Abstract
X-linked severe combined immunodeficiency (SCID-Xl) is a rare human inherited disorder in which early T and natural killer (NK) lymphocyte development is blocked. The genetic disorder results from mutations in the common γc chain that participates in several cytokine receptors including the interleukin-2 (IL-2), IL-4, IL-7, IL-9, and IL-15 receptors. We have shown in a previous report that γc gene transfer into SCID-Xl bone marrow (BM) cells restores efficient NK cell differentiation. In this study, we have focused on the introduction of the γc gene into SCID-Xl hematopoietic stem cells with the goal of obtaining differentiation into mature T cells. For this purpose, we used the in vitro hybrid fetal thymic organ culture (FTOC) system in which a combination of cytokines consisting of stem cell factor (SCF), Flt-3L, IL-7, IL-1, and IL-15 is added concomitantly. In this culture system, CD34+ marrow cells from two SCID-Xl patients were able to mature into double positive CD4+ CD8+ cells and to a lesser degree into CD4+ TCRβ+ single positive cells after retroviral-mediated γc gene transfer. In addition, examination of the output cell population at the TCR DJβ1 locus exhibited multiple rearrangements. These results indicate that restoration of the γc/JAK/STAT signaling pathway during the early developmental stages of thymocytes can correct the T-cell differentiation block in SCID-Xl hematopoietic progenitor cells and therefore establishes a basis for further clinical γc gene transfer studies.
THYMIC PROGENITORS acquire a number of cell surface molecules during their highly regulated maturation program. The earliest human precursor cells that seed the thymus from the fetal liver and bone marrow (BM) are CD34high CD38(−) and retain multipotential capacity.1-3 The loss of myeloid differentiation potential is heralded by upregulation of the CD45RA marker. This population has been shown to contain T, natural killer (NK), and dendritic cell precursor activity.1,4-6 The next step in maturation is the commitment to bipotential T/NK cells that express CD7, CD2, and cytoplasmic CD3. At this point, the β, γ, and δ loci are still in a germline configuration. Acquisition of CD1a marks the irreversible committment to the T-cell lineage. As these pre-T cells upregulate CD4 to become immature single positive cells (ISP) phenotypically characterized as CD34(−) CD38++CD45RO+ CD7+ CD2+ CD5+CD1+ CD4+, they gradually switch from CD45RA to CD45RO expression.4 After the progression to the CD4+ CD8+ double positive (DP) stage, thymocytes that express CD3/TCR at low levels undergo positive selection by interaction with the major histocompatibility complex (MHC), and it is during this process that TCRαβ+ DP cells develop into mature CD4+ or CD8+ single positive cells (SP).7,8 Furthermore, this positive selection signal induces the expression of CD69, Bcl-2, and CD27.9-15 Finally, CD1a is downregulated, and CD45RA is expressed immediately before these cells emigrate out of the thymus.
In addition to TCR/CD4/CD8-MHC class II/I interactions, this process involves cell-cell interactions between numerous different surface molecules on developing thymocytes and thymic cortical epithelial cells,16 as well as soluble factors. Thymocytes have indeed been shown to be responsive to cytokines in vitro.17 A variety of cytokines is produced by the thymic microenvironment including interleukin-1α (IL-1α), IL-3, IL-6, IL-7, IL-12, IL-15, granulocyte-macrophage colony-stimulating factor (GM-CSF), M-CSF, G-CSF, and stem cell factor (SCF).18 19 The role of IL-7 in T-cell development has been best defined in murine models.
The profound and early block observed in T and NK cell differentiation in patients with SCID-Xl,20 caused by mutations in the γc cytokine receptor gene,21 constitutes indirect evidence for the requirement of γc-binding cytokines, such as IL-7, during early thymocyte developmental stages. Nevertheless, the lack of signaling via the γc/Jak/STAT pathway could also be attributed to an inability to respond to other cytokines, which also use the γc chain such as IL-2, IL-4, IL-9, and IL-15.22 In a previous report, we have shown that γc gene transfer into SCID-Xl BM cells restores efficient NK cell differentiation in the presence of SCF and IL-15.23Function of the vector encoded γc chain was also demonstrated by restoration of high-affinity IL-2R expression and normal JAK3 activation in SCID-Xl B cell lines after γc gene transfer.24
To investigate the potential for T-cell development after γc gene transfer to CD34+ SCID-Xl BM cells, we have used the fetal murine thymic organ culture (FTOC) system as developed by Jenkinson et al.25 The murine FTOC environment can to some extent support human T lymphopoiesis from fetal sources or the postnatal thymus26-31 without the addition of mitogens or cytokines, yet we and others3 have found that the T-cell developmental potential of precursor cells isolated from cord blood (CB) or postnatal BM cells is very low and variable. While the CD34+CD38(−) population from human fetal BM contains both stromal and hematopoietic progenitors, the frequency of stromal progenitors decreases proportionally with increasing fetal gestational age.32 It is thus possible that the poor developmental potential in the FTOC system using CB or postnatal BM cells is related to the lack of stromal progenitors that have the potential to develop into cells capable of producing the human cytokines necessary for survival, growth, and subsequent T-cell development of the input cells. We have therefore defined FTOC culture conditions able to promote proliferation and T-cell differentiation of postnatal CD34+ CB cells. Using these defined conditions, we have shown that γc transduced CD34+ SCID-Xl BM cells can give rise to T lymphocyte differentiation in vitro with full phenotypic maturation. This experimental system, combining retroviral gene transfer and FTOC, constitutes a preclinical step towards the treatment of SCID-Xl patients with γc gene transfer.
MATERIALS AND METHODS
Cell samples and CD34+ cells purification.
All samples were obtained after parental informed consent. Human umbilical CB samples were collected immediately after delivery. SCID-Xl BM samples were obtained from two patients (S1 and S2) at the ages of 6 and 7 months, respectively, while under general anesthesia for central line insertion before undergoing BM transplantation.
The mutation detected in patient S1 in exon 3 (G355T) led to a glycine → valine substitution. In patient S2, a complex rearrangement resulting in a 2-bp deletion at position 836 and a 13-bp insertion at position 847 with a premature stop codon in exon 7 (amino acid 293). γc chain expression at the surface of both patients’ B lymphocytes was undetectable. In the peripheral blood of patient S1, the lymphocyte count was 612/μL, CD3+ 2%, CD19+ 73%, and CD56+ 1%. In patient S2, less than 1% CD3+cells were detected.
The mononuclear cell fraction from BM and CB samples was obtained by density gradient centrifugation (density 1.077 g/mL; Nycomed Pharma, Oslo, Norway) and for BM was cryopreserved before further processing. The mononuclear cell fraction was then enriched for primitive progenitors by immunoselection using the Ceprate LC34 cell separation system (Cell Pro, Bothell, WA). Enriched CD34+ cells were then incubated with phycoerythrin (R-PE) labeled anti-CD34 (PE-HPCA2; Becton Dickinson, San Jose, CA) and separated by cell sorting on a FACS-Star Plus cell sorter (Becton Dickinson). The purity of sorted cells, as assessed by analyzing after sorting, was greater than 99%.
Retroviral vector and packaging cell line.
Human γc chain cDNA extending from the initiation codon ATG (nucleotides numbered 1 to 1114) was generated by reverse transcription-polymerase chain reaction (RT-PCR) from mRNA of control B-cell lines. Forward primer: 5′-GCAAGCGACATGTTGAAGCC-3′, and reverse primer: 5′-GAGGATCCGGGTTCAGGTTTCAG-3′ contain an AFL III andBamHI site, respectively, allowing for γc chain insertion in the retroviral vector. Correct γc sequence was assessed by direct sequencing of the entire PCR amplified fragment. The human γc chain cDNA was then inserted into the Nco I and BamHI sites of the MFG B2 γc Mo-long terminal repeat (LTR) vector.33 MFG (B2) uses the Moloney murine leukemia virus (MO-MLV) LTRs for transcription of the viral genome and contains the B2 mutation corresponding to a single G to A transition at position +160 of the MO-MLV sequence.34 This plasmid, named MFG (B2)-γc, does not contain a selectable marker. Retroviral producer cell lines were then generated by cotransfecting the MFG (B2)-γc plasmid with the plasmid pSV2-neo into the amphotropic packaging cell line Ψ CRIP, as previously described.35 Two days later, the transduced cells were diluted 10 times and placed under G418 (Geneticin; GIBCO/BRL, Gaithersburg, MD) selection at 0.8 to 1 mg/mL active concentration until individual resistant colonies formed. Viral titering was performed by infecting 5 × 105 NIH3T3 cells with 0.5 mL of a 24-hour supernatant from the virus-producing clones in the presence of 8 μg/mL of Polybrene (Sigma Chemical Co, St Louis, MO). Thirty clones were screened for high-titer virus production by Southern blot analysis of the target cells. Southern blot analysis confirmed the presence of an unrearranged proviral genome from these producer clones and was used to determine the number of proviral copies integrated in the target population. The clone that produced the highest transfer efficiency of virus to NIH3T3 cells (0.5 copy of provirus per cell) was used in the subsequent transduction experiments.
Transduction of SCID-Xl mononuclear bone marrow cells (SCID-Xl BMC).
Purified CD34+ SCID-Xl BMC were prestimulated by culturing in the presence of SCF (100 ng/mL, kindly provided by Amgen, Thousand Oaks, CA), Flt3 ligand (Flt-3L; 100 ng/mL, kindly provided by Immunex, Seattle, WA), and IL-3 (20 ng/mL, kindly provided by Sandoz, Basel, Switzerland) at 37°C, 5% CO2 for 24 hours. The cells were then transduced on human recombinant fibronectin-coated wells (fragment CH296; Takara, Ohtsu, Japan) by culturing in vector conditioned media in the presence of the same cytokines and protamine sulfate (4 μg/mL) on 3 consecutive days. Viral supernatant was replaced every 24 hours during the 3-day transduction period.
Hybrid human/mouse FTOC.
Murine thymic lobes dissected from day 14 embryos (C57/Bl6) were treated with 1.35 mmol/L 2′deoxyguanosine (Sigma) for 5 to 6 days to remove endogenous thymocytes and hematopoietic cells before reconstitution. The thymic lobes were then washed extensively and cocultured in hanging drops in wells of a Terasaki plate with 1 to 3 × 104 fluorescence-activated cell sorter (FACS) Sorted CD34+ CB cells or 5 × 104 CD34+transduced SCID-Xl BM cells. Cells were allowed to seed the lobes during a 48-hour coculture. Thymi were then transferred to nucleopore filters (Costar, Cambridge, MA), which were layered over gelfoam sponge (Upjohn, Kalamazoo, MI) in 6-well plates (Costar). Culture medium consisted of RPMI (GIBCO/BRL) supplemented with 5% fetal calf serum (FCS) (Stem Cell Technologies, Vancouver, Canada) and 7% human AB serum. The following cytokines were used from the start of the hanging-drop culture throughout the FTOC: SCF 100 ng/mL (Amgen), Flt-3L 100 ng/mL (Immunex), IL-7 20 ng/mL (Genzyme), IL-1α 2 ng/mL (Genzyme), IL-15 20 ng/mL (Immunex), as indicated. Cultures were incubated at 37°C in 5% CO2 for 4 to 5 weeks. Afterwards, the thymic lobes were mechanically dispersed into a single cell suspension. Cells were enumerated and assessed for viability by trypan blue exclusion.
Liquid cultures in the presence of cytokines.
One hundred thousand sorted CD34+ CB cells were plated in flat-bottomed 96-well plates in the same complete RPMI medium supplemented with the same combination of cytokines used in T-cell differentiation assay (FTOC). After 30 days, growing cells were stained and analyzed by FACS.
Flow cytometry analysis.
Phenotypic analysis of human cells recovered from the hybrid mouse FTOC was performed using the following monoclonal antibodies (MoAbs): anti-CD34 (HPCA-2), anti-CD7 (Leu 9), anti-CD2 (Leu5b), anti-CD5 (Leu1), anti-CD4 (Leu 3a), anti-CD8 (Leu 2a), anti-CD3 (Leu 4), anti-CD56 (Leu 19), anti-CD16 (Leu 11b), anti-CD14 (Leu M3), anti-CD45RO, anti-CD45RA, (mouse antihuman PE or fluorescein isothiocyanate [FITC]-conjugated) all from Becton Dickinson, anti–TCR-αβ and anti–TCR-γδ from Immunotech (Marseille, France), rat antihuman γc (unconjugated) from Pharmigen (San Diego, CA) and anti-CD1a (unconjugated) from Becton Dickinson. Control isotype Ig FITC was obtained from Pharmigen. For conjugated antibodies, cells were stained for 30 minutes on ice and after two washings were subjected to flow cytometric analysis. For γc staining, cells were first incubated with unconjugated rat anti-human γc for 30 minutes, washed twice followed by staining with biotin-conjugated mouse antirat Ig (Jackson, Westgrove, PA). The second antibody was detected with PE-conjugated avidin (Caltag, San Francisco, CA). For CD1a staining, cells were first incubated with unconjugated mouse anti-CD1a and detected with FITC-conjugated rat antimouse Ig (Jackson, Baltimore, MD). Cells were suspended in phosphate-buffered saline (PBS) and subjected to flow cytometric analysis using a FACScan (Becton Dickinson). Between 5,000 and 20,000 events were collected per sample (depending on the availability of cells). For analysis, a live gate was set on forward and side scatter. Human cell origin was verified by staining for human CD45 expression.
Provirus integration study in thymocytes derived from CD34+ γc transduced cells.
To test for vector integration in the progeny of SCID-Xl CD34+ γc transduced cells, thymocytes were recovered and their DNA isolated. To specifically amplify γc proviral DNA, two primers were used, one within the retroviral backbone and one within γc gene sequence, respectively: PM-R 5′-GACCACTGATATCCTGTCTTCAAC-3′ and γc-F 5′-CCAGCCTACCAACCTCACT-3′. DNA was amplified in a 50-μL PCR reaction mixture using 30 cycles at an annealing temperature of 60°C. A 20-μL portion of the amplified product was separated on a 1% agarose gel and analyzed by ethidium bromide staining.
TCRβ gene rearrangement analysis.
Genomic DNA was prepared from human thymocytes recovered from organ cultures and PCR for DJβ amplification was conducted using the two primers TBF1 (upstream to the Dβ1 segment) 5′-TGGGAGGGGCTGTTTTTGTA-3′ and TBR1 (downstream to the Jβ1-6 element) 5′-TCCAGGTAAGAAGGGGTGAC-3′, and the hybridization probe was TBR3 5′-CTGACCTCCGTTCTTACACT-3′. PCR was performed with 30 cycles of 1 minute, 94°C; 2 minutes, 61°C; and 10 minutes, 72°C. The amplified product was separated on a 1% agarose gel, transferred to a nylon membrane, and probed with a radiolabeled TBR3 oligonucleotide.
RESULTS
Effect of different cytokine combinations on T-cell development from CD34+ CB cells.
To study the effects of different cytokine combinations on T-cell development from CD34+ cells derived from infants, we used CB CD34+ cells as an appropriate control for SCID-Xl BM CD34+ cells given the lack of availability of age-matched marrow cells.36 We compared several combinations of cytokines to establish the experimental conditions necessary for optimal cell proliferation and T-cell differentiation from CD34+ CB cells in the hybrid human/mouse FTOC.
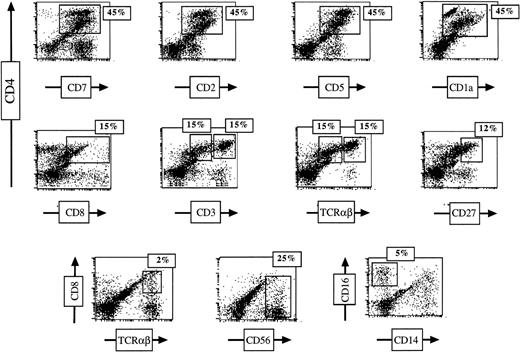
CD34+ CB cells were selected by an immunoaffinity column, further purified by FACS, and cultured together with mouse thymic lobes (10,000 to 30,000 cells per lobe) in the presence of SCF + Flt-3L + IL-7, SCF + Flt-3L + IL-7 + IL-1α, or SCF + Flt-3L + IL-7 + IL-1α + IL-15. After an incubation period of 4 to 5 weeks, recovered cells were enumerated and stained with antihuman CD45 to determine the total number of human cells. The cell expansion rate in FTOC was very low in the presence of SCF + Flt-3L + IL-7, with a less than twofold increase in the cell number per thymic lobe (Table1). CB CD34+ cells were shown to develop into double positive (DP), as well as CD4+TCRαβ+ mature T cells. However, the vast majority of the recovered cells had an immature (ISP) phenotype. CD56+ NK cells were also detected. The addition of IL-1α (2 ng/mL) considerably enhanced cell proliferation (Table 1), but did not result in differentiation beyond the DP CD4+CD8+ stage (Table 1). The recovered population expressed CD4, CD7, CD2, CD5, CD1a with or without CD8low (DP or ISP, respectively, Table 1). Leclercq et al19 have recently shown that the addition of a low concentration of IL-15 to FTOC colonized by murine fetal thymocytes increased cell proliferation. To address whether IL-15 could play a role in proliferation and/or differentiation of human CD34+ CB cells in mouse thymic lobes, T-cell differentiation was assessed in FTOC in the presence of this cytokine (20 ng/mL). Although some variability between experiments was observed, the thymic cell recovery was high (fivefold to sixfold increase) in this culture condition. Cells acquired surface expression of CD7, CD2, CD5, CD1a, and CD4. Although the ISP subset was predominant (Table 1), the next stages of DP and CD4+ SP mature T cells could also be detected. A typical experiment is depicted in Fig1, showing that 15% of cells were CD4+ SP TCR αβ+ cells. Fifteen percent were CD4+CD8+CD3low DP and 15% CD4+ ISP after 5 weeks of culture (not shown). All of these cell subsets coexpressed CD7, CD2, CD5, and CD1a. In addition, in this experiment, 2% of the TCR αβ+ cells were CD8+. However, in most experiments no CD8+ SP mature cells could be detected. As also shown in Fig 1, CD56+ and CD16+ CD14(−) NK cells were detectable. The CD4+ CD3high SP thymocyte subset expressed some features of cells that have responded to positive selection signals, as they were CD27 (Fig 1) and CD69 positive (not shown). However, these cells were still expressing CD1a as found in all other experiments. This finding is consistent with data published by Res et al31 indicating that another species-specific signal is required for the downregulation of CD1a in the further maturation of the CD3high TCR αβ+ cell subset. To exclude possible contamination of CD34+ sorted cells by mature T cells, an aliquot of sorted CD34+ CB cells used in the FTOC system was placed in liquid culture supplemented with the same cytokine combination. Three major subsets of cells emerged under these culture conditions: two populations bearing NK-cell markers CD56 and CD16, and a third population expressing the monocytic antigen CD14 (data not shown). This liquid culture failed to promote T lymphopoiesis, indicating that no expansion of contaminating T cells took place. Together, these data conclusively demonstrate that the addition of SCF, Flt-3L, IL-7, IL-1α, and IL-15 resulted in human T-cell differentiation from human CB CD34+ cell in murine FTOC. These culture conditions were therefore chosen for assessing γc gene transfer into CD34+ γc(−) cells.
Effect of Different Cytokine Combinations on Cell Yield and T-Cell Immunophenotype Acquisition by CD34+ Cells in Fetal Thymic Organ-Cultured Lobes
| Cytokines Added . | Cell Expansion . | Differentiation Stages (%) . | ||||
|---|---|---|---|---|---|---|
| ISP . | DP . | SP CD4 . | SP CD8 . | CD56 . | ||
| SCF + Flt-3L + Il-7 | ×2 | 54 ± 30 | 4 ± 3 | 3 ± 2 | 0 | 3 ± 2 |
| SCF + Flt-3L + Il-7 + Il-1α | ×6-7 | 50 ± 50 | 50 ± 50 | 0 | 0 | 0 |
| SCF + Flt-3L + Il-7 + Il-1α + Il-15 | ×5-6 | 35 ± 20 | 10 ± 5 | 10 ± 5 | 2 ± 2 | 29 ± 15 |
| Cytokines Added . | Cell Expansion . | Differentiation Stages (%) . | ||||
|---|---|---|---|---|---|---|
| ISP . | DP . | SP CD4 . | SP CD8 . | CD56 . | ||
| SCF + Flt-3L + Il-7 | ×2 | 54 ± 30 | 4 ± 3 | 3 ± 2 | 0 | 3 ± 2 |
| SCF + Flt-3L + Il-7 + Il-1α | ×6-7 | 50 ± 50 | 50 ± 50 | 0 | 0 | 0 |
| SCF + Flt-3L + Il-7 + Il-1α + Il-15 | ×5-6 | 35 ± 20 | 10 ± 5 | 10 ± 5 | 2 ± 2 | 29 ± 15 |
Numbers represent mean ± SD (4 experiments).
Abbreviations: ISP, immature single positive; DP, double positive; SP, single positive.
CD34+ cord blood cells in FTOC acquire a T-cell immunophenotype in the continuous presence of SCF, Flt-3L, IL-7, IL-1, and IL-15. CD34+ cells were sorted and cultured in fetal thymic lobes with the cytokines for 35 days. FTOC were then harvested, and T-cell differentiation was assessed by flow cytometric analysis using the indicated antibodies.
CD34+ cord blood cells in FTOC acquire a T-cell immunophenotype in the continuous presence of SCF, Flt-3L, IL-7, IL-1, and IL-15. CD34+ cells were sorted and cultured in fetal thymic lobes with the cytokines for 35 days. FTOC were then harvested, and T-cell differentiation was assessed by flow cytometric analysis using the indicated antibodies.
Retrovirus-mediated γc gene transfer into γc(−) CD34+ marrow cells from two SCID-Xl patients.
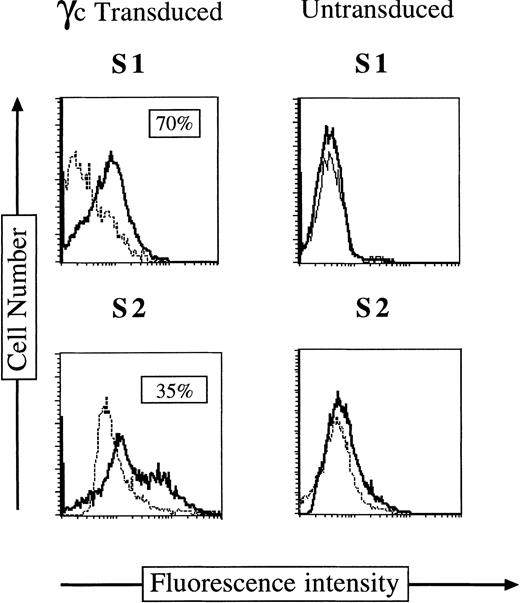
We have previously shown that cocultivation of bone marrow mononuclear cells23 or SCID-Xl EBV-transformed B-cell lines24 on the packaging cell line Ψ CRIP MFG (B2)γc allowed efficient γc gene transfer. To improve transduction efficiency under clinically applicable conditions, we used a strategy combining a standard supernatant transduction with the use of fibronectin fragment-coated culture wells.37-39 It has been shown previously that this fibronectin fragment colocalizes retroviral particles and target cells, optimizing gene transfer efficiency.39 Previously cryopreserved SCID X-l marrow mononuclear were thawed and cultured for 18 hours before CD34+ cell selection and sorting. The CD34+γc(−) sorted cells were prestimulated by culture for 24 hours in the presence of SCF, Flt3-L, and IL-3, followed by culture in MFG (B2)γc-conditioned supernatant for 3 cycles of 24 hours each on fibronectin-coated wells as described in Materials and Methods. As a control, CD34+ γc(−) cells were mock-transduced by culture under the same conditions without viral supernatant addition. As shown in Fig 2, under these experimental conditions, 35% to 70% of CD34+cells expressed γc on their surface immediately after the three cycles of infection.
γc Chain expression on CD34+ cells immediately after γc gene transfer. Immunofluorescence staining of surface γc chain on CD34+ S1 and S2 BM cells after γc transduction (left panel) or on uninfected CD34+ S1 and S2 cells (right panel).
γc Chain expression on CD34+ cells immediately after γc gene transfer. Immunofluorescence staining of surface γc chain on CD34+ S1 and S2 BM cells after γc transduction (left panel) or on uninfected CD34+ S1 and S2 cells (right panel).
Development of CD34+ γc(−) cells into T cells after γc gene transfer and culture in fetal thymic lobes.
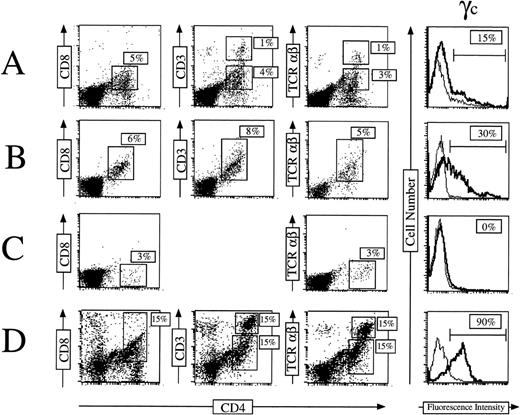
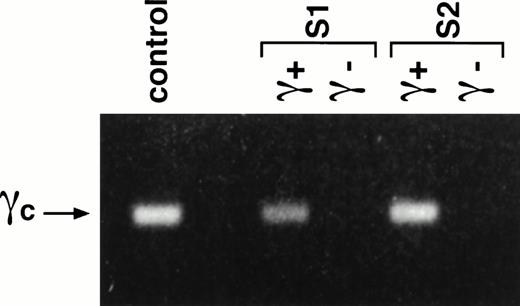
Fifty thousand CD34+γc transduced or mock-transduced cells (from the two patients) were used to reconstitute deoxyguanosine-treated fetal thymic lobes. The organ cultures were maintained 4 to 5 weeks in the continuous presence of SCF + Flt3-L + IL-7 + IL-1α + IL-15. Thymocytes were then recovered and assayed for γc and other cell surface markers expression. As shown in a representative experiment (Fig 3), 15% (patient S1) and 30% (patient S2) of recovered cells expressed γc at their surface (Fig 3A and B). These cells were all positive for CD45 staining showing their human origin. Conversely all CD45+cells were γc(+). γc(+) cells acquired the capacity for CD4 CD8 DP development in the thymic microenvironment. The latter subset represented 30% of γc(+) cells for S1 and 20% for S2. These data are consistent with those obtained from CD34+ CB cells (Fig3D). In addition to the DP subset, SP CD4+CD3high TCRαβ+ cells were also detected. Figure 3A shows that this population represented 6% of the human thymocytes. In some experiments, development of CD56+ NK cells was also observed (data not shown). In contrast, no cell differentiation could be achieved from CD34+γc(−) mock-transduced cells. Only a small population of γc(−) CD4low CD8(−) CD3(−) TCRαβ(−) was detected (Fig3C). It was not possible to further characterize this population because the harvest sample size was very low (<50,000 cells). γc Gene integration into thymocytes was analyzed by direct PCR. As shown in Fig 4, cells derived from S1- and S2-γc–transduced CD34+ cells contain the integrated γc gene after 5 weeks of organ culture. We could not detect the γc gene containing provirus in mock-transduced cells.
γc gene transfer restores T-cell differentiation of CD34+ SCID-Xl patients’ cells in FTOC. CD34+ γc transduced S1 (A), S2 (B) cells as well as untransduced CD34+ S1 cells (C) were cultured with the preestablished combination of cytokines (SCF, Flt-3L, IL-7, IL-1, IL-15) into fetal thymic lobes for 35 days. FTOC were harvested and cells were stained for expression of CD4, CD8, CD3, and TCRβ (dot plots) or for γc expression (histograms). T-cell differentiation obtained from CD34+ normal CB cells is indicated on panel (D) as a control.
γc gene transfer restores T-cell differentiation of CD34+ SCID-Xl patients’ cells in FTOC. CD34+ γc transduced S1 (A), S2 (B) cells as well as untransduced CD34+ S1 cells (C) were cultured with the preestablished combination of cytokines (SCF, Flt-3L, IL-7, IL-1, IL-15) into fetal thymic lobes for 35 days. FTOC were harvested and cells were stained for expression of CD4, CD8, CD3, and TCRβ (dot plots) or for γc expression (histograms). T-cell differentiation obtained from CD34+ normal CB cells is indicated on panel (D) as a control.
Presence of γc provirus gene in cells derived from transduced S1 and S2 CD34+ BM cells. Thymic lobes were seeded with transduced (γc+) or untransduced (γc−) S1 and S2 CD34+ BM cells. After 35 days of organ culture, genomic DNA was isolated from the thymocytes and analyzed by PCR using primers to amplify the γc gene. DNA from the retroviral producer cell line served as the positive control.
Presence of γc provirus gene in cells derived from transduced S1 and S2 CD34+ BM cells. Thymic lobes were seeded with transduced (γc+) or untransduced (γc−) S1 and S2 CD34+ BM cells. After 35 days of organ culture, genomic DNA was isolated from the thymocytes and analyzed by PCR using primers to amplify the γc gene. DNA from the retroviral producer cell line served as the positive control.
TCRβ rearrangements in control and γc transduced thymocytes.
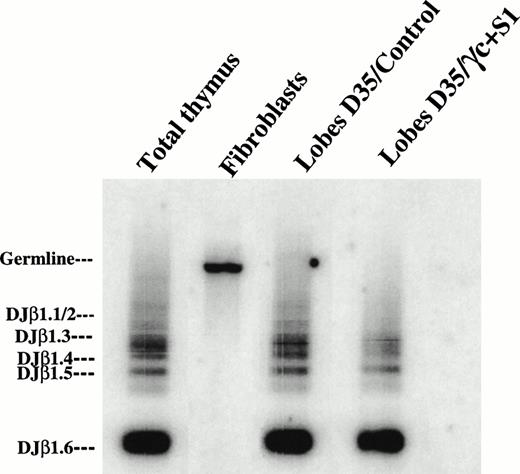
Previous studies have shown that human thymocytes developing in murine thymic organ cultures express a broad TCR repertoire.40 To analyze the rearrangement status at the TCR β locus, we studied DJβ rearrangement in the human T cells generated in the chimeric thymic organ cultures. Genomic DNA was isolated from differentiated CD34+ CB control cells or CD34+γc transduced SCID-Xl marrow cells after 5 weeks of culture and amplified by PCR to detect Dβ1-Jβ1 rearrangements (Fig 5). Human thymocytes and fibroblasts were used as positive and negative controls, respectively. As expected, human thymocytes generated in the FTOC system were characterized by the presence of distinct DJβ rearrangements. Multiple rearrangements of Dβ1 to the Jβ1.1-Jβ1.6, particularly those corresponding to short-sized products (DJβ1.6, DJβ1.5, DJβ1.4), were also detected in SP mature T cells generated from the γc transduced CD34+patient cells (Fig 5).
Fetal thymic organ cultured cells expressed multiple DJβ rearrangements. Genomic DNA was isolated from thymocytes generated from CD34+ CB cells (lobes D35/control) or from CD34+ γc transduced BM patient cells (lobes D35/γ + S1) after 35 days of organ culture. DNA was then amplified by PCR using TBF1 and TBR1 primers to detect Dβ1-Jβ1 rearrangements. PCR products were blotted and hybridized with the TBR3 probe. Total thymocytes and fibroblasts served as positive and negative controls, respectively. (Right) Positions of the genomic fragment and the specific rearrangements of Dβ1 to the Jβ1-1-Jβ1-6 elements.
Fetal thymic organ cultured cells expressed multiple DJβ rearrangements. Genomic DNA was isolated from thymocytes generated from CD34+ CB cells (lobes D35/control) or from CD34+ γc transduced BM patient cells (lobes D35/γ + S1) after 35 days of organ culture. DNA was then amplified by PCR using TBF1 and TBR1 primers to detect Dβ1-Jβ1 rearrangements. PCR products were blotted and hybridized with the TBR3 probe. Total thymocytes and fibroblasts served as positive and negative controls, respectively. (Right) Positions of the genomic fragment and the specific rearrangements of Dβ1 to the Jβ1-1-Jβ1-6 elements.
DISCUSSION
In this work, we have defined culture conditions required for human T-cell differentiation from CD34+ CB cells, and have used this system to show restoration of T-cell differentiation of CD34+γc(−) BM cells from SCID-Xl patients after retroviral-mediated γc gene transfer. The culture conditions required the additional cytokines SCF, Flt-3L, IL-7, IL-1α, and IL-15. The thymic stroma produces a number of cytokines, including IL-7, which have been shown to be necessary for early lymphoid development. This has been well demonstrated by the generation of mice mutant for IL-7,41 IL-7Rα,42 and γc,43 all of which display a dramatic decrease in thymic cellularity. The same phenomenon was observed in mice treated with anti–IL-744or anti–IL-7Rα.45 Furthermore, the use of these antibodies in vitro in FTOC resulted in the inhibition of T-cell development.46 IL-7 is also indispensable for human T-cell development. Plum et al30 described an early block at the CD34+ stage in T-cell differentiation in chimeric human/mouse FTOC treated with anti–IL-7 or anti–IL-7Rα MoAbs. Another receptor-ligand pair, cKit/SCF, has also been shown to drive expansion of immature thymocytes in the mouse.17,47Moreover, the recent report showing complete abrogation of thymocyte development in mice lacking both cKit and γc indicates essential and synergistic functions of these two distinct signaling pathways.48 As Flk-2/Flt-3 ligand (Flt-3L) has been shown to induce proliferation/expansion of the most immature CD4low murine thymocytes,49 we also decided to include Flt-3L in the cytokine combination. Our data indicate that human CB-derived CD34+ cells cultured in the presence of SCF + Flt-3L + IL-7, although being able to repopulate FTOC, poorly proliferate and differentiate along the T-cell lineage. Previous studies have shown that IL-1α is involved in murine lymphopoiesis.18 Zuniga-Pflücker et al50provided evidence that IL-1α (or TNFα) promotes the transition of the CD117+ CD25(−) subset to the CD25+ stage, which is a prerequisite for further maturation to the DP stage. When IL-1α was added in our FTOC culture system, it resulted in an increase in human thymic cellularity, while early thymic progenitors differentiated to the ISP/DP stages. In the presence of SCF + Flt-3L + IL-7 + IL-1α and IL-15, CD34+ cells developed mainly into CD4+ immature single positive (ISP), CD4+CD8+ (DP) and CD4+SP cells, as well as CD56, CD16 NK cells. Compared with cultures in which IL-15 was omitted, all of these subsets expanded more efficiently. The CD56+ NK cell subset expanded particularly well in the presence of IL-15. These results suggest that, in addition to the known role of IL-15 in cell differentiation towards the NK pathway, IL-15 in association with other cytokines promotes both proliferation and differentiation of progenitor cells towards the T-cell lineage.
The culture conditions we designed to study T-cell development of CD34+ progenitors from postnatal origin provided us with the tool to study restoration of human T-cell development after gene transfer and expression of the γc gene in the CD34+selected transduced SCID-Xl marrow cells. Although efficient progenitor cell transduction can be achieved by cocultivation with the packaging cell line, we used a viral supernatant transduction on wells coated with a recombinant human fibronectin fragment38,39 to both improve transduction efficiency and develop a clinically-applicable protocol. Under these conditions, high transduction levels were reached, with between 35% and 70% of the cells expressing γc. These progenitor cells were able to differentiate along the T-lymphoid lineage in the presence of SCF, Flt-3L, IL-7, IL-1α, and IL-15. Fifteen percent to 30% of harvested cells were shown to be of human origin. All of these cells expressed γc chain after a 4- to 5-week culture period. γc chain expression by SCID-Xl CD34+marrow cells enabled them to mature into DP cells and CD4+TRCαβ+ SP cells. Polyclonal DJ rearrangements of the TCR β locus were shown in the cell population. The inability of mock-transduced CD34+γc(−) cells placed in the same culture condition to acquire a mature T-cell phenotype confirms that, in humans, T-cell development is strictly dependent on γc chain expression. The almost complete failure of the generation of CD8+ SP T cells from both cord blood CD34+ and γc transduced SCID-Xl CD34+ cells could be attributed to the inability of murine MHC class I molecules to interact efficiently with human CD8+ T cells or to the requirement of other signals in addition to class I MHC.31 It is possible that a mutated form of γc may have been expressed in S1, which exerted no or only a limited influence on the function of the normal γc protein. It does not indicate, however, that other mutated products could not exert a dominant inhibitory effect.
The ability of γc-transduced CD34+ cells from SCID-Xl patients to mature into T cells (this study), as well as NK cells,24 sets the basis for a clinical study of ex vivo γc gene transfer into CD34+ cells from SCID-Xl patients. Despite the low efficiency of retrovirally-mediated gene transfer into human hematopoietic stem cells (HSC), it is indeed expected that a strong selective advantage will be conferred to the few transduced cells potentially able to differentiate into T, NK, and possibly B cells. The observation of a significant development of long-lasting T cells after γc gene reverse mutation into a T-cell precursor observed in a patient51 demonstrates that a strong positive pressure for survival, proliferation, and differentiation of γc(+) precursors does exist in vivo. In conclusion, this study shows that T-cell differentiation to mature CD4 T cells can be achieved in murine FTOC from CB and postnatal CD34 cells in the presence of a cytokine combination, which compensates for the lack of human stromal cells. In addition, this system has enabled us to provide evidence that after γc gene transduction, SCID-Xl CD34 cells can mature into T cells, paving the way for a clinical study of γc gene transfer in SCID-Xl patients.
ACKNOWLEDGMENT
We are indebted to the patients’ families for support in this study, to Dr Adrian Thrasher and Dr Bert Gerritsen for providing sample from one patient, to Dr Tony Troutt from Immunex, to Dr Setsuko Yoshimura (from Takara Shuzo Co) for providing useful reagents, to C. De Coene for excellent technical assistance to F. Selz and C. Garcia for cell sorting, and to Dr Jane Peake for checking the manuscript.
Supported by grants from INSERM, Association Française contre les Myopathies, Ministère de la Recherche et de la Technologie, Agence Française du Sang (contrat No. 9600 13 701 76) and Assistance Publique et Hôpitaux de Paris (AP-HP).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to S. Hacein-Bey, PhD, INSERM U 429, Hôpital Necker-Enfants Malades, 149, rue de Sèvres, 75743 Paris Cedex 15, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal