Abstract
Hematopoietic induction occurs on the ventral side ofXenopus gastrulae and is thought to be triggered by the growth factor bone morphogenetic protein 4 (BMP-4). To characterize this process, we developed a quantitative and sensitive assay for the induction of erythroid cells from totipotent ectoderm of the embryo. When high doses of BMP-4 were used in this explant assay, few erythroid cells were detected. In contrast, large numbers of differentiated erythroid cells were induced when ectoderm was treated with BMP-4 and the mesoderm inducers, activin, or fibroblast growth factor (FGF). Ectopic expression of GATA-1 also induced abundant erythroid cells in ectoderm treated with bFGF. This induction of erythroid cells by GATA-1 was blocked by coexpression with a dominant negative BMP-4 receptor, showing that GATA-1 requires the BMP signaling cascade to function. These results suggest that BMP-4 requires mesoderm induction to generate a program of gene expression, which regulates the specification of hematopoietic mesoderm by GATA factors.
HEMATOPOIESIS INVOLVES the proliferation and differentiation of hematopoietic stem cells (HSC) to yield erythroid, myeloid, and lymphoid lineages.1 In Xenopus, all HSCs (primitive or definitive) are derived from the ventral mesoderm of the gastrula2 and populate two sites of the embryo, the ventral blood island (VBI) and the dorsal lateral plate (DLP).3-7These sites are analogous to the yolk sac and AGM (aorta, gonads, mesonephros) region of higher vertebrates, respectively.8-11 Although factors involved in the proliferation and differentiation of hematopoietic lineages have been found and characterized, the factors that regulate the initial specification of HSCs during embryogenesis remain elusive. These factors presumably act downstream of early mesoderm patterning events in the embryo.
Bone morphogenetic protein 4 (BMP-4) is a transforming growth factor-β (TGF-β) family member that patterns ventral mesoderm. Injection of BMP-4 RNA or expression plasmids into embryos results in an expansion of the ventral and posterior region of the embryo and a decrease in dorsal and anterior structures.12-15 Ectopic expression of BMP-4 in totipotent ectodermal explants, called animal caps, induces expression of the hematopoietic-specific transcription factors GATA-1, GATA-2, and stem cell leukemia (SCL)16,17 and globin.17 Nevertheless, the dose of BMP-4 RNA required to produce erythroid cells or globin protein in these explants is extremely high and may not be physiologic.17-19 The explants may require other factors to cooperate with BMP-4 to regulate the early events of blood formation in vivo.
To test this hypothesis, we evaluated the role of BMP-4 and GATA-1 in embryonic hematopoiesis using an assay for the induction of erythroid cells from explants of primitive embryonic ectoderm. These explants were dispersed and stained with o-dianisidine to visualize single erythroid cells. This assay is sensitive, reproducible, and quantitative. BMP-4 overexpression induced low numbers of erythroid cells; however, in the presence of mesoderm inducers such as fibroblast growth factor (FGF) or activin, large numbers of erythroid cells were detected. GATA-1 overexpression induced abundant erythroid cells in the presence of FGF. Coinjection of GATA-1 and a dominant negative BMP receptor did not induce blood in animal explants treated with FGF, showing that GATA-1 requires the BMP signaling cascade to function. Blood formation during vertebrate embryogenesis may thus require at least three distinct functions: mesoderm induction (activin or FGF), mesoderm patterning (BMPs), and cell specification (GATA factors).
MATERIALS AND METHODS
DNA and RNA preparation.
cDNAs of human BMP-4,13XenopusGATA-1a,20Xenopus activin βB, andXenopus eFGF21 were cloned in the expression plasmid, pcDNA3 (Invitrogen, Carlsbad, CA), which uses a cytomegalovirus (CMV) promoter for expression in vertebrate cells. The GATA-1a plasmid is referred to as pcDNA3–GATA-1 in the rest of the article. eFGF cDNA was used instead of bFGF cDNA as eFGF has a signal sequence, unlike bFGF, and has an identical effect as bFGF on animal caps. pSP64T plasmids containing the dominant negative mouse BMP receptor (ΔmTFRII)22 and β-galactosidase (β-gal) cDNA were used in an in vitro transcription reaction with SP6 polymerase to generate capped RNA (Ambion, Austin, TX).
Growth factors.
Recombinant human activin A was obtained from the National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, MD) and used at 12 ng/mL. Recombinant human bFGF (Sigma Chemical Company, St Louis, MO) was used at 200 ng/mL. Recombinant human BMP-4 protein was used at 1 μg /mL and was a generous gift from Genetics Institute, Boston, MA.
Embryo injection and animal cap explant culture.
Xenopus laevis embryos were obtained as previously described23 and staged according to Nieuwkoop and Faber.24 Embryos for microinjection were placed in 0.5× Marc’s Modified Ringers (MMR; 1× MMR = 0.1 mol/L NaCl, 2 mmol/L KCl, 1 mmol/L MgSO4, 2 mmol/L CaCl2, 5 mmol/L HEPES, pH 7.8, 0.1 mmol/L EDTA), 3% Ficoll 400 (Sigma). Embryos were injected at the one-cell stage with either plasmid or synthetic RNA in a volume of 10 nL. The doses of plasmid and RNA used are described in the figure legends. Control injections used water or empty pcDNA3 plasmid. At stage 8, animal caps were explanted from the animal pole of the embryos and cultured for 2 days in cap culturing solution (0.5× MMR, 0.5 mg/mL bovine serum albumin (BSA), 50 μg/mL gentamycin, 100 U/mL penicillin, 100 μg/mL streptomycin), with or without growth factors until sibling whole embryos reached stage 35 (2 days postfertilization).
Dispersion and reaggregation of animal cap explants.
Animal caps were excised at stage 8 and incubated in calcium/magnesium-free media (CMFM).25 This caused the dispersion of the inner cap cells. The pigmented outer layer of the animal cap was discarded. Dispersed cells from 20 animal caps were exposed to various growth factors. After 1 to 2 hours of growth factor treatment, the dispersed cells were washed, reaggregated in cap culturing solution, and left to develop until control stage 35.
Isolation of erythroid cells from adult frogs and tadpoles.
Adult erythroid cells were collected in 10 U/mL heparin (Sigma), 0.5% BSA in 0.7× phosphate-buffered saline (PBS) from an adult frog wound site. The cells were spread on a slide and stained with Wright Giemsa. Embryonic erythroid cells were obtained from a stage 41 tadpole (3 days postfertilization) by cutting off the tail and collecting the cells that flowed out in a heparinized needle. The cells were concentrated onto a slide using a Cytospin 3 (Shandon, Pittsburgh, PA).
Western blot analysis for globin.
Animal caps were suspended in RIPA buffer (10 mmol/L Tris pH 7.5, 5 mmol/L EDTA, 1% Triton X-100, 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate [SDS], 10% glycerol, 1% aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF]) and incubated at 4°C with gentle rocking for 15 minutes. Cell debris was removed by centrifugation. Proteins were separated on a 15% SDS-polyacrylamide gel and transferred to nitrocellulose (Protran; Schleicher & Schuell, Keene, NH). One animal cap equivalent was loaded per lane. The resultant blot was blocked with 5% nonfat milk/Tris buffered saline + Tween (TBST) for 1 hour at room temperature and then incubated with a 1:7,500 dilution of a larval α-globin monoclonal antibody (generous gift from C.H. Katagiri, Hokkaido University, Japan) in TBST overnight at 4°C. The primary antibody was detected either by the method of alkaline phosphatase detection (Promega, Madison, WI; see Fig 6) or by chemiluminescence (Amersham, Arlington Heights, IL; see Fig 8).
o-Dianisidine staining of cells from whole embryos.
The procedure for detection of hemoglobin in Xenopus was adapted from a previously described protocol.26 Whole embryos and animal caps were treated without fixation in a 20:25:1 mixture of 0.14% o-dianisidine in 100% ethanol, 0.2 mol/L sodium acetate, and 30% hydrogen peroxide for 1 hour. Embryos and caps were washed with water, fixed in 4% paraformaldehyde, and stored in methanol. For viewing, the embryos or explants were cleared with benzyl benzoate:benzyl alcohol (1:2).
o-Dianisidine staining of cells from animal caps.
Three animal caps were dispersed in 200 μL of a collagenase B solution (2 mg/mL in 0.7× PBS). Twenty microliters of ano-dianisidine solution (10:1 mixture of 0.2%o-dianisidine [in 0.2% glacial acetic acid] and 30% hydrogen peroxide) was added to the cell suspension and incubated with the cells for 1 minute. The cells were pelleted using an Eppendorf (Madison, WI) centrifuge (4,000 rpm) and the supernatant removed. The pellets were resuspended in 250 μL of 0.7× PBS, and the cell suspensions were concentrated onto slides in a Cytospin 3 (Shandon) at 700 rpm for 3 minutes. The slides were fixed in methanol. Orange-brown color, indicative of globin expression was observed within individual erythroid cells under light microscopy.
RESULTS
Development of a quantitative assay for erythroid cell induction.
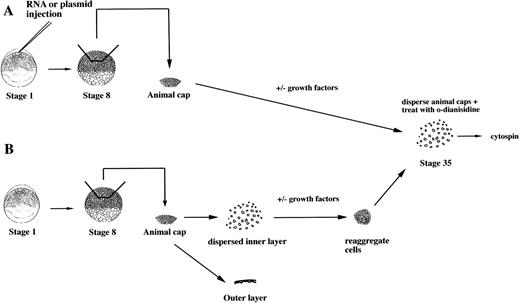
To study the induction of hematopoietic mesoderm, we developed a rapid, robust, and quantitative assay for erythroid cell induction inXenopus. This assay uses the totipotent primitive ectoderm from the animal pole of a midblastula embryo. RNAs or plasmids are injected into one cell embryos and animal caps excised at stage 8 (Fig 1A). RNAs are translated soon after injection, whereas plasmids are expressed after the midblastula transition when zygotic transcription initiates.23 To evaluate the effect of a particular RNA, plasmid, or growth factor on the induction of erythroid cells, the treated animal caps are cultured until control stage 35, when erythroid cells are detected in the VBI of sibling whole embryos (Fig 2). The cells of intact animal cap cells are dispersed, stained with the chemicalo-dianisidine, and concentrated onto a glass slide (Fig 1). In the stage-35 tadpole, only the cells of the VBI stain witho-dianisidine (Fig 2A), showing that it is a specific and sensitive indicator of differentiated erythroid cells26(Fig 2C). The visualization of discretely stained cells by microscopy allows for quantitation of the inductive assay. In our hands, this assay is more reproducible than reverse-transcription polymerase chain reaction (RT-PCR) analysis for globin mRNA expressed by the entire animal cap. In addition, o-dianisidine staining is indicative of both globin expression and hemebiosynthesis of erythroid cells.
An assay for the induction of erythroid cells from animal caps. (A) One-cell stage embryos are injected with RNA or plasmids. Animal caps are excised at stage 8 and cultured for 2 days under defined conditions and then dispersed by collagenase. The cells are incubated with o-dianisidine and immobilized onto a glass slide for analysis by microscopy. (B) Protein factors can be tested on dispersed animal caps cells. At stage 8, animal caps are excised and dispersed in CMFM. The outer pigmented layer is discarded. The dispersed inner layer cells are incubated with or without factors. After 1 to 2 hours the cells are washed and reaggregated in calcium/magnesium containing media and cultured for 2 days.o-dianisidine cells are detected as described in (A).
An assay for the induction of erythroid cells from animal caps. (A) One-cell stage embryos are injected with RNA or plasmids. Animal caps are excised at stage 8 and cultured for 2 days under defined conditions and then dispersed by collagenase. The cells are incubated with o-dianisidine and immobilized onto a glass slide for analysis by microscopy. (B) Protein factors can be tested on dispersed animal caps cells. At stage 8, animal caps are excised and dispersed in CMFM. The outer pigmented layer is discarded. The dispersed inner layer cells are incubated with or without factors. After 1 to 2 hours the cells are washed and reaggregated in calcium/magnesium containing media and cultured for 2 days.o-dianisidine cells are detected as described in (A).
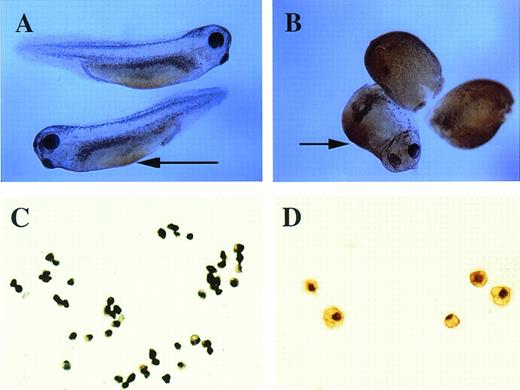
o-Dianisidine staining of whole embryos and erythroid cells. (A) Stage-35 whole embryo; the arrow points to the blood island that is stained orange-brown. (B) pcDNA3–BMP-4 (200 pg)– injected embryos; the ventralized embryos have diffuse orange-brown staining of the mesoderm. The arrow points to the enlarged blood island of a partially ventralized embryo. (C, D)o-dianisidine–positive cells from a dispersed stage-33 tadpole VBI. The cells in (C) were photographed at 10× original magnification, whereas the cells in (D) were photographed at 20× original magnification.
o-Dianisidine staining of whole embryos and erythroid cells. (A) Stage-35 whole embryo; the arrow points to the blood island that is stained orange-brown. (B) pcDNA3–BMP-4 (200 pg)– injected embryos; the ventralized embryos have diffuse orange-brown staining of the mesoderm. The arrow points to the enlarged blood island of a partially ventralized embryo. (C, D)o-dianisidine–positive cells from a dispersed stage-33 tadpole VBI. The cells in (C) were photographed at 10× original magnification, whereas the cells in (D) were photographed at 20× original magnification.
BMP-4 cooperates with mesoderm inducers to generate hematopoietic mesoderm.
BMP-4 has been thought to initiate the hematopoietic program during embryogenesis. An embryo ventralized by plasmid BMP-4 injection is stained with o-dianisidine in a radially symmetric pattern, corresponding to the expanded blood island (Fig 2B). We used the animal cap assay to study the role of BMP-4 in hematopoietic induction. BMP-4 RNA (0.5 to 1 ng) and BMP-4 cDNA (200 pg of a CMV-driven BMP-4 plamid, pcDNA3–BMP-4) were injected into embryos at the one cell stage, and animal caps were explanted at stage 8 and cultured until control stage 35. Few o-dianisidine–positive cells were detected (plasmid injection shown in Fig 3A). The activity of BMP-4 was also evaluated by exposing explanted animal caps to BMP-4 protein. To allow uniform access of growth factors to the animal cap cells, the caps were disaggreaged in CMFM at stage 8 and then treated with BMP-4 protein. After 1 to 2 hours the cells were washed, reaggregated, and cultured until control stage 35 (Fig 1B). As shown in Fig 3B, animal cap cells treated with BMP-4 protein alone (1 μg/mL) failed to differentiate into erythroid cells. Thus, even though forced BMP-4 expression can lead to enlarged ventral blood island formation12-14 (and this study). BMP-4 is unable to induce erythroid cells in animal caps. This suggests that there are additional factors in the embryo that cooperate with BMP-4 to regulate blood formation.
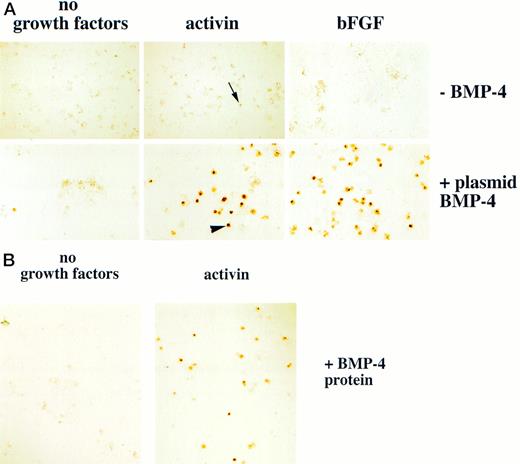
Erythroid cells are detected in animal caps treated with BMP-4 and mesoderm inducers. (A) For experiments using plasmid BMP-4 embryos are injected with control vector or pcDNA3–BMP-4 (200 pg) and treated with activin (12 ng/mL) or FGF (200 ng/mL). (B) For experiments using BMP-4 protein, animal caps are excised at stage 8 and dispersed in CMFM. The cell dispersion is treated with BMP-4 (1 μg/mL) with and without activin (12 ng/mL) in CMFM for 2 hours before the cells are washed and allowed to reaggregate in the calcium and magnesium containing cap culturing solution. In both experiments the cap cells are cultured until control stage 35 and analyzed foro-dianisidine–positive cells. The arrowhead points to an erythroid cell. The black arrow indicates a nonerythroid pigmented cell. The panels in (A) were photographed at 20× original magnification and the panels in (B) were photographed at 10× original magnification.
Erythroid cells are detected in animal caps treated with BMP-4 and mesoderm inducers. (A) For experiments using plasmid BMP-4 embryos are injected with control vector or pcDNA3–BMP-4 (200 pg) and treated with activin (12 ng/mL) or FGF (200 ng/mL). (B) For experiments using BMP-4 protein, animal caps are excised at stage 8 and dispersed in CMFM. The cell dispersion is treated with BMP-4 (1 μg/mL) with and without activin (12 ng/mL) in CMFM for 2 hours before the cells are washed and allowed to reaggregate in the calcium and magnesium containing cap culturing solution. In both experiments the cap cells are cultured until control stage 35 and analyzed foro-dianisidine–positive cells. The arrowhead points to an erythroid cell. The black arrow indicates a nonerythroid pigmented cell. The panels in (A) were photographed at 20× original magnification and the panels in (B) were photographed at 10× original magnification.
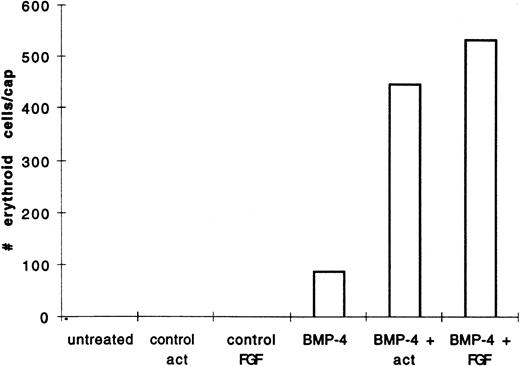
We hypothesized that BMP-4 patterns early mesoderm to a ventral fate and hence hematopoietic fate rather than directly inducing hematopoietic mesoderm. The absent or poor induction of erythroid cells in animal caps by BMP-4 alone may be due to the absence of direct mesoderm inducers. To test this hypothesis, pcDNA3–BMP-4–loaded caps, or dispersed caps incubated with BMP-4 protein, were treated with known mesoderm inducing factors, such as activin or bFGF (Fig 1A). Control caps treated with FGF or activin did not induce o-dianisidine stained cells (Fig 3A). Animal caps loaded with 200 pg of pcDNA3–BMP-4 and treated with activin or bFGF failed to elongate, confirming the ability of BMP-4 to override the induction of dorsal mesoderm.13 When these caps were dispersed, significantly higher numbers of cells expressing hemoglobin were evident than that seen in animal caps injected with pcDNA3–BMP-4 alone (Figs 3A and 4). On quantitation BMP-4 expression alone from pcDNA3–BMP4 yielded an average of 87 erythroid cells per cap, whereas no cells were detected in caps treated with activin or bFGF alone. BMP-4 plus activin yielded an average of 447 erythroid cells per cap, whereas BMP-4 plus FGF yielded an average of 536 erythroid cells per cap (averages are determined by counting the number of cells in six animal caps; Fig 4). In addition, BMP-4 protein (1 μg/mL) added to dispersed cells was able to induce erythroid cells in the presence of activin (Figs 1B and 3B). By Wright-Giemsa staining, the induced cells in the animal caps resemble primitive erythroid cells (Fig 5). These results show that BMP-4 cooperates with mesoderm inducers to induce erythroid cells.
BMP-4 and activin, or BMP-4 and FGF treatment of animal caps induce more of o-dianisidine–positive cells than BMP-4 treatment alone. The animal caps were treated as described in Fig 3. The o-dianisidine–positive cells were counted from two cytospins for each experimental condition in a representative experiment. As each cytospin represents three caps, the sum ofo-dianisidine–positive cells from two cytospins was divided by six to give the number of erythroid cells per cap.
BMP-4 and activin, or BMP-4 and FGF treatment of animal caps induce more of o-dianisidine–positive cells than BMP-4 treatment alone. The animal caps were treated as described in Fig 3. The o-dianisidine–positive cells were counted from two cytospins for each experimental condition in a representative experiment. As each cytospin represents three caps, the sum ofo-dianisidine–positive cells from two cytospins was divided by six to give the number of erythroid cells per cap.
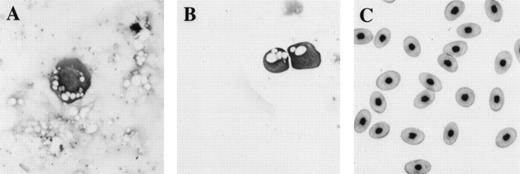
Comparison of morphology of erythroid cells induced in animal caps to those found in tadpoles and adult frogs. (A) An erythroid cell from an animal cap treated with BMP-4 and FGF; (B) circulating erythroid cells in a 3-day-old tadpole (stage 41); (C) adult erythroid cells. The erythroid cells are stained with Wright Giemsa. The primitive erythroid cells in (B) are 1 day older than and look slightly more differentiated than the cell shown in (A). Primitive erythroid cells are larger and more circular than definitive erythroid cells; they contain circular and less condensed nuclei and still possess yolk granules (compare B with C). The panels are photographed at 40× original magnification.
Comparison of morphology of erythroid cells induced in animal caps to those found in tadpoles and adult frogs. (A) An erythroid cell from an animal cap treated with BMP-4 and FGF; (B) circulating erythroid cells in a 3-day-old tadpole (stage 41); (C) adult erythroid cells. The erythroid cells are stained with Wright Giemsa. The primitive erythroid cells in (B) are 1 day older than and look slightly more differentiated than the cell shown in (A). Primitive erythroid cells are larger and more circular than definitive erythroid cells; they contain circular and less condensed nuclei and still possess yolk granules (compare B with C). The panels are photographed at 40× original magnification.
We next tested whether the cooperation observed between BMP-4 and activin, or BMP-4 and FGF, is dependent on the action of activin and FGF as mesoderm inducers. Animal caps failed to elongate when injected with CMV-driven activin or FGF plasmids, suggesting that the expression of activin and FGF from plasmid injections did not induce significant amounts of mesoderm (data not shown). This is likely to be due to insufficient accumulation of activin or FGF protein during the period of competence for mesoderm induction. Furthermore, cooperation between growth factors generated from CMV-driven activin or FGF and pcDNA3–BMP-4 was not detected in caps (data not shown). Taken together with the observation that dispersed animal cap cells differentiate to erythroid cells with only 1 hour of incubation with BMP-4 and activin (Fig 3B), these results suggest that FGF and activin act early as mesoderm inducers.
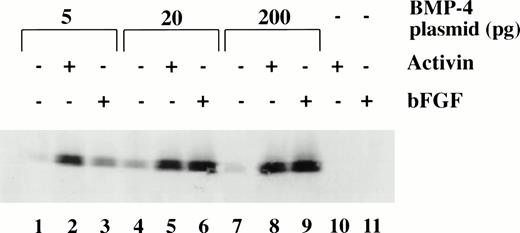
To determine the dose response of BMP-4 plasmid required for erythroid induction, larval globin expression was studied by Western blot analysis of animal caps. The expression of larval globin is indicative of primitive hematopoietic differentiation. Embryos were injected with three concentrations of pcDNA3–BMP-4 (5, 20, and 200 pg) and animal explants treated with or without activin or bFGF (Fig 6). Activin or bFGF treatment alone does not induce globin production (Fig 6, lanes 10 and 11). The induction of globin by pcDNA3–BMP-4 is cooperatively enhanced when the animal caps are treated with activin or bFGF. Note that in the presence of mesoderm inducers, 20 pg of pcDNA3–BMP-4 induces the same level of globin protein as 200 pg pcDNA3–BMP-4 (Fig 6, compare lanes 5 and 6 with 8 and 9, respectively). This indicates that 20 pg of pcDNA3–BMP-4 is capable of eliciting maximal globin induction. Doses as low as 0.2 pg pcDNA3–BMP-4 can induce erythroid cells in the presence of mesoderm inducers (data not shown) and doses of 5 to 20 pg of pcDNA3–BMP-4 induce a high amount of globin protein (Fig 6). The Western blot analysis is consistent with the o-dianisidine staining results, suggesting that low levels of BMP-4 RNA can cooperate with mesoderm inducers to induce erythroid cells.
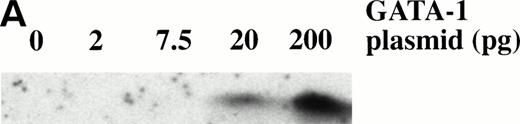
Dose response of BMP-4 plasmid for globin protein induction. Western blot analysis for globin expression in animal cap explants. Embryos were injected with 5, 20, and 200 pg of pcDNA3–BMP-4, and animal caps were explanted and cultured with or without activin (12 ng/mL) or bFGF (200 ng/mL) until sibling embryos were at stage 35. Protein extracts were made from the animal caps and separated on a SDS polyacrylamide gel. Proteins were transferred to nitrocellulose and incubated with a larval -globin monoclonal antibody. Each lane is loaded with one cap equivalence of protein extract. The finding that 5 pg of pcDNA3–BMP-4–loaded caps treated with activin (lane 2) induces more globin protein than FGF treatment (lane 3) is variable.
Dose response of BMP-4 plasmid for globin protein induction. Western blot analysis for globin expression in animal cap explants. Embryos were injected with 5, 20, and 200 pg of pcDNA3–BMP-4, and animal caps were explanted and cultured with or without activin (12 ng/mL) or bFGF (200 ng/mL) until sibling embryos were at stage 35. Protein extracts were made from the animal caps and separated on a SDS polyacrylamide gel. Proteins were transferred to nitrocellulose and incubated with a larval -globin monoclonal antibody. Each lane is loaded with one cap equivalence of protein extract. The finding that 5 pg of pcDNA3–BMP-4–loaded caps treated with activin (lane 2) induces more globin protein than FGF treatment (lane 3) is variable.
GATA-1 can induce erythroid cells without causing ventralization.
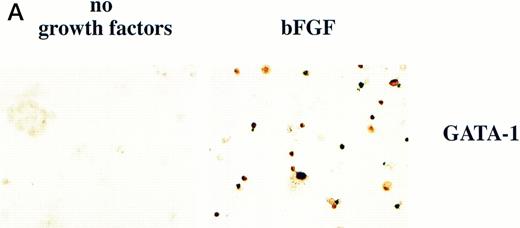
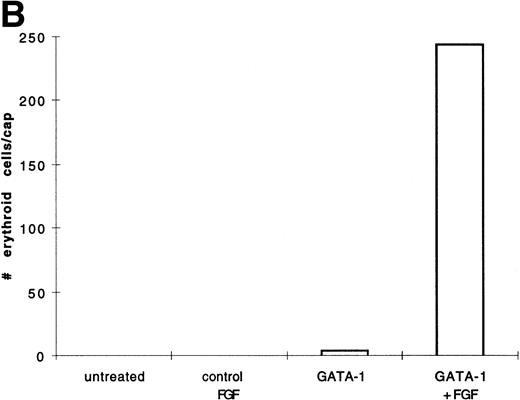
BMP-4 is known to induce the expression of the hematopoietic transcription factor, GATA-1.17 As GATA-1 expression is downstream of BMP-4 signaling, GATA-1 may specify ventral mesoderm to become erythroid cells. We tested this hypothesis by incubating pcDNA3–GATA-1 loaded caps with or without FGF, which has been shown to participate in ventral mesoderm induction.27 We found that pcDNA3–GATA-1 injection alone induces low amounts ofo-dianisidine cells, but in the presence of bFGF induces abundant erythroid cells (Fig7). Therefore GATA-1 can specify erythroid cells in cooperation with FGF.
GATA-1 induces erythroid cells in cooperation with bFGF. (A) Embryos are injected with pcDNA3–GATA-1 (200 pg) and treated with or without FGF (200 ng/mL). Animal caps are analyzed as described in Fig 1A. The panels are photographed at 20× original magnification. The dark cells in the panel of GATA-1 caps treated with no growth factors are pigmented cells that do not stain witho-dianisidine. (B) The o-dianisidine–positive cells were counted from two cytospins for each experimental condition in a representative experiment. The number of o-dianisidine cells per cap was determined as described in Fig 4.
GATA-1 induces erythroid cells in cooperation with bFGF. (A) Embryos are injected with pcDNA3–GATA-1 (200 pg) and treated with or without FGF (200 ng/mL). Animal caps are analyzed as described in Fig 1A. The panels are photographed at 20× original magnification. The dark cells in the panel of GATA-1 caps treated with no growth factors are pigmented cells that do not stain witho-dianisidine. (B) The o-dianisidine–positive cells were counted from two cytospins for each experimental condition in a representative experiment. The number of o-dianisidine cells per cap was determined as described in Fig 4.
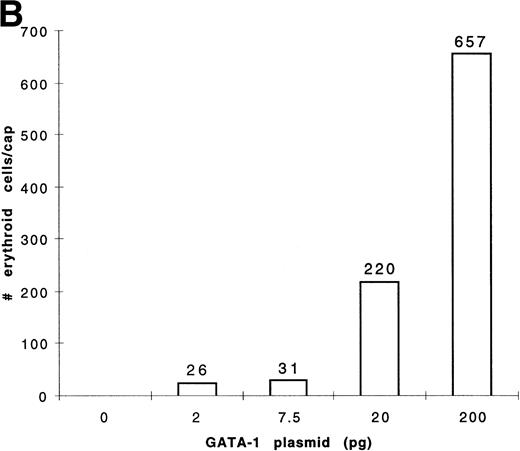
To determine the dose response of GATA-1 plasmid required for erythroid induction, embryos were injected with four concentrations of pcDNA3–GATA-1 (2, 7.5, 20, and 200 pg) and animal caps treated with bFGF (Fig 8). The animal caps were either analyzed for globin expression by Western analysis or for hemoglobin byo-dianisidine staining. Quantitation ofo-dianisidine–positive cells per animal cap induced from each dose shows that there is erythroid induction at the 2- and 7.5-pg doses of pcDNA3–GATA-1 (Fig 8B). The o-dianisidine assay is capable of detecting erythroid induction at levels that are undetected by Western analysis. This shows the sensitivity of the animal cap assay using o-dianisidine.
Dose response of GATA-1 plasmid for globin protein and erythroid cell induction. Embryos were injected with 2, 7.5, 20, and 200 pg of pcDNA3–GATA-1, and animal caps were explanted and cultured with bFGF (200 ng/mL) until sibling embryos were at stage 35. The animal caps were analyzed for globin induction by (A) Western analysis and (B) o-dianisidine staining. The Western analysis was performed as described in Fig 6. The number ofo-dianisidine–positive cells per cap represents the average of two cytospins for each dose of pcDNA3–GATA-1 and was determined as described in Fig 4.
Dose response of GATA-1 plasmid for globin protein and erythroid cell induction. Embryos were injected with 2, 7.5, 20, and 200 pg of pcDNA3–GATA-1, and animal caps were explanted and cultured with bFGF (200 ng/mL) until sibling embryos were at stage 35. The animal caps were analyzed for globin induction by (A) Western analysis and (B) o-dianisidine staining. The Western analysis was performed as described in Fig 6. The number ofo-dianisidine–positive cells per cap represents the average of two cytospins for each dose of pcDNA3–GATA-1 and was determined as described in Fig 4.
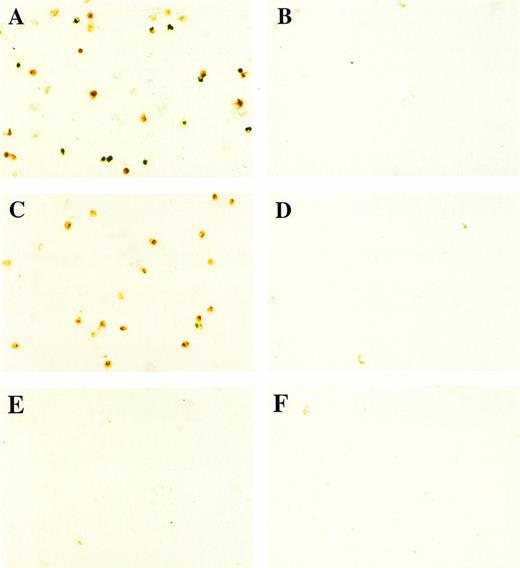
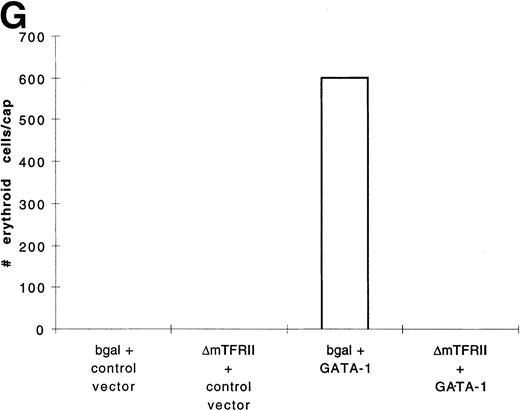
In contrast to BMP-4 overexpression, GATA-1 overexpression does not ventralize whole embryos.28 It is possible that GATA-1 could induce erythroid cells in the absence of BMP-4 signaling; however, the animal cap expresses low levels of BMP-4.12 To determine if signaling by this small amount of BMP-4 is required for GATA-1 function, we used a mutant type I BMP-2/4 receptor (ΔmTFRII) lacking the serine/threonine kinase domain. The mutant receptor has been shown to block BMP-4 signaling and ventralization.22Various doses of ΔmTFRII RNA were coinjected with 200 pg of pcDNA3–BMP-4 to establish a dose of mutant receptor RNA that would block induction of erythroid cells by BMP-4. A dose of 1 ng of ΔmTFRII RNA was found to block the ability of BMP-4 to induce erythroid cells (Fig 9A and B [see page 4134]). The injection of 1 ng of ΔmTFRII RNA alone does not prevent mesoderm from being induced. These animal caps treated with FGF show elongation, which is an indicator of mesoderm induction (data not shown). To determine if the mutant receptor could block the induction of erythroid cells by GATA-1, 200 pg of pcDNA3–GATA-1 with 1 ng of ΔmTFRII RNA was injected into animal caps. Erythroid cells were not detected (Fig 9C, D, and G), showing that BMP-4 signaling is involved in the GATA-1 induction of erythroid cells.
GATA-1 requires the BMP pathway to cooperate with bFGF to induce erythroid cells. Embryos were coinjected with (A) β-gal RNA (1 ng) and pcDNA3–BMP-4 (200 pg); (B) ▵mTFRII RNA (1 ng) and pcDNA3–BMP-4 (200 pg); (C) β-gal RNA (1 ng) and pcDNA3–GATA-1 RNA (200 pg); (D) ▵mTFRII RNA (1 ng) and pcDNA3–GATA-1 RNA (200 pg); (E) β-gal RNA (1 ng) and control vector (200 pg); and (F) ▵mTFRII RNA (1 ng) and control vector (200 pg). Animal caps were explanted, cultured with bFGF (20 ng/mL) until sibling embryos were at stage 35, and analyzed for o-dianisidine–positive cells (Fig 1A). The panels are photographed at 20× original magnification. (G) The number ofo-dianisidine–positive cells was counted in two cytospins for each experimental condition in a single experiment. The number ofo-dianisidine cells per cap was determined as described in Fig4.
GATA-1 requires the BMP pathway to cooperate with bFGF to induce erythroid cells. Embryos were coinjected with (A) β-gal RNA (1 ng) and pcDNA3–BMP-4 (200 pg); (B) ▵mTFRII RNA (1 ng) and pcDNA3–BMP-4 (200 pg); (C) β-gal RNA (1 ng) and pcDNA3–GATA-1 RNA (200 pg); (D) ▵mTFRII RNA (1 ng) and pcDNA3–GATA-1 RNA (200 pg); (E) β-gal RNA (1 ng) and control vector (200 pg); and (F) ▵mTFRII RNA (1 ng) and control vector (200 pg). Animal caps were explanted, cultured with bFGF (20 ng/mL) until sibling embryos were at stage 35, and analyzed for o-dianisidine–positive cells (Fig 1A). The panels are photographed at 20× original magnification. (G) The number ofo-dianisidine–positive cells was counted in two cytospins for each experimental condition in a single experiment. The number ofo-dianisidine cells per cap was determined as described in Fig4.
DISCUSSION
BMP-4 function in the induction of hematopoietic mesoderm.
BMP-4 acts after the initial steps of mesoderm induction to cause ventralization and erythroid induction.14,15,17 Our studies show that BMP-4 cooperates with FGF or activin to induce erythroid cells in animal cap assay, suggesting that BMP-4 patterns induced mesoderm to a ventral fate (Figs 3 and 4). Some studies propose that BMP-4 is a direct ventral mesoderm inducer. This is based on the detection of mesodermal markers in animal caps loaded with high doses of BMP-4 RNA.13,19,29-31 The ability of BMP-4 to act as a patterning molecule or a mesoderm inducer may be determined at the receptor level. BMP-4 signaling is mediated through the activation of type I and type II serine/threonine receptors.32,33 Type I and type II receptors of various TGF-β family members are known to oligomerize upon exposure to particular ligands, which may lead to qualitatively different signals. For example, the BMPRII receptor can bind to activinRI (ALK-1) as well as to BMPRIA and BMPRIB.34 BMP-4 prefers to bind BMPRIA/BMPRII and BMPRIB/BMPRII to generate a BMP signal. The endogenous levels of BMP-4 in the Xenopus embryo are not known, and it is quite possible that the level is high on the ventral side of the embryo. High levels of BMP-4 may force the formation of receptor combinations that generate an activin signal, which induces mesoderm. This mesoderm could then be patterned to form erythroid cells by that same high dose of BMP-4. Thus, although BMP-4 can perform both patterning and inductive functions in vitro, we believe that it is the patterning activity of BMP-4 on early mesoderm that results in the induction HSCs.
Activin and/or FGF have been previously postulated to be important in the induction and differentiation of the erythroid lineage. Activin was cloned as erythroid differentiation factor (EDF)35 and can induce in vitro differentiation of erythroleukemic cell lines at a concentration range of 0.5 to 10 ng/mL.36 Activin (and BMP-4) receptors are expressed on the erythroid cells of the developing blood island (J. Graff, personal communication, April 1995). Despite this, activin predominantly induces dorsal mesoderm in animal caps and is not capable of inducing globin expression. FGF signaling has been shown to be required for posterior-ventral mesoderm induction.27 FGF receptors have been detected in murine bone marrow cells, and bFGF can be found in the bone marrow extra-cellular matrix37; however, similar to activin, FGF is insufficient to induce erythropoiesis in animal caps.27,38 39 Our studies show an early role for activin and FGF as mesoderm inducers but do not exclude later roles for these factors during hematopoiesis.
Our studies in Xenopus extend the analysis of BMP-4 function in higher vertebrates. Targeted gene disruption of the BMP-4 ligand and type 1 BMP-2/4 receptor in mouse ES cells show an early requirement for BMP-4 signaling in the early proliferation of the epiblast.40,41 The most severe phenotype for both ligand- and receptor-deficient embryos is the lack of any mesodermal derivative, preventing an evalution of the role of BMP-4 signaling in hematopoietic induction. The BMP-4 mutants that undergo gastrulation develop with poorly organized posterior structures and a reduction in blood islands.41 These mutants are believed to be partially rescued by BMP-2. Our finding of cooperative effects of BMP-4 and activin, or BMP-4 and FGF, in the induction of hematopoietic mesoderm in Xenopus may also exist for higher vertebrates. These combinations may stimulate embryonic stem cell and primary yolk sac cultures to yield primitive colonies. BMP-4 has some ability to induce hematopoietic precursors when added to embryonic stem cells,42 and the addition of activin or FGF may lead to higher numbers of colonies. This would be an advance to studies of primitive erythroid cells since they are difficult to grow in vitro.
GATA function requires an active BMP cascade.
GATA-1 has been found to regulate most, if not all, erythroid-specific genes43 and is absolutely required for terminal erythroid differentiation.44,45 In contrast, GATA-2 is required for the maintenance or proliferation of hematopoietic progenitors.46 Our studies have shown that GATA-1 cooperates with FGF to induce erythroid cells. In these experiments, GATA-1 may have a similar activity as GATA-2. GATA-2 overexpression in animal caps is able to induce globin RNA expression.16Interestingly, GATA-3, which is involved in thymocyte development,47 and GATA-4, which participates in heart and gut,48 49 induce o-dianisidine–positive cells in our assay (T.H. and Y.Z., unpublished results, July 1996). As GATA factors share a highly conserved zinc finger-type DNA binding domain, they may activate the same genes when overexpressed in Xenopus embryos. Our results with GATA-1 therefore point to the involvement of the GATA binding proteins during HSC specification during embryogenesis.
GATA-1 does not ventralize whole embryos28 and is therefore not a patterning molecule. It is capable of cooperating with FGF to induce erythroid cells in animal caps (Fig 7). Based on the injections of ΔmTFRII, mesoderm that lacks an intact BMP signaling cascade cannot respond to GATA-1 (Fig 9). A variety of factors such as the homeobox genes Pv.1, Mix.1, Vent-1, Vent-2/Vox, and Xom are expressed ventrally in gastrula embryos and in response to BMP-4 in animal caps.50-55 The genes regulated by these homeobox genes may be essential for GATA-1 to induce erythroid cells. Understanding the requirement of BMP-4 and downsteam genes in erythroid induction might further establish later functions of BMP signaling cascade during hematopoiesis.
This work suggests a model for hematopoietic stem cell induction during vertebrate embryogenesis. Mesoderm induction at the late blastula and early gastrula stage establishes competence of cells to respond to BMP-4 to form HSCs. The endogenous mesoderm inducers may be FGF, activin-like molecules, or even BMP-4. The specific role of BMP-4 in hematopoietic induction is to pattern the mesoderm by affecting the program of gene expression. Ultimately, hematopoietic transcription factors, such as the GATA factors, are expressed that mediate the specification of the HSC fate.
ACKNOWLEDGMENT
We thank Chris Wright (Vanderbilt University) and Todd Evans (Albert Einstein College of Medicine) for BMP-4 cDNA, Jonathan Slack (University of Bath) for eFGF cDNA, Mitsugu Maeno (Niigata University, Japan) for ΔmTFRII cDNA, and CH Katagiri (Hokkaido University, Japan) for larval globin monoclonal antibody. BMP-4 protein was a generous gift from Genetics Institute, Boston, MA. We thank the members of the laboratory for helpful discussions and suggestions. We thank Drs Jeremy Green, Xi He, Barry Paw, Sadhana Agarwal, and David Ransom for their comments on this manuscript. Leonard Zon is an Associate Investigator of the Howard Hughes Medical Institute.
Supported by National Institutes of Health Grant No. 2RO1 HL48801-05 (L.I.Z.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Leonard I. Zon, MD, Division of Hematology/Oncology, Children’s Hospital, 300 Longwood Ave, Enders 650, Boston, MA 02115; e-mail: zon@rascal.med.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal