In this study we investigated the role of interleukin-15 (IL-15) in the immunobiology of cutaneous T-cell lymphoma (CTCL) cells. Using cell culture techniques, reverse transcriptase-polymerase chain reaction (RT-PCR), and immunhistochemistry we found that IL-15, like IL-7, is a growth factor for the Sézary cell line SeAx and that both cytokines prolonged the survival of malignant T cells directly isolated from Sézary syndrome (SS) patients. Both IL-15 and IL-7 were more potent than IL-2. IL-4 and IL-9, whose receptors share the same gamma chain with the receptors of IL-2, IL-7, and IL-15, did not sustain the growth of CTCL cells, indicating that signaling through the common gamma chain (γc) is not sufficient for continuous growth. IL-13 and tumor necrosis factor-α (TNF-α) had no effect. IL-7 and IL-15 also supported the growth of SeAx cells in the presence of the apoptosis inducing agents dexamethasone and retinoic acid. The analysis of patient Sézary cells and three CTCL cell lines by RT-PCR showed that all these cells expressed IL-15 mRNA, but only a few (25%) produced IL-7 mRNA. Immunohistological analyses of skin biopsy samples of SS and Mycosis fungoides patients showed immunoreactivity for IL-15 in basal cell layer keratinocytes and in the infiltrating lymphocytes. We conclude that IL-15 is a growth or viability factor for CTCL-derived cell lines or shortly cultivated Sézary cells. The findings that IL-15 mRNA can be detected in Sézary syndrome peripheral blood mononuclear cells and that the IL-15 protein is detected in skin sections from CTCL patients suggest that IL-15 plays an important role in the biology of CTCL.
CUTANEOUS T-CELL lymphoma (CTCL) is a heterogeneous group of lymphoproliferative disorders of the skin.1 The most frequent forms of CTCL are Mycosis fungoides (MF) and its leukemic counterpart the Sézary syndrome (SS). One striking feature of this disease is that the lymphocyte proliferations remain restricted to the skin. This fact implies that CTCL cells remain dependent on the specific cutaneous microenvironment, including cytokines and adhesion molecules. Keratinocytes produce a variety of T-cell growth supporting cytokines such as interleukin-7 (IL-7),2 which is necessary to cultivate freshly isolated Sézary cells and some established Sézary cell lines.3,4 Initially IL-7 has been identified as a growth factor for lymphocyte precursors and T cells and its high affinity receptor contains besides a specific α chain as well as the IL-2 receptor γ chain.5
IL-15 has been identified as a T- and B-cell growth stimulating cytokine6,7 produced by several cell types and tissues,6 including skin.8,9 The IL-15 receptor contains the β and γ chain of the IL-2 receptor and a recently identified specific α chain.10,11 IL-15 can replace IL-2 in several systems,6,8 10 but mostly it proved to be less effective than IL-2.
In this study we investigated whether IL-15 is a growth or viability factor for CTCL cells in vitro and whether it is synthesized by CTCL or other skin cells and may thus act as an autocrine or paracrine growth factor for CTCL cells.
MATERIALS AND METHODS
Cell culture.
The cell line HUT78 (SS) was obtained from European Collection of Animal Cell Cultures (Salisbury, UK). The cell lines MyLa (MF) and SeAx (SS) were kind gifts of Dr Keld Kaltoft (University of Aarhus, Denmark).12 HUT 78 and MyLa and patient Sézary cells were grown in HEPES-buffered RPMI 1640 medium with 2 mmol/L glutamine, supplemented with 10% fetal calf serum (FCS), 0.25 mg/mL amphotericin B, 100 U penicillin G, 100 U streptomycin, and 1 mmol/L pyruvate. SeAx cells were grown under the same conditions with the exception that 10% human serum (HS) instead of 10% FCS was used. The concentrations of the cytokines were as follows: IL-2, 50 ng/mL (100 U); IL-4, 20 ng/mL (100 U); IL-7, 5 ng/mL (10 U); IL-9, 10 ng/mL (10 U); IL-13, 600 ng/mL (100 U); IL-15, 10 ng/mL (10 U); tumor necrosis factor-α (TNF-α), 50 pg/mL (1 U). The concentrations of dexamethasone (DEX) and retinoic acid (RA) were 1 μmol/L. Recombinant cytokines (IL-2, IL-4, IL-7, IL-9, IL-13, TNF-α) were from R&D Systems Europe (Abingdon, UK), with the exception of IL-15, which was delivered by PeproTech EC Ltd (London, UK). DEX and RA were from Sigma Chemie (Buchs, Switzerland).
Blood and skin samples.
Skin biopsy and blood samples were taken primarily for diagnostic purposes with informed consent of the patients and the specimens used were surplus material available after all the routine diagnostic procedures. The average age of patients was 60.4 + 14.1 years and they were diagnosed as SS CTCL stage III (seven patients) and stage IVa (two patients). The assignment to a certain stage was done according to the recommendations of the European Organization for Research and Treatment of Cancer Cutaneous Lymphoma Project Group.13Sézary cells from patients' blood were isolated by Ficoll (Pharmacia, Uppsala, Sweden) gradient centrifugation and sorted by two-color fluorescence-activated cell sorter (FACS) using antibodies against CD4 and the vβ region of their T-cell receptor.14
RNA preparation DNA synthesis and reverse transcriptase-polymerase chain reaction (RT-PCR).
RNA from punch biopsy samples (4 mm), small spindles of skin (50 to 200 mg), or culture cells was prepared as described earlier.15 16 For cDNA preparation, 2 μg of total RNA was incubated for 1 hour at 37°C in a 20-μL reaction with 2 μL random hexamer primers (Boehringer Mannheim, Mannheim, Germany), 1 mmol/L nucleotide triphosphates (NTPs), 1 μL RNA-Guard (Pharmacia), 2 μL 10× RT-buffer, and 1 μL Moloney murine leukemia virus (Mo-MuLV) RT (New England Biolabs, Beverly, MA).
In a 20-μL reaction, 4 μL of the of cDNA reaction was incubated with 1 μmol/L upper and lower primer, 0.1 mmol/L NTPs, 2 μL 10× RT-buffer, and 0.2 μL Taq-polymerase (Boehringer Mannheim). The DNA was amplified in 35 cycles of 1 minute at 94°C (denaturation), 1 minute at 55°C (annealing), and 1 minute 30 seconds at 72°C (elongation), and the products were analyzed on an ethidium bromide–stained 2% Agarose gel (Boehringer, Mannheim). The sequences for the primers were the following: IL-15 up, GGATGGCTGCTGGAAACC; IL-15 low, GGGAGCCCTGCACTGAAA. The oligonucleotides were synthesized by Microsynth (Balgach, Switzerland).
PCR enzyme-linked immunosorbent assay (ELISA). For the PCR ELISA, a 20-nucleotide capture probe mapping within the amplified IL-15 cDNA was selected using the OLIGO 4.0 program (National Biosciences Inc, Plymouth, MN). This probe was synthesized as a single-stranded biotinylated oligonucleotide (Microsynth). PCR ELISA was performed according to kit directions (Boehringer Mannheim). The specific capture probe/PCR product hybrids were bound to streptavidin-coated microtiter plates via the biotin label of the probes. After washing, the immobilized hybrids were treated with antidigoxigenin peroxidase-conjugated antibody and ABTS, a substrate for the peroxidase. The plates were measured by reading with a photometer at a wavelength of 492 nm, and values that were higher than 2× the reading of the PCR reaction mix with water instead of cDNA were judged to be positive.
Immunohistochemistry.
All specimens were snap frozen in liquid nitrogen, embedded in tissue-tec (GIBCO, Grand Island, NY), and stored at −80°C until processing. Cryostat sections of 5 μm were cut onto gelatine-coated microscope slides and air-dried. Alkaline anti-alkaline phosphatase (APAAP)-immunohistochemistry (reagents from DAKO, Copenhagen, Denmark) was performed as published.14 The anti–IL-15 antibodies used are commercially available (PeproTech, London, UK). In addition, all samples were also examined for reactivity with anti-CD3, -CD4, and -CD8 (reagents from DAKO).
RESULTS
IL-15 and IL-7 can replace IL-2 as growth factor of SeAx cells and prolong the survival of patient Sézary cells in vitro.
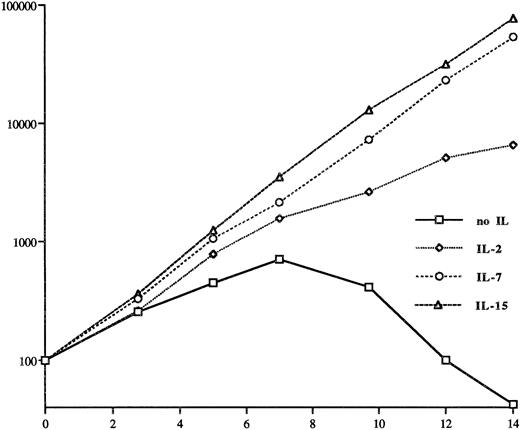
The fact that IL-2–dependent CTCL cell lines could be established12,17 18 points to the possibility that Ils which use receptors that share some common components with the IL-2 receptor may be growth factors for CTCL cells. Therefore, we tested first whether IL-7 and IL-15, whose receptor also uses the same β chain as the IL-2 receptor, are able to substitute IL-2 as a growth factor for the IL-2–dependent Sézary cell line SeAx. Figure 1 shows that IL-15 and IL-7 are better growth factors for SeAx cells than IL-2, although lower concentrations of these ILs (10 ng/mL IL-15 = 10 U, 5 ng/mL IL-7 = 10 U, 50 ng/mL IL-2 = 100 U) have been used. IL-15 increased the effect of IL-7 on the growth of SeAx when both were administered together, whereas IL-2 did not increase the growth of SeAx cells in combination with IL-7 (data not shown).
The effect of IL-2 (50 ng/mL = 100 U), IL-7 (5 ng/mL = 10 U), and IL-15 (10 ng/mL = 10 U) on the growth of the CTCL cell line SeAx. The withdrawal of these cytokines leads to cell death. The number of the cells is given in percent of the cell number used to start the cell culture (3 to 5 × 105/mL) on the y-axis in a logarithmic scale. The time of incubation is given in days on the x-axis. The data were obtained from four independent experiments and standard variations were between 10% and 30%. Note that the number of cells is given on a logarithmic scale, so that standard deviations of 10% to 30% are not visible.
The effect of IL-2 (50 ng/mL = 100 U), IL-7 (5 ng/mL = 10 U), and IL-15 (10 ng/mL = 10 U) on the growth of the CTCL cell line SeAx. The withdrawal of these cytokines leads to cell death. The number of the cells is given in percent of the cell number used to start the cell culture (3 to 5 × 105/mL) on the y-axis in a logarithmic scale. The time of incubation is given in days on the x-axis. The data were obtained from four independent experiments and standard variations were between 10% and 30%. Note that the number of cells is given on a logarithmic scale, so that standard deviations of 10% to 30% are not visible.
Ten units per milliliter of IL-7 or IL-15 was significantly more efficient than 5 U/mL IL-7 or IL-15; however, a further increase of both IL concentrations up to 100 U/mL had no significant growth-supporting effect. High IL-15 concentrations (>50 U/mL) may be even detrimental. IL-15 and IL-7 work through different receptors as an antibody against IL-15 reduced the effect of IL-15, but not that of IL-7 (data not shown).
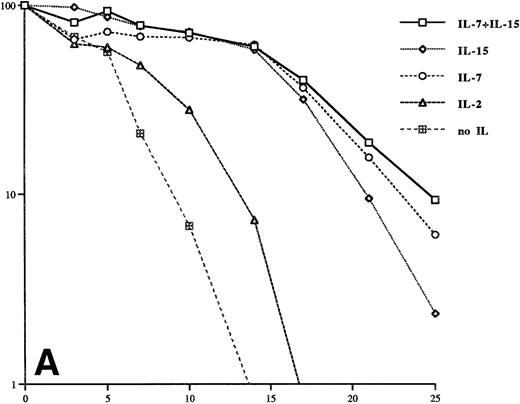
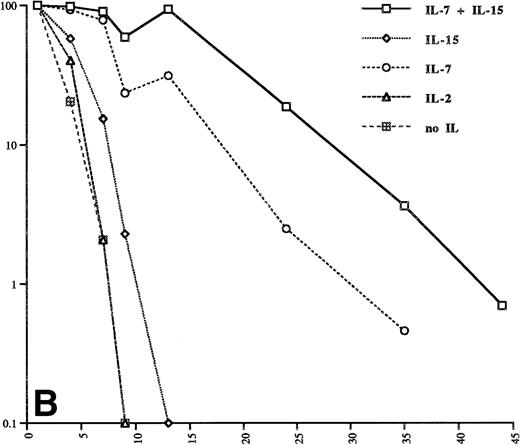
We next investigated whether IL-15 can sustain the survival of isolated Sézary cells freshly isolated from patients blood. Figure 2A and B show that the IL-15 and IL-7 concentrations used for SeAx cells can prolong the survival of these cells. Higher concentrations (20 ng/mL and 40 ng/mL, respectively) yielded an even longer survival of these cells (Fig 2B). IL-7 was more effective per se than IL-15 and, as in SeAx cells, the combination of IL-15 and IL-7 was more effective than each IL alone. These experiments show that IL-15 is a survival factor for Sézary cells in vitro, but IL-15 is not sufficient to maintain a continuous growth of these cells.
IL-7 and IL-15 prolong the survival of patient Sézary cells in vitro. The IL concentrations are the same as in Fig 1. The cells of patient 1 (A) were kept at these concentrations for the whole time of the experiment. The transient increase of the number of cells from patient 2 (B) on day 13 is caused by an increase of the concentrations of IL-7 and IL-15 to 20 ng/mL and 40 ng/mL, respectively. The data were obtained from three independent experiments.
IL-7 and IL-15 prolong the survival of patient Sézary cells in vitro. The IL concentrations are the same as in Fig 1. The cells of patient 1 (A) were kept at these concentrations for the whole time of the experiment. The transient increase of the number of cells from patient 2 (B) on day 13 is caused by an increase of the concentrations of IL-7 and IL-15 to 20 ng/mL and 40 ng/mL, respectively. The data were obtained from three independent experiments.
No increase of the growth rate of the IL-7/IL-15–independent cell lines HUT 78 and MyLa was observed when IL-7, IL-15, or both were added to the medium (data not shown).
The influence of IL-4, IL-9, and IL-13 on the survival of SeAx cells.
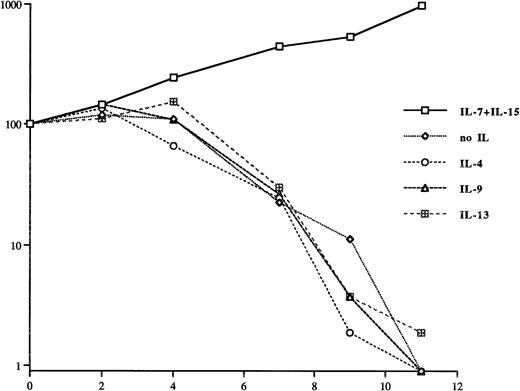
The γc chain of the IL-2 receptor is also shared by the receptors of IL-4 and IL-9. To see whether signaling through the γc chain is sufficient for the survival of SeAx cells, these two ILs were tested for whether they could sustain the growth of these cells. IL-13, whose receptor uses the same α chain as IL-4, was also included in this analysis. Figure 3 shows that none of these three ILs is able to sustain the growth of SeAx cells at the given concentrations, indicating that growth signals mediated through the γc chain alone are not sufficient for the growth of SeAx cells. No effects of IL-9 and IL-13 were seen even at concentrations of 1,000 U/mL, whereas 1,000 U of IL-4 could sustain the growth of SeAx cells. This may be either due to the activation of low affinity IL-4 receptors or unspecific effects.
IL-4, IL-9, and IL-13 are not growth factors for SeAx cells. The inefficiency of IL-4 and IL-9 indicates that signals through their common receptor γ chain which they also share with the receptors of IL-2, IL-7, and IL-15 are not sufficient to sustain the growth of this cell line. The concentrations for IL-7 and IL-15 were the same ones as in Fig 1. The concentrations for IL-4 (50 U), IL-9 (50 U), and IL-13 were 90 ng/mL (100 U), 50 ng/mL, and 600 ng/mL (100 U), respectively. The molecular weights of the ILs used are 13 kD (IL-15 and IL-13), 14 kD (IL-4 and IL-9), 15 kD (IL-2), and 17 kD (IL-7). The results have been obtained from three independent experiments.
IL-4, IL-9, and IL-13 are not growth factors for SeAx cells. The inefficiency of IL-4 and IL-9 indicates that signals through their common receptor γ chain which they also share with the receptors of IL-2, IL-7, and IL-15 are not sufficient to sustain the growth of this cell line. The concentrations for IL-7 and IL-15 were the same ones as in Fig 1. The concentrations for IL-4 (50 U), IL-9 (50 U), and IL-13 were 90 ng/mL (100 U), 50 ng/mL, and 600 ng/mL (100 U), respectively. The molecular weights of the ILs used are 13 kD (IL-15 and IL-13), 14 kD (IL-4 and IL-9), 15 kD (IL-2), and 17 kD (IL-7). The results have been obtained from three independent experiments.
IL-15 and IL-7 enable SeAx cells to survive in the presence of DEX and RA.
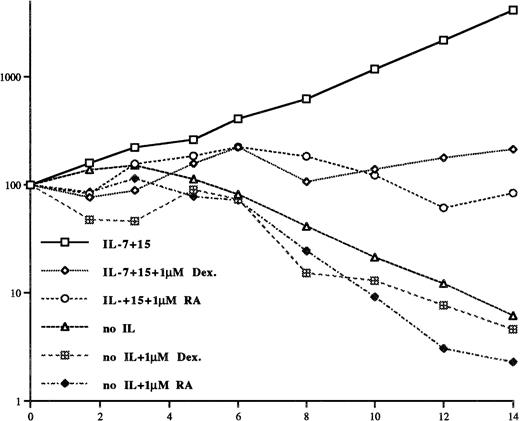
In early stages cutaneous T-cell lymphomas are treated with glucocorticoids and RA, which initially cause a regression of the skin lesions. Glucocorticoids and RA have been described as cell death–promoting agents and it may be that these agents are able to kill CTCL cells. To see whether IL-15 and IL-7 are able to sustain the growth of SeAx cells also in the presence of the synthetic glucocorticoid DEX and RA, the cells were incubated as previously described with 10 ng/mL IL-15, 5 ng/mL IL-7, and additionally with 1 μmol/L DEX and 1 μmol/L RA, respectively; ie, concentrations that have been shown to saturate cellular glucocorticoid and RA receptors.
Figure 4 shows that DEX and RA reduce the growth of SeAx cells in the presence of IL-7 and IL-15 and accelerate the cell death in the absence of these ILs. The addition of dexamethasone or RA (on days 0 and 6 in Fig 4) reduces the total number of cells only weakly in the presence of IL-15 and IL-7 and the cell number increases again 2 days after the addition of DEX and 4 to 6 days after the addition of RA. These experiments show that the effect of DEX and RA is only transient in the presence of IL-7 and IL-15.
The synthetic glucocorticoid DEX and RA reduce the growth of SeAx cells in the presence of IL-7 and IL-15 and quicken cell death in the absence of these cytokines. The decrease in cell number of the DEX- and RA-treated cells is caused by a second addition of another 1 μmol/L DEX or RA, respectively. The results have been obtained from three independent experiments.
The synthetic glucocorticoid DEX and RA reduce the growth of SeAx cells in the presence of IL-7 and IL-15 and quicken cell death in the absence of these cytokines. The decrease in cell number of the DEX- and RA-treated cells is caused by a second addition of another 1 μmol/L DEX or RA, respectively. The results have been obtained from three independent experiments.
The IL-15 gene is expressed in CTCL cells.
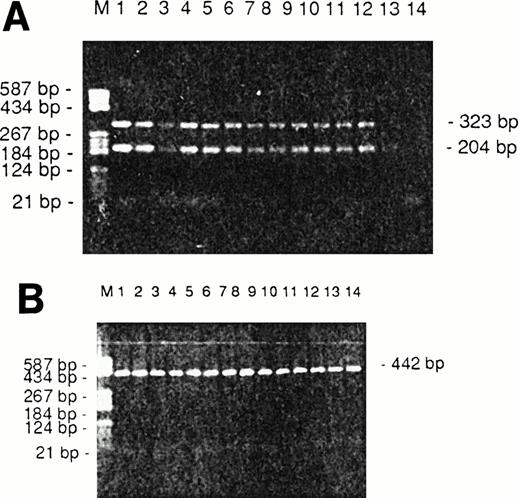
IL-independent cell lines have been established from several CTCL patients. One way this independence is established is that during tumor development mutant CTCL cells acquire the ability to produce enough IL-7 or IL-15 to sustain their own growth in an auto/paracrine way. Therefore, we tested the expression of the IL-15 and IL-7 genes by RT-PCR. We found that IL-15 was expressed in the IL-independent CTCL cell lines HUT 78 and MyLa, but also in SeAx cells and in all of the blood samples from nine different Sézary patients (Fig 5). For all CTCL samples we observed two bands of 204 and 323 bp (Fig 5) that correspond to the two described splice isoforms.19 The identity of the PCR products was confirmed by PCR ELISA with an internal IL-15 mRNA-specific capture probe and digests of the PCR product with the restriction enzymes PvuII, which cuts only the 305-bp isoform, and AflII, which cuts both forms (data not shown). The 305-bp signal of normal peripheral blood lymphocytes (PBLs) was weaker than those of the CTCL samples (Fig 5, lane 13) and any IL-15 band was absent in PBLs which had been depleted of monocytes (Fig 5, lane 14). IL-7 expression was only found in two cell lines and in samples of one patient (not shown).
(A) Representative IL-15 RT-PCR results from the three CTCL cell lines HUT78, MyLa, SeAx, and Sézary cells from nine patients. M, Boehringer marker V; the sizes of the most important marker bands are given on the left. Lanes 1 through 3, the cell lines MyLa, HuT 78, and SeAx; lanes 4 through 12, Sézary cells from nine patients; lane 13, PBLs of a healthy donor; lane 14, monocyte-depleted PBLs of a healthy donor. (B) Corresponding results of a β-actin PCR, which served as a control. The sizes of the RT-PCR products are given on the left.
(A) Representative IL-15 RT-PCR results from the three CTCL cell lines HUT78, MyLa, SeAx, and Sézary cells from nine patients. M, Boehringer marker V; the sizes of the most important marker bands are given on the left. Lanes 1 through 3, the cell lines MyLa, HuT 78, and SeAx; lanes 4 through 12, Sézary cells from nine patients; lane 13, PBLs of a healthy donor; lane 14, monocyte-depleted PBLs of a healthy donor. (B) Corresponding results of a β-actin PCR, which served as a control. The sizes of the RT-PCR products are given on the left.
To see whether the IL-7– and IL-15–independent MyLa and HUT 78 cells were able to secrete IL-15, the IL-15 concentrations in the media of these cells were measured by an IL-15 ELISA 48 hours after the last change of the medium. In all measurements the IL-15 levels were below the detection limit of the assay (50 pg/mL).
TNF-α is not a survival factor for Sézary cells.
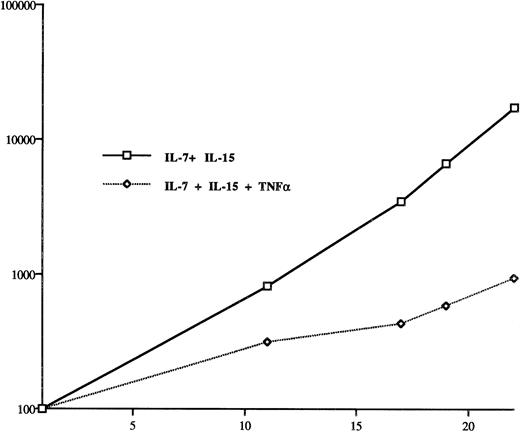
It has been recently reported that the Sézary cell line HUT 78 produces TNF-α and that TNF-α promotes the growth of these cells.20 Therefore, we tested whether TNF-α concentrations that have been reported for HUT 78 cells can promote the growth of SeAx cells. Figure 6 shows that TNF-α is detrimental for the growth of SeAx cells. A TNF-α concentration of 50 pg/mL strongly reduces the growth of SeAx cells despite the presence of IL-15 and IL-7, and 250 pg/mL TNF-α kills the SeAx cells within 2 weeks. Therefore, TNF-α cannot be considered to be a general growth factor for Sézary cells. The resistance of HUT78 cells to TNF-α may be explained by the assumption that this cell line represents a late stage of CTCL in which additional mutations have occurred.
TNF-α reduces the growth of SeAx cells. TNF-α has been reported to be an autocrine growth factor for the Sézary cell line HUT 78. The TNF-α concentration (50 pg/mL) used for the SeAx cells is the same as the one that has been reported to be secreted by HUT 78 cells. The results have been obtained from three independent experiments.
TNF-α reduces the growth of SeAx cells. TNF-α has been reported to be an autocrine growth factor for the Sézary cell line HUT 78. The TNF-α concentration (50 pg/mL) used for the SeAx cells is the same as the one that has been reported to be secreted by HUT 78 cells. The results have been obtained from three independent experiments.
Histological findings.
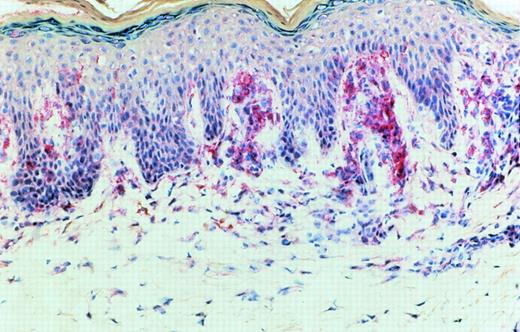
Immunoreactivity for IL-15 was detected in epidermal cells, especially in the basal layer of the epidermis, in cells with a dendritic morphology in the dermis and in mononuclear cells. This immunoreactivity could be blocked by exogenous recombinant IL-15. In early CTCL lesions (patches) (n = 5), immunoreactivity was strongest in the junction zone between epidermis and dermis where the lymphocytic infiltrates show their highest densities (Fig 7). In advanced lesions (plaques) (n = 5), immunoreactivity was also detected in deeper parts of the dermis in areas heavily infiltrated by mononuclear cells (Fig 8).
Detection of IL-15 in early CTCL lesion (patch stage) by an IL-15 antibody. The binding of the specific antibody to IL-15 was detected by an alkaline phosphatase (AP) coupled second antibody directed against the Fc region of the IL-15 antibody. Binding of the IL-15 antibody after APAAP staining was strongest in the junction zone between epidermis and dermis where CTCL cells show their highest densities. The specificity of the binding of the IL-15 antibody was tested by a competition experiment with recombinant IL-15 (not shown).
Detection of IL-15 in early CTCL lesion (patch stage) by an IL-15 antibody. The binding of the specific antibody to IL-15 was detected by an alkaline phosphatase (AP) coupled second antibody directed against the Fc region of the IL-15 antibody. Binding of the IL-15 antibody after APAAP staining was strongest in the junction zone between epidermis and dermis where CTCL cells show their highest densities. The specificity of the binding of the IL-15 antibody was tested by a competition experiment with recombinant IL-15 (not shown).
A more advanced CTCL lesion (plaque stage): immunoreactivity for IL-15 after APAAP staining was detected in the junctional area and in deeper parts of the dermis heavily infiltrated by mononuclear cells.
A more advanced CTCL lesion (plaque stage): immunoreactivity for IL-15 after APAAP staining was detected in the junctional area and in deeper parts of the dermis heavily infiltrated by mononuclear cells.
DISCUSSION
IL-15 has several common activities with IL-2 and IL-7, which are established growth factors for T cells. IL-7 is expressed in the skin and supports the growth of Sézary cells in vitro.2,3 Because IL-15 is also produced in the skin and the receptor of IL-15 shares the γc chain with the IL-2 and IL-7 receptors and a common β chain with the IL-2 receptor, we investigated whether IL-15 could be a growth factor for CTCL cells. Experiments with the Sézary cell line SeAx showed that IL-15 is sufficient to support the growth of the this cell line SeAx. IL-15 and IL-7 proved to be more efficient than IL-2. In contrast to IL-2, IL-7, and IL-15, IL-9 had no effect and IL-4 was only effective at very high concentrations, indicating that signaling through the common γ chain of their receptors is not sufficient and that additional signals through the α and β chains of the IL-2, IL-7, and IL-15 receptors are necessary. In this respect CTCL cells differ from peripheral blood T cells for which it already has been shown that IL-4 concentrations as low as 2 to 10 U/mL can protect them from cell death.21
IL-15 and IL-7 help SeAx cells to survive also in the presence of the apoptosis inducing agents DEX and RA, indicating that these two ILs are able to counteract the cell death promoting effects of these two agents by a still unknown way. The diminished growth of SeAx cells in the presence of DEX may be due to the fact that DEX can downregulate the expression of the IL-15 receptor α chain.22
The in vitro experiments with Sézary cell isolated from the blood of patients also show that IL-15 can support the survival of these cells. Under these conditions IL-7 was more effective than IL-15, but IL-15 was more effective than IL-2 and also could enhance the effect of IL-7.
Our RT-PCR analyses showed that the cell line SeAx and patients' Sézary cells express IL-15 mRNA. Despite this endogenous IL-15 gene transcription, these cells did not grow in the absence of IL-15 in vitro. An explanation may be that the amount of the produced IL-15 may be too low to sustain growth under in vitro conditions, because paracrine or autocrine IL-15 may be too strongly diluted by diffusion into the medium and, thus, too little IL-15 may bind to its receptors to have an effect on the cell.
Our immunohistological investigations detected IL-15 immunoreactivity restricted to the junction zone between epidermis and dermis in early lesions of MF (Fig 7). In this area keratinocytes, dermal dendritic cells, or lymphocytes could produce this cytokine. Because the area of IL-15 production includes the area where CTCL cells preferentially home, we propose that IL-15 is a paracrine/autocrine growth or viability factor and/or a chemotactic factor in early CTCL lesions.
In advanced lesions (plaques), IL-15 immunoreactivity was also detected in deeper parts of the dermis in areas heavily infiltrated by mononuclear cells (Fig 8). This can confirm our mRNA results, indicating that the dominant T-cell clone can produce the cytokine itself. It is possible that autocrine IL-15 production increases during tumor progression and that CTCL cells therefore lose their dependency on the cutaneous microenvironment.
Because IL-15 acts not only as a T-cell growth factor6 but also as a chemoattractant for T cells,23 IL-15 derived from keratinocytes and skin dendritic cells may attract CTCL cells and sustain their growth in skin. Thus, IL-15 may be a key molecule for the epidermotropism seen in CTCL. In late stages, CTCL may become IL-7/IL-15 independent either by autocrine IL-15 production or by a shortcut of the IL-7 and IL-15 signaling pathways. These cells will then lose their epidermotropism and settle other organs. Indirect evidence for a biological effect of IL-15 may be the production of IL-5 by CTCL cells,14 because it has been shown that IL-15 can promote the expression of IL-5 in human T-helper cells.24
The 5′ untranslated region (5′ UTR) of the IL-15 gene has an unusual structure because it contains 10 AUGs in front of the start of translation. It has been emphasized by Kozak25 that AUGs in front of the translation starting AUG can drastically reduce the efficiency of translation. The deletion of these upstream AUGs may lead to an increase of IL-15 production that may give cells with such deletions a growth advantage over nonmutated cells. Such a constellation has been found in the leukemia cell line HUT-102,26,27 where an incomplete human T- cell lymphotrophic virus I copy is integrated in the cellular genome in front of the IL-15 gene. This integration eliminated 8 of the 10 upstream AUGs and increased the IL-15 production in these cells.28 Whether such mutations occur in the IL-15 gene 5′ UTRs of cutaneous T-cell lymphoma cells has yet to be established. It has been recently shown that the leader peptides of both IL-15 splice forms lead only to inefficient IL-15 processing and secretion.29 Thus, it could theoretically be that the IL-15 gene in CTCL is expressed and translated into protein, but not secreted, and independence from IL-7 and IL-15 may be acquired by different mechanisms; eg, by mutations in the IL-7 or IL-15 signaling pathways that mimic constitutive stimulation. However, inefficient secretion does not mean no secretion, because Tagaya et al30 have found that the IL-15 form with the long leader peptide is sufficiently secreted to have a biological effect, although the secretion efficiency was very low. Our histological experiments with an IL-15 antibody clearly show that IL-15 is efficiently translated into IL-15 protein and even minor IL-15 secretion may be efficient at short distances. Mutations in the leader peptide regions of the IL-15 gene may lead to higher IL-15 production and secretion which, in turn, could stimulate CTCL cell growth in an autocrine way.
However, extracellular IL-15 may not be the only form of IL-15 that is important for CTCL cells, as Tagaya et al30 also showed that the short leader peptide form of IL-15 is retained in the cell and accumulates in the nucleus. This finding suggests that this form of IL-15 may be a ligand for an intracellular protein that may influence gene expression.
In summary, our results and the recent data on IL-15 gene regulation support the hypothesis that IL-15 together with interferon-γ inducible protein31 and IL-7 plays a key role in epidermotropism and tumor progression in CTCL.
ACKNOWLEDGMENT
The authors thank F. Bonvin, A. Flace, H. Grundmann, S. Manolio, and B. Müller for their excellent technical assistance, and M. Johnson and M. Bär for the preparation of the photographs.
Supported by the Swiss Cancer League, the Swiss National Science Foundation (Grants No. 3100-43244.95/1 and 31-43635.95 to G.B. and R.D.), and the Kanton of Zurich.
Address reprint requests to Udo Döbbeling, PhD, Department of Dermatology, University Hospital Zurich, Gloriastrasse 31, CH-8091 Zurich, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal