Abstract
A repeated selection of phages from a cyclic hexapeptide phage display library resulted in an enrichment of phages that bound to the monoclonal antibody (MoAb) 82D6A3 (an anti–von Willebrand Factor [vWF] antibody that inhibits binding of vWF to collagen). Two clones were selected that bound both to MoAb 82D6A3 and to rat tail collagen type I in a specific and dose-dependent manner. The two phage clones were further used in a two-direction competition experiment with vWF. vWF was able to displace phages from collagen in a dose-dependent manner with an IC50 of 35 μg/mL and phages were able to inhibit vWF binding to collagen. With the use of specific primers, the sequence of the cysteine-flanked hexapeptide inserts could be deduced. The two phage clones carried an almost identical sequence, CVWLWEQC and CVWLWENC, with a substitution of an N for a Q at position 6 of the hexapeptide. Sequence comparison with the known vWF sequence showed the presence of a comparable sequence at position 1129-1136 (VWTLPDQC), located between the collagen-binding A3-domain and the D4-domain. The two cyclic peptides, the putative corresponding vWF peptide, and a peptide with a scrambled cyclic sequence were synthesized. The two cyclic peptides inhibited vWF binding to rat tail collagen type I in a dose-dependent manner, whereas the linear vWF peptide and the scrambled cyclic peptide were inactive. For half maximal inhibition, 100 ± 12.7 μmol/L and 34.8 ± 8.59 μmol/L (mean ± SEM, n = 3) of the N- and the Q-peptide, respectively, were needed. The two cyclic peptides were also able to inhibit vWF binding to calfskin and human collagen type I, but effective concentrations were some 5 to 10 times higher.
PLATELET ADHESION TO subendothelial structures exposed on damage of the vessel wall is one of the first steps in a sequence of reactions that can lead to arterial thrombosis. One of the more abundant thrombogenic compounds to which platelets adhere are the collagens. Platelets bind to collagen both in a direct manner via their collagen receptors α2β1,1,2 glycoprotein IV3 and VI,4 and indirectly, with von Willebrand Factor (vWF) forming the bridge between collagen and its platelet receptor, the glycoprotein (GP) Ib/IX/V-complex.5Both binding via α2β1 and vWF is necessary to sustain platelet adhesion under high shear forces.6 7
Both α2β1 and vWF bind to collagen with their I domains, in vWF known as A-domains.8-14 I-domains form independent globular modules of some 200 amino acid residues. In vWF three such domains have been identified. The A1-domain (497-716) contains the binding site for GPIb,15,16sulfatides,17 heparin,18 and collagen VI,19 which constitutes the main reactive collagen in the extracellular matrix of endothelial cells. The A2-domain (717-909) has no clear binding function, but is relatively sensitive to enzymatic degradation, whereas the A3-domain (910-1111) contains the main binding site for fibrillar collagens such as type I and III.14,20 Recombinant A3-domain also binds to collagen,20 whereas deletion of A3 results in a vWF that binds 40 times less to collagen.14
Both direct and indirect vWF-mediated binding to collagen can be inhibited by the collagen binding leech products leech antiplatelet protein21,22 and Calin.23,24Furthermore, Calin was able to prevent platelet-rich thrombus formation in a hamster model. Apart from these products, an IgM apparently interacting with both the A1- and A3-domain and inhibiting vWF interaction with collagen was identified in a patient with an acquired bleeding disorder,25 and a number of murine anti-vWF monoclonal antibodies (MoAbs) have been described that also block the binding to collagen. One of these, MoAb 82D6A3,19 inhibits vWF binding to fibrillar collagens (type I and III) but not to collagen VI and also recognizes vWF after sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blotting. With this antibody, we identified a new collagen-binding eight amino acid sequence (cyclic hexapeptide). The selection was based on an anti-idiotypic approach to select phages from a cyclic hexamer phage display library on both MoAb 82D6A3 and on rat tail collagen type I.
MATERIALS AND METHODS
Products.
Rat tail collagen type I was purchased from Collaborative Biomedical Products (Bedford, MA), human type I and III, and calfskin type I from Sigma (St Louis, MO). The collagens were solubilized in 50 mmol/L acetic acid and subsequently dialyzed against phosphate-buffered saline PBS (48 hours, 4°C) to obtain fibrillar collagen. The phage display library with the random hexapeptides flanked by cysteine residues was obtained from Corvas (Gent, Belgium). MoAb 82D6A3 (a kind gift of Dr M. Hoylaerts, Leuven, Belgium), a MoAb against vWF, was purified from ascites by protein A chromatography. Peptides were custom synthesized by Ansynth (Roosendaal, The Netherlands). vWF was purchased from the Red Cross (Belgium).
Isolation of MoAb 82D6A3-binding phages.
Selection of phages was performed essentially as described.26 Briefly a high-binding polystyrene tube (Nunc, Roskilde, Denmark) was coated overnight with purified MoAb 82D6A3 (10 μg/mL, Tris-buffered saline [TBS]). After blocking the tubes with 3% milkpowder (2 hours, TBS), 2.1012 phages (0.25% Tween 20, 2% milkpowder, TBS) from the amplified cyclic hexapeptide library were added. After 150 minutes (90 minutes rotating, 60 minutes standing) the input phages were removed and the tube was washed seven times with TBS (0.4% Tween 20, 0.25% milkpowder) to remove the nonspecific binders. The bound phages were eluted with 0.1 mol/L glycine, pH 2.2, and the eluate was immediately neutralized with 1 mol/L Tris, pH 8. After amplification of the phages, two additional rounds of panning were performed. Phages were amplified by infection ofEscherichia coli TG1 cells and partially purified from the supernatant by polyethylene glycol precipitations. Phage DNA was prepared using the QIA-prep Spin M13 Kit (Westburg, Leusden, The Netherlands) and sequencing reactions were performed according to the autoread sequencing kit (Pharmacia, Roosendaal, The Netherlands) using the primer 5′-TGAATTTTCTGTATGAGG-3′ and the Automatic Laser Fluorescence Detection Unit (A.L.F. Sequencer; Pharmacia). After the third round of panning, individual phage-bearing E colicells were grown in a 96-well plate, and the supernatant was tested for the presence of phages, binding to both MoAb 82D6A3 and collagen.
Measurement of phage binding to MoAb 82D6A3 and to rat tail collagen type I.
A 96-well plate was coated overnight with purified MoAb 82D6A3 (10 μg/mL) or with rat tail collagen type I (50 μg/mL). After 2 hours blocking with 4% milkpowder, a dilution series of the individual phage clones in TBS with 0.3% milkpowder was added to the wells and phages were incubated at room temperature for 2 hours. Bound phages were detected after a 1-hour incubation with a polyclonal anti–M13-horseradish peroxidase (HRP)—conjugated antibody (Pharmacia) and visualization was performed with ortho-phenylenediamine (OPD, Sigma). The reaction was stopped with 4 mol/L H2SO4 and absorbance was determined at 490–630 nm. In between each incubation step the plates were washed three to nine times with TBS (0.1% Tween 20).
Inhibition of vWF binding to collagen by phages or peptides.
Collagen was coated at a concentration of 30 to 50 μg/mL (overnight, 4°C). After blocking the plates with 3% milkpowder, a dilution series of phages in TBS with 0.3% milkpowder or peptides in PBS with 0.3% milkpowder was added. After a preincubation for 30 minutes, a constant amount of vWF was added to the wells and mixed with the peptide or phage solution. Unbound vWF was removed after 90 minutes and bound vWF was detected with a polyclonal anti–vWF-HRP—conjugated antibody (Dako, Glostrup, Denmark). Visualization was performed with OPD. The reaction was stopped with 4 mol/L H2SO4 and absorbance was determined at 490–630 nm. In between each incubation step the plates were washed three to nine times with TBS (0.002% Tween 80).
RESULTS
Selection of phages that bind to the MoAb 82D6A3 and to collagen.
A repeated selection of phages from the cyclic hexapeptide phage display library resulted in an enrichment of phages that bound to the MoAb 82D6A3. Because this anti-vWF MoAb inhibits the binding of vWF to collagen, then, in line with the anti-idiotypic theory, a phage that binds to MoAb 82D6A3 could be a mimic of vWF and in this case could bind to collagen. Therefore, after the third round of panning individual colonies were tested for binding both to MoAb 82D6A3 and to rat tail collagen.
Two double-positive clones were identified and amplified for further characterization. The two selected clones bound both to MoAb 82D6A3 (Fig 1A) and to rat tail collagen type I (Fig1B) in a specific and dose-dependent manner, whereas they did not bind to uncoated wells. The two clones were further used in a two-direction competition experiment with vWF. We found that vWF displaced phages from collagen in a dose-dependent manner (Fig 2A) with an IC50 of 35 μg/mL at 5 × 1011 phages/mL. Moreover, these phages were also able to inhibit vWF binding to collagen (Fig2B). However, because of limitations in the number of phages that can be used, no full inhibition curve could be constructed.
Binding of two different phage clones (▴, Q-phage; •, N-phage) to microtiterplates coated with 10 μg/mL MoAb 82D6A3 (A) or with 50 μg/mL rat tail collagen type I (B). The figures are representative of three experiments each.
Binding of two different phage clones (▴, Q-phage; •, N-phage) to microtiterplates coated with 10 μg/mL MoAb 82D6A3 (A) or with 50 μg/mL rat tail collagen type I (B). The figures are representative of three experiments each.
(A) Inhibition of the binding of 5 × 1011N-phages/mL to rat tail collagen type I by increasing concentrations of vWF. (B) Inhibition of the binding of 25 μg/mL vWF to rat tail collagen type I by increasing concentrations of the N-phage.
(A) Inhibition of the binding of 5 × 1011N-phages/mL to rat tail collagen type I by increasing concentrations of vWF. (B) Inhibition of the binding of 25 μg/mL vWF to rat tail collagen type I by increasing concentrations of the N-phage.
Sequence determination and comparison.
With the use of specific primers, the sequence of the Cys-flanked hexapeptide inserts could be deduced: the two collagen binding phage clones carried an almost identical sequence, with a substitution of an N (N-peptide) for a Q (Q-peptide) at position 6 of the hexapeptide (Fig3). Sequence comparison with the known vWF sequence showed the presence of a comparable sequence at position 1129-1136 (Fig 3). This vWF sequence also contained a cysteine, which in the phage peptide is expected to be part of a disulphide bond.
Sequence comparison of the Q- and N-peptide and the scrambled peptide with vWF. Identical and similar amino acids are given in bold and in italics, respectively. A gap is introduced in the Q- and N-peptide to optimize the alignment.
Sequence comparison of the Q- and N-peptide and the scrambled peptide with vWF. Identical and similar amino acids are given in bold and in italics, respectively. A gap is introduced in the Q- and N-peptide to optimize the alignment.
Peptide analysis.
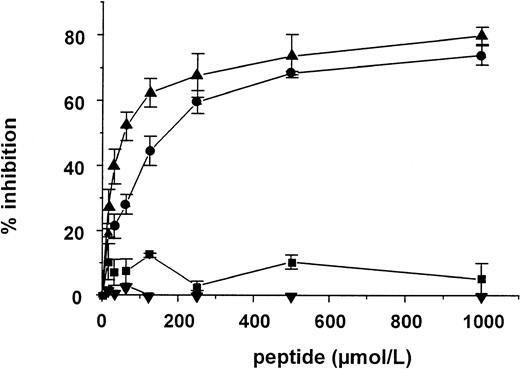
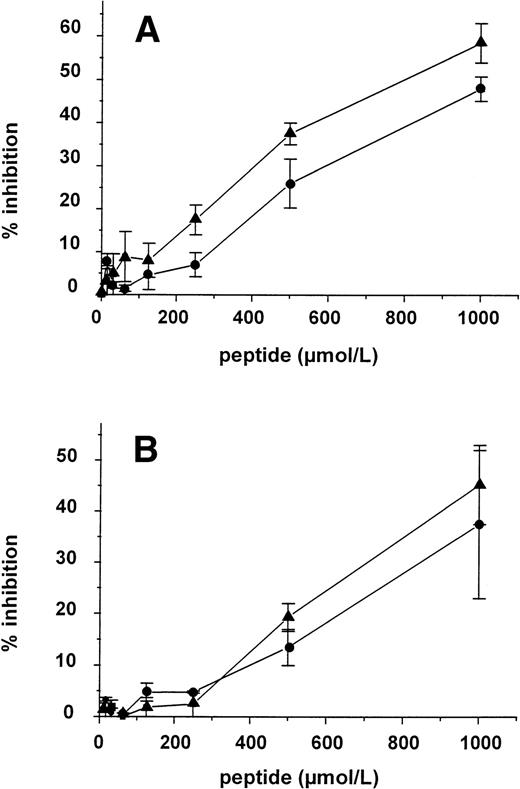
Four peptides were synthesized based on the two cyclic sequences, the putative corresponding vWF sequence, and a scrambled cyclic sequence, containing the same amino acids as the Q-peptide, but in a random sequence. Whereas the peptides were not able to compete with vWF for the binding to the MoAb, the two cyclic peptides inhibited vWF binding to rat tail collagen type I in a dose-dependent manner. The linear vWF peptide and the scrambled cyclic peptide on the other hand were inactive (Fig 4). Concentrations needed for halfmaximal inhibition of the binding of 25 μg/mL vWF to rat tail collagen type were 100 ± 12.7 μmol/L and 34.8 ± 8.59 μmol/L (mean ± SEM, n = 3) for the N- and the Q-peptide, respectively. Next, the inhibitory effect of the two cyclic peptides and the scrambled peptide was tested on different types of collagen, such as calfskin type I and human type I and type III. Both the N- and the Q-peptide were able to inhibit vWF binding to calfskin type I collagen (Fig 5A) and to human type I collagen (Fig5B) but inhibitory concentrations were 5 to 10 times higher than the ones needed for inhibition of vWF binding to rat tail collagen type I. We were not able to detect any inhibition on human type III collagen. Finally the inhibitory effect of those peptides was tested in an experiment where vWF in diluted plasma was used. Also, in this test both peptides showed an inhibitory effect with halfmaximal concentrations in the same range as in the inhibition assays described above.
Inhibition of the binding of 25 μg/mL vWF to rat tail collagen type I by increasing concentrations of peptide. (▴, Q-peptide; •, N-peptide; ▪, linear vWF peptide; and ▾, scrambled peptide) (mean ± SEM, n = 3).
Inhibition of the binding of 25 μg/mL vWF to rat tail collagen type I by increasing concentrations of peptide. (▴, Q-peptide; •, N-peptide; ▪, linear vWF peptide; and ▾, scrambled peptide) (mean ± SEM, n = 3).
Inhibition of the binding of 150 ng/mL vWF to calfskin collagen type I (A) and of 50 ng/mL vWF to human collagen type I (B) by increasing concentrations of Q- (▴) and N-peptide (•) (mean ± SEM, n = 3).
Inhibition of the binding of 150 ng/mL vWF to calfskin collagen type I (A) and of 50 ng/mL vWF to human collagen type I (B) by increasing concentrations of Q- (▴) and N-peptide (•) (mean ± SEM, n = 3).
DISCUSSION
In an attempt to identify peptides that may interfere with vWF binding to collagen, phages from a phage display library were selected on MoAb 82D6A3, an anti-vWF MoAb that prevents vWF binding to fibrillar collagen.19 A hexapeptide phage display library was used, in which the hexapeptides were flanked by two cysteines, thus potentially forming a disulphide-linked octapeptide loop, with a restricted conformational freedom.
After three rounds of panning, nearly all selected phages bound to MoAb 82D6A3 in a phage enzyme-linked immunosorbent assay. A more stringent selection criterion was next applied: if MoAb 82D6A3 recognizes a collagen-binding sequence in vWF, then some of the selected phages may mimic vWF and should bind to collagen. This “anti-idiotypic–like” approach resulted in the identification of two phage clones that bound to both the antibody and to collagen. That they indeed were binding to similar sites on collagen was further shown by competition experiments: vWF could displace the phages and vice versa.
Sequence analysis of the phage hexapeptide inserts showed two nearly identical sequences, and a similar sequence could be identified in the vWF sequence at amino acids 1129-1136. The sequence found is not part of the known collagen binding areas of vWF, but is located 18 amino acid residues C-terminal of the A3-domain (910-1111). Although it is quite possible that this linear sequence in vWF represents the actual epitope of the antibody, especially because the antibody recognizes denatured vWF in Western blots, this still is unclear because neither the synthetic cyclic peptide nor the linear corresponding vWF peptide were able to prevent vWF binding to the antibody. However, part of the reason for this could be that the affinity of the antibody for vWF is quite high: antibody concentrations at or below 1 μg/mL only are needed to observe binding as well as inhibitory effects (19 and own observations).
Furthermore, the synthetic linear vWF peptide also was not able to inhibit vWF binding to collagen. This is probably because of the fact that the peptide may not represent the active conformation found in vWF. On the other hand, synthetic cyclic peptides with sequences corresponding to either of the phage peptides inhibited vWF binding to rat tail, calfskin, and human collagens type I, but were inactive when vWF binding to human collagen type III was studied. Whereas so far it is known that different domains in vWF are involved in its binding to fibrillar collagens type I or III (A3-domain) and to the microfibrillar collagen type VI (A1-domain), the present findings also imply that differences in the binding mechanisms of vWF to collagens type I and III may exist.
That the vWF A3-domain is involved in the binding of vWF to fibrillar collagens comes from the observation that an A3-deletion mutant only minimally interacts with collagen, whereas a recombinant A3-fragment binds to collagen.14,20 However, the binding of the fragment was rather weak, which was believed to be because of a higher avidity of the multimeric vWF for collagen as compared with the monovalent A3-domain.20 With the identification of a possible additional collagen-binding sequence within vWF, we hypothesize that the A3-domain and this sequence, which is situated some 18 amino acids downstream of the A3-domain, may be both necessary for and cooperate in the binding of vWF to collagen type I.
To analyze whether the vWF sequence 1129-1136 is really important for the binding to collagens, we are constructing a mutant vWF and an extended recombinant A3-domain. At this moment preliminary evidence seems to indicate that residues 1129-1136 of vWF may indeed be involved in the binding of vWF to rat tail collagen type I.27
Whatever the further results will be with these constructs, the two cyclic peptides that we have identified nevertheless inhibit vWF binding to collagen and therefore may be useful in the development of new antiadhesive compounds.
ACKNOWLEDGMENT
We thank Dr G. Vlasuk (Corvas San Diego) and Dr M. Hoylaerts (Center for Molecular and Vascular Biology, KU Leuven) for the generous gift of the cyclic hexapeptide library and MoAb 82D6A3, respectively.
Supported by the following grants: National Fonds voor Wetenschappelijk Onderzoek 3.0270.95, OT/95/11, VIS/96/15, and FWO-PDM/97/59 (to A.V.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to H. Depraetere, PhD, Laboratory for Thrombosis Research, IRC, KU Leuven Campus Kortrijk, E Sabbelaan 53, B-8500 Kortrijk, Belgium; e-mail: Hilde.Depraetere@kulak.ac.be.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal