All-trans-retinoic acid (ATRA) downregulates the expression of two cellular procoagulants, tissue factor (TF) and cancer procoagulant (CP), in human promyelocytic leukemia cells. To evaluate whether or not changes of the procoagulant activities (PCAs) may share mechanisms with the ATRA-induced cyto-differentiation process, we have characterized the effect of ATRA on the TF and CP expression by NB4 cells, an ATRA maturation-inducible cell line, and two NB4-derived cell lines resistant to ATRA-induced maturation, the NB4.306 and NB4.007/6 cells. Next, we evaluated the effect on the PCAs of the NB4 parental cells of three synthetic retinoid analogues, ie: AM580 (selective for the retinoic acid receptor [RAR] α), capable to induce the granulocytic differentiation of NB4 cells; and CD2019 (selective for RARβ) and CD437 (selective for RARγ), both lacking this capability. Cells were treated with either ATRA or the analogues (10−6 to 10−8 mol/L) for 96 hours. The effect on cell differentiation was evaluated by morphologic changes, cell proliferation, nitro blue tetrazolium reduction assay, and flow cytometry analysis of the CD33 and CD11b surface-antigen expression. PCA was first measured in 20 mmol/L Veronal Buffer cell extracts by the one-stage clotting assay of normal and FVII-deficient plasmas. Further TF and CP have been characterized and quantified in cell-sample preparations by chromogenic and immunological assays. In the first series of experiments, ATRA downregulates both TF and CP in NB4 parental cells, as expected. However, in the differentiation-resistant cell lines, it induced a significant loss of TF but had little or no effect on CP. In a second series of experiments, in the NB4 parental cells, the RARα agonist (AM580) induced cell maturation and reduced 91% CP expression, whereas CD437 and CD2019 had no cyto-differentiating effects and did not affect CP levels. On the other hand, in the same cells the TF expression was reduced by ATRA and AM580, but also by the RARβ agonist CD2019, which did not induce cell maturation. These data indicate that in NB4 cells, ATRA modulation of CP occurs in parallel with signs of cell differentiation, while the regulation of TF appears to be at least in part independent from these processes, and involves both α and β nuclear retinoid receptors.
ALL-TRANS-RETINOIC ACID (ATRA) induces the differentiation of acute promyelocytic leukemia (APL) cells into mature granulocytes. APL is a variety of acute myeloid leukemia (AML-M3 in the French-American-British classification) characterized by the association with a life-threatening coagulation/bleeding syndrome1,2 and by the balanced reciprocal t(15;17) chromosomal translocation with breakpoints in the retinoic acid α receptor (RARα) gene on chromosome 17 and the promyelocyte (PML) gene on chromosome 15. A PML/RARα chimeric gene encoding a fusion PML/RARα protein is formed as a result of the translocation. The presence of PML/RARα confers to leukemic cells a unique sensitivity to ATRA-induced cyto-differentiation. ATRA therapy for remission induction of APL represents one of the most important advances in the field of leukemia therapy, because it induces the complete remission in greater than 90% of APL patients and a simultaneous rapid resolution of the bleeding symptoms.3-5
Among the mechanisms responsible for the intravascular clotting activation in APL is the release of procoagulant activities (PCA) by the promyelocytic blast cells. APL cells possess at least two procoagulants: (1) tissue factor (TF), a transmembrane glycoprotein of normal and malignant cells which forms a complex with factor VII (FVII) to activate coagulation factor X (FX)6-8; and (2) cancer procoagulant (CP), a cysteine proteinase procoagulant from fetal and malignant tissues, which directly activates FX in the absence of FVII.9-12 ATRA treatment significantly depresses both TF and CP expression in human APL cells in vitro and in vivo.13-16 Furthermore, in APL patients, the decrement of the bone marrow (BM) cells' PCA parallels the improvement of clotting parameters, including the increase of platelet count and plasma fibrinogen, and the decrease of circulating markers of hypercoagulation/hyperfibrinolysis.15 This suggests that modulating blast cell PCA may have a role for the control of the coagulopathy.
Mechanisms of ATRA-induced changes of APL cell PCAs are not clarified. We have designed this study to define whether these changes correlate to ATRA-induced cell differentiation. To this purpose we have followed changes in the expression of TF and CP and in cell differentiation features : (1) in response to ATRA treatment in APL cell lines sensitive and resistant to ATRA-induced cyto-differentiation; and (2) in response to a series of retinoid analogues with a different capacity to induce differentiation in ATRA-sensitive cells.
First, we have compared the effect of ATRA on the expression of TF and CP by NB4 cells, a human APL cell line sensitive to ATRA-induced maturation (S-NB4),17 to the effect on TF and CP expressed by two NB4-derived cell lines, resistant to the ATRA-induced maturation (R-NB4), NB4.306, and NB4.007/6 cell lines.18,19 S-NB4 and R-NB4 cells contain the t(15;17) chromosome translocation and show the PML/RARα hybrid DNA; however, they differ in that R-NB4 cells do not possess the complete form of the PML/RARα protein.18 19
Second, we have exposed S-NB4 cells to three retinoid derivatives, selective for each of the nuclear RAR subfamily members, RARα, RARβ, and RARγ.20,21 ATRA does not have any selectivity for the RAR subtypes. The use of these compounds has shown that the RARα agonist AM580 is a potent inducer of the granulocytic differentiation of leukemic promyelocytes, whereas CD2019 and CD437, the RARβ and γ agonists, respectively, lack this capacity.22 The effect of AM580, CD2019, and CD437 on CP and TF expression by S-NB4 cells was evaluated. The results of this study show that while CP downregulation by retinoids correlates with phenotype differentiation features, TF modulation occurs at least in part independently from these processes and involves α and β retinoic acid receptors. The different regulation of these procoagulant proteins by ATRA suggests new implications for ATRA/PCA interactions in human malignancy.
MATERIALS AND METHODS
Cell Lines and Reagents
The following human leukemic cell lines were used: (1) The NB4 parental line, kindly provided by Dr M. Lanotte's laboratory (St Louis Hopital, Paris, France). This line, established in vitro from an APL patient,17 shows the typical t(15;17) chromosomal translocation, expresses the PML/RARα fusion protein, and is sensitive to ATRA-induced cell differentiation (S-NB4). (2) Two cell lines derived in vitro from S-NB4, which are resistant to ATRA-induced differentiation (R-NB4): (a) NB4.306 cell line, obtained by mutagenesis with low-dose radiation and then selected with increasing ATRA concentrations (10−8 to 10−6mol/L),18 and (b) NB4.007/6 cell line, obtained by long-term culture with increasing ATRA concentrations (10−8 to 10−6 mol/L).19NB4.306 and NB4.007/6 cells contain the t(15;17) chromosome translocation, but express no detectable amount of the intact PML/RARα protein, a property considered relevant for the resistence to ATRA.18 19
Cells were maintained in RPMI 1640 containing 10% fetal calf serum, penicillin (100 U/mL), and streptomycin (100 μg/mL). In the first series of experiments, S- and R-NB4 cells were resuspended in fresh medium (2 × 105 cells/mL) and cultured for 96 hours in the absence or presence of 10−6 mol/L ATRA (Hoffman-La Roche, Basel, Switzerland), the optimal condition to induce cell differentiation.17 In the second series of experiments, S-NB4 cells were cultured for 96 hours either with ATRA or the following synthetic agonists of RARs: (1) the RARα agonist AM580 {[4-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl) carboxamido] benzoic acid), (2) the RARβ agonist CD2019 {6-[3-(1-methylcyclohexyl)-4-methoxyphenyl]-2-naphthoic acid}, and (3) the RARγ agonist CD437{6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthoic acid] (CIRD Galderma, Sophia Antipolis, Valbonne, France).20-22 The RARα agonist AM580 induces the granulocytic differentiation of NB4 cells,22 whereas both the RARβ agonist CD2019 and RARγ agonist CD437 lack this capacity. In most of the experiments the three compounds were used at a final concentration of 10−8mol/L, the highest concentration showing selective binding for the receptors and not affecting cell viability.
In some experiments RARα was blocked by the RARα synthetic inhibitor Ro 41-5253 (p-[(E)-2-[3′,4′-dihydro-4′,4′-dimethyl-7′-(heptyloxy)-2′H-1-benzothiopyran-6′-yl]propenyl] benzoic acid 1′, 1′-dioxide) (Hoffman-La Roche),23 while RARβ was blocked by an RARβ antagonist CD2665 (CIRD Galderma). S-NB4 cells were cultured with 10−6 mol/L Ro 41-5253 or 10−6 mol/L CD2665 or both plus 10−8 mol/L ATRA or 10−8 mol/L AM580. Stock solutions of the various retinoids (10−2 mol/L) were prepared in dimethylsulfoxide under dimmed light, stored at −80°C, and protected from light until use. After treatment with ATRA or RARs-agonists and antagonists, cell viability, evaluated by Tripan Blue (Crle Erbe, Milan, Italy) exclusion dye test, was more than 95%.
Analysis of Cell Differentiation
Cell diffentiation was evaluated after 96 hours treatment with ATRA or retinoids or the vehicle by different criteria. (1) Morphological changes (appearance of nuclear segmentation and granules formation) were analyzed by microscopic examination of histologic slides after May-Grünwald-Giemsa and cytochemical stainings. (2) Cell proliferation was evaluated by direct cell counting (trypan blue exclusion method) using a hematocytometer chamber. (3) The capability to reduce nitro blue tetrazolium (NBT), a property associated with myeloid cell maturation, was evaluated spectrophotometrically at 540 nm, as previously described.24 (4) Analysis of myeloid cell-surface markers included the quantification of the number of CD33+ and CD11b+ cells using a FACScan analyzer (Becton Dickinson, Mountain View, CA). CD33 and CD11b are cellular surface antigens of early and late myeloid differentiation, respectively.25 Increase in CD11b and decrease in CD33 expressions correlate with cell differentiation in S-NB4 and R-NB4 cells.17-19,22,24 26 Determination of the surface markers was performed by a direct immunofluorescence assay using the following fluorescence-conjugated monoconal antibodies (MoAbs) (Becton Dickinson): phycoerythrin (PE)-conjugated Leu15 (anti-CD11b) and Leu M9 (anti-CD33).
Samples
After 96 hours of treatment with ATRA or the retinoids, cells were washed three times in sterile phosphate-buffered saline (PBS) and different types of samples were ad hoc prepared according to the optimal assay conditions required for each type of procoagulant (CP or TF). The following samples were prepared:
Cell extracts in Veronal buffer (VB).
Sample preparation consisted of protein extraction in a low ionic strength aqueous buffer (Veronal, BDH Chemicals ltd, Poole, UK), which is the optimal condition to extract CP, as described.9-13 Cells (40 × 106cells/mL) were extracted in two changes of 20 mmol/L VB, pH 7.8, at 4°C.
VB cell extracts were centrifuged at 10,000g for 10 minutes and the supernatants were assayed for: (1) total and factor VII–independent PCA by the coagulation assay; (2) CP activity by the CP functional chromogenic assay; and (3) CP antigen by the CP immunological assay.
Cell lysates.
Cell lysates were prepared to measure the activity of TF, a membrane glycoprotein, by the TF functional chromogenic assay. Specifically, cells (33 × 106 cells/mL) were resuspended in 10 mmol/L HEPES buffer, pH 7.45 (containing 137 mmol/L NaCl, 4 mmol/L KCl, 11 mmol/L α-D glucose, 5 mg/mL ovalbumin, 2.5 mmol/L CaCl2), and lysed by three cycles of freezing/thawing.27
Cell extracts in Tris Buffer/Triton (TBT).
TBT cell extracts consisted of cell membrane solubilization with Triton. This type of sample was to quantify TF antigen, as is the most efficient condition for the recovery of this antigen.27 Cells (20 × 106 cells/mL) were resuspended in Tris Buffer, pH 7.5 (containing 50 mmol/L Tris, 100 mmol/L NaCl, 1% Triton X-100 [Merck-Darmstadt, Frankfurt, Germany]), disrupted by three cycles of freezing/thawing and extracted on ice for 3 hours. Samples were centrifuged at 10,000g for 10 minutes and supernatants assayed for TF antigen content by the TF immunological assay.
Coagulation Assay
Total PCA of VB cell extracts was measured by the one-stage recalcification assay of normal human plasma (NHP, containing the coagulation factors, including factor VII [FVII]), as previously described.9-13 Briefly, 0.1 mL of cell extract was incubated with 0.1 mL of NHP for 1 minute at 37°C. The reaction was started by the addition of 0.1 mL of 25 mmol/L CaCl2 and the clotting time was recorded. To identify an FVII-independent PCA, a characteristic of CP, the recalcification assay of human plasma congenitally deficient of FVII (FVII-DP; Behringwerke, AG, Marburg, Germany) was performed. PCA was expressed as Russell's Viper Venom (RVV) units per milligram of protein. Units were calculated on a calibration curve obtained with different dilutions (from 10−1 to 10−6) of RVV (Sigma Chemical Co, St Louis, MO); 1 unit = activity of 1 mEq/mL RVV in the one-stage clotting assay.9-13 Protein content was determined by the Bradford assay method.
To enzymatically characterize the VB cell extract PCA, an inhibition study was conducted by assaying the samples' clotting activity in the presence of three cysteine proteinase inhibitors, including HgCl2 (Sigma), Iodoacetic acid (Sigma), and Z-Ala-Ala-peptidyl diazomethyl-ketone (Z-Ala-Ala-CHN2; Enzyme System Products, Dublin, CA), which inhibit CP, and of two TF inhibitors, including Concanavalin A (Con A) and anti-TF MoAb (anti-human TF TF9-9B4; American Diagnostica Inc, Greenwich, CT). Cell extracts were incubated with 0.1 mmol/L HgCl2, 1 mmol/L Iodoacetic acid and 0.2 mmol/L Z-Ala-Ala-CHN2 and with anti-TF (0.1 mg/mL final concentration) for 30 minutes at 25°C, and with 200 μg/mL Con A for 50 minutes at 37°C, before the coagulation assay, as described.9-13 27
CP Functional Assay
CP functional activity was determined by a three-stage chromogenic assay.28 VB cell extract (100 μL) was mixed with 10 μL of bovine FX (100 mg/mL; Sigma) and 30 μL of 25 mmol/L CaCl2 in 50 mmol/L bis-Tris propane buffer (pH 6.7). After 30 minutes of incubation, 10 μL of bovine prothrombin (1 mg/mL; Enzyme Research Laboratories Inc) and 30 μL of rabbit brain cephalin (RBC)/Ca2+ mix were added to the samples [RBC/Ca2+ mix = 1 part of a 1:10 dilution RBC + 1 part of 50 mmol/L CaCl2 + 2 parts of 100 mmol/L bis-Tris propane buffer (pH 7.8)]. After a further 30-minute incubation at 37°C, 200 μL 50 mmol/L Tris buffer, pH 7.8, and 50 μL thrombin substrate Sar-Pro-Arg-p-nitroanilide (2 mmol/L in 10% dimethyl sulfoxide [DMSO; Sigma]) were added. Color development at 405 nm was recorded in the time (from 0 to 30 minutes). Samples' CP content was expressed as mUnits per milliliter (1 Unit = the amount of enzyme responsible for releasing 1 μmol of p-nitroaniline from the subtrate in 1 minute). RVV, a serine proteinase FX activator, was the standard control to calibrate the assay.
Under these conditions, thrombin formation is totally inhibited by incubation of the VB extracts (30 minutes at 25°C) with 1 mmol/L HgCl2.
CP Antigen Assay
CP antigen was measured with a standard dot-blot analysis against calibrated CP standards purified from human amnion-chorion tissue.29 Five CP standard concentrations (from 0 to 41 μg of CP/mL) and cell extract samples were diluted to 0.4 mL in VB and 100-μL samples were added to the wells of the dot-blot apparatus containing nitrocellulose. After 30 minutes' incubation of the sample solution with the nitrocellulose, remaining solution was removed by applying a vacuum. Then the nitrocellulose sheet was removed and blocked overnight by Tris-bufferd saline (pH 7.6) (TBS) containing 10% nonfat dried milk. The membrane was first incubated with anti-CP IgG in TBS-0.1% Tween 20 (TBST) containing 1% nonfat dried milk for 2 hours at 25°C, and then, after washings with TBST, was incubated with an antimurine IgG alkaline phosphatase conjugate goat antibody for 2 hours at 25°C. Finally, after washing again, the nitrocellulose was incubated in BCIT/NBT alkaline phosphatase substrate at room temperature until the color development was of satisfactory intensity. The nitrocellulose image was scanned into a computer and the dot intensity was determined by SigmaScan. CP antigen content was calculated from a calibration curve obtained with the different concentrations of pure CP.
TF Functional Assay
TF activity of cell lysates was measured as the rate of FX hydrolysis using a spectrophotometric assay for FXa, as described.27Briefly, 30 μL of lysed cells were 1:6 diluted with 10 mmol/L HEPES buffer (pH 7.45) and incubated with 1 nmol/L FVII for 10 minutes at 37°C to allow binding to TF. The reaction of FX hydrolysis was started by the addition of 0.1 μmol/L FX. After 0, 2.5, 5, 10, and 20 minutes, 30-μL sub-samples were taken from the reaction mixture and diluted in 20μL ice-cold 50 mmol/L Tris/EDTA (pH 7.5) stopping buffer. To measure FXa content, each sub-sample was 1:1 diluted with 50 mmol/L Tris-NaCl buffer and the reaction started by adding 0.2 mmol/L S-2337 chromogenic substrate (Ortho Diagnostic System, Milan, Italy). After 30 minutes, the reaction was stopped by 50 mmol/L benzamidine and the 405 nm absorbance was recorded. The assay was calibrated by active site-titrated FXa and results expressed as rate of FX hydrolysis (pmol FXa/min/106 cells).
Under these conditions, the FXa formation was completely dependent on the presence of FVII and totally inhibited by antibody against human TF (0.1 mg/mL final concentration MoAb anti-human TF TF9-9B4, American Diagnostica Inc).
TF Antigen Assay
TF antigen was measured in a Tris/NaCl buffer-1% Triton X-100 (TBT) solubilized cell samples by a double-antibody enzyme-linked immunosorbent assay (ELISA).27 Briefly, microtiter plates were coated with the anti-TF MoAb TF8-10H10 (American Diagnostica Inc) and incubated overnight at 4°C (0.25 μg/well). After three washings, 100 μL sample or standard TF dilutions (0 to 400 pmol/L; Recomboplastin S, Baxter Diagnostica Inc, Deerfield, IL) were added to each well and incubated overnight at 4°C. Plates were then washed and 100 μL of biotinylated anti-TF MoAb TF9-9B4 (2 μg/mL/well; American Diagnostica Inc) were added. After 4 hours of incubation at 25°C, a 1:1,000 dilution Avidine-horse radish peroxidase conjugate (Sigma) was added. After 3 hours at 25°C, bound peroxidase activity was detected by the substrate tetra-methyl benzidine (100 μg/mL). The color reaction was stopped after 10 minutes by 100 μL 4 N H2SO4 and the absorbance at 450 nm was read. Results were calculated on the reference curve of standard TF and expressed as femtomoles of TF per 106 cells.
In this case the results were expressed per cell number, as appropriate for this type of sample and because protein could not be technically determined on account of interference by the detergent (Triton) in this sample.
Statistical Analysis
Statistical analysis of the data was performed by the unpaired and paired Student's t-tests.
RESULTS
Untreated and ATRA-treated S-NB4 and the two R-NB4 cells (NB4.306 and NB4.007/6) were first assayed for PCA by the coagulation assay (Fig 1). Total PCA is the activity measured with the clotting assay using NHP, and FVII-independent (CP-like) PCA is the activity measured with the clotting assay using FVII-DP. All the cell lines were assayed for both total and FVII-independent PCA. Treatment of S-NB4 cells with 10−6 mol/L ATRA for 96 hours significantly decreased (59%) the total PCA from the control level of 12.2 ± 6.2 RVV units/mg protein to the ATRA-treated level of 5 ± 4 RVV units/mg protein (P < .01); the total PCA of the NB4.306 and NB4.007/6 cells decreased 44% and 42%, respectively (P < .05) (Fig 1, left panel). In contrast, ATRA treatment virtually abolished the FVII-independent PCA of S-NB4 cells from a control level of 5.46 ± 2.83 RVV units/mg protein to an ATRA-treated level of 0.006 ± 0.024 RVV units/mg protein, while there was little or no effect on the activity of the FVII-independent PCA of ATRA-resistant cells (NB4-306 showed a 15% decrease and NB4-007/6 showed no decrease in PCA) (Fig 1, right panel).
PCA of normal human plasma (A) and FVII-deficient plasma (B) of cell extracts from NB4 cells, sensitive to ATRA-induced differentiation, and two NB4-derived cell lines, resistant to ATRA-induced differentiation (ie, NB4.306 and NB4.007/6). Cells were cultured with 10−6 mol/L ATRA (▪) or the vehicle (□) for 96 hours. Results, expressed as RVV units/mg total proteins, are the mean of at least three experiments. Statistical analysis of PCA of untreated versus treated samples was performed by the paired Student'st-test; *P < .05, **P < .01.
PCA of normal human plasma (A) and FVII-deficient plasma (B) of cell extracts from NB4 cells, sensitive to ATRA-induced differentiation, and two NB4-derived cell lines, resistant to ATRA-induced differentiation (ie, NB4.306 and NB4.007/6). Cells were cultured with 10−6 mol/L ATRA (▪) or the vehicle (□) for 96 hours. Results, expressed as RVV units/mg total proteins, are the mean of at least three experiments. Statistical analysis of PCA of untreated versus treated samples was performed by the paired Student'st-test; *P < .05, **P < .01.
The PCA of untreated and ATRA-treated samples was further characterized by testing the sensitivity to three cysteine proteinase inhibitors, known to inhibit CP (ie, HgCl2, Iodoacetic acid and Z-Ala-Ala-CHN2), and to Con A and anti-TF MoAb, as TF inhibitors (Fig 2). As previously observed,13 in S-NB4 cells, the PCA of untreated samples was highly sensitive to HgCl2 (P < .0001), Iodoacetic acid (P < .05), and Z-Ala-Ala-CHN2(P < .005), while the ATRA-treated counterparts were unaffected by the cysteine proteinase inhibitors. In addition untreated and ATRA treated S-NB4 cells were significantly affected by Con A (26% and 15% inhibition, respectively) and anti-TF MoAb (65% and 93%, respectively). In contrast, in R-NB4 cell extracts the PCA of either untreated or ATRA-treated samples were significantly inhibited by the three cysteine proteinase inhibitors and the anti-TF MoAb (Fig 2).
Sensitivity to three cysteine proteinase inhibitors (0.1 mmol/L HgCl2, 0.2 mmol/L ZAA-diazomethyl-ketone, and 1 mmol/L IodoAcetic acid [IA]), and to the TF inhibitor anti-TF MoAb (0.1 mg/mL final concentration) of the PCA of S-NB4 (left) and R-NB4-306 cells (right) treated with 10−6 mol/L ATRA (▪) or the vehicle (□) for 96 hours. VB cell extracts were incubated with cysteine proteinase inhibitors or the anti-TF MoAb for 30 minutes or the vehicle (control), before the clotting assay. Statistical analysis compares the inhibitor-treated sample with the untreated (vehicle treated) corresponding control. *P < .05, **P < .01.
Sensitivity to three cysteine proteinase inhibitors (0.1 mmol/L HgCl2, 0.2 mmol/L ZAA-diazomethyl-ketone, and 1 mmol/L IodoAcetic acid [IA]), and to the TF inhibitor anti-TF MoAb (0.1 mg/mL final concentration) of the PCA of S-NB4 (left) and R-NB4-306 cells (right) treated with 10−6 mol/L ATRA (▪) or the vehicle (□) for 96 hours. VB cell extracts were incubated with cysteine proteinase inhibitors or the anti-TF MoAb for 30 minutes or the vehicle (control), before the clotting assay. Statistical analysis compares the inhibitor-treated sample with the untreated (vehicle treated) corresponding control. *P < .05, **P < .01.
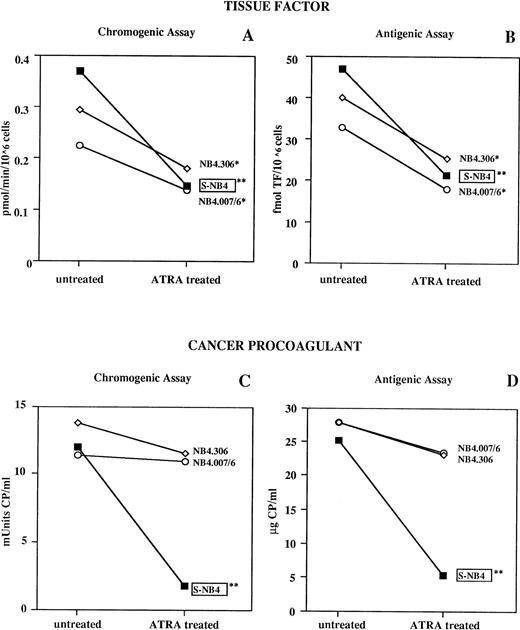
These results prompted us to further characterize the effect of ATRA on the different procoagulants. Thus, we measured CP and TF activities and antigens by specific chromogenic and immunological assays in optimized experimental conditions. Untreated and ATRA-treated S- and R-NB4 cells were subdivided in aliquots to prepare different samples for the separate analysis of CP and TF activities and antigens. Figure 3 shows TF chromogenic activity of cell lysates (3A) and TF antigen content of TBT extracts (3B) from S-NB4, R-NB4.306, and R-NB4.007/6 cells. TF chromogenic activity was differently, but significantly, downregulated by ATRA treatment in all three cell lines (61%, 39%, and 39% decrease in TF activity, respectively). A similar profile was observed for TF antigen reduction (55%, 38%, and 45% reduction in TF antigen, respectively). Measurements of CP chromogenic activity and antigen in VB extracts from the same cell lines are shown in Fig 3C and D. After ATRA treatment, there was a significant decrease of 85% in the CP activity of the S-NB4 cells (P < .01), but there was little or no reduction in the two R-NB4 lines. Accordingly, CP antigen showed a 79% reduction in the S-NB4 cells (P > .01) but no significant changes in the R-NB4 cell lines.
Effect of ATRA on cellular procoagulants analyzed by chromogenic and immunological assays for TF and CP expressed by S-NB4 cells and the two R-NB4 cell lines, NB4.306 and NB4.007/6, cultured for 96 hours with 10−6 mol/L ATRA or the vehicle. TF functional activity (A) was measured as rate of FX hydrolysis by the TF/FVII complexes in cell lysates. TF antigen (B) was measured in TBT cell extracts by ELISA, using an anti-human TF MoAb. CP functional activity (C) was measured in VB cell extracts by a three-stage chromogenic assay. CP antigen (D) was immunologically identified by a dot-blot assay using a pure anti-CP monoclonal IgG. The results are the mean of at least three experiments. Statistical analysis as in Fig 1. *P < .05, **P < .01.
Effect of ATRA on cellular procoagulants analyzed by chromogenic and immunological assays for TF and CP expressed by S-NB4 cells and the two R-NB4 cell lines, NB4.306 and NB4.007/6, cultured for 96 hours with 10−6 mol/L ATRA or the vehicle. TF functional activity (A) was measured as rate of FX hydrolysis by the TF/FVII complexes in cell lysates. TF antigen (B) was measured in TBT cell extracts by ELISA, using an anti-human TF MoAb. CP functional activity (C) was measured in VB cell extracts by a three-stage chromogenic assay. CP antigen (D) was immunologically identified by a dot-blot assay using a pure anti-CP monoclonal IgG. The results are the mean of at least three experiments. Statistical analysis as in Fig 1. *P < .05, **P < .01.
ATRA-induced cell maturation of NB4 cells was evaluated by microscopic examination, cell proliferation, NBT reduction assay, and the expression of CD33 and CD11b surface antigens, the two markers of early and late myeloid differentiation,25 respectively. In NB4 parental cells, ATRA treatment caused growth inhibition (fold increase: untreated v treated cells: 4.174 ± 2.28 v2.02 ± 0.77; P < .01). The analysis of cell morphology, evaluated on May-Grünwald-Giemsa–stained cells, showed that ATRA treated S-NB4 cells became morphologically similar to metamyelocytes and polynuclear neutrophils, as described.17 Morphologic maturation corresponded to increased capacity to reduce NBT and to changes in myeloid differentiation marker expression. The results of the NBT reduction assay and the cell-surface markers analysis are reported in Table 1. In all the experiments, in the NB4 parental cells, ATRA increased the capacity of reducing NBT (from 32 ± 4 to 110 ± 10 Δ/h; P< .01) and the number of CD11b+ cells (control, 5.8% ± 3%; +ATRA, 31.6% ± 5%, P < .01), whereas it significantly decreased the CD33+ cells (control, 96.8% ± 4.8%; +ATRA, 83.0% ± 8.85%, P < .01). The same treatment did not induce significant NBT reduction capacity and surface antigen changes in the two R-NB4 cell lines.
Effect of ATRA and Selective RAR Agonists on NB4 Cell Differentiation
| Cell Line . | Treatment . | NBT Reduction (ΔOD/h) . | CD11b (% positive cells) . | CD33 (% positive cells) . |
|---|---|---|---|---|
| S-NB4 | Control | 32 ± 4 | 5.8 ± 3.0 | 96.8 ± 4.8 |
| ATRA | 110 ± 10-150 | 31.6 ± 5.0-150 | 83.0 ± 8.8-150 | |
| R-NB4.306 | Control | 34 ± 6 | 4.9 ± 1.4 | 98.6 ± 1.2 |
| ATRA | 35 ± 3 | 5.6 ± 0.2 | 98.2 ± 1.1 | |
| R-NB4.007/6 | Control | 31 ± 4 | 4.7 ± 2.8 | 94.0 ± 7.2 |
| ATRA | 34 ± 8 | 5.9 ± 4.9 | 94.3 ± 4.4 | |
| S-NB4 | Control | 29 ± 6 | 5.8 ± 3.0 | 96.8 ± 4.8 |
| AM580 | 190 ± 12-150 | 56.21 ± 5-150 | 73.2 ± 7.6-150 | |
| CD2019 | 30 ± 7 | 6.1 ± 4.3 | 91.8 ± 4.9 | |
| CD437 | 27 ± 5 | 5.3 ± 4.1 | 91.9 ± 6.2 |
| Cell Line . | Treatment . | NBT Reduction (ΔOD/h) . | CD11b (% positive cells) . | CD33 (% positive cells) . |
|---|---|---|---|---|
| S-NB4 | Control | 32 ± 4 | 5.8 ± 3.0 | 96.8 ± 4.8 |
| ATRA | 110 ± 10-150 | 31.6 ± 5.0-150 | 83.0 ± 8.8-150 | |
| R-NB4.306 | Control | 34 ± 6 | 4.9 ± 1.4 | 98.6 ± 1.2 |
| ATRA | 35 ± 3 | 5.6 ± 0.2 | 98.2 ± 1.1 | |
| R-NB4.007/6 | Control | 31 ± 4 | 4.7 ± 2.8 | 94.0 ± 7.2 |
| ATRA | 34 ± 8 | 5.9 ± 4.9 | 94.3 ± 4.4 | |
| S-NB4 | Control | 29 ± 6 | 5.8 ± 3.0 | 96.8 ± 4.8 |
| AM580 | 190 ± 12-150 | 56.21 ± 5-150 | 73.2 ± 7.6-150 | |
| CD2019 | 30 ± 7 | 6.1 ± 4.3 | 91.8 ± 4.9 | |
| CD437 | 27 ± 5 | 5.3 ± 4.1 | 91.9 ± 6.2 |
Cells were grown in the presence of 10−6 mol/L ATRA or 10−8 mol/L RAR agonists (AM580 = RARα agonist; CD2019 = RARβ agonist; CD437 = RARγ agonist) or the vehicle (control) for 96 hours. Washed cells were analyzed for NBT reduction activity and surface-marker CD11b and CD33 expression. Results are mean ± SD of five experiments (determinations in duplicate).
P < .01 compared with controls. Statistics for S-NB4 adjusted for multiple comparisons.
In further experiments we focused our attention on the effect of three synthetic RAR agonists (RARα, RARβ, and RARγ) on the PCA expression by S-NB4 cells. The RARα agonist AM580 induces granulocytic differentiation of NB4 cells,22 while neither the RARβ agonist CD2019 nor the RARγ agonists CD437 have the capacity to induce differentiation. Table 1 also shows the effect of AM580, CD2019, and CD437 on the NBT reduction capacity and the expression of the surface differentiation antigens, CD11b and CD33, by S-NB4 cells. Only AM580 was able to significantly affect both the NBT assay (from 29 ± 6 to 190 ± 12 ΔOD/h; P < .01), and the CD11b and CD33 expression. A low percentage (ranging from 5.3% to 6.1%) of the undifferentiated cells expressed CD11b; the RARα agonist induced the CD11b expression up to 56%, a ninefold increase over the control NB4 cells (P < .01). The expression of CD33 in the undifferentiated cells (ranging from 97% to 92% of the cells) decreased to 73% with the agonist AM580 (P < .01).
S-NB4 cell maturation induced by AM580 was accompanied by a significant 91% loss (P < .01) of CP chromogenic activity (from 11.48 ± 2.9 mU/mL to 1 ± 0.8 mU/mL), while treatment of the NB4 cells with CD2019 or CD437 did not modulate CP expression (Fig 4A). In contrast, cellular TF chromogenic activity was significantly decreased not only by the RARα agonist AM580 (58% decrease, P < .01), but also by the RARβ agonist CD2019 (36% decrease, P < .05); CD437 (RARγ agonist) had no effect (Fig 4B).
Effect of AM580 (RARα agonist), CD2019 (RARβ agonist), and CD437 (RARγ agonist) on CP chromogenic activity (A) and TF activity (B) in S-NB4 cells. Cells were cultured ATRA (10−6 mol/L) or synthetic retinoids (10−8mol/L) for 96 hours. (□), Untreated; (▪), treated. The results are the mean of at least three experiments. Statistical analysis as in Fig 1. *P < .05, **P < .01.
Effect of AM580 (RARα agonist), CD2019 (RARβ agonist), and CD437 (RARγ agonist) on CP chromogenic activity (A) and TF activity (B) in S-NB4 cells. Cells were cultured ATRA (10−6 mol/L) or synthetic retinoids (10−8mol/L) for 96 hours. (□), Untreated; (▪), treated. The results are the mean of at least three experiments. Statistical analysis as in Fig 1. *P < .05, **P < .01.
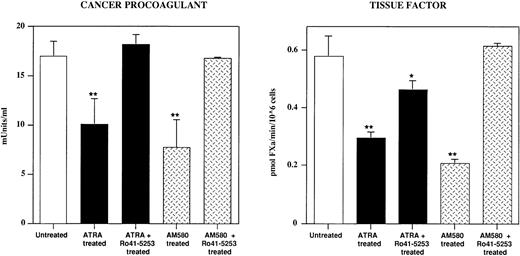
To evaluate the role of RARα in the downregulation of the two procoagulants, in some experiments RARα was blocked by the RARα antagonist Ro 41-5253 (Fig 5). Treatment of S-NB4 cells with Ro 41-5253 + ATRA completely prevented the ATRA-induced CP downregulation, and only partially counteracted the TF downregulation, confirming a role of other receptor(s) in the regulation of TF expression. In contrast, the treatment of S-NB4 cells with Ro 41-5253 in addition to AM580 completely prevented the AM580-induced downregulation of both CP and TF. In these conditions, CP chromogenic activity was 100% inhibited by treatment of the sample with HgCl2 and TF chromogenic activity was 100% abolished by the anti-TF MoAb.
Effect of the RARα antagonist Ro 41-5253 in blocking the ATRA-induced and AM580-induced reduction of CP and TF expression in S-NB4 cells. The cells were cultured for 96 hours with 10−8 mol/L ATRA ± 10−6 mol/L Ro 41-5253 or with 10−8 mol/L AM580 ± 10−6 mol/L Ro 41-5253. Results are the mean of at least three experiments. Statistical analysis to compare untreated and treated samples as in Fig1. *P < .05; **P < .01 versus untreated control samples.
Effect of the RARα antagonist Ro 41-5253 in blocking the ATRA-induced and AM580-induced reduction of CP and TF expression in S-NB4 cells. The cells were cultured for 96 hours with 10−8 mol/L ATRA ± 10−6 mol/L Ro 41-5253 or with 10−8 mol/L AM580 ± 10−6 mol/L Ro 41-5253. Results are the mean of at least three experiments. Statistical analysis to compare untreated and treated samples as in Fig1. *P < .05; **P < .01 versus untreated control samples.
To further investigate the role of RARβ in the modulation of TF, we have studied the effect of ATRA and AM580 in the presence of RARα and RARβ antagonists. As shown in Table 2, the RARβ antagonist slightly counteracted the action of ATRA, whereas the simultaneous presence of both the RARα and RARβ antagonists completely abolished its effect. The RARβ antagonist did not affect the activity of AM580.
Downregulation of TF Expression by ATRA and RARα Agonist AM580 in S-NB4: Inhibition of the Effect by RARα and RARβ Selective Antagonists
| Treatment . | TF Activity (% of control) . | Pv Control . |
|---|---|---|
| Untreated control | 100 ± 0 | |
| ATRA* | 51.3 ± 3.6 | .0001 |
| ATRA + anti-RARα | 80.1 ± 5.3 | .001 |
| ATRA + anti-RARβ | 59.4 ± 1 | .05 |
| ATRA + anti-RARα + anti-RARβ | 98 ± 1.6 | NS |
| AM580 (RARα agonist)* | 35.7 ± 2.6 | .0001 |
| AM580 + anti-RARα | 100 ± 2 | NS |
| AM580 + anti-RARβ | 31.7 ± 1 | .0001 |
| Treatment . | TF Activity (% of control) . | Pv Control . |
|---|---|---|
| Untreated control | 100 ± 0 | |
| ATRA* | 51.3 ± 3.6 | .0001 |
| ATRA + anti-RARα | 80.1 ± 5.3 | .001 |
| ATRA + anti-RARβ | 59.4 ± 1 | .05 |
| ATRA + anti-RARα + anti-RARβ | 98 ± 1.6 | NS |
| AM580 (RARα agonist)* | 35.7 ± 2.6 | .0001 |
| AM580 + anti-RARα | 100 ± 2 | NS |
| AM580 + anti-RARβ | 31.7 ± 1 | .0001 |
Cells were incubated with ATRA 10−8 mol/L or RARα agonist (AM580, 10−8 mol/L) for 96 hours in the presence of 10−6 mol/L retinoid receptor antagonists: anti-RARα (Ro 41-5253) or anti-RARβ (CD2665). TF activity was measured by a spectrophotometric assay for FXa. Results are expressed as percent of untreated control cells (=100%). Statistical analysis was done versus the untreated control by the paired Student's t-test.
Abbreviation: NS, not significant.
Comparison between the effects of the two agonists, by the unpaired Student's t-test, also showed that AM580 was significantly more potent than ATRA (P < .05) in reducing TF activity.
DISCUSSION
Several studies have shown ATRA's capability to downregulate the PCA of APL cells.13-16 This property may be one of the mechanisms by which ATRA facilitates a beneficial effect on the coagulopathy associated with the early phase of APL. We have observed that both procoagulants, CP (a cysteine proteinase) and TF (a membrane glycoprotein), are decreased by ATRA in the BM cells of patients under treatment.15 However, whether this decrease is produced by a direct mechanism or is a part of the differentiation process is not known. To address this question, we have designed this study primarily to follow the changes in the expression of the two procoagulants in response to retinoids, in the presence or absence of retinoids-induced cyto-differentiation. Specifically, two experimental conditions were exploited, the availability of ATRA maturation-resistant NB4-derived cell lines18,19 and the use of synthetic retinoid analogues with different granulocytic differentiating capacity on NB4 cells.20-22 The results show that CP downregulation parallels cell differentiation, whereas TF occurs, at least in part, independently from this mechanism.
All of the experiments to evaluate the modifications of CP and TF expression were uniformly conducted treating the cells with retinoids for 96 hours to allow the time necessary for cell differentiation.17,22 The effect of retinoid treatment on cell maturation was confirmed by the study in the same cells of morphological changes, growth arrest, NBT reduction capacity, and by the analysis of surface expression of CD11b and CD33 markers of myeloid differentiation.25 The surface CD11b increase and CD33 decrease induced by retinoids highly correlate, in previous studies, with morphological changes, growth arrest, and NBT reduction capacity in S- and R-NB4 cells.17-19,22,24,26 They also correlate with the expression of alkaline phosphatase, another marker of granulocytic differentiation, in NB4 cells treated with ATRA.22
PCA was identified in the study by four different criteria: (1) the clotting activity by the one-stage clotting assay of NHP and FVII-DP; (2) selective inhibition by agents known to block either TF or CP PCA; (3) the chromogenic assays for CP and TF activity; (4) the immunological identification of the two procoagulants by specific anti-TF and anti-CP MoAbs. The clotting assay of NHP and FVII-DP allows identification of total PCA (including all possible procoagulants present) and the proportion of FVII-independent PCA. We choose to perform these plasma assays in the VB extracts because these conditions have been used in previous studies to characterize PCA9-13,15 and, therefore, permit comparisons of the results. In agreement with our previous findings in the same cells13 and in cells from APL patients,10,12 15we found that in NB4 cells, before treatment, a large proportion (45%) of total PCA is FVII independent. The use of inhibitors of cysteine proteinases or of TF provides a sensitive way to identify the presence of CP or TF or both.
In the first part of the study, after ATRA, the total PCA was significantly reduced in all cell lines, regardless of their sensitivity to ATRA-induced differentiation. However, the FVII-independent CP-like PCA was virtually abolished only in the S-NB4 cells that underwent differentiation, but not in the resistant cells that did not differentiate. The assay of PCA's sensitivity to inhibitors confirmed the persistence, after ATRA, of a cysteine proteinase procoagulant in the R-NB4, but not in the S-NB4, cells.
This first observation suggested that ATRA might differently affect the two procoagulants. Therefore, we further characterized the two procoagulants in the treated and untreated cell samples by specific chromogenic and immunological assays. Because the VB aqueous extract is the optimal condition for the CP recovery, ad hoc samples from S- and R-NB4 cells were prepared to optimize the detection of TF as well. Specifically, cell lysates were prepared for the chromogenic assay of TF activity and cell extract in Tris/NaCl buffer containing 1% Triton were prepared for the ELISA of TF; these are the most efficient condition for TF antigen recovery.15,27 These further analyses, by different assays, confirmed the occurrence of CP reduction in association with cell differentiation features, while TF modulation was, at least in part, independent from this process. TF was downregulated by ATRA in both S- and R-NB4 cells. Because NB4.306 and NB4.007/6 contain no or very low levels of the PML/RARα protein,18 19 these data indicate that the reduction of CP by retinoids requires the presence of detectable PML/RARα protein levels.
These results are in agreement with known characteristics of the two procoagulants. TF is a procoagulant of malignant cells, but it is also the cellular procoagulant found in normally differentiated cells that activates normal blood coagulation.6,30 CP has been described in extracts of neoplastic cells or in amnion-chorion tissues, but not in extracts of normally differentiated cells.10-12,31-33 In patients with acute myeloid leukemias, CP was detected in the BM mononuclear cells at the onset of the disease, but not in samples from the same subjects during complete remission.12 All of these findings support the thesis that CP may be expressed by undifferentiated fetal and dedifferentiated malignant cells and, once normal differentiation occurs, the expression of this enzyme is repressed. On the other hand, TF has been shown to be downregulated by ATRA in leukemic cells other than APL, not expressing the PML/RARα and not sensitive to ATRA-induced cyto-differentiation,34 and also in normal human endothelial cells and monocytes.35-37
To confirm the relation to cell maturation of the two procoagulants modulating mechanisms, we used a second experimental approach, the treatment of NB4 cells with retinoic acid analogues that selectively act on the RAR subtypes and possess different cyto-differentiating capacity. The RAR gene family comprises three subtypes α, β, and γ.20 The classification of these receptors is based on differences in amino acid sequence, responsiveness to different retinoids, and the ability to modulate the expression of different target genes. ATRA binds to all members of this family with the same affinity. Therefore, the multiple effects of retinoic acid are better analyzed by using ligands selective for the known receptors.21 Using this strategy RARα has been shown to play a major role in the granulocytic differentiation of leukemic promyelocytes.22 In our study the compound AM580, which selectively binds to RARα and induces cell differentiation, produced a significant reduction of CP levels, whereas the CD2019 and CD437 retinoid-analogues, which selectively bind to RARβ and RARγ, respectively, neither showed an effect on modulation granulocyte differentiation nor affected the expression of CP in the NB4 cells. In contrast, TF was modulated not only by AM580, but also by CD2019, regardless of the cell maturation status. The RARγ agonist CD437 had no effect on either TF or CP regulation. These data suggest a major role for RARα in CP downregulation by retinoids, whereas both RARα and RARβ appear to be implicated in the regulation of TF expression in promyelocytic leukemia cells. A major role for RARα in the CP downregulation by retinoids is also shown by the experiments with the RARα antagonist Ro41-5253. Cotreatment of cells with this compound completely blocked the downregulation of CP induced by both ATRA and AM580. On the other hand, the same agent completely prevented the reduction of TF induced by AM580, the RARα agonist, but counteracted only partially the reduction of TF induced by ATRA, which binds to all RARs. The cotreatment of cells with both the RARα and RARβ antagonists completely blocked the ATRA-induced TF downregulation (Table 2). This provides evidence for a role of RARβ in the downregulation of TF by retinoids. The involvement of RARβ in the regulation of TF (in leukemic cells and normal endothelial cells) has also been recently demonstrated by Shibakura et al.38
It is known that AM580 is a more powerful cyto-differentiating agent than ATRA in NB4 cells.22 In our study AM580 is more potent than ATRA in downregulating TF in the S-NB4 cells (see Table 2). In addition, treatment of NB4 cells with AM580 results in a significant reduction of TF expression at concentrations 100-fold lower than those necessary to obtain the same effect with ATRA (as shown in Fig 4). In contrast, AM580 and ATRA have the same effect in the R-NB4.306 line (data not shown). These data confirm that, while AM580 is an active retinoid in cells expressing RARα, its potential is enhanced in cells containing PML/RARα.
The different pathways of regulation of the cell procoagulants, CP and TF, by ATRA, have important new implications for these proteins during ATRA therapy in human malignancy. It supports the concept that CP is a differentiation dependent protein that is expressed by malignant and fetal cells. Further, we postulate that the CP loss, besides reducing the procoagulant capacity of APL cells, might provide an important new tool (marker) to monitor leukemic cell (and possibly other cancer cells) maturation. Furthermore, the TF loss even in non-ATRA maturation-sensitive tumor cells might help to improve clotting complications in other malignant diseases different from APL treated with ATRA.
ACKNOWLEDGMENT
The authors thank Dr M. Lanotte (INSERM U-301, Hopital Saint-Louis, Paris, France) for providing the NB4 cells, and Drs B. Shroot and U Reichert (CIRD Galderma, Sophia Antipolis, Valbonne, France) for providing the synthetic retinoid analogues.
M.M. is the recipient of a fellowship from the Associazione Italiana Ricerca sul Cancro (AIRC).
Address reprint requests to A. Falanga, MD, Hematology Division, Ospedali Riuniti, Largo Barozzi 1, 24100 Bergamo, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 2. Sensitivity to three cysteine proteinase inhibitors (0.1 mmol/L HgCl2, 0.2 mmol/L ZAA-diazomethyl-ketone, and 1 mmol/L IodoAcetic acid [IA]), and to the TF inhibitor anti-TF MoAb (0.1 mg/mL final concentration) of the PCA of S-NB4 (left) and R-NB4-306 cells (right) treated with 10−6 mol/L ATRA (▪) or the vehicle (□) for 96 hours. VB cell extracts were incubated with cysteine proteinase inhibitors or the anti-TF MoAb for 30 minutes or the vehicle (control), before the clotting assay. Statistical analysis compares the inhibitor-treated sample with the untreated (vehicle treated) corresponding control. *P < .05, **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/92/1/10.1182_blood.v92.1.143.413k18_143_151/5/m_blod41318002x.jpeg?Expires=1769094629&Signature=x8T3lDNIpHCAvisVdimjizUawZf7yPaAmysOwWEE3MSzOiKuQ8GHUdFuNN4oZdq0CMZdah06T4TsuSBC6V-8j9-5S-3VpaMgi0M2NbzmZ6r3WGatwXlBsOnk5x3UO32qweqt6-gFOG4qtSEvtmmgbpYDXanmQzyIAfxP1zinWYFlU6NjORRvXPjoUMu6uimnLlSqEkDggwHRYcaDWViAq2a~SuBa2UfQklwMFDflf0Wj4BmwV2g8RhqAwZQWDGIDdqyJRmZE53YYiUbiHKLlB9HVisv-fFKDRMLSmWY3vHspJgSOd9Dqx5iCP9VAciBJZClduV25U-JSwEJuWUywqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal