), and HDL (3) were solubilized with 3% SDS and analyzed by immunoblot (on 12% polyacrylamide gel) with CD55 (B), CD59 (C) MoAbs. Bands at 19 (B) and 60 kD (C) correspond to CD59 and CD55, respectively. (D) Immunoblots of four commercial HDL preparations (labeled 1 through 4) stained with CD59 MoAb (on 14% polyacylamide gel). A band at 19 kD corresponds to CD59.
(A) Flow cytometric analysis of RBC microvesicles shed from stored RBC. Two units of packed RBC units, stored for 35 days at 4°C, were washed with PBS. RBC microvesicles were gated by forward scatter/side scatter to include microparticles, smaller than RBCs, staining with antiglycophorin; scattergrams with gates can be seen on the right. Gated microvesicles of the two units (above and below) stained with CD59 and antiglycophorin are demonstrated on the left. (B and C) Immunoblot of RBC eluate, microvesicles, and HDL. Samples of RBC eluates (1), microvesicles (2), and HDL (3) were solubilized with 3% SDS and analyzed by immunoblot (on 12% polyacrylamide gel) with CD55 (B), CD59 (C) MoAbs. Bands at 19 (B) and 60 kD (C) correspond to CD59 and CD55, respectively. (D) Immunoblots of four commercial HDL preparations (labeled 1 through 4) stained with CD59 MoAb (on 14% polyacylamide gel). A band at 19 kD corresponds to CD59.
Transfer of CD55 and CD59 to deficient RBC.
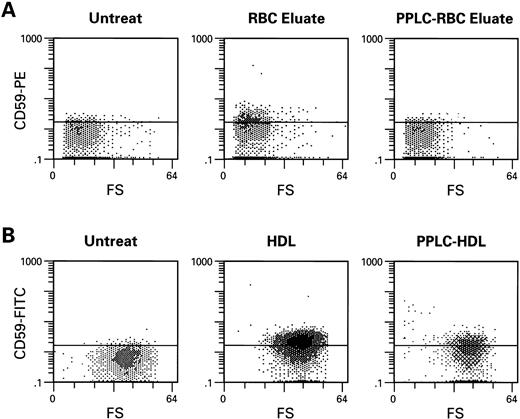
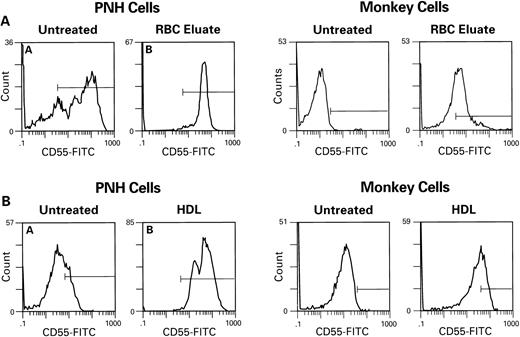
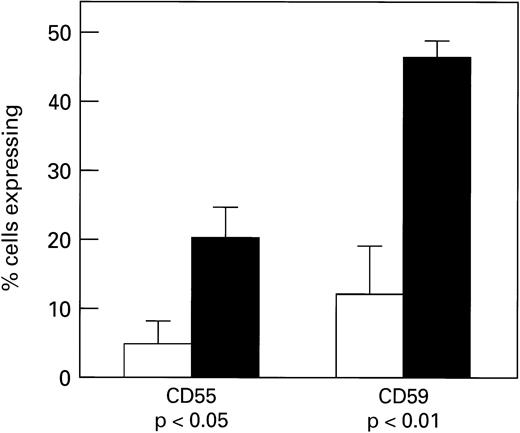
We next determined if GPI-anchored proteins could be transferred to GPI-protein-deficient RBC, using RBC eluates, microvesicles, or HDL. When CD55 and CD59-deficient erythrocytes of patients with PNH (n = 5) or simian RBC (n = 15) (which lack proteins cross-reactive with human CD55 and CD59 antigens) were incubated with complement-inactivated RBC eluates (n = 7), microvesicles (n = 6), or HDL (n = 11), both types of target cells showed increased cell surface membrane content of CD59 (Fig 2) and CD55 (Fig 3); example of microvesicle transfer is seen in Fig 4. Composite rhesus data on HDL transfer is seen in Fig 5. When deficient PNH patient samples were treated with HDL (n = 5), there was an average increase of 20% in the number of cells expressing CD59 and 10% in CD55 (five patients, 13 HDL preparations). Transfer of CD55 and CD59 after treatment with RBC eluates varied greatly depending on the RBC eluate preparation used; efficiency of transfer appeared to be related to the concentration of CD55 and CD59 in the preparation as determined by immunoblot; this dose dependence is demonstrated in Fig 6; an intact phospholipid anchor appeared to be necessary for transfer, as pretreatment of HDL, RBC eluate, and microvesicles with phosphotidylinositol-specific–phospholipase C (PI-PLC) eliminated transfer (Fig 2).
Transfer of CD59 from RBC eluates (A) and HDL (B) to monkey RBC lacking cross reactive CD59 antigen. Monkey RBC was washed in PBS, incubated with RBC eluate or HDL for 1 hour at 37°C, then subsequently washed and stained with CD59-FITC. PI-PLC (1 U/mL) for 1 hour at 37°C treatment of samples of RBC eluate and HDL significantly decreased transfer.
Transfer of CD59 from RBC eluates (A) and HDL (B) to monkey RBC lacking cross reactive CD59 antigen. Monkey RBC was washed in PBS, incubated with RBC eluate or HDL for 1 hour at 37°C, then subsequently washed and stained with CD59-FITC. PI-PLC (1 U/mL) for 1 hour at 37°C treatment of samples of RBC eluate and HDL significantly decreased transfer.
Transfer of CD55 to PNH cells and to monkey cells by RBC eluate (A) and HDL (B) after 1 hour of incubation. Procedures are as described above in Fig 2.
Transfer of CD55 to PNH cells and to monkey cells by RBC eluate (A) and HDL (B) after 1 hour of incubation. Procedures are as described above in Fig 2.
Deficient monkey RBCs were incubated with RBC microvesicles for 2 hours. Treated cells were then washed with PBS and stained with CD55-PE and CD59-FITC.
Deficient monkey RBCs were incubated with RBC microvesicles for 2 hours. Treated cells were then washed with PBS and stained with CD55-PE and CD59-FITC.
Transfer of CD55 and CD59 to monkey erythrocyes by HDL preparations (n = 11). After a 2-hour incubation at 37°C, cells were stained using CD55-FITC or CD59-FITC and analyzed by flow cytometry. Untreated samples are indicated by (□), treated as (▪).
Transfer of CD55 and CD59 to monkey erythrocyes by HDL preparations (n = 11). After a 2-hour incubation at 37°C, cells were stained using CD55-FITC or CD59-FITC and analyzed by flow cytometry. Untreated samples are indicated by (□), treated as (▪).
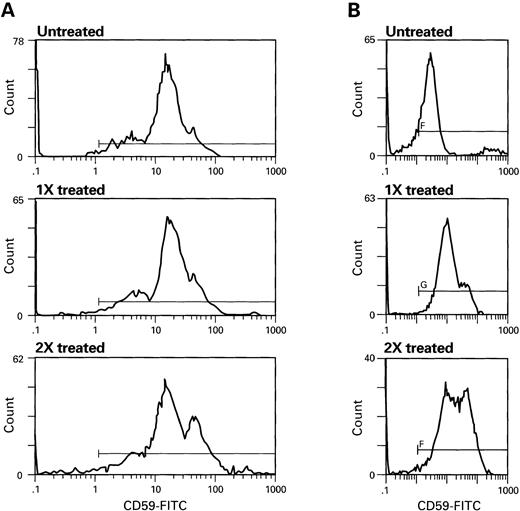
Dose-dependent transfer of CD59 from RBC eluate (A) and HDL (B). Increasing concentrations, (denoted as 1X, 2X) of RBC eluate or HDL were incubated with PNH cells. RBC were washed and stained with CD59-FITC. Scattergrams of RBCs treated with RBC eluate (A) HDL (B) are seen above.
Dose-dependent transfer of CD59 from RBC eluate (A) and HDL (B). Increasing concentrations, (denoted as 1X, 2X) of RBC eluate or HDL were incubated with PNH cells. RBC were washed and stained with CD59-FITC. Scattergrams of RBCs treated with RBC eluate (A) HDL (B) are seen above.
Restoration of functional properties of reconstituted cells.
To examine the functional consequences of transfer of GPI-linked proteins, we tested the effect of CD59 transfer on complement-mediated lysis of erythrocytes derived from PNH patients, as measured in the Ham test. Treatment of PNH RBC with eluate or HDL conferred relative resistance to complement-mediated lysis, as evidenced by a significant decrease in hemolysis in the Ham test (example seen in Fig 7).
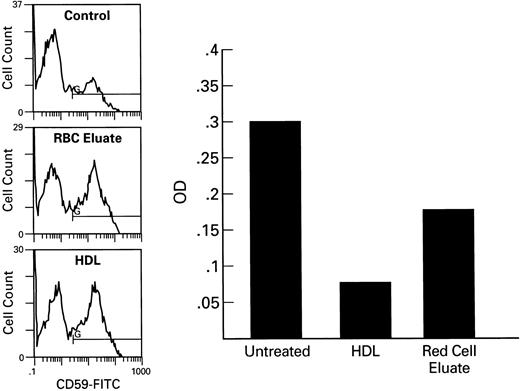
Ham test of RBC treated RBC. Samples of PNH RBC were incubated for 1 hour with RBC eluate, HDL, or microvesicles at 37°C, washed with PBS, and incubated with acidified serum as a source of complement. Optical density measurements at 540 nm are shown on the right. Histograms of corresponding samples stained with CD59-FITC are seen at the left. CD55 expression was greater on the RBC incubated with HDL than on those incubated with RBC eluate (data not shown). Untreated samples showed 30% hemolysis, HDL treated 7%, RBC eluate treated 15%.
Ham test of RBC treated RBC. Samples of PNH RBC were incubated for 1 hour with RBC eluate, HDL, or microvesicles at 37°C, washed with PBS, and incubated with acidified serum as a source of complement. Optical density measurements at 540 nm are shown on the right. Histograms of corresponding samples stained with CD59-FITC are seen at the left. CD55 expression was greater on the RBC incubated with HDL than on those incubated with RBC eluate (data not shown). Untreated samples showed 30% hemolysis, HDL treated 7%, RBC eluate treated 15%.
DISCUSSION
Cell-to-cell transfer of GPI-linked protein transfer has been reported in a variety of systems. In the first report, the variable surface glycoprotein (VSG),14 which is attached to trypanosomes through a GPI-anchor, was transferred to the human erythrocyte by coincubation. Human CD59, expressed by transgenic mouse erythrocytes, could also be transferred to the intact mouse vascular endothelial cells.7 In our laboratory, we have shown transfer of CD24 from normal embryonic stem cells (ESC) to a PIG-A−(GPI−AP−) knock-out ESC.8 Embryoid bodies composed only of PIG-A− cells did not form hematopoietic colonies, but chimeric bodies containing GPI−AP+ ECS exhibited PIG-A− knock-out–derived hematopoiesis.
In the current study, we demonstrate that RBC eluates and microvesicles prepared from outdated RBC concentrates, as well as HDL, contain CD55 and CD59 and can transfer both GPI-linked proteins to deficient RBC. Transferred CD59 appears to be functional, as it is capable of inhibiting hemolysis in susceptible cells. Successful transfer of both CD55 and CD59 was dependent on the presence of an intact GPI-anchor, as cleavage of that anchor by PI-specific phospholipase C decreased the efficiency of transfer as determined by flow cytometry. Although investigators have previously shown that HDL acts as a carrier of GPI-linked CD5910 and is capable of transferring the protein from RBC to RBC and from RBC to endothelium, they never demonstrated retention of function of protein after transfer. HDL has also not previously been reported to act as a carrier for CD55.
The Ham test, broadly, correlated with the flow cytometry data; the Ham test showed differences in RBC eluate and HDL hemolysis, despite similar CD59 expression (but greater CD55 expression) measured by flow cytometry. This discordance may be explained by the fact that only major differences in CD59 and CD55 expression are detected by flow cytometry. Small differences in transfer may be reflected in large differences in sensitivity to hemolysis. It may also be possible that additional proteins (including CD55), capable of conferring resistance to acidified complement in vitro, are present (and transferred) in the HDL preparations but not in eluates. The amount of GPI-linked protein expression required to prevent hemolysis is also unclear and the pathophysiology of hemolysis may be complex. Lysis of cells during incubation with GPI-rich preparations cannot be excluded in samples of PNH cells in which transfer was performed. However, each HDL and RBC eluate preparation underwent heat treatment to inactivate complement, and RBC numbers were similar before and after incubation.
The amount of transfer was dependent on the length of incubation and the concentration of the transferring protein. Histograms showing a phenotype intermediate between normal and abnormal could be seen in samples exposed to lesser concentrations for shorter times. The figures chosen for this report show only samples exposed for optimal times and concentrations to demonstrate optimal transfer. The absence of linear transfer in some instances may be related to the complexities involved in protein and lipid transfer. GPI-linked proteins may be located within caveolae structures and delivery of the GPI-linked protein to the membrane structure may be related to multiple factors, which allow fusion of this structure to the membrane. Alternatively, initial membrane acquisition of GPI-linked proteins may affect the probability of subsequent transfer events.
It is not surprising that outdated RBC stored for transfusion contain material rich in GPI-linked proteins. Long et al15 reported loss of both CD55 and CD59 from the RBC membrane during a 6-week storage. RBC microvesicles are formed during RBC storage and a number of investigators16 have demonstrated enrichment of microvesicles in GPI-linked proteins including CD55 and CD59.
Cell-to-cell transfer or reuptake of proteins from shed membrane fragments of RBC or from membrane-free proteins, which probably exist in the form of micelles, are all potential avenues of transfer of GPI-linked proteins. Both HDL and RBC eluates could potentially provide convenient vehicles for protein transfer to deficient cells and either could provide successful therapeutic interventions for patients with PNH and incapacitating hemolysis. We have shown convincing in vitro evidence that hemolysis of PNH cells is inhibited by transfer. Previous data demonstrating that PNH cells expressing subnormal amounts of CD59 have prolonged in vivo survival compared with PNH cells expressing no protein,17 suggests that even partial transfer of protein may have real clinical consequences. Whether significant transfer occurs in vivo between RBC lacking GPI-linked proteins and those expressing them is unknown and is currently being investigated. Transfer of GPI-linked proteins in vivo is supported by the fact that flow cytometry examination of cells from PNH patients rarely shows two discrete populations of cells. Often a substantial population of cells with CD55 and CD59 expression that is intermediate between normal and negative cells can be detected. Even in the presence of a nonsense mutation, totally eliminating functional expression of PIG-A proteins, affected cell clones would be expected to exhibit GPI expression intermediate between normal and negative cells if transfer occurred. Furthermore, one group of investigators reported that although reticulocytes of a group of PNH patients were almost all GPI-deficient, most cases demonstrated a significant population of RBC exhibiting GPI-linked proteins intermediate between normal and deficient (type II cells).18
Transfusions of normal RBC to PNH patients may provide a source of GPI-enriched proteins to the deficient cells and could potentially lead to increased survival of PNH cells if significant transfer from transfused cells to PNH cells occurred. An advantage of cell-free GPI-rich preparation is that protein solutions can potentially be made infection-free by filtration and heat sterilization and do not result in iron-overload. A number of factors including albumin concentration, temperature, and salt concentration may affect transfer in vitro,18 and it is difficult to predict what conditions would pertain in vivo.
Our study was designed to determine if GPI-linked proteins could be transferred from HDL and from RBC eluates and microvesicles obtained from outdated RBC units stored for transfusion. The feasibility of using these modes of transfer may have important consequences for therapy of PNH, as well as to understanding of the pathophysiology of the disease.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
This is a US government work. There are no restrictions on its use.
REFERENCES
Author notes
Address reprint requests to Elaine M. Sloand, MD, 31 Center Dr, MSC 2490, Bldg 31, Room 4A11, Bethesda, MD 20892-2490.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal