Peripheral T-cell lymphomas (PTCL) have been generally reported to have a worse prognosis than B-cell lymphomas (BCL). Because of their heterogeneity and scarcity, the outcomes of the different histological subtypes have not been compared. From October 1987 to March 1993, 1,883 patients with diffuse aggressive non-Hodgkin's lymphomas (NHL) included in the LNH87 protocol could be assessed for both morphology and immunophenotyping. Among them, 288 (15%) had PTCL and 1,595 (85%) had BCL. According to the Kiel classification, most PTCL were classified as angioimmunoblastic (AIL; 23%), pleomorphic medium and large T-cell (PML; 49%), or anaplastic large cell (ALCL; 20%) lymphomas. Comparing PTCL with BCL patients, the former had more disseminated disease (78% v 58%), B symptoms (57% v40%), bone marrow involvement (31% v 17%), skin involvement (21% v 4%), and increased β2-microglobulin (50% v 34%), whereas BCL patients had more bulky disease (41% v 26%). According to the International Prognostic Index (IPI), PTCL and BCL scores were, respectively: 0 factors, 13% and 15%; 1 factor, 17% and 22%; 2 factors, 24% and 25%; ≥3 factors, 45% and 37% (P = .09). For BCL and PTCL, respectively, complete remission rates were 63% and 54% (P = .004); the 5-year overall survival (OS) rates were 53% and 41% (P = .0004) and event-free survival (EFS) rates were 42% and 33% (P < .0001). Comparison of the different histological subtypes of lymphoma showed that the 5-year OS rate for T-ALCL (64%) was superior to those of other PTCL (35%) as well as diffuse large B-cell (53%) NHL. When multivariate analysis was applied using the IPI score as one factor, nonanaplastic PTCL remained an independent parameter (P = .0004). Although the poor prognosis of non-ALCL PTCL could be due in part to the presence of adverse prognostic factors at diagnosis, this study shows that the T-cell phenotype is an independent significant factor, which should be incorporated into the definition of prognostic groups.
T-CELL NON-HODGKIN'S lymphomas (NHL) are rare in Europe and the United States, where they constitute about 15% to 20% of aggressive lymphomas.1-4 They are more common in Taiwan and Japan5 and are frequently associated with human T-lymphotropic virus-I.6,7 They include T-cell lymphomas expressing an immature prethymic or thymic phenotype, classified as lymphoblastic lymphomas and T-cell lymphomas of peripheral origin, with a post-thymic CD1−, TdT−phenotype that have been selected for the study. According to morphologic and immunological criteria, all cases have been classified according to the updated Kiel classification,8and the Revised European-American Lymphoma (REAL) classification has also distinguished other entities based on the site of origin, and/or additional phenotyping or cytogenetic features.9
The prognostic significance of the immunophenotype has been explored in several studies,2,10-12 and conflicting results have been reported concerning the outcome of peripheral T-cell lymphomas (PTCL) compared with that of B-cell lymphomas (BCL). PTCL patients were found to have equivalent10,5 or poorer prognoses than patients with BCL.11,12 We previously reported2 that PTCL were associated with a poor prognosis and that immunophenotype per se could be an independent adverse prognostic parameter for event-free survival (EFS). PTCL represent a heterogeneous group of lymphomas, and a wide variety of different histological subtypes have been recognized.8,9,13-16 According to the Kiel classification, in our series the most common PTCL is the pleomorphic medium and large-cell lymphoma (PML),8 followed by anaplastic large cell lymphoma (ALCL),17 angioimmunoblastic lymphoma (AIL),18,19 and lymphoepithelioid lymphoma. Although it was suggested that PTCL had low-grade or high-grade histological features, the prognostic significance of such a distinction has not been established.8
The International Prognostic Index (IPI) Project20 did not evaluate the influence of immunophenotype on overall survival (OS), primarily because of the limited amount of phenotypic data available and the difficulty of prospectively studying a cohort of PTCL patients large enough to ensure statistical significance. Recently, the prognostic value of immunophenotype, using the REAL classification, was evaluated with stratification of other prognostic factors.3Although the T-cell phenotype was an independent and significant prognostic factor, it was not possible to show a statistically significant effect on OS among the different histological subtypes.
To better define the clinical outcomes of the different subtypes of T-cell lymphoma, 288 patients with a confirmed T-cell immunophenotype included in the prospective LNH87 protocol were compared with 1595 BCL patients of comparable histological grades. The aim of this study was to evaluate the prognoses of the different entities defined in the Kiel classification and to compare them with BCL patients with similar clinical characteristics according to the IPI score.
MATERIALS AND METHODS
Patient Selection
This series included all patients with the diagnosis of PTCL confirmed by pathological review and who were registered in the LNH87 study. The LNH87 trial, conducted by the Groupe d'Etude des Lymphomes de l'Adulte (GELA), recruited from 50 participating French and Belgian centers patients who were at least 15 years old and had intermediate- or high-grade NHL, according to the Working Formulation, but were seronegative for the human immunodeficiency virus and had no previous cancer, cardiac disease, uncontrolled diabetes mellitus, or kidney failure. Patients were staged according to the Ann Arbor system. Disease dissemination was evaluated before treatment by physical examination, bone marrow (BM) biopsy, and computerized tomography scan of chest and abdomen.
The number of extranodal sites (ENS) and the largest tumor mass diameter were also determined. Performance status (PS) was based on the World Health Organization scale. The serum lactate dehydrogenase (LDH) level was expressed as the ratio over the maximal normal value. From October 1st, 1987 to April 1st, 1993, 3,232 patients were included in the LNH87 trial. At the time of analysis, 88% of the slides were available for pathological review. At the present time, 288 patients have been given the diagnosis of peripheral T-cell NHL after review of the histopathological slides and immunohistochemical analyses. Similarly, 1,595 patients were considered to have BCL, and most of them, 1,390, were diffuse large B-cell lymphomas of the categories G and H of the Working Formulation (ie, centroblastic and immunoblastic type according to the updated Kiel classification). Follicular large cell and mantle cell lymphoma were excluded from the comparison.
Treatment and Assessment of Response
Group 1.
This group included 77 PTCL and 351 BCL patients less than 70 years old without any adverse prognostic factors. Patients were randomly assigned to receive 8 cycles every 3 weeks of mBACOD (Adriamycin 45 mg/m2, cyclophosphamide 600 mg/m2, vincristine 1 mg/m2, bleomycin 10 mg/m2 d1, methotrexate 200 mg/m2 d8 and d15, Decadron [Merck Sharp Dohme, Paris, France] d1-5) or 3 courses every 2 weeks of ACVB (Adriamycin [Pharmacia, Guyancourt, France] 75 mg/m2 d1, cyclophosphamide 1,200 mg/m2 d1, vindesine 2 mg/m2 d1, d5, bleomycin 10 mg d1 and d5, prednisone 60 mg/m2 d1-5) followed by the consolidation and maintenance chemotherapies of the LNH84 protocol.21
Group 2.
This group included 91 PTCL and 520 BCL patients, 15 to 55 years old, with at least one of the adverse prognostic factors. Patients were randomly assigned to receive 4 cycles of ACVB or 4 courses of NCVB (same as ACVB but with mitoxantrone 12 mg/m2 d1 instead of Adriamycin). Those in complete remission (CR) were randomized between autologous BM transplantation using CVB (cyclophosphamide 1,500 mg/m2 d1-4, Vepesid [Novartis-Pharma, Revil-Malmaison, France] 250 mg/m2 d1-4, Carmustine [BCNU] 300 mg/m2 d4) as conditioning regimen or the classical consolidation and maintenance therapy of the LNH84 protocol.22
Group 3.
Group 3 included 80 PTCL and 451 BCL patients, 55 to 70 years old, with at least one adverse prognostic factor. Patients were randomized to receive 4 cycles of ACVB followed by the consolidation and maintenance therapy of LNH84 protocol or 4 alternating induction cycles every 3 weeks of VIM3 (mitoxantrone 10 mg/m2 d1, ifosfamide 1,000 mg/m2 d1-3, Methyl-GAG 300 mg/m2 d1 and d5, Vehem [Novartis-Pharma] 100 mg/m2 d1 and d5, prednisone 60 mg/m2 d1-5, methotrexate 1,500 mg/m2 d14) and ACVB then a maintenance therapy with 4 alternating courses every 3 weeks of VIM (mitoxantrone 10 mg/m2 d1, Vepesid 150 mg/m2 d1-3, ifosfamide 1,000 mg/m2 d1-3) and ACVM (Adriamycin 50 mg/m2 d1, cyclophosphamide 750 mg/m2 d1, vindesine 2 mg/m2 d1, methotrexate 200 mg/m2 d7, d15).23
Group 4.
Group 4 included 40 PTCL and 273 BCL patients over 70 years old. They were randomly assigned to receive CVP (cyclophosphamide 750 mg/m2 d1, Vehem 75 mg/m2 d1, prednisone 40 mg/m2 d1-3) or CTVP (same as CVP plus tetrahydropyranyladriamycin 50 mg/m2 d1) for 6 cycles.24
Response to therapy was evaluated after induction therapy, which generally consisted of 4 cycles of chemotherapy. A CR was defined as the disappearance of all clinical evidence of disease and the normalization of all laboratory values, radiographs, scans, and biopsies that were abnormal before therapy. Additionally, patients with persistent computed tomographic abnormalities but regression greater than 75% of the initial tumor volume were deemed to be in CR when in CR of all other parameters. Partial remission (PR) was defined as reduction of 50% to 75% of the initial tumor volume. Treatment failure was defined by a lower response, progressive disease, or a treatment-related death.
Histological and Immunophenotypic Studies
The slides of all patients included in the LNH87 protocol that were initially diagnosed as having PTCL by three independent pathologists were reviewed again by two other independent pathologists (J.D., P.G.). Disagreements were resolved by consensus discussions, usually after additional immunostaining tests. The aims of this second review were (1) to confirm the diagnosis of PTCL and (2) to subclassify them according to the categories of the updated Kiel classification to assess their potential prognostic significance. In addition, some categories of extra nodal T-cell lymphomas recently recognized in the REAL classification mostly based on the site of origin were identified among the included PML category.9
The diagnosis of PTCL was based on both morphologic examination of slides from routinely paraffin-embedded samples stained with hematoxylin-eosin, Giemsa, and Gordon-Sweet's staining and immunophenotyping results. In all cases, immunophenotyping was performed with a panel of antibodies comprising CD20/L26, CD3, and CD45R0/UCHL1 (Dako, Glostrup, Denmark) to determine the B- or T-cell lineage, respectively, using an indirect immunoperoxidase or alkaline phosphatase antialkaline phosphatase (APAAP) method. Depending on histological subtypes, the expression of activation (CD30/BerH2, EMA, Dako), cytotoxic (TIA-1; Coulter, Hialeah, FL), Epstein-Barr virus (EBV; latent membrane protein LMP-1, CS 1-4; Dako) antigens were also tested, as well as the presence of follicular dendritic cells (CNA-42 antibody, kindly given by G. Delsol, Hopital Purpan, Toulouse, France). Furthermore, additional immunophenotyping was obtained on frozen sections for one third of the cases or for some cases by flow cytometry.
The diagnosis of the different subcategories of PTCL according to the updated Kiel classification was based on histological and phenotypic criteria.8 Lymphoblastic lymphoma, mycosis fungoides, and Sezary syndrome were excluded from the study. Briefly, the diagnosis of AIL-like required morphological and/or phenotypic evidence of follicular dendritic cells (CNA-42+) in addition to other classical cytological criteria. The diagnosis of lymphoepithelioid (Lennert's) lymphoma was assigned when the proliferation consisted of slightly pleomorphic T cells admixed with epithelioid cells but without morphological evidence of Reed Sternberg cells and follicular dendritic cells. PML included all cases characterized by some degree of pleomorphism and a large cell component. This category included a few cases with a predominance of medium-sized cells. Some of the PML cases were with extranodal presentation, which were further subclassified according to the entities of the REAL classification. For the diagnosis of T-ALCL, anaplastic morphology, T-cell phenotype, and the expression of CD30 on virtually all tumor cells was required, as previously reported.25 After additional immunostaining, 11 new cases from the LNH87 study were diagnosed as T-ALCL and put together with the 49 patients reported by Tilly et al.25The histological distribution of the 288 cases of PTCL according to the updated Kiel classification is reported in Table1.
Histological Distribution According to the Kiel Classification of the 288 PTCL Lymphomas
| Histology . | Number . | % . |
|---|---|---|
| T-zone | 0 | 0 |
| Lymphoepithelioid (Lennert's) | 12 | 4 |
| Pleomorphic small cell | 3 | 1 |
| Angioimmunoblastic | 68 | 24 |
| Pleomorphic medium and large* | 142 | 49 |
| Immunoblastic | 3 | 1 |
| Anaplastic | 60 | 21 |
| Total | 288 | 100 |
| Histology . | Number . | % . |
|---|---|---|
| T-zone | 0 | 0 |
| Lymphoepithelioid (Lennert's) | 12 | 4 |
| Pleomorphic small cell | 3 | 1 |
| Angioimmunoblastic | 68 | 24 |
| Pleomorphic medium and large* | 142 | 49 |
| Immunoblastic | 3 | 1 |
| Anaplastic | 60 | 21 |
| Total | 288 | 100 |
*PML subcategory included extranodal lymphomas belonging to subcategories of the REAL classification, ie, angiocentic nasopharyngeal (n = 12), intestinal (n = 7), subcutaneous panniculitis (n = 6), and hepatosplenic γδ (n = 4) T-cell lymphomas.
Statistical Analysis
Patient characteristics and CR rates were compared using the chi-squared test. Because of the low number of patients in some morphological subgroups, AIL, lymphoepithelioid, and pleomorphic small cell, which all show a predominance of small/medium-sized cell populations, were analyzed together. The three cases of immunoblastic lymphoma were included in the PML category.
Overall survival time was calculated from the date of randomization until death or last follow-up examination. EFS was calculated from the date of randomization until disease progression, relapse, or last follow-up visit.
Survival curves were estimated using the product-limit method of Kaplan-Meier26 and were compared using the log-rank test. Univariate analyses were made using the chi-squared test and log-rank test. Stepwise Cox proportional-hazards regression analysis with the OS as the dependent variable was used to adjust the effect of the T-cell phenotype for potential independent prognostic factors.27
RESULTS
The main clinical and biological characteristics of the 288 PTCL patients with the 1,595 patients with diffuse BCL are listed in Table2. The median age of the PTCL patients enrolled in the study was 56 years and that of BCL patients was 57 years. Several clinical parameters were significantly more prevalent in PTCL patients; male gender, advanced stage with multiple-node involvement, B symptoms, BM involvement, hepatosplenomegaly, and skin lesions. BCL patients had more localized stage and bulky disease. Significantly more PTCL patients presented with anemia, hypereosinophilia, and hypergammaglobulinemia. A comparison of the main clinical characteristics of the 228 non-ALCL PTCL patients with the 60 patients with T-ALCL are reported in Table3. These latter tend to have more localized stage with a more favorable IPI score.
Clinical Characteristics of PTCL and Diffuse BCL Patients
| Parameter . | PTCL (n = 288) (%) . | BCL (n = 1,595) (%) . | P Value . |
|---|---|---|---|
| Age ≤60 yr | 64 | 59 | .1 |
| Male | 69 | 53 | .001 |
| Female | 31 | 47 | |
| Stage | |||
| I-II | 22 | 42 | .001 |
| III-IV | 78 | 58 | |
| B-symptoms | 57 | 40 | .001 |
| PS >1 | 28 | 25 | .1 |
| ENS >1 | 37 | 30 | .01 |
| BM positive | 31 | 17 | .001 |
| Bulk >10 cm | 26 | 41 | .001 |
| Hepatomegaly | 21 | 11 | .001 |
| Splenomegaly | 34 | 20 | .001 |
| Skin lesions | 21 | 4 | .001 |
| LDH >NI | 51 | 57 | .05 |
| β2-microglobulin >NI | 50 | 34 | .001 |
| Hemoglobin <10 g/dL | 21 | 13 | .001 |
| Platelets <100 × 109/L | 9 | 3 | .001 |
| Eosinophils ≥0.8 × 109/L | 14 | 2 | .001 |
| Gammaglobulin >20 g/L | 8 | 3 | .001 |
| IPI score*: | |||
| 0 factors | 13 | 15 | .09 |
| 1 factor | 17 | 22 | |
| 2 factor | 24 | 25 | |
| ≥3 factors | 45 | 37 |
| Parameter . | PTCL (n = 288) (%) . | BCL (n = 1,595) (%) . | P Value . |
|---|---|---|---|
| Age ≤60 yr | 64 | 59 | .1 |
| Male | 69 | 53 | .001 |
| Female | 31 | 47 | |
| Stage | |||
| I-II | 22 | 42 | .001 |
| III-IV | 78 | 58 | |
| B-symptoms | 57 | 40 | .001 |
| PS >1 | 28 | 25 | .1 |
| ENS >1 | 37 | 30 | .01 |
| BM positive | 31 | 17 | .001 |
| Bulk >10 cm | 26 | 41 | .001 |
| Hepatomegaly | 21 | 11 | .001 |
| Splenomegaly | 34 | 20 | .001 |
| Skin lesions | 21 | 4 | .001 |
| LDH >NI | 51 | 57 | .05 |
| β2-microglobulin >NI | 50 | 34 | .001 |
| Hemoglobin <10 g/dL | 21 | 13 | .001 |
| Platelets <100 × 109/L | 9 | 3 | .001 |
| Eosinophils ≥0.8 × 109/L | 14 | 2 | .001 |
| Gammaglobulin >20 g/L | 8 | 3 | .001 |
| IPI score*: | |||
| 0 factors | 13 | 15 | .09 |
| 1 factor | 17 | 22 | |
| 2 factor | 24 | 25 | |
| ≥3 factors | 45 | 37 |
*Based on data from 261 PTCL and 1,433 BCL patients.
Clinical Characteristics of T-Nonanaplastic Large-Cell Lymphoma and T-Anaplastic Large-Cell Lymphoma Patients
| Parameter . | Non-ALCL-T (n = 228) (%) . | ALCL-T (n = 60) (%) . | P Value . |
|---|---|---|---|
| Age ≤60 yr | 60 | 75 | .04 |
| Male | 68 | 73 | .4 |
| Female | 32 | 26 | |
| Stage | |||
| I-II | 14 | 30 | .001 |
| III-IV | 85 | 50 | |
| B-symptoms | 48 | 56 | .2 |
| PS >1 | 30 | 19 | .08 |
| ENS >1 | 40 | 23 | .01 |
| BM positive | 36 | 13 | .001 |
| Bulk >10 cm | 28 | 19 | .1 |
| Hepatomegaly | 23 | 15 | .1 |
| Splenomegaly | 39 | 13 | .001 |
| Skin lesions | 19 | 26 | .2 |
| LDH >NI | 55 | 34 | .004 |
| β2-microglobulin >NI | 55 | 32 | .01 |
| Hemoglobin <10 g/dL | 21 | 18 | .5 |
| Platelets <100 × 109/L | 7 | 11 | .3 |
| Eosinophils ≥0.8 × 109/L | 15 | 5 | .1 |
| Gammaglobulin >20 g/L | 10 | 0 | .001 |
| IPI score*: | |||
| 0 factors | 7 | 39 | .001 |
| 1 factor | 15 | 24 | |
| 2 factor | 26 | 14 | |
| ≥3 factors | 51 | 22 |
| Parameter . | Non-ALCL-T (n = 228) (%) . | ALCL-T (n = 60) (%) . | P Value . |
|---|---|---|---|
| Age ≤60 yr | 60 | 75 | .04 |
| Male | 68 | 73 | .4 |
| Female | 32 | 26 | |
| Stage | |||
| I-II | 14 | 30 | .001 |
| III-IV | 85 | 50 | |
| B-symptoms | 48 | 56 | .2 |
| PS >1 | 30 | 19 | .08 |
| ENS >1 | 40 | 23 | .01 |
| BM positive | 36 | 13 | .001 |
| Bulk >10 cm | 28 | 19 | .1 |
| Hepatomegaly | 23 | 15 | .1 |
| Splenomegaly | 39 | 13 | .001 |
| Skin lesions | 19 | 26 | .2 |
| LDH >NI | 55 | 34 | .004 |
| β2-microglobulin >NI | 55 | 32 | .01 |
| Hemoglobin <10 g/dL | 21 | 18 | .5 |
| Platelets <100 × 109/L | 7 | 11 | .3 |
| Eosinophils ≥0.8 × 109/L | 15 | 5 | .1 |
| Gammaglobulin >20 g/L | 10 | 0 | .001 |
| IPI score*: | |||
| 0 factors | 7 | 39 | .001 |
| 1 factor | 15 | 24 | |
| 2 factor | 26 | 14 | |
| ≥3 factors | 51 | 22 |
*Based on data from 261 PTCL and 1,433 BCL patients.
Response to Treatment
The CR rates were 54% and 63% for T- and B-cell NHL, respectively (P = .005). Partial responses and failures were observed in 20% and 14% of T-cell and 18% and 10% of B-cell NHL. During the induction phase, respectively, 11% and 9% of the patients died. According to univariate analysis, factors affecting the response rate were similar for the T and B subgroups. For T- and B-cell NHL, the CR rates according to the prognostic subgroups defined by the IPI score were similar except for patients with greater than or equal to 3 factors 35% and 52%. Comparisons between other subgroups are reported in Table 3. It should be noted that T-ALCL patients had the best CR rate (72%), which was significantly different from that of the non-ALCL PTCL (49%) (P = .002).
Survival
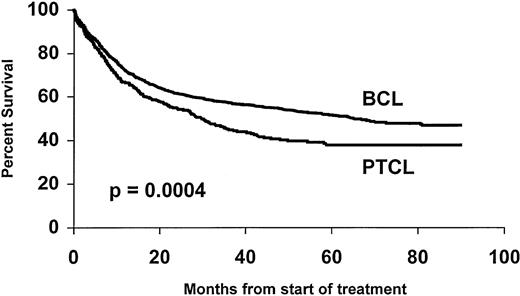
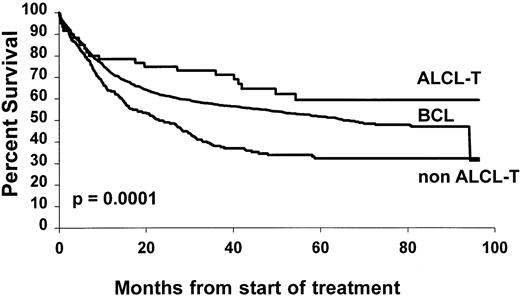
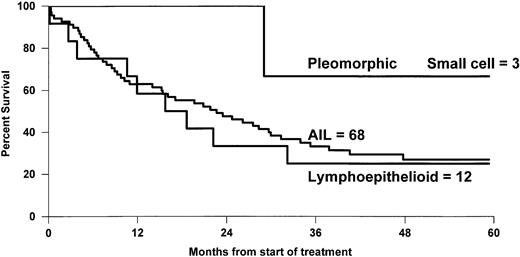
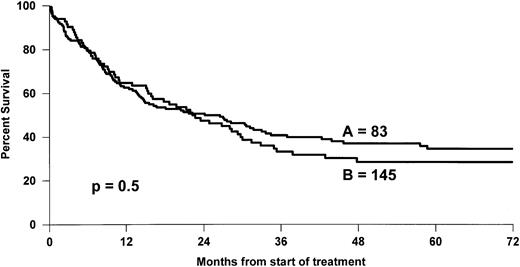
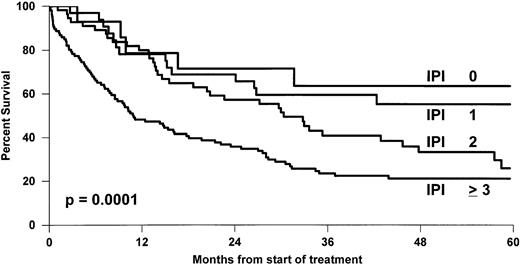
The overall 5-year survival rates (Fig 1)differed between PTCL (41%) and BCL (52%) (P = .0004). Comparison between B- and T-cell lymphoma patients was made within the different subgroups of the international prognostic index, and the results are reported in Table 3. A better survival was observed for BCL patients, but differences were significant only for patients with greater than or equal to 2 factors. Comparison between stage III to IV was also significant with a 5-year survival rate of 43% and 33% for BCL and PTCL, respectively (P = .01), but was not significant for stage I to II, 66% and 69% (P = .6). Survival was also analyzed according to the histological subtypes defined by the Kiel classification (Table 4). First, ALCL had a 5-year OS of 64%, which was better than that of any subgroup of T- or B-cell NHL and was superior to non-ALCL PTCL (Fig2). Second, PML and immunoblastic T-cell lymphoma had significantly lower survival rates when compared with diffuse large (centroblastic and immunoblastic) BCL patients. Third, the remaining subgroup including AIL-like, lymphoepitheliod, and pleomorphic small-cell lymphomas with a 31% probability of survival (Fig 3) had a worse outcome than diffuse mixed B lymphomas (46%). However, this survival was similar to that of the other PML T-cell lymphoma patients (Fig4). Using non-ALCL T-cell lymphoma as the variable, the international prognostic index was used to stratify the patients (Fig 5). For non-ALCL PTCL patients the OS probability for patients with IPI scores of 0, 1, 2, or ≥3 were, respectively, 64%, 56%, 34%, and 22%. The difference was significant when compared with BCL patients except for an IPI 1 score. The 5-year EFS was 32% for PTCL and 45% for BCL (P = .0001).
Overall survival of 288 PTCL patients compared with 1,595 diffuse BCL patients.
Outcome of PTCL Categories Compared With BCL Patients
| Histology . | Cell Type . | n . | CR (%) . | P Value . | 5-yr OS (%) . | P Value . |
|---|---|---|---|---|---|---|
| BCL | B | 1,595 | 63 | .005 | 53 | .0004 |
| PTCL | T | 288 | 54 | 41 | ||
| All other non-ALCL | T | 228 | 49 | .002 | 35 | .0001 |
| Anaplastic | T | 60 | 72 | 64 | ||
| Large cell | B | 1,390 | 62 | .02 | 52 | .0005 |
| PML | T | 145 | 53 | 37 | ||
| Diffuse mixed | B | 79 | 43 | .9 | 46 | .003 |
| AIL, LE | T | 83 | 42 | 31 | ||
| AIL, LE | T | 83 | 42 | .1 | 31 | .7 |
| PML | T | 145 | 53 | 37 | ||
| IPI score3-150 | ||||||
| 0 factors | B | 216 | 81 | .7 | 84 | .1 |
| T | 35 | 82 | 77 | |||
| 1 factor | B | 319 | 71 | .7 | 63 | .7 |
| T | 45 | 73 | 60 | |||
| 2 factors | B | 359 | 63 | .4 | 53 | .01 |
| T | 63 | 58 | 36 | |||
| 3 factors | B | 539 | 52 | .001 | 35 | .03 |
| T | 118 | 35 | 23 |
| Histology . | Cell Type . | n . | CR (%) . | P Value . | 5-yr OS (%) . | P Value . |
|---|---|---|---|---|---|---|
| BCL | B | 1,595 | 63 | .005 | 53 | .0004 |
| PTCL | T | 288 | 54 | 41 | ||
| All other non-ALCL | T | 228 | 49 | .002 | 35 | .0001 |
| Anaplastic | T | 60 | 72 | 64 | ||
| Large cell | B | 1,390 | 62 | .02 | 52 | .0005 |
| PML | T | 145 | 53 | 37 | ||
| Diffuse mixed | B | 79 | 43 | .9 | 46 | .003 |
| AIL, LE | T | 83 | 42 | 31 | ||
| AIL, LE | T | 83 | 42 | .1 | 31 | .7 |
| PML | T | 145 | 53 | 37 | ||
| IPI score3-150 | ||||||
| 0 factors | B | 216 | 81 | .7 | 84 | .1 |
| T | 35 | 82 | 77 | |||
| 1 factor | B | 319 | 71 | .7 | 63 | .7 |
| T | 45 | 73 | 60 | |||
| 2 factors | B | 359 | 63 | .4 | 53 | .01 |
| T | 63 | 58 | 36 | |||
| 3 factors | B | 539 | 52 | .001 | 35 | .03 |
| T | 118 | 35 | 23 |
Abbreviation: LE, lymphoepithelioid.
Based on data from 261 PTCL and 1,433 BCL patients.
Overall survival of 228 non-ALCL cell and 60 T-ALCL lymphoma patients compared with 1,595 diffuse BCL patients.
Overall survival of 228 non-ALCL cell and 60 T-ALCL lymphoma patients compared with 1,595 diffuse BCL patients.
OS of 68 AIL patients, 12 lymphoepithelioid, and 3 pleomorphic small-cell lymphoma patients.
OS of 68 AIL patients, 12 lymphoepithelioid, and 3 pleomorphic small-cell lymphoma patients.
Comparison of OS of the two main groups of PTCL: (A) n = 83; AIL, lymphoepithelioid, pleomorphic small-cell lymphoma patients and (B) n = 145 PML, immunoblastic patients.
Comparison of OS of the two main groups of PTCL: (A) n = 83; AIL, lymphoepithelioid, pleomorphic small-cell lymphoma patients and (B) n = 145 PML, immunoblastic patients.
OS of non-ALCL T-cell lymphoma patients grouped according to the IPI score.
Because of the better survival advantage for T-ALCL, multivariate analysis was performed only on patients with non-ALCL PTCL and BCL. Five factors adversely and significantly influenced survival: age greater than 60 years, disseminated stage, elevated LDH, performance status, and non-ALCL T-cell lymphoma (P = .0001). Treatment arm was not a significant factor. A second multivariate analysis was done using the IPI (age, stage, LDH, PS) as one factor. Non-ALCL T-cell lymphoma remained highly significant (P = .0004).
DISCUSSION
This large series of PTCL further emphasizes the morphological heterogeneity of these neoplasms. Classification on the basis of morphology and immunophenotyping is still subject to controversy because of numerous entities included under the name of PTCL. The clear identification of ALCL T-cell lymphomas on the basis of morphology, CD30 antigen expression,17 a common cytotoxic profile,28,29 and a better outcome25 clarifies the situation by separating these lymphomas from the others. Since its recognition in 1978 as an immune disorder, there is also evidence that AIL-like T-cell lymphoma constitutes a distinct PTCL entity, with morphological, phenotypic, and clinical features and a peculiar pattern of EBV30 and cytokines expression.31 In the present study, we further confirm that lymphoepithelioid (Lennert's) lymphoma and T-zone lymphoma are very rare morphological subtypes, often difficult to distinguish from epithelioid-cell–rich AIL or other categories of PTCL.9 About half of the present cases were classified as PML based on the predominance of medium and/or large neoplastic cells. This PML category included several cases of more recently recognized extranodal lymphomas, eg, nasal T-natural killer (NK)-cell lymphomas,32,33 intestinal T-cell lymphomas,34 subcutaneous panniculitis-like T-cell NHL,35 and hepatosplenic gamma-delta T-cell lymphoma.36 37 Altogether, these subgroups represented about 20% of the PML category.
The cytological distinction between high- and low-grade malignancies did not translate into a different outcome for non-ALCL T-cell lymphoma8 in this large series representative of the lymphoma population encountered by clinicians in Europe. However, the selection of cases eliminated T-CLL, mycosis fungoides, and Sezary syndrome.
Using the updated Kiel classification, only three major subgroups (PML, AIL, and ALCL) could be considered sufficiently common to be analyzed in clinical trials. For the other described entities, their prognoses seemed to be rather poor, whether or not they presented with nodal or extranodal disease. In the absence of new data, they should be treated in the same way as the major subgroups, PML or AIL.
Analysis of clinical parameters showed that T-cell NHL more often presented with disseminated disease and extranodal presentation than B-cell NHL. When patients were stratified according to their IPI score, 45% of T-lymphoma patients had three or more adverse prognostic factors, which was significantly different from the distribution in BCL (37%). The high degree of BM involvement (31%) was associated with anemia and thrombopenia. Eosinophilia and hypergammaglobulinemia were more often encountered in AIL. Severe disease at the time of presentation had previously been described2,3 and might explain the lower CR rate observed in non-ALCL T-cell lymphoma as compared with B-cell NHL. Not surprisingly, this poorer response was translated into shorter OS and EFS. These results are in agreement with our previous study2 but were not found in other retrospective studies.5,10,11 In fact in the study of Kwak et al10 based on 21 T-cell and 77 B-cell NHL, there was a trend favoring the better outcome of T-cell lymphomas.
In our study, patients were enrolled in a prospective randomized trial, and 88% of histological material was available for immunophenotyping. Because of the size of this population, it was possible to separate and analyze each of the major subgroups defined by the updated Kiel classification and to compare them with B-cell NHL. Only ALCL T-cell lymphomas had significantly better CR rates and survival, and thus should be clearly separated from the other groups for the analysis of the significance of immunophenotype as a prognostic factor. A similar trend was observed by Melnyk et al, although it could not reach a statistically significant difference with only 10 patients with T-ALCL.3 With risk stratification according to the IPI score, the OS was significantly worse for non-ALCL T lymphomas, but this difference was mainly found with an IPI score greater than or equal to 2, even when all PTCL were analyzed together. In multivariate analysis, non-ALCL T-cell character appeared to be an independent adverse prognostic factor. Association in Cox's analysis with other well-known parameters such as age, performance status, and disseminated stage lead us to further investigate non-ALCL T-cell phenotyping and to consider IPI as a single parameter in a stratified analysis. Non-ALCL T-cell lymphoma remained significant, thereby allowing it to be considered as an independent adverse prognostic factor.
The poor prognosis of peripheral T-cell lymphoma has been reported in most series.2,3,5,11,38 With the intensive regimens used in the LNH87 study, the 41% 5-year survival rate is similar to those reported by others.2,10,11,39,40 The same control arm ACVB was used until 70 years of age, and there was no difference between treatment arms in each group for PTCL. However, the 23% 5-year survival rate for patients with three or more adverse prognostic factors cries out for new strategies. In one arm of the LNH87 trial already reported,22 autologous stem-cell transplantation was performed after CR. Interestingly, for 16 PTCL patients submitted to this procedure the probability of survival was not different from that of BCL patients. However, the low CR rate hampered such an approach for the majority of them. Various chemotherapy regimens have been tested39,40 and, until now, have proven to be unsuccessful in modifying outcome. Curiously, other agents such as interferon-α,41 13-cis-retinoid acid,42 interleukin-2,43 or cyclosporine44 have shown some degree of transient activity in refractory disease. Nevertheless, treatment of patients with several adverse prognostic factors remains unsolved, and most PTCL should be included in prospective trials testing innovative approaches.
ACKNOWLEDGMENT
The authors acknowledge Catherine Balmale and Catherine Belorgey for their invaluable technical assistance, Catherine Barli for preparing the manuscript, and they thank the following clinicians and pathologists who actively participated in the study: I. Abdalsamad, M.F. d'Agay, C. Allard, R. Angonin, J. d'Anjou, B. Audhuy, J. Audouin, G. Auzanneau, A.C. Baglin, C. Bailly, Y. Bastion, E. Baumelou, P. Bensimon, F. Berger, P. Biron, A.M. Blaise, M. Blanc, F. Boman-Ferrand, A. Boehn, J. Boniver, M. Bordes, D. Bordessoule, A. Bosly, R. Bouabdallah, S. Boucheron, J. Bouvier, P. Brice, J. Brière, N. Brousse, P. Brousset, P.A. Bryon, D. Caillot, J.P. Carbillet, R.O. Casasnovas, T. Caulet, D. Cazals, F. Charlotte, L. Charvillat, A.M. Chesneau, B. Christian, B. Coiffier, T. Conroy, J.F. Cordier, C. Cordonnier, J.P. Clauvel, E. Deconinck, M. Delage, A. Delannoy, M. Delos, G. Delsol, A. Devidas, J. Diebold, M. Diviné, H. Dombret, C. Doyen, H. Duplay, B. Dupriez, C. Duval, J.C. Eisenmann, J.M. Emberger, B. Epardeau, B. Fabiani, P. Felman, J.P. Fermand, C. Fermé, A. Ferrand, M. Ffrench, M. Fievez, Y. Fonck, N. Froment, J. Gabarre, P. Galian, O. Gasser, P. Gaulard, C. Gisselbrecht, B. Gosselin, H. Guy, D. Guyotat, C. Haioun, J. Hamels, R. Herbrecht, O. Hopfner, N. Horschowski, F. Huguet, P. Jacomy, J. Jaubert, R. Jeandel, Y. Kerneis, J.P. Knopf, M. Kuentz, E. Labouyrie, B. Lancien, G. Laurent, A. Lavergne, C. Lavignac, V. Leblond, M. Lecomte-Houke, P. Léderlin, F. Lejeune, M.B. Leger-Ravet, R. Loire, R. Marcellin, J.P. Marolleau, G. Marit, C. Martin, C. Marty-Double, A. De Mascarel, S. Méhaut, J.P. Merlio, C. Merignargues, J.M. Micléa, J.L. Michaux, M. Monconduit, P. Morel, F. Morvan, J.F. Mosnier, G. Nédellec, C. Netter-Pinon, H. Noel, C. Nouvel, M. Patey, P.Y. Peaud, G. Perie, M. Peuchmaur, T. Petrella, B. Pignon, C. Platini, M. Pluot, J.P. Pollet, E. Pujade-Lauraine, M. Raphael, M.C. Raymond-Gelle, J. Reiffers, F. Reyes, M. Rochet, J.F. Rossi, A.M. Roucayrol, A. Rozenbaum, G. Salles, H. Schill, C. Sebban, M. Simon, P. Solal-Céligny, P. Straub, E. Suc, L. Sutton, M. Symann, G. Tertian, S. Thiebaut, A. Thyss, H. Tilly, P. Travade, V. Trillet, J.P. Vernant, D. Wendum, and L. Xerri.
Supported in part by the Ligue Contre le Cancer.
Address reprint requests to Christian Gisselbrecht, MD, Institut d'Hématologie, Hôpital Saint-Louis, 1, Avenue Claude-Vellefaux, 75475 Paris Cedex 10, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal