Abstract
Mice with a null mutation of the βc chain of the granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5 receptors (βc-null mice) develop an alveolar proteinosis-like lung disease. The pathogenesis of this disease is uncertain and, although a defect in alveolar macrophage function has been postulated, no previous analysis of mature hematopoietic cells in mice with alveolar proteinosis has been reported. Therefore, we undertook a functional analysis of the mature hematopoietic cell compartment in βc-null mice. In addition, we reexamined the roles of the GM-CSF receptor chain and the βc chain in signaling by GM-CSF. Neutrophils and macrophages from βc-null mice were capable of normal survival and phagocytosis in the absence of stimulus and of similar levels of nitric oxide production in response to interferon-γ and lipopolysaccharide. GM-CSF–mediated augmentation of survival, phagocytosis, and hydrogen-ion production were absent in neutrophils from βc-null mice. Interestingly, we were unable to show any ability of the GM-CSF receptor -chain alone to mediate glucose transport in these cells. In keeping with the βc-null mice lung pathology, examination of lavage fluid from the lungs of βc-null mice showed increased cellularity. This was caused by an increase in the number of lymphocytes, neutrophils, and macrophages. Large foamy cells in the lavage fluid from βc-null mice were identified as macrophages using immunohistochemistry. Functional analysis showed that these βc-null alveolar macrophages were capable of phagocytosis but uptake of colloidal carbon and cellular adhesion were reduced. In summary, mature hematopoietic cells with a null mutation of the βc receptor were unable to perform GM-CSF–mediated hematopoietic cell functions including glucose transport, but responded normally to a range of other ligands.
THE HEMATOPOIETIC cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5 stimulate proliferation and differentiation of hematopoietic progenitor cells. These cytokines also play a role in mature hematopoietic cell functions, including mature cell survival and phagocytosis.1 They act on target cells via a receptor complex composed of an alpha (α) and a beta (β) chain. The α subunit for each cytokine is unique and binds the ligand with low affinity, while the β chain converts the interaction with ligand to one of high affinity and is required for intracellular signaling. In both mice and humans a common β subunit is used by GM-CSF, IL-3, and IL-5 receptors and is known as the common beta chain (βc).2-4 In the mouse, an additional IL-3–specific β chain exists, known as βIL-3,5 which is used in preference to βc for signaling by IL-3.6
The requirement for the βc chain in mediating the actions of GM-CSF has been formally demonstrated by the creation of βc-null mice,7,8 and mutational analysis of βc has shown that multiple different signaling pathways are initiated from distinct regions of the cytoplasmic domain of the βc receptor.9,10In contrast to the βc chain, the only signaling function so far attributed to the GM-CSF receptor α chain (GM-CSF Rα) alone is GM-CSF–mediated glucose transport.11 12 This function was shown in Xenopus oocytes transfected with the human GM-CSF Rα and it was postulated that such α chain–mediated involvement in glucose transport may be associated with prolongation of cell survival.
Mice with a null mutation for the βc chain (βc-null)7,8have normal baseline hematopoiesis except for a low basal circulating eosinophil level. The eosinopenia is similar to that observed in mice with a null mutation of the IL-5 gene13 or the IL-5 receptor α chain gene.14 Like mice with a null mutation of the gene for GM-CSF,15,16 the βc-null mice have lung disease, the pathology of which is reminiscent of the human disease pulmonary alveolar proteinosis (PAP). The alveolar spaces of the lungs progressively accumulate surfactant and GM-CSF null mice have been shown to have markedly reduced alveolar clearance and catabolism of surfactant protein A (SP-A).17 This pulmonary lesion, thought to be due at least in part to defective function of alveolar macrophages,18 can be cured by bone marrow transplantation19 and, in GM-CSF null mice, by expression of a GM-CSF transgene in type II pneumocytes.20
To date, analysis of the functional activity of mature hematopoietic cells in mice lacking the βc receptor has been limited to an examination of the resident and elicited peritoneal populations.21 A more complete examination of mature hematopoietic cell function in these mice is required to explore the pathogenesis of the PAP-like disease.
In this study we used the βc-null mice to reexamine the role of the GM-CSF Rα in influencing cell survival. We also report experiments in which we assessed the function of granulocytes and macrophages from these mice using a variety of parameters including cell survival, phagocytosis, cell adhesion, and nitric oxide production.
MATERIALS AND METHODS
Mice.
129/Sv βc-null7 or wild-type (WT) control mice were used for the majority of experiments. In some experiments C57BL/6 x 129/Sv mice were used. No significant differences were seen comparing the two strains. Mice were 6 to 12 weeks old for neutrophil function experiments and were 8 to 12 weeks old for peritoneal and alveolar macrophage studies. As indicated, selected experiments were performed on 2-week-old mice.
Cytokines.
Lyophilized recombinant human G-CSF (rhG-CSF; AMRAD Melbourne, Australia) was dissolved in sterile water and diluted in sterile normal saline for injection with 5% bovine calf serum (BCS; Hyclone, Logan, UT). Recombinant murine (rm) GM-CSF was produced inEscherichia coli or Saccharomyces cerevisiae and purified by conventional chromatography (specific activity 108 U/mg). rmIL-3 was from Peprotech (Rocky Hill, NJ). Interferon-γ (IFN-γ) was from Genzyme Corp (Cambridge, MA). E coli lipopolysaccharide was from Sigma Chemical Co (St Louis, MO).
G-CSF–elicited neutrophil preparation.
Mice were injected with 2.5 μg of rhG-CSF twice daily at 8 AM and 7 PM for 5 days. All analyses were commenced at 9 AM on the morning following the last evening injection. Mice were anesthetized and retro-orbital plexus blood and axillary vessel blood was collected. White blood cell counts and differential cell counts were performed as previously described.22 Blood obtained from the axilla was subjected to hypotonic lysis for 10 minutes followed by washing in Dulbecco’s modified Eagle’s medium with 10% BCS (10% DME BCS). After this, 200 cell differential counts were performed on cytocentrifuge preparations stained with May-Grünwald-Giemsa. The purity of neutrophils in the preparations was similar for both WT and βc-null mice (WT 87% ± 4% n = 5 mice, βc-null 83% ± 6%, n = 5 mice). Radioiodination of rmGM-CSF, binding assays, and Scatchard analysis were performed as described.23
Neutrophil survival assay.
Survival assays were performed by culturing neutrophils in 60-well Lux 5260 microtiter plates (Nunc, Naperville, IL) according to a method previously described.24 Each well contained 10 μL 10% DME BCS and 200 cells. Serial dilutions of 5 μL of cytokine-containing preparations were added to quadruplicate microwells before the addition of target cells. Cultures were incubated at 37°C in a fully humidified atmosphere of 10% CO2 in air. Cultures were examined in replicate wells at intervals and cells that were highly refractile with a clearly defined cell border were counted as viable using an inverted microscope at 200× magnification.
Neutrophil phagocytosis assay.
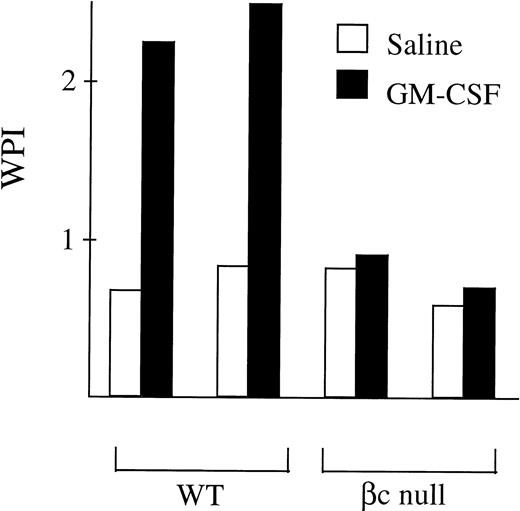
Cells were plated at a cell density of 105 cells/mL in 24-well plates (Falcon, Becton Dickinson, Lincoln Park, NJ) in 10% DME BCS. 106 latex beads conjugated with a fluorescein dye (Flouresbrite carboxy YG microspheres; Polysciences Inc, Northampton, PA) were then added, with 50 μL of carrier with or without rmGM-CSF. After 6 hours of incubation at 37°C cells were detached with 100 μL of 0.1 mol/L EDTA. Cytocentrifugation of 200 μL of cells and beads was performed. After staining with May-Grünwald-Giemsa, enumeration of beads per neutrophil was performed for 200 consecutive neutrophils. The Weighted Phagocytic Index (WPI)25 was calculated by multiplying the number of neutrophils with 1, 2 to 3, 4, or ≥5 associated beads by 1, 2, 3, and 4, respectively, and dividing the total score by the number of neutrophils examined.
Cytosensor analysis.
105 neutrophils were analyzed per point on the Cytosensor Microphysiometer (Molecular Devices Corp, Sunnyvale, CA) according to a method previously described.26 The extracellular acidification rate (ECAR) was measured in microvolts per second and normalized in running buffer (DME without bicarbonate buffering, 0.1% bovine serum albumin [BSA], endotoxin free) before exposure of the cells to ligand. The change in ECAR versus time was documented over 1 to 2 hours after exposure to cytokine for 6 minutes.
Peritoneal and bronchoalveolar lavage.
Resident peritoneal cells were washed from the peritoneal cavities of sacrificed mice by injecting 5 mL phosphate-buffered saline (PBS), gently massaging the abdominal wall, then aspirating the lavage, first with a syringe and 18-gauge needle, then with a glass pasteur pipette inserted through the peritoneum, and again after exposure of the peritoneal cavity. Alveolar cells were lavaged from murine lungs after peritoneal lavage. The trachea was exposed transthoracically and a piece of fine bore peristaltic tubing inserted to just above the carina and secured. The lungs were then lavaged with 10 × 1-mL aliquots of PBS with 0.5 mmol/L EDTA. The percentage of macrophages was determined by staining with crystal violet (to examine nuclear morphology) and cell counts were performed with eosin (to assess cell viability). Cells were washed and suspended at 106 per mL in RPMI 1640 with 10 mmol/L HEPES pH 7.3, and 10% BCS (heat-inactivated at 56°C for 60 minutes). Cells, 2 × 104, were cytocentrifuged and stained with May-Grünwald-Giemsa or stored at −70°C. Frozen cell preparations were brought slowly to room temperature in a mixture of acetone and methanol (50:50 vol/vol).
Immunohistochemistry.
Cytospin preparations were washed in PBS containing 0.1% vol/vol Triton X-100 (BDH-Merck, Darmstadt, Germany) and treated with a solution of 2% normal rabbit serum, 2% normal mouse serum, and 2% BCS in PBS for 30 minutes. Cytospin preparations were incubated for 90 minutes in monoclonal antibody (MoAb), PBS, or isotype-matched control MoAb, and endogenous peroxidase activity was blocked as previously described.27 The cells were incubated with primary peroxidase conjugated secondary antibody for 45 minutes, washed, and then incubated with 0.5 mg/mL diaminobenzidine (Polysciences Inc) and 0.024% H2O2 in 10 mmol/L imidazole, pH 7.4. Sections were counterstained in Mayers hematoxylin and mounted using DePeX (BDH, Poole, Dorset, UK).
Antibodies.
The following MoAbs raised in rats and directed against mouse antigens were used as hybridoma supernatants for immunohistochemistry: F4/8028 recognizes a 160-kD antigen of unknown function; 5C629 was used to recognize the β2 integrin, complement receptor type 3 (CR3); IC2 recognizes sialoadhesin30; FA-1131 recognizes the major wheat germ agglutinin-binding lectin of murine macrophages, macrosialin; 2F8 recognizes the murine macrophage scavenger receptor (mMSR)32; 8D2 recognizes the hyaluronan receptor CD44/pgp-133; and TIB 120, which recognizes class II major histocompatibility complex (MHC).34
Adhesion assays.
Cells were resuspended in RPMI 1640 with 10% BCS, plated at a density of 3 × 105 macrophages per well in flat-bottom tissue culture plastic (TCP) 96-well plates, and incubated with MoAb (5 μg/mL) and/or chelator (5 mmol/L EDTA) as previously described.35 In some assays cells were plated in 10-μL volumes in microtiter plates and adherent cells were enumerated under phase-contrast microscopy, counting selected regions of a grid (average number of cells per area) for a minimum of three independent wells.
Macrophage phagocytosis assays.
For colloidal carbon phagocytosis, cells were cultured at a density of 105 cells per well in flat-bottom TCP 96-well plates in RPMI 1640 with 10% BCS and allowed to adhere for 90 minutes at 37°C. After one wash, 5 μL of 5% colloidal carbon (Pelikan Ink, Gunther Wagner, Germany) in combination with MoAb and/or chelator was added to test wells and incubated for 1 hour at 37°C. After washing gently three times in PBS, cells were viewed by phase-contrast microscopy and the percentage of phagocytic cells was enumerated. For latex bead phagocytosis, cells were cultured at 2 × 104 cells per well in flat-bottom 96-well TCP and adhered overnight at 37°C. Latex beads, 2 × 105, were added and incubated for 12 hours at 37°C. After one gentle wash in PBS adherent cells were fixed using 100 μL 1% glutaraldehyde and the number of beads per cell determined for 100 consecutive cells using phase-contrast microscopy. For sheep red blood cell (RBC) phagocytosis, cells were cultured as for latex bead phagocytosis. Thirty microliters of sheep RBCs (prepared by incubation with or without anti-sheep RBC serum and suspended at 2 × 107cells/mL) was added and incubated for 2 hours (in selected experiments for ½, 1, or 2 hours) at either 4°C or 37°C. After gentle washing of all wells and hypotonic lysis of selected wells, adherent cells were fixed, the number of sheep RBCs per macrophage enumerated for 100 consecutive macrophages, and the WPI calculated as for neutrophil latex bead phagocytosis. For endocytosis of acetylated low-density lipoprotein (AcLDL) labeled with 1,1′-dioctadecyl-1-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; PerImmune, Inc, Rockville, MD),32 cells were adhered overnight on glass coverslips, washed three times, and incubated with 10 μg/mL DiI-AcLDL for 30 minutes at 37°C. Coverslips were then washed three times for 5 minutes each with PBS and mounted on glass slides. Uptake was detected by confocal microscopy using rhodamine excitation and emission filters. Where phagocytosis studies involved βc-null alveolar macrophages, all test samples were washed very gently to minimize cell loss caused by the poor adhesion of these cells to TCP.
Nitric oxide assays.
105 cells per well were plated in U-bottom 96-well TCP in RPMI 1640 with 10% BCS and allowed to adhere for 3 hours at 37°C. After washing, medium containing saline with or without IFN-γ (102 U/mL final concentration) and lipopolysaccharide (LPS) (0.1 μg/mL) was added and cells were incubated for 48 hours at 37°C. Culture supernatants were assayed for nitrite content.36 Fifty microliters was reacted for 10 minutes at room temperature with an equal volume of the colorimetric Griess reagent [0.5% sulfanilimide and 0.05%N-(1-napthyl)ethylenediamine dihydrochloride in phosphoric acid]. The absorbance at 540 nm was measured and the nitrite content was quantified by comparison with a standard curve generated with NaNO2 in the range of 0 to 100 μmol/L. Nitric oxide (NO) production by resident peritoneal macrophages and peritoneal macrophages obtained 4 days after intraperitoneal injection of 2 mL of 3% thioglycollate broth was examined. NO produced in response to Listeria monocytogenes was assessed as follows: peritoneal cells were cultured at a concentration of 2 × 106 cells/mL in 24-well TCP (Nunc, Roskilde, Denmark) in the presence or absence of 2 × 108heat-killed Listeria organisms/mL for 24 hours.37Culture supernatant was assayed for NO production as described above.
Glucose uptake assay.
Uptake of 2-deoxy-D-glucose (2-DOG) was performed as previously described.11,38 39 Bone marrow cells were washed three times in a serum-free, glucose-free buffer (15 mmol/L HEPES/135 mmol/L NaCl/5 mmol/L KCl/1.8 mmol/L CaCl2/0.8 mmol/L MgCl2 pH 7.4). Cells, 2 × 106, were incubated in 1-mL cultures with 5% serum/saline with or without cytokine and 2-DOG (0.01 mmol/L final concentration; Sigma) for 50 minutes. 2-deoxy-D-(1,2-3H)glucose (3H-2-DOG, 1 μCi; Amersham, Buckinghamshire, UK) was then added to each culture for exactly 10 minutes. Three 10-mL washes of ice-cold 5 mmol/L D-glucose were performed, followed by solubilization with 1% sodium dodecyl sulfate (SDS) and addition to 2 mL of aqueous scintillant (Starscint; Packard, Groningen, The Netherlands). Incorporated radioactivity was then determined. Cytochalasin B or E (10 μmol/L final concentration; Sigma) were added to selected cultures 10 minutes before the addition of 3H-2-DOG.
RESULTS
Neutrophil function in βc-null mice.
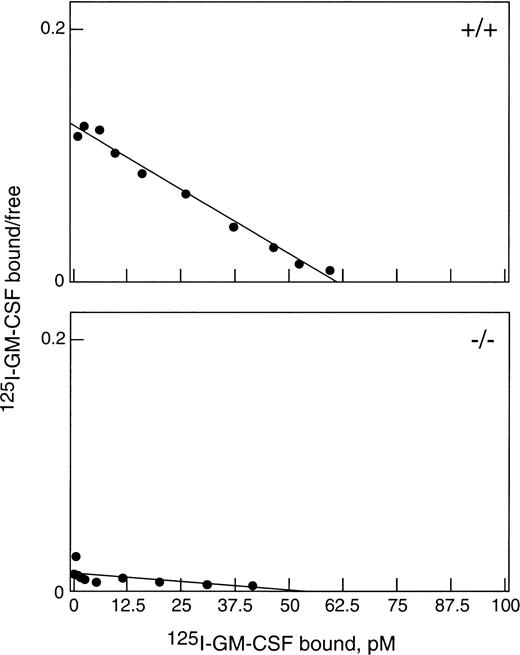
To obtain sufficient neutrophils for this study, WT and βc-null mice were injected with 2.5 μg rhG-CSF subcutaneously twice daily for 5 days. Both βc-null and WT mice responded with a comparable leukocytosis (βc-null 53.2 ± 1.2 × 106/mL [mean ± SD] and WT 55.6 ± 5.7 × 106/mL, n = 3 to 4 mice per group and 82% and 81% neutrophilia, respectively) and increase in circulating progenitor cells (βc-null 7,700 ± 775 cells/mL, and WT 7,000 ± 556 cells/mL).22There was also a similar increase in splenic weight, cellularity, and number of splenic progenitor cells in both groups. As shown in Fig 1, top panel, G-CSF–elicited blood neutrophils from WT mice bound 125I-GM-CSF with a single class of high-affinity binding (kd = 500 pmol/L, n = 1,700 receptors). Cells from βc-null mice (Fig 1, bottom panel) bound GM-CSF with only low affinity (kd 4.2 nmol/L, n = 2,100 receptors per cell) as was previously reported for bone marrow cells from untreated βc-null mice.6 7 Thus, βc-null mice responded normally to administration of G-CSF and in vivo exposure to G-CSF did not alter the GM-CSF binding characteristics of elicited cells.
Binding of GM-CSF to G-CSF–elicited blood neutrophils from βc-null and WT mice. Cells were incubated with125I-GM-CSF for 3 hours at 4°C and assayed for binding. Data were corrected for nonspecific binding and are shown plotted in the Scatchard coordinate system (•). (Top) Neutrophils (87% pure) from WT mice bound with a single class of high-affinity binding: kd = 500 pmol/L, n = 1,700 per cell. (Bottom) Neutrophils (83% pure) from βc-null mice bound GM-CSF only with low affinity: kd = 4.2 nmol/L, n = 2,100 per cell.
Binding of GM-CSF to G-CSF–elicited blood neutrophils from βc-null and WT mice. Cells were incubated with125I-GM-CSF for 3 hours at 4°C and assayed for binding. Data were corrected for nonspecific binding and are shown plotted in the Scatchard coordinate system (•). (Top) Neutrophils (87% pure) from WT mice bound with a single class of high-affinity binding: kd = 500 pmol/L, n = 1,700 per cell. (Bottom) Neutrophils (83% pure) from βc-null mice bound GM-CSF only with low affinity: kd = 4.2 nmol/L, n = 2,100 per cell.
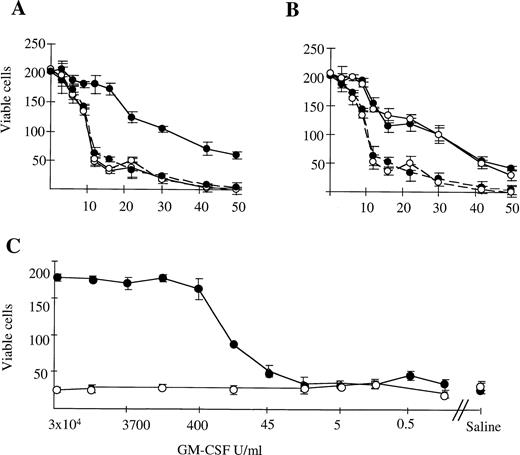
We examined the survival of murine G-CSF–elicited neutrophils in microwell cultures. Survival of neutrophils from WT mice was prolonged by the addition of GM-CSF, G-CSF, or IL-3 (Fig 2A and B; data for G-CSF not shown). Neutrophils from βc-null animals exhibited equivalent baseline survival in saline and prolongation of survival in the presence of IL-3 and G-CSF but not GM-CSF. Increasing the concentration of GM-CSF did not result in prolongation of survival of βc-null neutrophils (Fig2C). This was observed even when the concentration of GM-CSF was increased up to 106 U/mL, equivalent to a calculated receptor occupancy of 99% for the low-affinity GM-CSF Rα. For WT neutrophils, the concentration of GM-CSF at which 50% neutrophil survival was seen was 100 U/mL. This was higher than previously observed for human neutrophils24 and may be caused by the in vivo exposure to G-CSF. A plateau-effect in prolongation of cell survival was seen at concentrations greater than 500 U/mL of GM-CSF for the WT cells. Results obtained for incubation of cells in IL-3 were similar for both WT and βc-null cells, with an IL-3 dose-response relationship demonstrable for both (data not shown). Thus, there was a lack of survival response of βc-null neutrophils to GM-CSF, while responsiveness to IL-3 was unaltered compared with WT cells. No evidence was seen for acceleration of cell death in the absence of βc (timepoints: 3 to 16 hours, n = 2 mice; >16 hours, n = 9 mice).
The survival of G-CSF elicited peripheral blood neutrophils (87% ± 2% pure) from WT (•) and βc-null (○) mice in in vitro cultures containing (A) 3 × 104 U/mL mGM-CSF (continuous line) or saline (broken line). (B) 4 × 103U/mL mIL-3 (continuous line) or saline (broken line). Cells were placed in microtiter trays (200 cells per well) and the number of viable cells was counted in four replicate wells at various time points thereafter (hours). (C) Dose-response relationship for neutrophils from WT (•) and βc-null (○) mice in mGM-CSF, starting concentration 3 × 104U/mL with fivefold dilutions. Results are the means of four wells. Error bars represent SD. One of six similar experiments.
The survival of G-CSF elicited peripheral blood neutrophils (87% ± 2% pure) from WT (•) and βc-null (○) mice in in vitro cultures containing (A) 3 × 104 U/mL mGM-CSF (continuous line) or saline (broken line). (B) 4 × 103U/mL mIL-3 (continuous line) or saline (broken line). Cells were placed in microtiter trays (200 cells per well) and the number of viable cells was counted in four replicate wells at various time points thereafter (hours). (C) Dose-response relationship for neutrophils from WT (•) and βc-null (○) mice in mGM-CSF, starting concentration 3 × 104U/mL with fivefold dilutions. Results are the means of four wells. Error bars represent SD. One of six similar experiments.
The ability of neutrophils from βc-null and WT animals to phagocytose latex beads was examined. With WT neutrophils, baseline phagocytic activity increased after incubation in GM-CSF (fold change 3.9 ± 1.2, n = 9 mice, Fig 3). In the βc-null mice, baseline levels of neutrophil phagocytosis were normal. However, no increase in phagocytic ability was seen in βc-null neutrophils incubated in GM-CSF (fold change 0.9 ± 0.2, n = 9 mice). The lack of response in terms of phagocytic activity in βc-null cells was also evident with higher doses of GM-CSF (up to 50,000 U/mL, equivalent to a calculated occupancy of 85% for the GM-CSF Rα; data not shown).
Phagocytosis of latex beads by G-CSF–elicited peripheral blood neutrophils from WT and βc-null mice. 105neutrophils were incubated in 1-mL cultures with 106 beads and either carrier or mGM-CSF, 4,000 U/mL, for 6 hours. Cytospin preparations were stained and 200 consecutive neutrophils were scored for number of cell-associated beads. The WPI was derived for each mouse by multiplying the number of neutrophils with 1, 2 to 3, 4, or ≥5 associated beads by 1, 2, 3, or 4, respectively, and dividing the total score by the number of neutrophils examined. Results for two mice of each genotype are shown and similar results were obtained in a further seven mice.
Phagocytosis of latex beads by G-CSF–elicited peripheral blood neutrophils from WT and βc-null mice. 105neutrophils were incubated in 1-mL cultures with 106 beads and either carrier or mGM-CSF, 4,000 U/mL, for 6 hours. Cytospin preparations were stained and 200 consecutive neutrophils were scored for number of cell-associated beads. The WPI was derived for each mouse by multiplying the number of neutrophils with 1, 2 to 3, 4, or ≥5 associated beads by 1, 2, 3, or 4, respectively, and dividing the total score by the number of neutrophils examined. Results for two mice of each genotype are shown and similar results were obtained in a further seven mice.
When cultured in vitro, cells excrete acidic metabolites into the culture medium. The production of acid metabolites can be quantitated and may increase in response to certain stimuli, such as exposure to cytokines.40 Figure 4 shows the ECAR after cytokine treatment of G-CSF–elicited neutrophils. GM-CSF induced an increase in the ECAR of cells from WT animals, with maximal levels achieved within 6 minutes. This gradually returned to baseline over several hours. This increase was absent when cells from βc-null animals were exposed to GM-CSF. The lack of response was maintained at concentrations of GM-CSF up to 106 U/mL. However, both WT and βc-null cells responded to IL-3 to a comparable degree.
Acidification responses of WT and βc-null G-CSF–elicited peripheral blood neutrophils in response to stimulation with cytokine. The change in ECAR versus time following normalization in running buffer (DME without bicarbonate buffering, 0.1% BSA, endotoxin free) and exposure to cytokine for 6 minutes is shown. Results for mIL-3, 102 U/mL (dashed line); WT (○), βc-null#1 (□), and βc-null#2 (◊). Results for mGM-CSF (continuous line) (102 U/mL, WT [•] and 104U/mL βc-null animal #1 [▪] and βc-null animal #2 [⧫]) and buffer alone (continuous line), WT (○), βc-null#1 (□). The cycle time was 2 minutes, and the pump speed was 120 μL/min with a pump-off time of 30 seconds. Three additional separate experiments were performed with similar results using a concentration of mGM-CSF in 0.1% BCS of 102 U/mL for both WT and βc-null mice with other conditions being unchanged.
Acidification responses of WT and βc-null G-CSF–elicited peripheral blood neutrophils in response to stimulation with cytokine. The change in ECAR versus time following normalization in running buffer (DME without bicarbonate buffering, 0.1% BSA, endotoxin free) and exposure to cytokine for 6 minutes is shown. Results for mIL-3, 102 U/mL (dashed line); WT (○), βc-null#1 (□), and βc-null#2 (◊). Results for mGM-CSF (continuous line) (102 U/mL, WT [•] and 104U/mL βc-null animal #1 [▪] and βc-null animal #2 [⧫]) and buffer alone (continuous line), WT (○), βc-null#1 (□). The cycle time was 2 minutes, and the pump speed was 120 μL/min with a pump-off time of 30 seconds. Three additional separate experiments were performed with similar results using a concentration of mGM-CSF in 0.1% BCS of 102 U/mL for both WT and βc-null mice with other conditions being unchanged.
Bronchoalveolar lavage of βc-null mice.
In WT mice the number of cells obtained by bronchoalveolar lavage was 0.23 ± 0.24 × 106 cells per animal (n = 40 mice). Macrophages were the predominant cell type (76% ± 22%) with lymphocytes (14% ± 6%) and neutrophils (10% ± 16%) also present. The number of cells in lavage fluid from βc-null animals was greatly increased (15.26 ± 7.78 × 106 cells per animal, n = 19 mice). Differential cell counts showed increased numbers of neutrophils (28% ± 13%), lymphocytes (29% ± 9%), and cells with typical macrophage morphology (27% ± 10%). In addition, there were cells with an atypical macrophage-like morphology, with increased amounts of foamy cytoplasm (16% ± 7%). Although there was a decrease in the percentage of alveolar macrophages in βc-null mice, the absolute number of typical alveolar macrophages was increased 20-fold (WT 0.18 × 106 per animal, βc-null 4.12 × 106). We also examined the bronchoalveolar lavage fluid of 2-week-old WT and βc-null mice to determine if changes in the cellular content were present at this young age. Again, neutrophils (56% ± 5%) were present and large atypical cells were seen (8% ± 1%) in lavage fluid from βc-null mice. However, the cellular yield was not increased compared with WT mice (βc-null 0.27 ± 0.35 × 106 per animal, n = 6 mice; WT 0.31 ± 0.2 × 106, n = 8 mice). Immunohistochemistry was performed to determine whether the large, foamy cells present in lung lavage fluid were macrophages (Table 1). Peritoneal macrophages from both WT and βc-null mice stained positively for the macrophage markers F4/80, macrosialin, and murine macrophage scavenger receptor (mMSR). Alveolar cells with typical macrophage morphology from WT and βc-null animals were positive for the macrophage surface markers macrosialin, sialoadhesin, and mMSR, and, as expected from previous reports, were negative for F4/80 and CR3.41 The large foamy alveolar cells were positive for macrophage markers (F4/80, macrosialin, sialoadhesin, and mMSR), the adhesion marker CR3 (β2 integrin), and for MHC class II, which when present on macrophages is a marker of activation. Thus, the foamy cells from bronchoalveolar lavage showed surface markers consistent with their being of macrophage origin.
Immunohistochemical Staining of Peritoneal and Bronchoalveolar Lavage Specimens From WT and βc Null Mice
| . | Peritoneal Macrophages . | Alveolar Macrophages . | |||
|---|---|---|---|---|---|
| WT . | βc Null . | WT . | βc Null . | ||
| Typical . | Large . | ||||
| F4/80 | + | + | − | + | + |
| Macrosialin/FA-11 | +/− | + | + | + | + |
| Sialoadhesin/IC2 | − | − | + | +/− | +/− |
| mMSR/2F8 | + | + | + | + | + |
| CR3/β2 integrin/5C6 | + | + | − | + | + |
| CD44/8D2 | + | +/− | ND | + | + |
| MHC class II/TIB 120 | − | − | − | − | + |
| . | Peritoneal Macrophages . | Alveolar Macrophages . | |||
|---|---|---|---|---|---|
| WT . | βc Null . | WT . | βc Null . | ||
| Typical . | Large . | ||||
| F4/80 | + | + | − | + | + |
| Macrosialin/FA-11 | +/− | + | + | + | + |
| Sialoadhesin/IC2 | − | − | + | +/− | +/− |
| mMSR/2F8 | + | + | + | + | + |
| CR3/β2 integrin/5C6 | + | + | − | + | + |
| CD44/8D2 | + | +/− | ND | + | + |
| MHC class II/TIB 120 | − | − | − | − | + |
Antibodies against a range of macrophage markers and adhesion molecules were tested. In each case samples incubated with the isotype control antibody were unstained.
Abbreviations: −, no staining detected; ND, not done.
Macrophage function in βc-null mice.
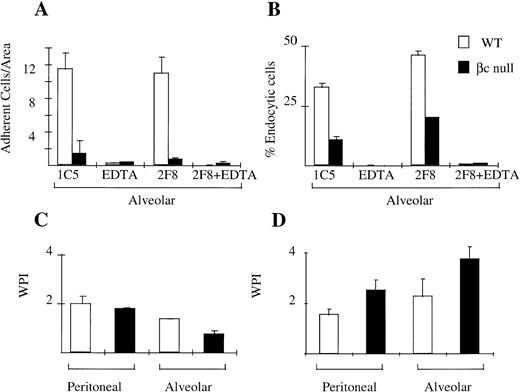
Cellular adhesion is an important function of macrophages which allows them to display site-preference and which facilitates endocytosis/phagocytosis. We assessed the adhesion of WT and βc-null peritoneal and alveolar macrophages in the presence and absence of EDTA and using the 2F8 antibody that blocks the function of mMSR. Peritoneal macrophages from βc and WT animals adhered normally to TCP. This adhesion was mediated via both divalent cation-dependent mechanisms and via mMSR (data not shown). Alveolar macrophages from WT animals remained adherent to TCP in the absence of inhibitors. This adhesion was substantially divalent cation-dependent. Alveolar macrophages from βc-null animals, however, displayed markedly reduced adhesion (Fig 5A).
Cellular adhesion and phagocytosis of three agents by WT and βc-null macrophages. (A) Alveolar macrophage adhesion (average cell number per area, derived as shown in Materials and Methods) with incubation in IgG2b isotype control antibody (IC5), chelator EDTA, 2F8 (MoAb to mMSR), or combination of EDTA plus 2F8. (B) Alveolar macrophage endocytosis of colloidal carbon in presence or absence of EDTA or 2F8. (C) Phagocytosis of latex beads. (D) Phagocytosis of opsonized sheep RBCs. Mean results of 2 to 4 wells, incubated at 37°C. No significant phagocytosis was observed in duplicate assays performed at 4°C. Error bars represent SD. One of three similar experiments.
Cellular adhesion and phagocytosis of three agents by WT and βc-null macrophages. (A) Alveolar macrophage adhesion (average cell number per area, derived as shown in Materials and Methods) with incubation in IgG2b isotype control antibody (IC5), chelator EDTA, 2F8 (MoAb to mMSR), or combination of EDTA plus 2F8. (B) Alveolar macrophage endocytosis of colloidal carbon in presence or absence of EDTA or 2F8. (C) Phagocytosis of latex beads. (D) Phagocytosis of opsonized sheep RBCs. Mean results of 2 to 4 wells, incubated at 37°C. No significant phagocytosis was observed in duplicate assays performed at 4°C. Error bars represent SD. One of three similar experiments.
We examined the phagocytic ability of WT and βc-null peritoneal and alveolar macrophages using four different agents: colloidal carbon (alveolar macrophages only), opsonized sheep RBCs, latex beads, and DiI-AcLDL. Adherent peritoneal macrophages from both WT and βc-null were able to phagocytose all agents tested (Fig 5C and D, data for endocytosis of DiI-AcLDL not shown). Alveolar macrophage phagocytosis assays were performed by modifying the washing procedure as described in Materials and Methods. There was no significant difference in the ability of adherent alveolar macrophages from WT and βc-null mice to take up latex beads or sheep RBCs (Fig 5C and D). However, there was a reduction in the uptake of colloidal carbon (Fig 5B) by adherent βc-null alveolar macrophages. Neither the adhesion defect nor the colloidal carbon uptake defect was altered by addition of IL-3 or G-CSF to the assays (data not shown).
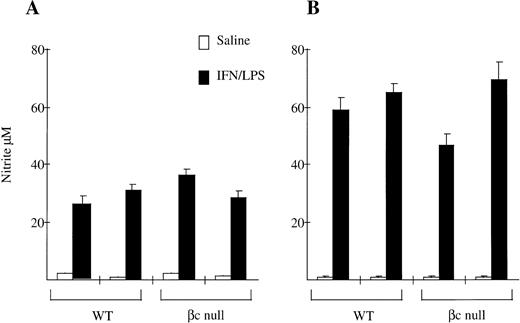
We also examined NO production by peritoneal macrophages. As shown in Fig 6, NO production in response to IFN-γ and LPS by resident peritoneal macrophages from βc-null animals was the same as for macrophages from WT animals (WT 28 ± 4 μmol/L nitrite; βc-null 32 ± 5, n = 2 mice). There was no consistent difference between cells from WT and βc-null mice in five experiments, although considerable inter-experimental variability was observed. NO production by thioglycollate-elicited peritoneal macrophages from βc-null animals was also similar to WT (WT 61 ± 4; βc-null 57 ± 16, n = 2 mice, similar results in three experiments). We then examined NO production in vitro by peritoneal macrophages in response to heat killed L monocytogenes and did not detect a difference between βc-null and WT macrophages (data not shown). We were not able to examine NO production using βc-null alveolar macrophages because of the adhesion defect described above. In summary, in these assays the peritoneal macrophages from βc-null mice showed normal adhesion, phagocytic ability, and nitric oxide production. In contrast, the βc-null pulmonary macrophages showed reduced cellular adhesion. However, the macrophages that did adhere sufficiently to be examined were able to phagocytose latex beads, opsonized sheep RBCs, and DiI-AcLDL normally, but displayed a reduction in the ability to take up colloidal carbon.
Nitrite production by (A) resident peritoneal macrophages and (B) thioglycollate-elicited peritoneal macrophages from WT and βc-null mice in response to saline or IFN-γ/LPS after 48 hours of incubation. Mean of 4 to 5 wells. Error bars represent SD. Results shown are for two mice per genotype. Five experiments performed with similar results.
Nitrite production by (A) resident peritoneal macrophages and (B) thioglycollate-elicited peritoneal macrophages from WT and βc-null mice in response to saline or IFN-γ/LPS after 48 hours of incubation. Mean of 4 to 5 wells. Error bars represent SD. Results shown are for two mice per genotype. Five experiments performed with similar results.
Glucose transport in bone marrow cells from βc-null mice.
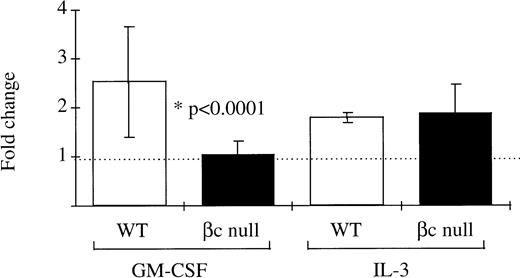
It has been previously suggested that the GM-CSF Rα alone may mediate signaling for glucose transport.11 12 Therefore, this hypothesis was examined using βc-null bone marrow cells which express only the GM-CSF Rα. In normal WT bone marrow cells the fold increase over control in uptake of 3H-2-DOG after incubation in GM-CSF was 2.53 ± 1.13 (n = 14 mice, GM-CSF concentrations ranging from 500 U/mL to 50,000 U/mL: Fig 7). However, in bone marrow cells from βc-null animals there was no significant increase in glucose uptake after incubation in GM-CSF (fold increase in 3H-2-DOG uptake 1.02 ± 0.30, n = 22 mice examined). At the highest concentration of GM-CSF used, the calculated receptor occupancy of the low-affinity receptor was 85%. In contrast, a similar increase in uptake of 3H-2-DOG was seen after incubation in IL-3 for both WT cells (fold change 1.85 ± 0.12, n = 4 mice) and βc-null cells (2.00 ± 0.69, n = 8 mice). GM-CSF– or IL-3–stimulated uptake of 3H-2-DOG was inhibited by cytochalasin B, an inhibitor of facilitative glucose transport (average percent inhibition 91% ± 6%, n = 11 samples) but not by the inactive analogue, cytochalasin E (average percent inhibition 33% ± 20%, n = 2 samples). These data showed that the GM-CSF Rα alone was insufficient to mediate GM-CSF signaling for increased glucose transport in hematopoietic cells from mice lacking the βc chain.
The ability of mouse bone marrow cells from WT and βc-null mice to take up the glucose analogue 3H-2-DOG after incubation in GM-CSF (concentration range, 500 to 50,000 U/mL) or carrier. Cells were incubated in 2-DOG and then 3H-2-DOG, 1 μCi per 1 mL culture, was added for 10 minutes’ incubation at 37°C. The fold change in glucose uptake after incubation in GM-CSF compared with carrier was calculated for each cell preparation. Results are means of 14 WT mice and 22 βc-null mice. Error bars represent SD.
The ability of mouse bone marrow cells from WT and βc-null mice to take up the glucose analogue 3H-2-DOG after incubation in GM-CSF (concentration range, 500 to 50,000 U/mL) or carrier. Cells were incubated in 2-DOG and then 3H-2-DOG, 1 μCi per 1 mL culture, was added for 10 minutes’ incubation at 37°C. The fold change in glucose uptake after incubation in GM-CSF compared with carrier was calculated for each cell preparation. Results are means of 14 WT mice and 22 βc-null mice. Error bars represent SD.
DISCUSSION
GM-CSF enhances the activity of a range of mature hematopoietic cell functions, including mature cell survival,24phagocytosis,42,43 leukocyte adhesion,44 and proliferation and activation of alveolar macrophages.45-47To date, the function of mature blood cells in hematopoietic cytokine and cytokine receptor null mice has largely been examined by assessing the response to infection with parasitic and bacterial organisms.48-51 In this study we used a range of in vivo and in vitro assays to examine the function of neutrophils and macrophages from βc-null mice.
We administered G-CSF to βc-null and WT mice to obtain a neutrophil-rich cell population for analysis.22 The in vivo exposure to G-CSF did not impair the ability of cells to bind GM-CSF. In both WT and βc-null cell preparations neutrophil responses at baseline or in response to IL-3 were normal for survival, phagocytosis, and hydrogen ion secretion. In βc-null cells a GM-CSF–mediated increase in these functions was not seen.
A role for the GM-CSF Rα in mediating glucose uptake has been described in two studies. In one, Xenopus laevis oocytes were injected with RNA encoding human GM-CSF Rα, and in another melanoma cell lines that endogenously expressed only low-affinity receptors for GM-CSF were used.11 12 In contrast with these studies, an increase in glucose uptake in response to GM-CSF was not seen in βc-null neutrophils expressing only the low-affinity GM-CSF Rα chain. Moreover, the lack of an extracellular acidification response to GM-CSF in neutrophils that express GM-CSF Rα alone suggests that occupancy of GM-CSF Rα leads to little change in the metabolic state of the cells.
The in vitro survival of βc-null neutrophils cultured in the presence of saline or IL-3 did not differ from WT. Iversen et al52 53 have shown that the GM-CSF analog E21R, which binds normally to the GM-CSF Rα but abnormally to βc, causes apoptosis of hematopoietic cells in the presence of the high-affinity GM-CSF receptor. These studies, together with our observations, demonstrate that the GM-CSF Rα alone is insufficient to mediate a survival signal in hematopoietic cells.
In keeping with the pathological findings, examination of the bronchoalveolar lavage fluid from βc-null mice showed increased total cellularity and the presence of large foamy cells. In this study we used immunohistochemistry to demonstrate that these large cells were of the macrophage lineage. In in vitro assays of macrophage function, we found that adhesion and phagocytosis of colloidal carbon by βc-null alveolar macrophages were reduced. Immunohistochemistry showed the βc-null macrophages did express CR3 and mMSR, which have been implicated in these macrophage functions.35 The defects may be secondary to the lung disease, because a similar adhesion defect has been described in patients with human pulmonary alveolar proteinosis.54 Overall, we were unable to demonstrate defects in macrophage function that could account for the alveolar proteinosis-like disease seen in these mice. To address this further, we are now undertaking studies examining the surfactant catabolism by βc-null alveolar macrophages.
Production of NO in response to IFN-γ/LPS was similar in both WT and βc-null peritoneal macrophages. Similarly, the production of NO by βc-null peritoneal macrophages in response to heat-killed L monocytogenes was normal, consistent with reports that the response of βc-null mice to infection with this organism is similar to that of WT.48 NO production by βc-null alveolar macrophages was unable to be assessed because of the adhesion defect of these cells. These results are similar to those in a recent study in which the NO response of peritoneal macrophages from GM-CSF null mice, when stimulated with IFN-γ/LPS, was similar to that of WT.55The investigators did observe a decrease in NO production when peritoneal macrophages from GM-CSF null mice were stimulated with LPS alone.
In summary, this study showed that neutrophils and peritoneal macrophages from βc-null mice were capable of normal survival, phagocytosis, and NO production. In contrast, alveolar macrophages, implicated in the lung disease alveolar proteinosis, showed impaired adhesion and reduced ability to phagocytose colloidal carbon. No GM-CSF–elicited responses were seen in cells from βc-null mice. In particular, the GM-CSF Rα alone was unable to mediate glucose transport in hematopoietic cells lacking the βc chain.
ACKNOWLEDGMENT
We thank Drs Yifan Zhan and Christina Cheers for performing theListeria experiments, and Bette Papaevangeliou for technical assistance. We are grateful to Prof Donald Metcalf for comments on the manuscript.
Supported by the National Health and Medical Research Council, Canberra, the Anti-Cancer Council of Victoria, National Institutes of Health Grant No. CA22556, and the Australian Government Cooperative Research Centres Scheme.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to C.L. Scott, MD, The Walter and Eliza Hall Institute of Medical Research, PO Royal Melbourne Hospital, Victoria 3050 Australia.

![Fig. 4. Acidification responses of WT and βc-null G-CSF–elicited peripheral blood neutrophils in response to stimulation with cytokine. The change in ECAR versus time following normalization in running buffer (DME without bicarbonate buffering, 0.1% BSA, endotoxin free) and exposure to cytokine for 6 minutes is shown. Results for mIL-3, 102 U/mL (dashed line); WT (○), βc-null#1 (□), and βc-null#2 (◊). Results for mGM-CSF (continuous line) (102 U/mL, WT [•] and 104U/mL βc-null animal #1 [▪] and βc-null animal #2 [⧫]) and buffer alone (continuous line), WT (○), βc-null#1 (□). The cycle time was 2 minutes, and the pump speed was 120 μL/min with a pump-off time of 30 seconds. Three additional separate experiments were performed with similar results using a concentration of mGM-CSF in 0.1% BCS of 102 U/mL for both WT and βc-null mice with other conditions being unchanged.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/11/10.1182_blood.v92.11.4119/5/m_blod42340004x.jpeg?Expires=1770959773&Signature=sOPZ5bsEYi6VPoJ769Lc6RSj~Topt4lmrhzK84ra-DciFlmIQqtdctAABEHRm6jbQxpCATur-zQ84o6--rII5v3ykAsPIp47q0iTiay~BN8TTvLo4Wp2JZG8BNC5q~OAW-JzAe2LbsWbx4-e-b2Y9Sv6ihIMuy3wh~3HB6yVjQggRoSF9ptOfJkGBmUaTBpI3vY5TJKBCUfYfznqOAsbek14xWilXOFsx~U1C26uppErFVdtmg5kA1eaLt53GqyHPUkCAh6iBxZNCukSrJOit~uCThXDZqYxTOEcPvTR9MInk4nAC4uZ3dsUx4I4FEsbfdbPainCqgjvgvTScqMLOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal