Abstract
Autologous leukemia cells engineered to express immune-stimulating molecules may be used to elicit antileukemia immune responses. Gene delivery to human B-precursor acute lymphoblastic leukemia (ALL) cells was investigated using the enhanced green fluorescent protein (EGFP) as a reporter gene, measured by flow cytometry. Transfection of the Nalm-6 and Reh B-precursor ALL leukemia cell lines with an expression plasmid was investigated using lipofection, electroporation, and a polycationic compound. Only the liposomal compound Cellfectin showed significant gene transfer (3.9% to 12% for Nalm-6 cells and 3.1% to 5% for Reh cells). Transduction with gibbon-ape leukemia virus pseudotyped Moloney murine leukemia virus (MoMuLV)-based retrovirus vectors was investigated in various settings. Cocultivation of ALL cell lines with packaging cell lines showed the highest transduction efficiency for retroviral gene transfer (40.1% to 87.5% for Nalm-6 cells and 0.3% to 9% for Reh cells), followed by transduction with viral supernatant on the recombinant fibronectin fragment CH-296 (13% to 35.5% for Nalm-6 cells and 0.4% to 6% Reh cells), transduction on human bone marrow stroma monolayers (3.2% to 13.3% for Nalm-6 cells and 0% to 0.2% Reh cells), and in suspension with protamine sulfate (0.7% to 3.1% for Nalm-6 cells and 0% for Reh cells). Transduction of both Nalm-6 and Reh cells with human immunodeficiency virus–type 1 (HIV-1)–based lentiviral vectors pseudotyped with the vesicular stomatitis virus-G envelope produced the best gene transfer efficiency, transducing greater than 90% of both cell lines. Gene delivery into primary human B-precursor ALL cells from patients was then investigated using MoMuLV-based retrovirus vectors and HIV-1–based lentivirus vectors. Both vectors transduced the primary B-precursor ALL cells with high efficiencies. These studies may be applied for investigating gene delivery into primary human B-precursor ALL cells to be used for immunotherapy.
ACUTE LYMPHOBLASTIC leukemia (ALL), usually of B-precursor lymphoblastic origin, is the most frequently occurring cancer in childhood. The overall event-free survival at 5 years varies between 70% and 80%.1 However, the prognosis of patients with certain high-risk forms of ALL or patients who relapse after conventional chemotherapy is poor. This group of patients accounts for a significant number of children with cancer.
B-precursor ALL cells are inefficient antigen-presenting cells and thereby evade the body’s immune surveillance system.2Based on previous preclinical studies in solid tumor therapy,3,4 the expression of immunomodulatory factors by B-precursor ALL cells may facilitate the presentation of tumor-associated antigens to the immune system and cause an immune response against the leukemia. The possibility of introducing various immunomodulatory genes into neoplastic cells has been successfully documented.4-6 Recently, disabled single-cycle virus–herpes simplex virus (DISC-HSV) vectors have been shown to transduce human malignant hematopoietic cells transiently.7However, there has been no systematic comparison of different methods of gene transfer to ALL cells reported in the scientific literature.
Gene therapy for ALL will require efficient gene delivery procedures. We investigated gene delivery to the human B-precursor ALL cell lines Nalm-6 and Reh. The enhanced green fluorescent protein (EGFP) was used as a reporter gene to measure gene transfer efficiency. Methods evaluated included direct DNA delivery of an expression plasmid using electroporation, lipofection, and the polycationic compound SuperFect. Gene delivery using Moloney murine leukemia virus (MoMuLV)-based retroviral vectors and human immunodeficiency virus–type 1 (HIV-1)–based lentiviral vectors was also investigated. The efficiency of the MoMuLV vectors and the HIV-1–based vectors in transducing leukemia cells was evaluated with or without the use of the recombinant fibronectin fragment CH-296 as a support matrix.8
Gene transfer to Nalm-6 and Reh cells by electroporation was minimal and variable and there was significant cell mortality. There was no measurable gene transfer with SuperFect. Of the cationic lipids evaluated, Cellfectin alone produced reproducible, low levels of gene transfer. MoMuLV-based retroviral vectors transduced Nalm-6 cells more efficiently than Reh cells. High levels of stable gene transduction were achieved in Nalm-6 cells by cocultivation with murine packaging cells and by using cell-free virus supernatant on either human bone marrow stroma or CH-296 as a support matrix. The highest levels of gene transfer in both Nalm-6 and Reh cells were achieved using lentivirus vectors pseudotyped with the vesicular stomatitis virus-G (VSV-G) envelope. Primary B-precursor ALL cells from patients were efficiently transduced by both MoMuLV-based and HIV-1–based vectors pseudotyped with the VSV-G envelope.
MATERIALS AND METHODS
Cells.
The human ALL cell lines used in this study were Nalm-6 (provided by Dr Dario Campana, St Jude Children’s Research Hospital, Memphis, TN) and Reh (obtained from American Type Culture Collection, Rockville, MD), which have been previously characterized as B-precursor ALL cells.9 10 Both cells express the early B-lymphoid antigen CD19 on greater than 95% of cells. The cells were cultured in RPMI 1640 medium with L-glutamine (BioWittaker, Walkersville, MD), 10% fetal bovine serum (FBS), and penicillin/streptomycin (50 U/mL) (leukemia medium) at 37°C, 5% CO2. Experiments were performed when the cells were in the exponential growth phase. PG13 vector producing fibroblasts and 293T, 293A, and HT-29 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; BioWittaker, Walkersville, MD), 10% FBS, L-glutamine (2 mmol/L), and penicillin/streptomycin (50 U/mL) at 37°C and 5% CO2. Primary B-precursor ALL cells were obtained from cryopreserved cells remaining from diagnostic bone marrow aspirates taken from pediatric ALL patients with marrows that were more than 90% replaced with ALL blasts. The cells were cryopreserved in 10% dimethyl sulfoxide (DMSO) and 90% FBS. These cells were thawed at 37°C using a thawing medium containing AIM-V serum-free medium (GIBCO/BRL, Grand Island, NY), 30% FBS, 20 U/mL heparin, and 0.2 U/mL DNAse. Primary ALL cells were cultured in AIM-V serum-free medium containing 0.5 to 1.5 μg/mL of CD40L (Immunex, Seattle, WA) on monolayers of irradiated (3,000 cGy) allogeneic primary human bone marrow stroma (2 × 104 cells per well) at a cell density of 1 × 105 cells per well in 48-well flat-bottom plates at 37°C, 5% CO2 on the day before transduction.
Reporter constructs.
The reporter gene EGFP (Clontech Laboratories, Palo Alto, CA) was used for the detection of transfected/transduced leukemia cells by fluorescence-activated cell sorting (FACS) analysis. To make the expression plasmid VR-EGFP, EGFP was inserted into the expression plasmid VR-1012 under transcriptional control of the CMV I/E promoter (provided by Dr Robert H. Zougg, Vical Inc, San Diego, CA; Fig 1). Plasmids for transfection were prepared using Qiagen Endotoxin free Plasmid Maxi kit (Qiagen, Valencia, CA).
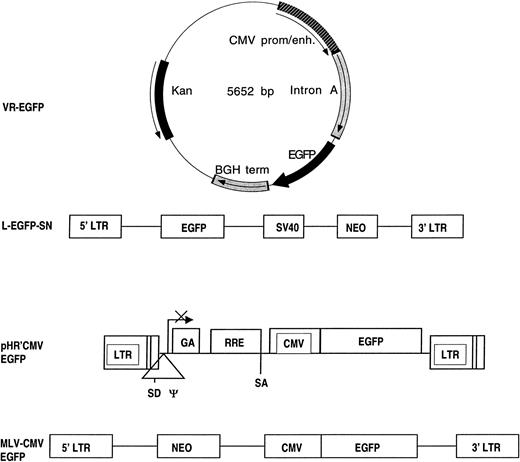
Diagrams of the plasmid and vectors expressing EGFP. Cartoons of the expression plasmid VR-EGFP, the MoMuLV-based vector encoding EGFP used in the packaging cell line PG-13, and the reporter gene vectors pHR′-CMV-EGFP and MLV-CMV-EGFP used in the transient packaging system. VR-EGFP contains a CMV promoter/enhancer (CMV prom/enh) and intron from CMV, the EGFP gene and bovine growth hormone polyadenylation signal (BGH term) in a plasmid-encoding kanamycin resistance (Kan). L-EGFP-SN is an MoMuLV-based vector containing the 5′LTR and 3′LTR from MoMuLV, the EGFP gene, an SV40 early promoter, and the bacterial neomycin phosphotransferase gene (NEO). pHR′-CMV-EGFP is a lentiviral vector containing HIV-1–derived elements, including the 5′LTR and 3′LTR, the splice donor (SD) and splice acceptor (SA), the packaging signal (Ψ), a portion of the gag encoding region (GA), a CMV prom/enh (CMV), and the EGFP gene. MLV-CMV-EGFP is another MoMuLV-based vector containing the 5′LTR and 3′LTR of MoMuLV, the bacterial neomycin phosphotransferase gene (NEO), the CMV prom/enh (CMV), and the EGFP gene.
Diagrams of the plasmid and vectors expressing EGFP. Cartoons of the expression plasmid VR-EGFP, the MoMuLV-based vector encoding EGFP used in the packaging cell line PG-13, and the reporter gene vectors pHR′-CMV-EGFP and MLV-CMV-EGFP used in the transient packaging system. VR-EGFP contains a CMV promoter/enhancer (CMV prom/enh) and intron from CMV, the EGFP gene and bovine growth hormone polyadenylation signal (BGH term) in a plasmid-encoding kanamycin resistance (Kan). L-EGFP-SN is an MoMuLV-based vector containing the 5′LTR and 3′LTR from MoMuLV, the EGFP gene, an SV40 early promoter, and the bacterial neomycin phosphotransferase gene (NEO). pHR′-CMV-EGFP is a lentiviral vector containing HIV-1–derived elements, including the 5′LTR and 3′LTR, the splice donor (SD) and splice acceptor (SA), the packaging signal (Ψ), a portion of the gag encoding region (GA), a CMV prom/enh (CMV), and the EGFP gene. MLV-CMV-EGFP is another MoMuLV-based vector containing the 5′LTR and 3′LTR of MoMuLV, the bacterial neomycin phosphotransferase gene (NEO), the CMV prom/enh (CMV), and the EGFP gene.
Retroviral vectors.
An MoMuLV vector encoding EGFP (L-EGFP-SN) was constructed by inserting the Bgl II-Not I fragments containing the EGFP gene into the Hpa I site in the polylinker of LXSN11(Fig 1). L-EGFP-SN was packaged by transfecting the vector plasmid into PG13 cells, which produce virus particles pseudotyped with the gibbon-ape leukemia virus (GALV) envelope.12 Transfected cells were selected in G418 and high-titer clones were selected by sorting EGFP-positive single-cell clones using a flow cytometry-assisted automatic cell deposition unit. The titer of these clones was assessed by transduction of HT-29 cells and subsequent G418 selection. Viral supernatant from high-titer clones was collected after incubation of confluent plates for 48 hours at 32°C, 5%CO2. Cell-free supernatant from a high-titer clone (106 infectious units/mL) was used in the transduction experiments.
Virus particles containing an MoMuLV-based vector encoding EGFP under control of an internal CMV promoter (MLV-CMV-EGFP; Fig 1)13and pseudotyped with the VSV-G envelope were produced by cotransfecting 293T cells with the vector plasmid, packaging plasmid pHIT 60,14 and the VSV-G protein encoding plasmid pMDG13 using the calcium phosphate method and collecting viral supernatant for a period of 72 hours, starting 24 hours after transfection. Viral supernatants were concentrated by ultracentrifugation at 19,000 rpm for 2 hours and 20 minutes at 22°C and were titered by assessing expression of EGFP in transduced 293A cells by flow cytometry. Briefly, 1 × 105 293A cells were plated in each well of a 6-well plate. The following day, cells from three representative wells were trypsinized and counted, and the average number of cells was determined. Three dilutions (1/500, 1/5,000, and 1/50,000) of concentrated viral supernatant were used to infect the 293A cells. Polybrene (10 μg/mL) was used as a transduction adjuvant. Medium was changed after 24 hours and cells were analyzed for the percentage of cells expressing EGFP 48 hours after transduction. Titer was calculated using the formula: percentage of cells expressing EGFP × dilution factor × average number of cells per well/100.15 Multiplicities of infection (MOI) for leukemia cells were calculated as infectious units as determined on 293A cells divided by the number of leukemia cells exposed to the vector.
Lentiviral vectors.
The HIV-1–based lentiviral vectors used were those described by Naldini et al (kindly provided by Drs Didier Trono and Romain Zuffrey [University of Geneva, Geneva, Switzerland] and Dr Luigi Naldini [Cell Genesys, Foster City, CA]).12,16 A vector containing the EGFP gene under control of the CMV promoter (pHR′-CMV-EGFP) was produced by transient cotransfection of 293T cells with plasmids encoding the vector; the HIV-1 gag, pol, tat, and rev genes (Δr 8.91); and the VSV-G envelope (pMDG; Fig 1) using the calcium phosphate method.13 The viral supernatants were concentrated and titered as described above. Viral supernatant was used to infect cells in transduction experiments.
Gene transfer methods.
For direct DNA delivery, two methods were used. (1) Electroporation of leukemia cells was performed using the Gene Pulser II RF module from Bio-Rad (Hercules, CA). Leukemia cells were washed once with Hank’s Balanced Salt Solution (HBSS) and resuspended at a concentration of 107/mL in HEPES buffered media (15 mmol/L potassium phosphate buffer, pH 7.2, 1 mmol/L magnesium chloride, 2 mmol/L HEPES, 250 mmol/L mannitol) with 12 μg/mL of VR-EGFP plasmid DNA. Four-hundred–microliter samples were placed in cuvettes and experiments were performed by varying the voltage (50, 100, and 150 V) and burst interval (0.2, 0.5, and 1 second) while keeping the percentage of modulation (100%), radio frequency (40 KHz), burst duration (2 milliseconds), and the number of bursts (5) constant. Cuvettes were placed on ice immediately after electroporation for 5 minutes. The electroporated cells were then placed in leukemia medium and cultured for 48 hours before analysis of transfection. (2) The cationic lipids used for transfection were Lipofectin, Lipofectamine, Cellfectin, and Dimrie/C (GIBCO/BRL). Transfection was performed following the manufacturer’s instructions initially and then optimized in later experiments. Briefly, Lipofectin (10 μL), Lipofectamine (10 μL), Cellfectin (15 μL), and Dimrie/C (5 μL) were added to 250 μL of AIM-V serum-free medium (GIBCO/BRL). These samples were added to the first column of a 24-well plate that had 250 μL of AIM-V in every well and incubated at room temperature for 30 minutes. Two hundred fifty microliters of VR-EGFP plasmid DNA at a concentration of 12.8 μg/mL of AIM-V was added to the first well in each column and 5 twofold dilutions were performed. Leukemia cells were washed twice with AIM-V and resuspended to obtain a cell density of 8 × 106/mL. Fifty microliters of cells was added to each well containing the lipid-DNA complexes and incubated for 4 hours at 37°C, 5% CO2. Fresh AIM-V medium (0.6 mL) was then added to each well and incubated for 48 hours in the same conditions. Cells were then washed and analyzed for transfection. SuperFect (Qiagen) transfection reagent was also used following the manufacturer’s instructions. Leukemia cells were washed once with HBSS and then plated at a concentration of 2.5 × 106 cells in 2.5 mL of leukemia medium in each well of a 6-well plate. VR-EGFP plasmid DNA (2.5 μg) was diluted in 75 μL of AIM-V and 10 μL of SuperFect reagent was added to the DNA solution. The mixture was vortexed for 10 seconds and then incubated at room temperature for 10 minutes to allow complex formation. Transfection complexes were then added dropwise to each well while gently swirling the plate to ensure uniform distribution of the complexes to the cells.
Retroviral- and lentiviral-mediated gene transfer.
Retroviral- and lentiviral-mediated gene transduction of leukemia cells was evaluated using allogeneic human bone marrow or the recombinant fibronectin fragment CH-296 (RetroNectin; Takara Shuzo Co, Ltd, Otsu, Shiga, Japan) as a support matrix, with bovine serum albumin (BSA) as a control. Six-well plates were coated with 2 mL/well of 50 μg/mL CH-296 for 2 hours at room temperature and then blocked for 30 minutes with 2% BSA in sterile water. Coated wells were then washed with HBSS (BioWittaker) containing 2.5 mmol/L vol/vol HEPES. Control wells were coated with 2% BSA for 30 minutes and then washed with the HEPES/Hank’s solution. Leukemia cells (1 × 106) were plated in each well in 1 mL of leukemia medium. Viral supernatant was diluted to give the calculated MOI, added to each well, and then incubated at 37°C, 5% CO2 for 24 hours. Leukemia cells were mobilized using enzyme-free cell dissociation buffer (GIBCO/BRL), washed twice with leukemia medium, and then incubated for 24 to 48 hours at 37°C, 5% CO2 before FACS analysis. Transduction experiments on primary human B-precursor ALL cells were performed under culture conditions described earlier. Retroviral and lentiviral vector supernatants were added at the indicated MOI and the cells were cultured for 48 hours at 37°C, 5% CO2. Two days after addition of the vector, leukemia cells were harvested for analysis of transduction.
FACS analysis.
For analysis of EGFP expression, cells were washed once with phosphate-buffered saline, resuspended at 107/mL, and then fixed with 1% paraformaldehyde. Detection of EGFP expression was accomplished with a FACScan cytometer equipped with a 488-nm argon laser for excitation of the reporter protein and a 530/30-nm bandpass filter for monitoring the fluorescent emissions. To establish background for fluorescence and to set gates for data acquisition, sham-transduced or sham-transfected cells were used. Primary ALL cells were stained with a monoclonal antibody to the B-precursor cell antigen CD19 and conjugated to phycoerthrin (CD19-PE; Becton Dickinson, San Jose, CA), followed by fixation in 1% paraformaldehyde and FACS analysis. Care was taken to analyze cells that fell in the lymphocyte gate based on forward and side scatter characteristics and that were positive for the expression of CD19. Mean fluorescence intensity was used to calculate levels of EGFP expression.
RESULTS
Stability of EGFP expression.
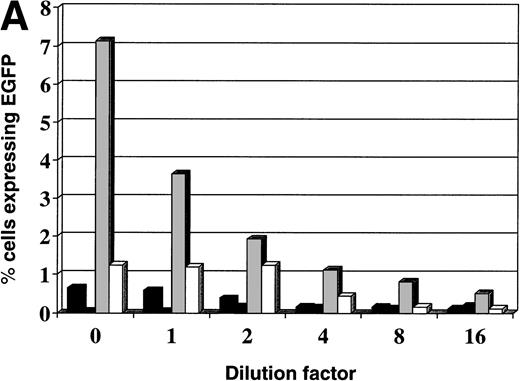
Leukemia cells transduced with L-EGFP-SN by cocultivation on vector producing fibroblasts or on CH-296 were sorted by FACS for those expressing EGFP and then placed in culture. Leukemia cells were split 1:10 every 3 to 4 days. Cell aliquots were then cryopreserved every week. At the completion of 2 months of culture, leukemia cells frozen at various time points were thawed and placed in culture for 24 hours and then simultaneously analyzed by flow cytometry. The level of expression of EGFP remained between 82.54% and 94.53% for leukemia cells transduced with the L-EGFP-SN vector for Nalm-6 and Reh cells (Fig 2A and B). Leukemia cells transduced with the lentiviral vectors but not sorted by FACS also showed stable expression of EGFP for at least 3 weeks in culture (Fig 2A and B). The cells grew well in culture, suggesting that EGFP was a stable and nontoxic reporter gene in these cells. Leukemia cells transiently transfected with the expression plasmid VR-EGFP using Cellfectin showed relatively stable expression of EGFP for approximately 1 week in culture before falling to undetectable levels (Fig 2A and B). These cells were not FACS sorted.
Stability of EGFP expression in leukemia cells in culture. Stability of EGFP expression in leukemia cells in culture after transfection with the expression plasmid (▴) VR-EGFP and transduction by the retroviral vector (▪) L-EGFP-SN and the lentiviral vector (•) pHR′-CMV-EGFP. The transfected cells and the lentiviral-transduced cells were not FACS sorted before culture, but the retroviral-transduced cells were. Day 0 is 48 hours after transfection or transduction. (A) Nalm-6 cells; (B) Reh cells.
Stability of EGFP expression in leukemia cells in culture. Stability of EGFP expression in leukemia cells in culture after transfection with the expression plasmid (▴) VR-EGFP and transduction by the retroviral vector (▪) L-EGFP-SN and the lentiviral vector (•) pHR′-CMV-EGFP. The transfected cells and the lentiviral-transduced cells were not FACS sorted before culture, but the retroviral-transduced cells were. Day 0 is 48 hours after transfection or transduction. (A) Nalm-6 cells; (B) Reh cells.
Direct DNA delivery.
Electroporation resulted in massive cell destruction (70% cell death). Of the viable cells recovered, gene transfer was negligible as assessed by expression of EGFP by flow cytometry. In one experiment, there were approximately 0.5% to 1% of the cells transfected (data not shown). SuperFect, a polycationic compound, also had no efficacy in transfecting the leukemia cell lines.
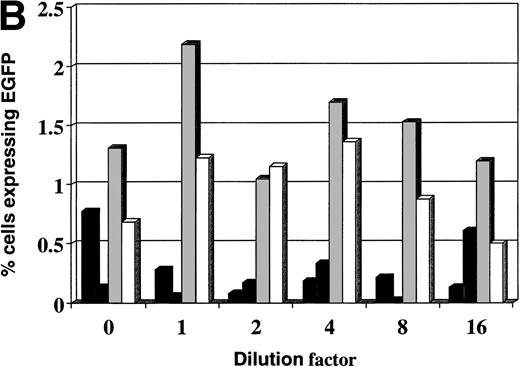
Four lipofection compounds (Lipofectin, Lipofectamine, Cellfectin, and Dimrie/C) were screened for transfection of the leukemia cell lines. Only Cellfectin produced significant results. At the optimal dilution, Cellfectin produced 7.2% transfection of Nalm-6 cells (Fig 3A) and 2.2% of Reh cells (Fig 3B).
Transfection of leukemia cells using different lipofectants. Transfection of leukemia cells with (▪) Lipofectin, (▧) Lipofectamine, (▧) Cellfectin, and (□) Dimrie-C. 0 dilution corresponds to 10 μL Lipofectin, 10 μL Lipofectamine, 15 μL Cellfectin, and 5 μL Dimrie/C. (A) Nalm-6 cells; (B) Reh cells.
Transfection of leukemia cells using different lipofectants. Transfection of leukemia cells with (▪) Lipofectin, (▧) Lipofectamine, (▧) Cellfectin, and (□) Dimrie-C. 0 dilution corresponds to 10 μL Lipofectin, 10 μL Lipofectamine, 15 μL Cellfectin, and 5 μL Dimrie/C. (A) Nalm-6 cells; (B) Reh cells.
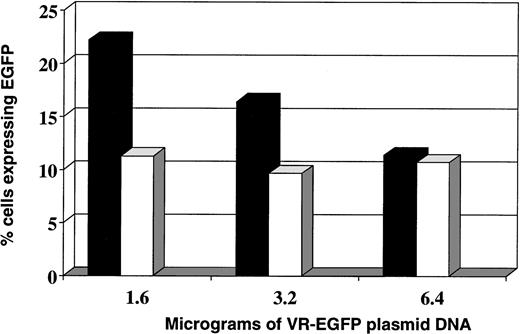
Experiments were then designed using Cellfectin at various concentrations (15, 7.5, 3.25, and 1.625 μL) together with varied amounts of plasmid DNA (1.6, 3.2, and 6.4 μg). Cellfectin at 3.25 μL and 1.6 μg of plasmid DNA per 100,000 leukemia cells resulted in transfection of 22% of the Nalm-6 cells and 11% of the Reh cells (Fig 4). The intensity of EGFP fluorescence was brighter for Nalm-6 cells than for REH cells (data not shown).
Transfection of leukemia cell lines using various quantities of VR-EGFP plasmid DNA. Transfection of (▪) Nalm-6 and (□) Reh cells with cellfectin using different amounts of VR-EGFP plasmid DNA.
Transfection of leukemia cell lines using various quantities of VR-EGFP plasmid DNA. Transfection of (▪) Nalm-6 and (□) Reh cells with cellfectin using different amounts of VR-EGFP plasmid DNA.
MoMuLV-based retroviral gene transfer.
Cocultivation of Nalm-6 cells with the fibroblast packaging cell line PG13 L-EGFP-SN resulted in gene transfer efficiency of 41.5%. Reh cells were transduced less well under similar conditions, resulting in a gene transfer efficiency of 0.71% (Table1). Varying concentrations of protamine sulfate (1, 2, 4, and 8 μg/mL) were used as transduction adjuvants together with cell-free retroviral supernatants. Protamine sulfate at 2 μg/mL produced the most efficient gene transfer in both Nalm-6 and Reh cells (Table 1).
Transduction of Leukemia Cell Lines With L-EGFP-SN
| Transduction Method . | % Cells Expressing EGFP . | |
|---|---|---|
| Nalm-6 . | Reh . | |
| Cocultivation | 41.5 | 0.71 |
| Supernatant (μg/mL protamine) | ||
| 1 | 9 | 0 |
| 2 | 15.8 | 0.3 |
| 4 | 11 | 0.01 |
| 8 | 7.6 | 0.1 |
| Fibronectin (50 μg/mL) | 43.5 | 2.4 |
| BSA (2%) | 13.3 | 0.2 |
| Transduction Method . | % Cells Expressing EGFP . | |
|---|---|---|
| Nalm-6 . | Reh . | |
| Cocultivation | 41.5 | 0.71 |
| Supernatant (μg/mL protamine) | ||
| 1 | 9 | 0 |
| 2 | 15.8 | 0.3 |
| 4 | 11 | 0.01 |
| 8 | 7.6 | 0.1 |
| Fibronectin (50 μg/mL) | 43.5 | 2.4 |
| BSA (2%) | 13.3 | 0.2 |
Transduction was next evaluated using the recombinant fibronectin fragment CH-296 (50 μg/mL) as a support matrix or BSA (2%) as a control. The results are shown in Table 1. A total of 43.5% of Nalm-6 cells were transduced on fibronectin compared with 13.3% on BSA. A total of 2.4% of Reh cells were transduced on fibronectin compared with 0.2% on BSA. Three rounds of transduction on 3 consecutive days was more efficient than three rounds of transduction on a single day (Table 2).
Effect of Multiple Rounds of Transduction on Gene Transfer to Leukemia Cells With L-EGFP-SN
| Transduction Method . | % of Cells Expressing EGFP . | |||
|---|---|---|---|---|
| Nalm-6 . | Reh . | |||
| Fibronectin . | BSA . | Fibronectin . | BSA . | |
| 3 rounds, 1 day | 20.1 | 2.1 | 1.3 | 0.8 |
| 3 rounds, 3 days | 33.6 | 2.7 | 2.1 | 0.5 |
| Transduction Method . | % of Cells Expressing EGFP . | |||
|---|---|---|---|---|
| Nalm-6 . | Reh . | |||
| Fibronectin . | BSA . | Fibronectin . | BSA . | |
| 3 rounds, 1 day | 20.1 | 2.1 | 1.3 | 0.8 |
| 3 rounds, 3 days | 33.6 | 2.7 | 2.1 | 0.5 |
Fibronectin was used at 50 μg/mL and BSA was used at 2%.
In another experiment, transduction of Nalm-6 and Reh cells was also evaluated comparing cell-free viral supernatant alone, viral supernatant with protamine sulfate, viral supernatant with protamine sulfate on allogeneic human bone marrow stroma, and viral supernatant on recombinant CH-296 or on BSA. The results are shown in Table 3. Recombinant CH-296 provided the best support matrix and produced the highest gene transfer in both cell lines. Varying concentrations of fibronectin (25, 50, 75, and 96 μg/mL) used to coat the culture dishes were also evaluated for the effect on gene transfer efficiency. No significant differences were noted (data not shown).
Comparison of Various Support Matrices and Transduction of Leukemia Cells
| Transduction Method . | % Cells Expressing EGFP . | |
|---|---|---|
| Nalm-6 . | Reh . | |
| PG13-LN3-150 | 0 | 0 |
| PG13-LEGFP3-150 + protamine sulfate (4 μg/mL) | 7.6 | 0.1 |
| PG13-LEGFP3-150 + protamine sulfate (4 μg/mL) on bone marrow stroma | 13.7 | 0.9 |
| PG13-LEGFP3-150 on fibronectin (50 μg/mL) | 42.1 | 1 |
| PG13-LEGFP3-150 on BSA (2%) | 5.1 | 0.1 |
| Transduction Method . | % Cells Expressing EGFP . | |
|---|---|---|
| Nalm-6 . | Reh . | |
| PG13-LN3-150 | 0 | 0 |
| PG13-LEGFP3-150 + protamine sulfate (4 μg/mL) | 7.6 | 0.1 |
| PG13-LEGFP3-150 + protamine sulfate (4 μg/mL) on bone marrow stroma | 13.7 | 0.9 |
| PG13-LEGFP3-150 on fibronectin (50 μg/mL) | 42.1 | 1 |
| PG13-LEGFP3-150 on BSA (2%) | 5.1 | 0.1 |
MOI of 20.
HIV-1–based lentiviral gene transfer.
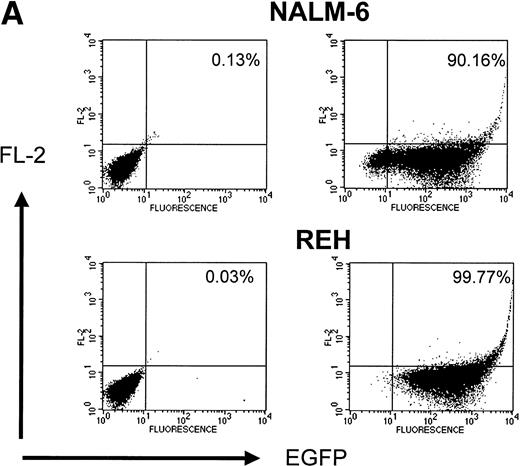
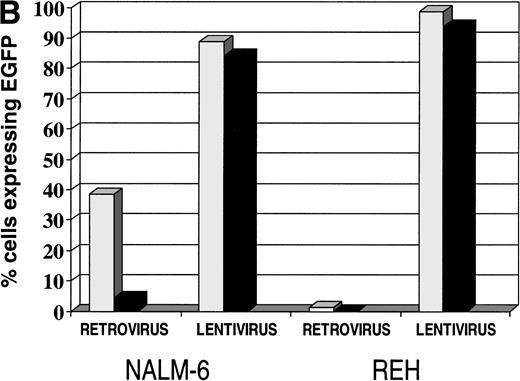
Lentiviral vectors produced very efficient gene transfer into both Nalm-6 and Reh cells. A total of 90.16% of Nalm-6 cells and 99.77% of Reh cells were transduced at an MOI of 30 (Fig 5A). Transduction of the leukemia cell lines with GALV-pseudotyped MoMuLV and lentiviral vectors was compared on fibronectin, with BSA as a control. Gene transfer was enhanced on recombinant CH-296 when compared with BSA for the GALV-pseudotyped MoMuLV vector but not for the lentiviral vector (Fig 5B).
(A) Transduction of leukemia cell lines with HIV-1–based lentiviral vectors. Transduction of Nalm-6 and Reh cells with the lentiviral vector pHR′-CMV-EGFP. (Left panels) untransduced cells; (right panels) transduced cells; (upper panels) Nalm-6 cells; (lower panels) Reh cells. (B) Comparison of retroviral- and lentiviral-mediated gene transfer in leukemia cell lines on (▧) fibronectin (50 μg/mL) and (▪) BSA (2%).
(A) Transduction of leukemia cell lines with HIV-1–based lentiviral vectors. Transduction of Nalm-6 and Reh cells with the lentiviral vector pHR′-CMV-EGFP. (Left panels) untransduced cells; (right panels) transduced cells; (upper panels) Nalm-6 cells; (lower panels) Reh cells. (B) Comparison of retroviral- and lentiviral-mediated gene transfer in leukemia cell lines on (▧) fibronectin (50 μg/mL) and (▪) BSA (2%).
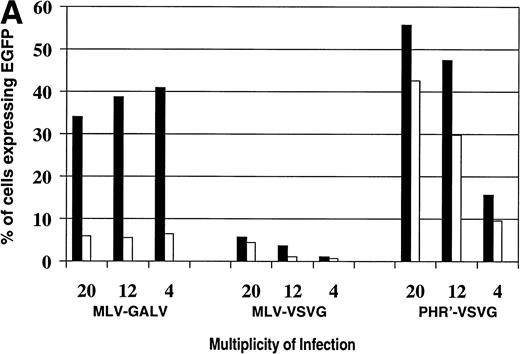
To evaluate whether the difference in gene transfer was due to the vector backbone or due to the viral envelope, gene transduction experiments were performed with viral supernatants containing MoMuLV vectors pseudotyped with the GALV envelope, MoMuLV vectors pseudotyped with the VSV-G envelope, and the HIV-based vector pseudotyped with the VSV-G envelope. We were consistently unable to produce lentiviral vectors with a GALV pseudotype (Xu and Kohn, unpublished observation). A range of MOI was used. As before, transduction of Nalm-6 cells by the GALV-pseudotyped MoMuLV vector was markedly enhanced by the presence of a fibronectin support matrix (Fig 6A). In contrast, fibronectin only moderately enhanced transduction by the VSV-G–pseudotyped vectors. No increase in transduction occurred as the MOI of the GALV-pseudotyped MoMuLV vector was increased from 4 to 20. Gene transfer was MOI dependent for the vectors pseudotyped with the VSV-G envelope. The VSV-G–pseudotyped MoMuLV vector transduced Nalm-6 cells poorly. In contrast, the VSV-G–pseudotyped lentiviral vector efficiently transduced the Nalm-6 cells, with a maximal transduction of 57% at the highest MOI tested (20).
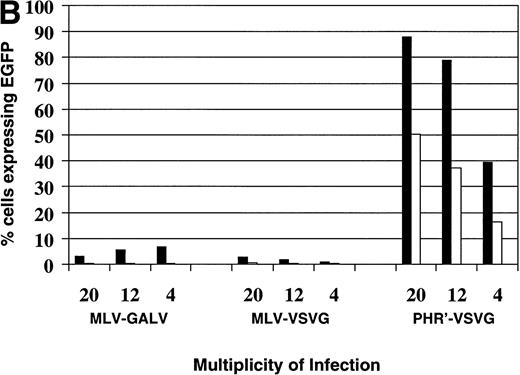
Comparison of vector backbone and viral envelope in transducing leukemia cells. Transduction of leukemia cells with MLV-GALV, MLV-VSVG, and pHR′-VSVG vectors encoding EGFP at different MOI on (□) fibronectin (50 μg/mL) and (▪) BSA (2%). (A) Nalm-6 cells; (B) Reh cells.
Comparison of vector backbone and viral envelope in transducing leukemia cells. Transduction of leukemia cells with MLV-GALV, MLV-VSVG, and pHR′-VSVG vectors encoding EGFP at different MOI on (□) fibronectin (50 μg/mL) and (▪) BSA (2%). (A) Nalm-6 cells; (B) Reh cells.
Similar, although more striking results were seen with the Reh cells (Fig 6B). Transduction of Reh cells by the GALV-pseudotyped MoMuLV vector was very poor, although enhanced by fibronectin. No increase in transduction occurred as the MOI was increased. The VSV-G–pseudotyped MoMuLV vector transduced even more poorly. However, the VSV-G–pseudotyped lentiviral vector transduced 88% of the Reh cells at an MOI of 20. As shown earlier, increasing the MOI to 30 could transduce almost 100% of the cells.
Primary human B-precursor ALL cells.
Primary B-precursor ALL cells were studied for transduction, comparing the VSV-G–pseudotyped MoMuLV vector and the HIV-1 vector. Cells were transduced by a single exposure to cell-free vector supernatant and analyzed after 2 days for EGFP expression. Because the cells were cultured on human allogeneic bone marrow stromal monolayers to preserve leukemia cell viability,17 we costained cells for the expression of the B-precursor cell antigen CD19 to exclude any stromal cells that may have been recovered from the culture with leukemia cells.
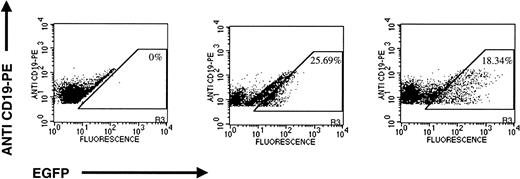
Both MoMuLV-based and HIV-1–based vectors transduced primary B-precursor ALL cells as measured by expression of EGFP. Five patient samples were analyzed in four different experiments using various MOIs. Because of the limited availability of primary ALL cells, transduction could be repeated in two of the five patient samples. CD40L was used only in experiments no. 1 and 2. There was a large variation in transduction with both the MoMuLV vectors (2.81% to 74.05%) and HIV-1 vectors (2.2% to 43.35%; Table 4). HIV-1–based vectors transduced primary human pre-B ALL cells with higher efficiency than the MoMuLV-based vectors at an MOI of 3,000, the highest MOI tested. ALL cells transduced by the HIV-1–based vector had a higher mean fluorescence intensity of EGFP expression than those transduced by the MoMuLV-based vector (Fig7).
Transduction of Primary Human B-Precursor ALL Cells With Retroviral and Lentiviral Vectors
| Experiment No.4-150 . | Patient No. . | MOI . | % Cells Expressing EGFP . | |
|---|---|---|---|---|
| MLV-CMV-EGFP . | PHR′-CMV-EGFP . | |||
| 1 | 1 | 30 | 2.75 | 2.2 |
| 300 | 25.69 | 18.34 | ||
| 2 | 30 | 2.81 | 2.35 | |
| 300 | 21.71 | 19.44 | ||
| 2 | 1 | 3,000 | 8.11 | 28.68 |
| 3 | 3,000 | 19.55 | 29.1 | |
| 3 | 1 | 100 | 31.08 | 5.03 |
| 500 | 61.29 | 15.47 | ||
| 3 | 100 | 48.09 | 9.57 | |
| 500 | 74.05 | 15.28 | ||
| 4 | 4 | 3,000 | 14.53 | 43.35 |
| 5 | 3,000 | 4.09 | 34.92 | |
| Experiment No.4-150 . | Patient No. . | MOI . | % Cells Expressing EGFP . | |
|---|---|---|---|---|
| MLV-CMV-EGFP . | PHR′-CMV-EGFP . | |||
| 1 | 1 | 30 | 2.75 | 2.2 |
| 300 | 25.69 | 18.34 | ||
| 2 | 30 | 2.81 | 2.35 | |
| 300 | 21.71 | 19.44 | ||
| 2 | 1 | 3,000 | 8.11 | 28.68 |
| 3 | 3,000 | 19.55 | 29.1 | |
| 3 | 1 | 100 | 31.08 | 5.03 |
| 500 | 61.29 | 15.47 | ||
| 3 | 100 | 48.09 | 9.57 | |
| 500 | 74.05 | 15.28 | ||
| 4 | 4 | 3,000 | 14.53 | 43.35 |
| 5 | 3,000 | 4.09 | 34.92 | |
CD40L was used in experiments no. 1 and 2.
Transduction of patient no. 1 primary B-precursor ALL cells with MLV-CMV-EGFP and pHR′-CMV-EGFP. X-axis, EGFP fluorescence; Y-axis, anti–CD19-PE. (Left panel) Mock-transduced cells; (center panel) cells transduced with MLV-CMV-EGFP; (right panel) cells transduced with pHR′-CMV-EGFP.
Transduction of patient no. 1 primary B-precursor ALL cells with MLV-CMV-EGFP and pHR′-CMV-EGFP. X-axis, EGFP fluorescence; Y-axis, anti–CD19-PE. (Left panel) Mock-transduced cells; (center panel) cells transduced with MLV-CMV-EGFP; (right panel) cells transduced with pHR′-CMV-EGFP.
DISCUSSION
For clinical application of immunostimulatory gene therapy of ALL, there is a need to achieve a balance between high efficiency gene transfer and survival of primary human ALL cells, which may be fragile and poorly tolerant of extended culture. Genetically modified leukemia cells used as vaccines will probably be irradiated to prevent their growth in vivo and, therefore, will only express introduced gene products for a few days. Thus, gene transfer methods that lead to stable or transient gene expression may be adequate for this approach. In this series of experiments, gene delivery to human B-precursor ALL cell lines was evaluated using a number of transfection and transduction protocols.
Electroporation was ineffective in transfecting the leukemia cells lines and also produced high cell mortality. Primary leukemia cells are prone to undergo apoptosis in vitro; hence, electroporation will probably be ineffective as a tool for gene transfer to primary cells. Cellfectin was the only transfection reagent that produced some gene transfer. Nalm-6 cells were better transfected than Reh cells. Even though the level of gene transfer by Cellfectin was relatively low and expression was transient, it may modify primary ALL cells for a time sufficient to elicit an immune response in vivo.
MoMuLV vectors have been used in clinical trials for certain genetic and neoplastic diseases. The potential for use of MoMuLV-based vectors in ALL will be dependent on whether a patient’s primary ALL cell sample can be transduced efficiently. MoMuLV-based vectors used in the presence of recombinant fibronectin fragment CH-296 were moderately efficient in transducing Nalm-6 cells but were inefficient in transducing Reh cells. The difference in gene transfer to the cell lines could possibly reflect the heterogeneity of primary ALL cells. One possible reason could be the different expression of certain adhesion molecules on ALL cells. Nalm-6 cells expressed high levels of both VLA-4 and VLA-5, whereas Reh cells expressed only VLA-4 (unpublished observation). These integrins are required for the binding of the cells to the recombinant fibronectin fragment, CH-296, and helps colocalize the cells and retrovirus.8 It is also possible that the Nalm-6 cells express higher levels of the GALV receptor than Reh cells, leading to better transduction of the former with the GALV-pseudotyped MoMuLV vector.
Lentiviral vectors efficiently transduced both Nalm-6 and Reh cells. These vectors overcame the differences between the cell lines seen with retroviral transduction, making them possibly the best agents for gene transfer into primary ALL cells. Because the cell lines are continuously dividing, the high transduction is probably not secondary to the ability of lentiviral vectors to transduce quiescent cells. The better transduction may be related to more efficient reverse transcription and integration in the human leukemia cell genome.
We have extended these studies in the cell lines to analysis of transduction or primary B-precursor ALL cells from pediatric patients. Primary ALL cells are very fragile and under the optimal conditions there is essentially no increase in cell number, although the cells can be sustained for a few days. Both MoMuLV-based retroviral and HIV-1–based lentiviral vectors showed reproducible transduction of these cells. Even though there was no increase in gene transfer with increasing the MOI across patient samples, there was an increase in gene transfer within patient samples. To the best of our knowledge, these experiments for the first time show that primary ALL cells can be transduced by retroviral and lentiviral vectors. We are currently studying methods to increase transduction efficiency of primary ALL cells, examining the efficacy of multiple exposures to vector, the use of higher MOI, or the use of spinoculation protocols. Better culture conditions for primary human ALL cells may improve gene transfer with retroviral and lentiviral vectors and also allow ex vivo expansion of transduced cells. We will also test the delivery and expression of candidate therapeutic genes such as CD80 and granulocyte-macrophage colony-stimulating factor (GM-CSF) in primary B-precursor ALL cells. If successful, these modified cells could be used in patients with high-risk disease to stimulate an autologous immune response against leukemia. Even though lentiviral vectors have so far been shown to be free of replication competent virus, there are still some safety concerns before they can be used in clinical trials. Development of safer lentiviral vectors will aid in making this approach feasible.
ACKNOWLEDGMENT
The authors acknowledge the help of Karen A. Pepper of the Vector Core Facility at CHLA for construction of the vectors, Lora W. Barsky for help with the FACS analysis, and Tanja A. Gruber and Dianne C. Skelton for their critique of the manuscript.
Supported by a grant from an anonymous donor to the USC Norris Cancer Center and Childrens Hospital Los Angeles for studies of ALL and by a Translational Grant from the Leukemia Society of America (6211-98). R.S. is a recipient of the Childrens Hospital Los Angeles Research Institute Career Development Fellowship.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Donald B. Kohn, MD, Childrens Hospital Los Angeles, 4650 Sunset Blvd, Mailstop #62, Los Angeles, CA 90027; e-mail:dkohn@chla.usc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal