We have recently established the culture system to generate dendritic cells (DCs) from murine Lin−c-kit+ bone marrow hematopoietic progenitor cells (HPCs) in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) + stem cell factor (SCF) + tumor necrosis factor-α (TNF-α). We present here the identification of two DC precursor subsets originated from HPCs with the phenotype of CD11b−/dullCD11c+ and CD11b+hiCD11c+ that develop independently at early time points (days 4 to 6) in the same culture conditions. Both of CD11b−/dullCD11c+ and CD11b+hiCD11c+ precursors could differentiate at day 10 to 14 into CD11b−/dullCD11c+ mature DCs with typical morphology, phenotype, and the ability to stimulate allogenic mixed leukocyte reaction (MLR). However, the endocytic capacity of fluorescein isothiocyanate-dextran was markedly reduced during the differentiation. CD11b−/dullCD11c+precursors expressed high levels of Ia, CD86, CD40, and E-cadherin molecules, but not c-fms transcript, and mature DCs derived from this precursor subset continue to express abundant E-cadherin antigen, a discernible marker for Langerhans cells. In contrast, CD11b+hiCD11c+ precursors expressed c-fms mRNA, but low levels of Ia, CD86, and E-cadherin, whereas CD40 was undetectable. CD11b−/dullCD11c+mature DCs differentiated from these precursors displayed abundant c-fms mRNA and nonspecific esterase activity. Interestingly, CD11b+hiCD11c+precursors, but not CD11b−/dullCD11c+precursors, may be bipotent cells that can be induced by M-CSF to differentiate into macrophages. All of these results suggest that CD11b−/dullCD11c+ and CD11b+hiCD11c+ cells are distinct DC precursors derived from Lin−c-kit+ HPCs, which differentiate into mature DCs through bifurcated and independent DC differentiation pathways.
DENDRITIC CELLS (DCs) that have the capacity to initiate immune responses as professional antigen-presenting cells consist of heterogeneous cell populations and are distributed in nonlymphoid as well as lymphoid organs.1,2 Phenotypic differences between murine thymic and splenic DCs have been based on the expression of BP-1 and Thy-1 antigens that are exclusively expressed on thymic DCs but not splenic ones.3-7 In the skin, epithelial Langerhans cells (LCs) express Birbeck granules+ and E-cadherin+, whereas dermal dendrocytes do not have Birbeck granules, but express factor XIIIa+.8,9 Functionally different subsets of DCs have been also identified based on phenotypic criteria. In human peripheral blood (PB), the CD11c− and CD83− subsets have been shown to be immature DCs, whereas CD11c+ and CD83+ markers identify mature DCs displaying considerable T-cell–stimulatory capacity.10,11Murine thymic DCs can be further subdivided into major histocompatibility complex (MHC) class IIlo and MHC class IIhi DC subsets which represent immature and mature DCs, respectively.12 Moreover, DCs with CD8α antigen induce apoptosis of activated T cells in contrast to CD8α−DCs.13 Taken together, all these findings indicate that DCs are not only phenotypically but also functionally heterogeneous.
DCs generated from cultured hematopoietic progenitor cells (HPCs) in vitro also consist of heterogeneous populations. Human cord blood CD34+ HPCs have been reported to differentiate in vitro into at least two DC precursor subsets by distinct pathways based on their CD1a and CD14 expression.14CD1a+CD14−c-fms−precursors can give rise to Birbeck granule+ DCs resembling epithelial LCs, whereas CD1a−CD14+c-fms+precursors can generate Birbeck granule−factor XIIIa+ blood or dermal dendrocytic DCs as well as macrophages. The most striking difference is the unique capacity of CD14+ precursor-derived DCs to induce naive B cells to differentiate into IgM-secreting cells.15 These findings suggest that DC progenitor cells can differentiate into functionally distinct DC subtypes and that different DC precursor subsets may exist for DCs distributed in various tissues.
Multiple DC subpopulations have been identified in vivo in the spleen of Flt3 ligand (Flt3L)-treated mice based on the expression of CD11c, CD11b, and CD8α.16,17CD11bdullCD11c+ and CD11b−CD11c+ DC subsets abundantly express CD8α, whereas CD11bbrightCD11c+ DCs are negative for this molecule. These three DC subsets all localize to different sites in the spleen of Flt-3L–treated mice.17Interestingly, in vivo treatment with Flt3L only induced an increase in the CD11b−CD11c+ DC subset, but not others, in the thymus,17 suggesting that distinct differentiation pathways also exist in Flt3L-treated mice. However, it remains unknown whether these multiple DC subsets might differentiate from a common precursor or simply represent an immature stage in the development of DC population. We have recently established a culture system to generate DCs from murine bone marrow (BM) Lin−c-kit+ HPCs in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF), and tumor necrosis factor-α (TNF-α).7Using the same culture system, here we investigate the cellular basis of DC differentiation from Lin−c-kit+ HPCs, and find that CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors represent two distinct intermediate-stage cell subsets with the capacity to differentiate into mature DCs.
MATERIALS AND METHODS
Cytokines and antibodies.
Recombinant murine SCF and GM-CSF were kindly provided by Kirin Brewery Co (Tokyo, Japan) and Dr T. Sudo (Basic Research Institute of Toray Co, Kanagawa, Japan), respectively. M-CSF was kindly provided by Morinaga Milk Industry Cooperation (Morinaga, Japan). Mouse TNF-α was produced as described previously.18 Endotoxin was not detectable in these cytokine preparations using a Toxicolor assay kit (Seikagaku-Kogyo, Tokyo, Japan). These cytokines were used at the optimal concentrations as previously described.7 An anti–c-kit antibody (ACK-2) was kindly provided by Dr T. Sudo (Toray, Kanagawa, Japan) and conjugated with biotin by using an NHS-Biotin kit (Pharmacia-Biotech, Uppsala, Sweden) according to the manufacturer's instructions.19 A rat monoclonal antibody (MoAb) to murine dendritic cell marker, DEC-205 (NLDC145), was a generous gift of Dr R.M. Steinman (Rockefeller University, New York, NY).20 21 MoAb to mouse E-cadherin was purchased from Dainipon Pharmaceutical Co (Tokyo, Japan). Other MoAbs and reagents used for immunostaining were obtained from PharMingen (San Diego, CA), unless otherwise indicated.
Mice.
C57BL/6 and Balb/c mice were obtained from Kurea Animal Co (Tokyo, Japan) and maintained under pathogen-free conditions in the Animal Facility of Department of Molecular Preventive Medicine, School of Medicine, the University of Tokyo (Tokyo, Japan). All animal experiments complied with the standards set out in the Guideline for Care and Use of Laboratory Animals of the University of Tokyo.
Suspension culture of Lin−c-kit+ HPCs.
BM cells were obtained by aspirating femurs and tibiae of 8- to 10-week-old female mice. Lin−c-kit+HPCs were isolated from nonadherent BM mononuclear cells (MNCs) using an EPICS ELITE cell sorter (Coulter Electronics, Hialeah, FL) as previously described.7,22 23 In brief, nonadherent MNCs were stained with an indirect staining composed of biotin-conjugated anti–c-kit MoAb and phycoerythrin (PE)-labeled streptavidin followed by a set of fluorescein isothiocyanate (FITC)-labeled MoAbs to CD3 (145-2C11), CD4 (H129.19), CD8 (53-6.7), B220 (RA3-6B2), Gr-1 (Ly-6G), CD11a (2D7), and CD11b (M1/70). The contamination by other types of cells in these preparations was consistently less than 0.5% as shown by an immunofluorescence analysis.
Purified Lin−c-kit+ HPCs were incubated as previously described7 at a cell concentration of 1 × 104 cells/mL in Iscove's modified Dulbecco's medium (IMDM; GIBCO, Rockville, MD) supplemented with 10% fetal bovine serum (FBS), 5 × 10−5 mol/L 2-mercaptoethanol, penicillin G (100 U/mL), and streptomycin (100 μg/mL) in the presence of GM-CSF + SCF + TNF-α. Optimal conditions were maintained by splitting these cultures at day 4 with medium exchange containing fresh cytokines. For most experiments cells were collected after 6 days of culture for cell sorting.
Isolation of CD11b−/dullCD11c+and CD11b+hiCD11c+ DC precursors by a cell sorter.
After 6 days of culture in the presence of GM-CSF, SCF, and TNF-α, cells were collected, labeled with FITC-conjugated anti-CD11b and PE-conjugated anti-CD11c (HL3), and sorted into CD11b−/dullCD11c+ and CD11b+hiCD11c+ cell subsets. In some experiments, the CD11b−CD11c− cell fraction was also sorted. All the staining and sorting procedures were performed in the presence of 1 mmol/L EDTA to avoid cell aggregation. Reanalysis of the sorted populations showed a purity higher than 98%. Sorted cells were routinely incubated in medium containing GM-CSF + TNF-α or M-CSF for an additional 5 to 8 days. The cultured cells were collected between days 10 and 14.
Immunofluorescence analysis.
Immunofluorescence analyses were performed as previously described.7,22 23 In three-color analyses, 4 × 105 cells were incubated with biotinylated hamster anti-CD11c MoAbs and rat anti-CD11b MoAbs, followed by staining with Cy-Chrome (CY)-labeled streptavidin and PE-conjugated goat anti-rat IgG (Fab′)2 antibody. The cells were then stained with various FITC-conjugated MoAbs. In some experiments, the cells were first stained with rat anti–E-cadherin MoAb and biotinylated anti-CD11c, followed by staining with PE-conjugated goat anti-rat IgG(Fab′)2 antibody and CY-labeled streptavidin, and then stained with FITC-conjugated anti-CD11b. In other experiments, the cells were first stained with biotinylated antibodies and revealed by CY-conjugated streptavidin, followed by staining with PE-labeled anti-CD11c and FITC-conjugated anti-CD11b. The instrument compensation was set in each experiment using single-color and/or two-color stained samples.
Reverse transcription-polymerase chain reaction (RT-PCR).
Total RNAs were extracted from 1 × 105 indicated cells using RNAzol B (Biotex Laboratories Inc, Houston, TX), according to the manufacturer's instructions. First-strand cDNA was synthesized in a 25-μL reaction volume using an RT-PCR kit (Takara Shuzo, Kyoto, Japan) with random primers. Thereafter, cDNA was amplified for 25 cycles consisting of 94°C for 30 seconds, 57°C for 1 minute, and 72°C for 1.5 minutes with the c-fms–specific oligonucleotide primers (5′-CCAGAACTGGTTGTAGAGCC-3′ and 5′-CAGCTTGCTAGGCTCCAATT-3′), which specifically result in a 500-bp cDNA encoding c-fms.24 As a control, mouse β-actin transcript was amplified in parallel as previously described.7 The PCR products were fractionated on 1.5% agarose gel and visualized by ethidium bromide staining.
Endocytosis.
The endocytosis experiments were performed as previously reported.25 In brief, cells were incubated with 0.1 mg/mL FITC-Dextran (FITC-DX; 4,000 daltons; Sigma Chemical Co, St Louis, MO) at 37°C or 0°C for 60 minutes. Uptake was stopped by adding ice-cold phosphate-buffered saline (PBS) containing 5% bovine serum albumin (BSA) and 0.02% sodium azide. After staining with biotinylated anti-CD11c and rat anti-CD11b MoAbs, the cells were further incubated with CY-labeled streptavidin and PE-conjugated goat anti-rat IgG (Fab′)2 antibody followed by flow cytometric analysis.
Mixed leukocyte reaction (MLR).
Splenic MNCs were prepared from allogenic mice (Balb/c) as described previously.7 The adherent cells were first removed by incubating them at 37°C for 60 minutes in IMDM containing 10% fetal calf serum (FCS). To obtain highly purified T cells, the nonadherent splenic MNCs were incubated with rat anti-B220 and mouse anti-Ia MoAbs followed by staining with anti-rat IgG and anti-mouse IgG conjugated magnetic beads to deplete B220+ and Ia+ cells using Dynal-beads (Dynal, Oslo, Norway). After treatment with mitomycin C (MMC; 15 μg/mL),26 the indicated stimulator cells (from 100 to 3 × 104 cells) were added to T cells (3 × 105) in each well of 96-well round-bottomed microtest tissue-culture plates (Nunc, Roskilde, Denmark). After incubating at 37°C for 4 to 5 days, cell proliferation was determined using 3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT; Sigma Chemical Co). In brief, 15 μL of MTT (5 mg/mL in PBS) was added into each well and the plates were incubated at 37°C for an additional 4 hours. The resultant absorbance at 550 nm was read by a microplate immunoreader.
Electron microscopy.
Cultured cells sampled after 7 and 14 days of culture were fixed in 2% glutaraldehyde, postfixed in 1% osmium tetroxide, and embedded in Epon 812 (E. Fullan, Inc, Latham, NY). Ultrathin sections were then cut and stained with uranyl acetate and lead citrate, and examined with an electron microscope, HITACHI H-800 (HITACHI, Tokyo, Japan).27
Statistical analysis.
Differences were evaluated using the Student's t-test.P values of <.05 were considered to be statistically significant.
RESULTS
Differentiation of two DC precursor subsets from Lin−c-kit+ HPCs.
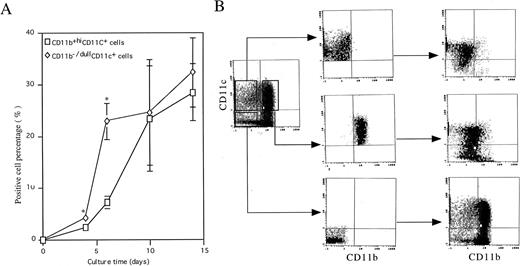
To elucidate the cellular basis of the development of DC, we followed the kinetics of CD11b and CD11c expression during differentiation of murine Lin−c-kit+ HPC stimulated with GM-CSF + SCF + TNF-α. At day 0, murine Lin−c-kit+ HPCs did not express CD11b or CD11c (Fig 1A). However, two cell populations characterized by the expression of CD11b−/dullCD11c+ and CD11b+hiCD11c+ emerged independently in the cultures at day 4. At day 6, a distinct population of CD11b−/dullCD11c+ cells could be identified (7.2% ± 1.2%) which increased rapidly thereafter and reached the maximum levels by days 10 to 14. Although CD11b+hiCD11c+ cells (22.9% ± 3.5%) were usually dominant over the CD11b−/dullCD11c+ cells in the cultures at day 6, they did not increase thereafter during more prolonged culture periods (Fig 1A), implying that CD11b+hiCD11c+ cells may contribute to the later generation of CD11b−/dullCD11c+cells in the cultures. To examine this possibility, the three cell populations CD11b−/dullCD11c+, CD11b+hiCD11c+, and CD11b−CD11c− were routinely sorted at day 6 (Fig 1B) and cultured in the presence of GM-CSF + TNF-α for an additional 5 to 8 days. Most of the CD11b+hiCD11c+ cells could differentiate into cells with the CD11b−/dullCD11c+ (51.0% ± 10.4%; n = 15) phenotype at days 12 to 14. In contrast, CD11b−/dullCD11c+-derived cells did not change their phenotype at days 10 to 12 (81.7 ± 11.2%, n = 15) and did not revert into a CD11b+hi phenotype. Interestingly, the CD11b−CD11c− cell population generated both of CD11b−/dullCD11c+ (8.4% ± 2.1%, n = 4) and CD11b+hiCD11c+(25.1% ± 3.4%, n = 4) cells at a similar rate and extent as Lin−c-kit+ HPC cultures at day 6 (Fig 1B). These results suggest that Lin−c-kit+ HPC may generate CD11b−/dullCD11c+ cells through at least two independent differentiation pathways: one may directly differentiate from Lin−c-kit+ HPCs at the early stage, while another one is an intermediate stage for CD11b+hiCD11c+ cells at the latter stage of culture.
Generation of DC precursors and DCs from murine Lin−c-kit+ HPCs stimulated with GM-CSF + TNF-α. (A) Murine Lin−c-kit+ HPCs were cultured in the presence of GM-CSF + SCF + TNF-α for 6 to 7 days and then in the presence of GM-CSF + TNF-α for another 7 days. At the indicated timepoint, independent aliquots of cells were recovered and processed for analyses of CD11b and CD11c expression by using double-color immunostaining with anti–CD11b-FITC and anti–CD11c-PE. The data represent mean value ± SD of percentage of the two subpopulations observed in more than five experiments. *P < .05 significance as compared with CD11b−/dullCD11c+ cells at the indicated timepoint. (B) The cells were routinely sorted from cultures of GM-CSF + SCF + TNF-α–stimulated murine Lin−c-kit+ HPCs at day 6 into CD11b−/dullCD11c+, CD11b+hiCD11c+, and CD11b−CD11c− cell populations using EPICS ELITE cell sorter (middle panel). The sorted cells were cultured again in the presence of GM-CSF + TNF-α for an additional 5 to 8 days and reanalyzed for the expression of CD11b and CD11c by double-color immune staining (right panel). Quads were set up on the isotype-matched control dot plot and the results are representative of more than 15 experiments.
Generation of DC precursors and DCs from murine Lin−c-kit+ HPCs stimulated with GM-CSF + TNF-α. (A) Murine Lin−c-kit+ HPCs were cultured in the presence of GM-CSF + SCF + TNF-α for 6 to 7 days and then in the presence of GM-CSF + TNF-α for another 7 days. At the indicated timepoint, independent aliquots of cells were recovered and processed for analyses of CD11b and CD11c expression by using double-color immunostaining with anti–CD11b-FITC and anti–CD11c-PE. The data represent mean value ± SD of percentage of the two subpopulations observed in more than five experiments. *P < .05 significance as compared with CD11b−/dullCD11c+ cells at the indicated timepoint. (B) The cells were routinely sorted from cultures of GM-CSF + SCF + TNF-α–stimulated murine Lin−c-kit+ HPCs at day 6 into CD11b−/dullCD11c+, CD11b+hiCD11c+, and CD11b−CD11c− cell populations using EPICS ELITE cell sorter (middle panel). The sorted cells were cultured again in the presence of GM-CSF + TNF-α for an additional 5 to 8 days and reanalyzed for the expression of CD11b and CD11c by double-color immune staining (right panel). Quads were set up on the isotype-matched control dot plot and the results are representative of more than 15 experiments.
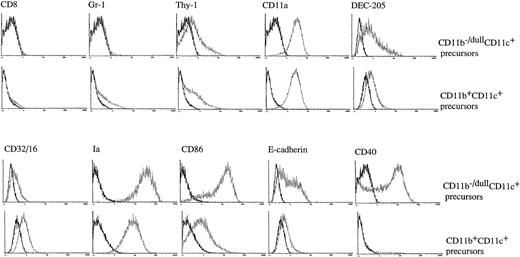
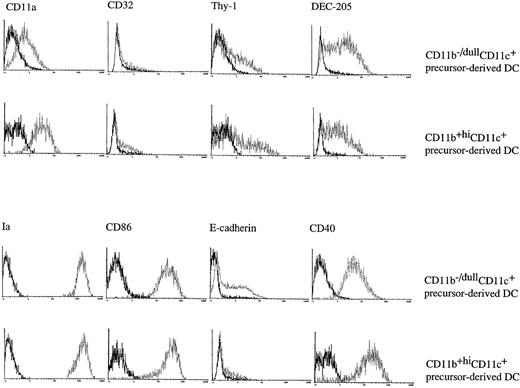
To further characterize the phenotype of CD11b−/dullCD11c+ and CD11b+hiCD11c+ cells generated in the cultures at day 6, three-color immunofluorescence analyses were performed. These showed that both of the populations were negative for CD4 and B220 (data not shown), CD8, and Gr-1 (Fig 2). Although both of the subsets expressed comparable levels of Thy-1, CD11a, and CD32/16, CD11b−/dullCD11c+ cells expressed much higher levels of Ia, CD86, E-cadherin, and DEC-205 than CD11b+hiCD11c+ cells. Interestingly, CD11b−/dullCD11c+ cells also expressed high levels of CD40 that were undetectable on CD11b+hiCD11c+ cells at this time point (Fig2). Taken together, these data would indicate that the two populations differ phenotypically from each other in the expression of a number of surface markers.
The CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors display a different immunophenotype. Murine Lin−c-kit+ HPCs were cultured in presence of GM-CSF + SCF + TNF-α for 6 days. The phenotype of the CD11b−/dullCD11c+ and CD11b+hiCD11c+ precursors was determined by three-color immune staining using uncoupled test MoAbs shown by PE-conjugated anti-rat Ig and biotinylated anti-CD11c MoAb shown by CY-conjugated streptavidin, and the third color was shown by FITC-conjugated anti-CD11b MoAb. In some experiments FITC-conjugated anti-CD11b and PE-conjugated anti-CD11c MoAbs were used, while the test biotinylated MoAbs were shown by CY-conjugated streptavidin. In some experiments, uncoupled CD11b and biotinylated CD11c were shown by PE-conjugated anti-rat IgG and CY-conjugated streptavidin and finally FITC-conjugated test MoAbs. Histograms shown in the figures are gated on CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors. Solid and dotted lines indicate the immunofluoresecence intensity of cells stained with a control and the test antibodies, respectively. Representative results from three or more independent experiments are shown.
The CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors display a different immunophenotype. Murine Lin−c-kit+ HPCs were cultured in presence of GM-CSF + SCF + TNF-α for 6 days. The phenotype of the CD11b−/dullCD11c+ and CD11b+hiCD11c+ precursors was determined by three-color immune staining using uncoupled test MoAbs shown by PE-conjugated anti-rat Ig and biotinylated anti-CD11c MoAb shown by CY-conjugated streptavidin, and the third color was shown by FITC-conjugated anti-CD11b MoAb. In some experiments FITC-conjugated anti-CD11b and PE-conjugated anti-CD11c MoAbs were used, while the test biotinylated MoAbs were shown by CY-conjugated streptavidin. In some experiments, uncoupled CD11b and biotinylated CD11c were shown by PE-conjugated anti-rat IgG and CY-conjugated streptavidin and finally FITC-conjugated test MoAbs. Histograms shown in the figures are gated on CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors. Solid and dotted lines indicate the immunofluoresecence intensity of cells stained with a control and the test antibodies, respectively. Representative results from three or more independent experiments are shown.
Because both CD11b−/dullCD11c+ and CD11b+hiCD11c+ cells can differentiate into cells of the CD11b−/dullCD11c+phenotype, a previously demonstrated DC specific phenotype,3-7,16 17 we therefore designated these two cell populations sorted from Lin−c-kit+ HPC cultures at day 6 as CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors, respectively.
Both CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors can differentiate into mature phenotypically distinct DC-like cells.
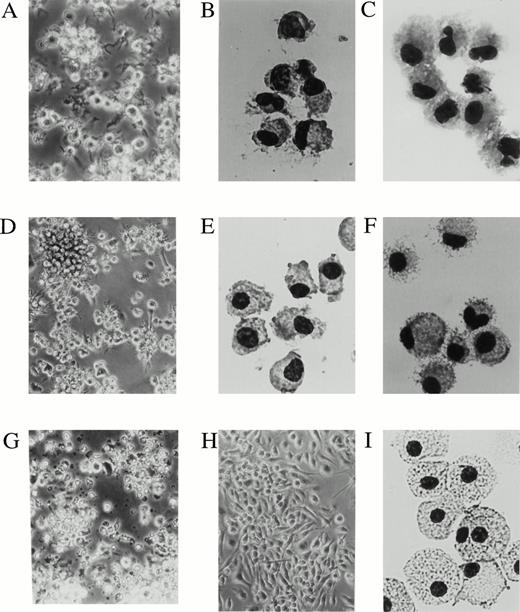
To better understand the phenotypic differences between the two DC precursors and their derived mature DCs, CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors were routinely sorted at day 6 and recultured in the presence of GM-CSF + TNF-α. Stimulation with GM-CSF + TNF-α could not again induce proliferation of CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors (data not shown). Upon culture with GM-CSF + TNF-α, CD11b−/dullCD11c+ DC precursors developed into a relatively homogeneous, plastic nonadherent cell population that were irregular in shape and congregated into large aggregates of DC surrounded by cells with long spiny processes at days 10 to 12 (Fig 3A through C). In contrast, most CD11b+hiCD11c+ DC precursors appeared to be adherent cells, medium to large in size that could form large DC aggregates at later time points of days 12 to 14 under the same culture conditions (Fig 3D and E). These cells could be easily detached and had the morphological characteristics of DC-like cells (Fig 3F).
The day 6 DC precursors differentiate into cells displaying a dendritic cell morphology at days 10 to 14. CD11b−/dullCD11c+ and CD11b+hiCD11c+ precursors were sorted from murine Lin−c-kit+ HPC cultures stimulated with GM-CSF + SCF + TNF-α for 6 days. A phase contrast microscopical observation (A, D, G, and H) and May-Grünwald-Giemsa staining (B, C, E, F, and I) were performed on sorted CD11b−/dullCD11c+ and CD11b+hiCD11c+ precursors or after culture for 5 to 8 additional days in the presence of GMCSF + TNF-α or M-CSF, respectively. (A and C) CD11b−/dullCD11c+ precursors cultured for 5 to 6 days in the presence of GM-CSF + TNF-α; (B) sorted CD11b−/dullCD11c+ precursors; (D and F) CD11b+hiCD11c+precursors cultured for additional 5 to 8 days in presence of GM-CSF + TNF-α; (E) sorted CD11b+hiCD11c+ precursors; (G) CD11b−/dullCD11c+ precursors cultured in the presence of M-CSF for 3 days; (H and I) cultured CD11b+hiCD11c+ precursors in the presence of M-CSF for 5 to 8 days. Original magnifications: (A) ×200; (D, G, and H) ×160; (B, C, E, F, and I) ×400.
The day 6 DC precursors differentiate into cells displaying a dendritic cell morphology at days 10 to 14. CD11b−/dullCD11c+ and CD11b+hiCD11c+ precursors were sorted from murine Lin−c-kit+ HPC cultures stimulated with GM-CSF + SCF + TNF-α for 6 days. A phase contrast microscopical observation (A, D, G, and H) and May-Grünwald-Giemsa staining (B, C, E, F, and I) were performed on sorted CD11b−/dullCD11c+ and CD11b+hiCD11c+ precursors or after culture for 5 to 8 additional days in the presence of GMCSF + TNF-α or M-CSF, respectively. (A and C) CD11b−/dullCD11c+ precursors cultured for 5 to 6 days in the presence of GM-CSF + TNF-α; (B) sorted CD11b−/dullCD11c+ precursors; (D and F) CD11b+hiCD11c+precursors cultured for additional 5 to 8 days in presence of GM-CSF + TNF-α; (E) sorted CD11b+hiCD11c+ precursors; (G) CD11b−/dullCD11c+ precursors cultured in the presence of M-CSF for 3 days; (H and I) cultured CD11b+hiCD11c+ precursors in the presence of M-CSF for 5 to 8 days. Original magnifications: (A) ×200; (D, G, and H) ×160; (B, C, E, F, and I) ×400.
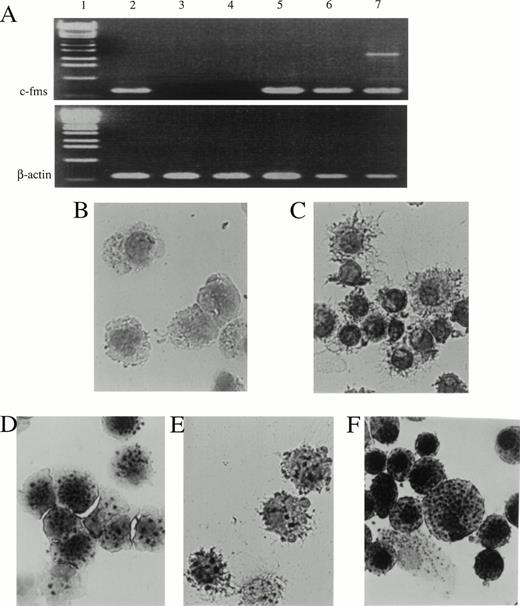
During the differentiation of these two DC precursors, c-fmswas exclusively expressed by CD11b+hiCD11c+ DC precursors and their mature offspring, but not by CD11b−/dullCD11c+ DC precursors and their mature CD11b−/dullCD11c+ offspring (Fig4A). Interestingly, most of the CD11b+hiCD11c+ DC precursors (Fig 4D) and their mature offspring (Fig 4E) also displayed nonspecific esterase activity in contrast with the CD11b−/dullCD11c+ DC precursors (Fig4B) and their mature offspring (Fig 4C) in which the nonspecific esterase activity was undetectable.
Expression of c-fms mRNA and nonspecific esterase activity in CD11b−/dullCD11c+, CD11b+hiCD11c+ DC precursors, and their derived DCs. (A) Expression of c-fms mRNA was examined in the indicated cells using RT-PCR. Total RNAs were extracted from 1 × 105 indicated cells. The β-actin transcripts were used as control. Lane 1, 1-kb DNA ladder; 2, Lin−c-kit+ HPC; 3, CD11b−/dullCD11c+ precursor; 4, CD11b−/dullCD11c+ mature DC derived from CD11b−/dullCD11c+ precursors; 5, CD11b+hiCD11c+ precursor; 6, CD11b−/dullCD11c+ mature DC derived from CD11b+hiCD11c+ precursors; 7, macrophage derived from M-CSF–induced CD11b+hiCD11c+ DC precursors. (B through F) The cultured cells were sorted and processed for nonspecific esterase staining. (B) CD11b−/dullCD11c+precursors; (C) CD11b−/dullCD11c+precursor-derived CD11b−/dullCD11c+mature DCs; (D) CD11b+hiCD11c+ DC precursors; (E) CD11b+hiCD11c+ DC precursor-derived CD11b−/dullCD11c+ mature DCs; (F) macrophages derived from M-CSF–induced CD11b+hiCD11c+ DC precursors. Original magnification ×400.
Expression of c-fms mRNA and nonspecific esterase activity in CD11b−/dullCD11c+, CD11b+hiCD11c+ DC precursors, and their derived DCs. (A) Expression of c-fms mRNA was examined in the indicated cells using RT-PCR. Total RNAs were extracted from 1 × 105 indicated cells. The β-actin transcripts were used as control. Lane 1, 1-kb DNA ladder; 2, Lin−c-kit+ HPC; 3, CD11b−/dullCD11c+ precursor; 4, CD11b−/dullCD11c+ mature DC derived from CD11b−/dullCD11c+ precursors; 5, CD11b+hiCD11c+ precursor; 6, CD11b−/dullCD11c+ mature DC derived from CD11b+hiCD11c+ precursors; 7, macrophage derived from M-CSF–induced CD11b+hiCD11c+ DC precursors. (B through F) The cultured cells were sorted and processed for nonspecific esterase staining. (B) CD11b−/dullCD11c+precursors; (C) CD11b−/dullCD11c+precursor-derived CD11b−/dullCD11c+mature DCs; (D) CD11b+hiCD11c+ DC precursors; (E) CD11b+hiCD11c+ DC precursor-derived CD11b−/dullCD11c+ mature DCs; (F) macrophages derived from M-CSF–induced CD11b+hiCD11c+ DC precursors. Original magnification ×400.
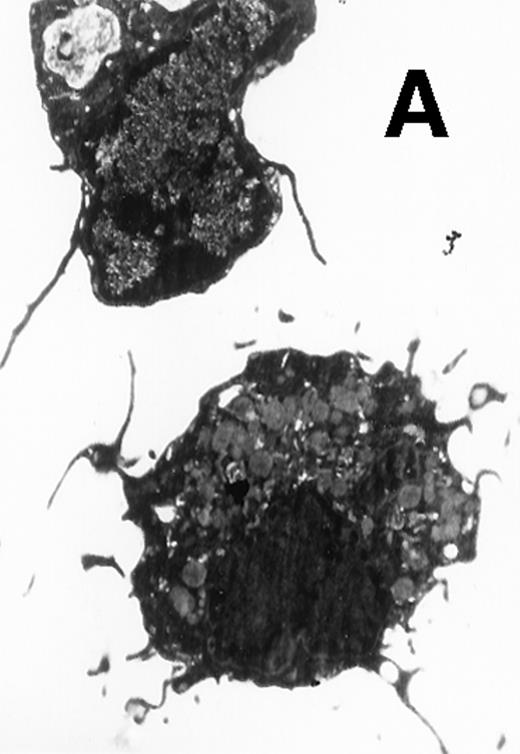
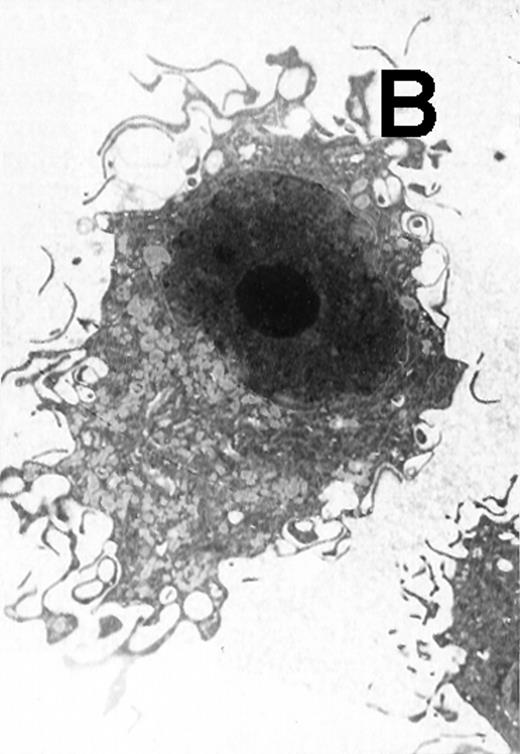
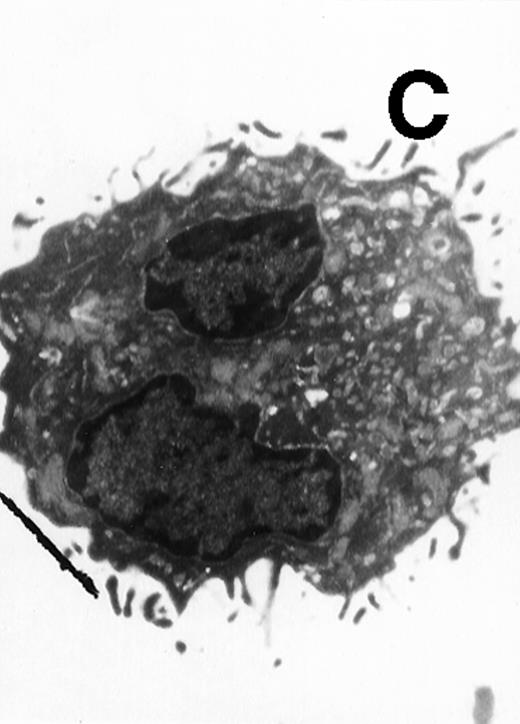
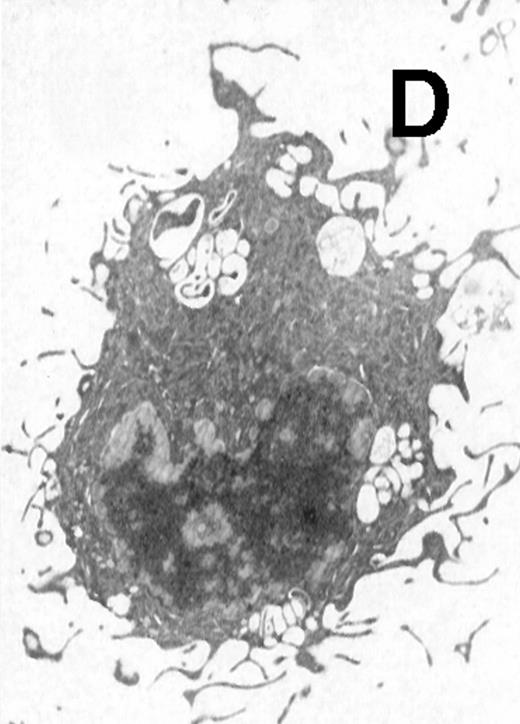
Ultrastructural observation showed that both CD11b−/dullCD11c+ (Fig5A) and CD11b+hiCD11c+ DC precursors (Fig 5B) were small and mostly round in shape. They contained mitochondria and rough endoplasmic in the cytoplasm and projected a few short cytoplasmic processes. After culture for an additional 5 to 8 days, the cells became larger and showed well-developed Golgi apparatus, mitochondria, rough endoplasmic reticulum (RER), and a few small lysosomes. The cells also developed abundant long cytoplasmic processes, a tubulovesicular system, vesicles, and multivesicular bodies near the nucleus, particularly adjacent to the Golgi apparatus in the cytoplasmic (Fig 5C and D). There were no significant morphological differences between CD11b−/dullCD11c+ DC precursor-derived mature DCs and CD11b+hiCD11c+ precursor-derived ones.
Electron microscopy of typical CD11b−/dullCD11c+ precursor and its derived CD11b−/dullCD11c+ mature DCs. The CD11b−/dullCD11c+ precursors and its derived CD11b−/dullCD11c+ mature DCs were sorted at culture day 6 and day 12, respectively, and processed for electron microscopy staining as described in Materials and Methods. (A) A representative of CD11b−/dullCD11c+precursor sorted at day 6. Original magnification ×5,300. (B) A representative of CD11b−/dullCD11c+precursor-derived CD11b−/dullCD11c+ mature DC sorted at day 12. Original magnification ×5,300. (C) A representative of CD11b+hiCD11c+ precursor sorted at day 6. Original magnification ×9,600. (D) A representative of CD11b+hiCD11c+ precursor-derived mature DC with the phenotype of CD11b−/dullCD11c+sorted at day 12. Original magnification ×9,600.
Electron microscopy of typical CD11b−/dullCD11c+ precursor and its derived CD11b−/dullCD11c+ mature DCs. The CD11b−/dullCD11c+ precursors and its derived CD11b−/dullCD11c+ mature DCs were sorted at culture day 6 and day 12, respectively, and processed for electron microscopy staining as described in Materials and Methods. (A) A representative of CD11b−/dullCD11c+precursor sorted at day 6. Original magnification ×5,300. (B) A representative of CD11b−/dullCD11c+precursor-derived CD11b−/dullCD11c+ mature DC sorted at day 12. Original magnification ×5,300. (C) A representative of CD11b+hiCD11c+ precursor sorted at day 6. Original magnification ×9,600. (D) A representative of CD11b+hiCD11c+ precursor-derived mature DC with the phenotype of CD11b−/dullCD11c+sorted at day 12. Original magnification ×9,600.
To characterize the immunophenotype of the mature DCs differentiated from the two DC precursor subsets, three-color immunofluorescence analyses were performed. As shown in Fig6,CD11b−/dullCD11c+ mature DCs derived from either CD11b−/dullCD11c+ or CD11b+hiCD11c+ DC precursors expressed higher levels of Ia, CD86, CD40, and DEC-205, characteristic of active mature DC. However, E-cadherin antigen, a discernible marker for LCs,28 was exclusively expressed only on CD11b−/dullCD11c+ DC precursor-derived mature DCs but not by CD11b+hiCD11c+ precursor-derived ones. All these results indicate that CD11b+hiCD11c+ and CD11b−/dullCD11c+ DC precursors and their derived mature DCs share a combination of different phenotypes and cannot be converted by each other, even though their mature offspring have the common phenotype of CD11b−/dullCD11c+.
The day 6 DC precursors differentiate into cells with typical DC markers. CD11b−/dullCD11c+and CD11b+hiCD11c+ precursors were sorted from murine Lin−c-kit+ HPC cultures stimulated with GM-CSF + SCF + TNF-α for 6 days and were cultured in the presence of GMCSF + TNF-α for an additional 5 to 8 days. At days 12 to 14, the phenotype of the CD11b−/dullCD11c+ DC-like cells derived from either CD11b−/dullCD11c+ or CD11b+hiCD11c+ precursors was determined using three-color analyses as described in the legend to Fig 2.
The day 6 DC precursors differentiate into cells with typical DC markers. CD11b−/dullCD11c+and CD11b+hiCD11c+ precursors were sorted from murine Lin−c-kit+ HPC cultures stimulated with GM-CSF + SCF + TNF-α for 6 days and were cultured in the presence of GMCSF + TNF-α for an additional 5 to 8 days. At days 12 to 14, the phenotype of the CD11b−/dullCD11c+ DC-like cells derived from either CD11b−/dullCD11c+ or CD11b+hiCD11c+ precursors was determined using three-color analyses as described in the legend to Fig 2.
Mature DCs derived from CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors each stimulate allogenic MLR.
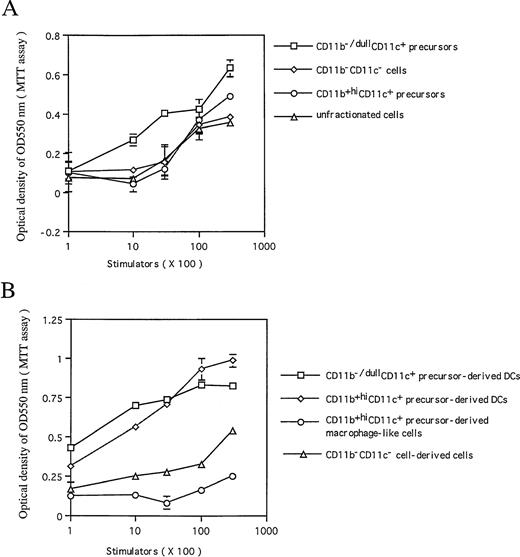
As examined by allogenic MLR, CD11b−/dullCD11c+ DC precursors, but not CD11b+hiCD11c+ ones, could effectively enhance allogenic MLR, suggesting that CD11b−/dullCD11c+ cells may be functionally active in presenting antigen as well as stimulating T-cell proliferation (Fig 7A). However, after culture in the presence of GM-CSF + TNF-α for an additional 5 to 8 days, CD11b−/dullCD11c+ mature DCs derived from either CD11b+hiCD11c+ or CD11b−/dullCD11c+ precursors each became equally potent in their capacity to stimulate in vitro T-lymphocyte proliferation (Fig 7B). In comparison with CD11b−/dullCD11c+ DCs matured at days 12 to 14, CD11b−/dullCD11c+ DC precursors were less potent in enhancing allogenic MLR, indicating that both CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors can differentiate into functional mature DCs in response to GM-CSF + TNF-α at days 12 to 14, while CD11b−/dullCD11c+ DC precursors are still functionally immature at day 6, even though they express active markers of DC.
The capacity of the cultured cells to enhance allogenic MLR. Allogenic MLR was performed using purified T cells (3 × 105 cells per well in 96 round-well plates) as responder cells. (A) The day 6 sorted CD11b−/dullCD11c+, CD11b+hiCD11c+, and CD11b−CD11c− precursors, and unfractionated cells from Lin−c-kit+ HPC cultures were treated with MMC and used as stimulators at the indicated cell number. (B) Sorted mature CD11b−/dullCD11c+ mature DCs derived from CD11b−/dullCD11c+, CD11b+hiCD11c+ DC precursors at days 12 to 14 and macrophages derived from M-CSF–induced CD11b+hiCD11c+ DC precursors were used as stimulator cells at the indicated cell number. The proliferation of T cells was measured by MTT assay after 4 days of culture. Results are expressed as mean ± 1 SD of triplicate cultures. Results of each panel are representative of three independent experiments.
The capacity of the cultured cells to enhance allogenic MLR. Allogenic MLR was performed using purified T cells (3 × 105 cells per well in 96 round-well plates) as responder cells. (A) The day 6 sorted CD11b−/dullCD11c+, CD11b+hiCD11c+, and CD11b−CD11c− precursors, and unfractionated cells from Lin−c-kit+ HPC cultures were treated with MMC and used as stimulators at the indicated cell number. (B) Sorted mature CD11b−/dullCD11c+ mature DCs derived from CD11b−/dullCD11c+, CD11b+hiCD11c+ DC precursors at days 12 to 14 and macrophages derived from M-CSF–induced CD11b+hiCD11c+ DC precursors were used as stimulator cells at the indicated cell number. The proliferation of T cells was measured by MTT assay after 4 days of culture. Results are expressed as mean ± 1 SD of triplicate cultures. Results of each panel are representative of three independent experiments.
Decrease in endocytic ability during CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursor maturation.
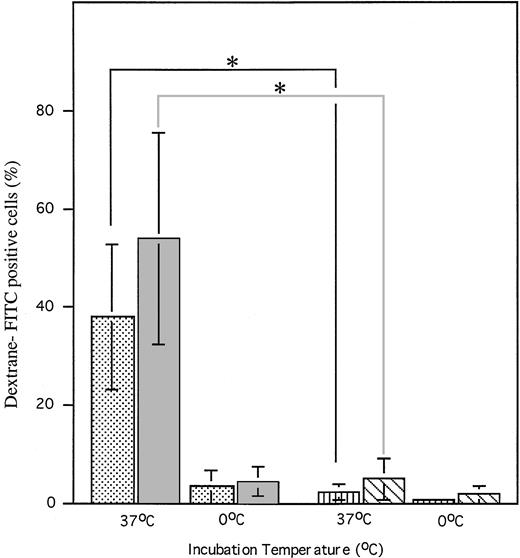
During maturation, DCs gradually lose their endocytic ability.25,29 30 As shown in Fig8, either CD11b−/dullCD11c+ or CD11b+hiCD11c+ precursors could efficiently take up FITC-DX at 37°C, but this was blocked by incubating them at 0°C. This capacity was significantly reduced when these cells differentiated into CD11b−/dullCD11c+ mature DCs induced by GM-CSF + TNF-α by days 12 to 14. These data suggest that micropinocytosis is highly reduced during maturation and differentiation of DC precursors.
FITC-DX uptake by CD11b−/dullCD11c+ precursors (▧) and CD11b+hiCD11c+ DC precursors (▧) at day 6 and their derived CD11b−/dullCD11c+ mature DCs at days 12 to 14. Cells were first exposed to 0.1 mg/mL of FITC-DX at 0°C and 37°C, respectively, for 60 minutes. After washing twice, the cells were stained with rat–anti-mouse CD11b and biotinylated hamster–anti-mouse CD11c and then shown by PE-conjugated anti-rat Ig and CY-conjugated-streptavidin. A three-color immunofluorescence analysis was performed to show the capacity of FITC-DX uptake by these cells as indicated. *P < .01 significance compared with CD11b−/dullCD11c+ (▥) and CD11b+hiCD11c+ (□) DC precursor-derived mature DCs. Results are representative of three experiments.
FITC-DX uptake by CD11b−/dullCD11c+ precursors (▧) and CD11b+hiCD11c+ DC precursors (▧) at day 6 and their derived CD11b−/dullCD11c+ mature DCs at days 12 to 14. Cells were first exposed to 0.1 mg/mL of FITC-DX at 0°C and 37°C, respectively, for 60 minutes. After washing twice, the cells were stained with rat–anti-mouse CD11b and biotinylated hamster–anti-mouse CD11c and then shown by PE-conjugated anti-rat Ig and CY-conjugated-streptavidin. A three-color immunofluorescence analysis was performed to show the capacity of FITC-DX uptake by these cells as indicated. *P < .01 significance compared with CD11b−/dullCD11c+ (▥) and CD11b+hiCD11c+ (□) DC precursor-derived mature DCs. Results are representative of three experiments.
CD11b+hiCD11c+ but not CD11b−/dullCD11c+ DC precursors differentiate into macrophage when stimulated with M-CSF.
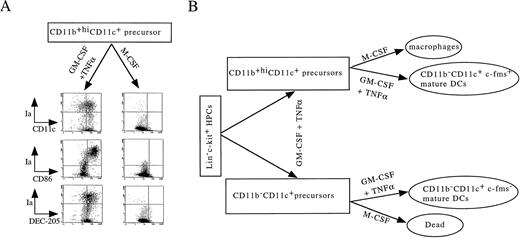
Upon stimulating with M-CSF for an additional 5 to 8 days, all of the CD11b+hiCD11c+ precursors differentiated uniformly into macrophages with numerous vacuoles and nonspecific esterase activity (Fig 3H and I, Fig 4F). These M-CSF–induced cells expressed moderate to high levels of CD11b but not Ia, CD11c, CD86, or DEC-205 molecules (Fig 9A) and were incapable of stimulating allogeneic MLR (Fig 7B). In contrast, M-CSF did not induce CD11b−/dullCD11c+ DC precursors to develop into macrophages and all of them died within 3 days in the cultures (Fig 3G). This is consistent with the fact that c-fms transcripts were selectively expressed in CD11b+hiCD11c+ DC precursors but not in CD11b−/dullCD11c+ precursors (Fig 4A). These results indicate that CD11b+hiCD11c+ precursors may have dual potential to differentiate into either mature DCs or macrophages depending on the supplemented growth factors, whereas CD11b−/dullCD11c+ precursors have already committed to DC lineage, as illustrated in Fig 9B.
The dual differentiation potential of CD11b+hiCD11c+ precursors. (A) Day 6 sorted CD11b+hiCD11c+ DC precursors from Lin−c-kit+ HPCs were cultured in presence of GM-CSF + TNF-α and M-CSF, respectively, for 5 to 8 additional days. At days 12 to 14, the phenotypes of cells were reanalyzed using double-color immunofluorecence as indicated. For double-color immunostaining, PE-conjugated anti-Ia was used, whereas biotinylated anti-CD11c and CD86 were shown by FITC-conjugated streptavidin and anti–DEC-205 was shown by FITC-labeled anti-rat IgG. The representative expression shown here is one of more than five experiments. (B) A schematic DC differentiation model in vitro from Lin−c-kit+ HPCs.
The dual differentiation potential of CD11b+hiCD11c+ precursors. (A) Day 6 sorted CD11b+hiCD11c+ DC precursors from Lin−c-kit+ HPCs were cultured in presence of GM-CSF + TNF-α and M-CSF, respectively, for 5 to 8 additional days. At days 12 to 14, the phenotypes of cells were reanalyzed using double-color immunofluorecence as indicated. For double-color immunostaining, PE-conjugated anti-Ia was used, whereas biotinylated anti-CD11c and CD86 were shown by FITC-conjugated streptavidin and anti–DEC-205 was shown by FITC-labeled anti-rat IgG. The representative expression shown here is one of more than five experiments. (B) A schematic DC differentiation model in vitro from Lin−c-kit+ HPCs.
DISCUSSION
Previous studies have shown the generation of heterogeneous mature DCs in the cultures of mouse BM hematopoietic cells.7,27,31 32However, it remains to be established whether the heterogeneous DC subsets may differentiate from a distinct precursor and/or the same precursor committed to DC at a different maturation stage. The present investigation shows that Lin−c-kit+ HPCs generate mature DCs in vitro in response to GM-CSF + TNF-α through the bifurcated DC differentiation pathways CD11b−/dullCD11c+and CD11b+hiCD11c+ DC precursors, respectively (Fig 9B). The CD11b−/dullCD11c+ DC precursors expressed the high levels of CD40, Ia, and CD86. Morphologically, the CD11b−/dullCD11c+ DC precursors were small and mostly round in shape with less cytoplasmic projections than mature DCs as shown by electron microscopy observation. These cells differentiated into mature DCs, but not other myeloid cells, and became large in size with abundant long cytoplasmic processes and multivesicules. During maturation, the endocytic capacity was reduced while the capability of stimulating the proliferation of allogeneic T cells was significantly enhanced. All these features suggest that CD11b−/dull CD11c+ DC precursors are committed DC precursors at a functionally immature stage, which makes them distinguishable from CD11b−/dullCD11c+mature DCs.
In contrast, CD11b+hiCD11c+ DC precursors expressed high levels of myeloid antigens CD11b and c-fms, but low to moderate levels of Ia and CD86, implying that they are myeloid precursor-derived cells. In response to GM-CSF and TNF-α, they differentiated into mature DCs with the phenotype of CD11b−/dullCD11c+ and obtained the expression of high levels of CD40 and other markers of mature DC. As with their precursors, these mature DCs continuously displayed abundant c-fms mRNA and nonspecific esterase activity. Interestingly, CD11b+hiCD11c+ DC precursors could differentiate into macrophages in response to M-CSF, indicating that CD11b+hiCD11c+ DC precursors may be an intermediate-stage cell population of myeloid origin rather than being restricted to DC commitment cells at the immature stage.
The observations by electron microscopy indicate that both CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors contained mitochondria and rough endoplasmic reticulum in the cytoplasm, while their mature DC offspring had well-developed Golgi apparatus, mitochondria, RER, and tubulovesicular system, particularly vesicles and multivesicular bodies near the Golgi apparatus. As previously described,27,33 the tubulovesicular system and vesicles in DCs may be an intracellular storage compartment of MHC class II molecules which has connections to the lysosomal apparatus in the same region within the cells. For successful expression of antigenic peptide-MHC class II complexes on the cell surface, the endocytosed antigen needs to be in proximity to newly synthesized class II MHC molecules in the specialized compartments of the endosome/lysosome system.34 Most recently it has been shown that DCs can modulate these parameters to control antigen presentation, and maturation of DC engages the appropriate cellular component to stimulate the trafficking of MHC class II molecules onto the cell surface.29,30,34 However, several questions remain to be clarified: (1) the molecular basis of the changes in MHC class II trafficking after forming the complexes with loaded antigens; (2) the signals responsible for constitutive membrane ruffling and endocytosis in immature DCs; and (3) the mechanisms for downregulating this response. All of these questions may be critical for designing DC-based immunotherapy.29,30,34 35 The existence of distinct differentiation pathways mediated by CD11b−/dullCD11c+ and CD11b+hiCD11c+ DC precursors implies that heterogeneous DCs might develop the capacity for driving MHC class II and antigenic peptide complexes to the cell surface in a distinct manner or at a distinct rate. Our findings may provide an important tool for elucidating these questions by using purified and well-defined differentiation or maturation stage of DC precursors based on immunophenotypings; eg, the expression of CD11b and CD11c in our system.
Based on the phenotype and differentiation potential, the DC precursor subpopulations described here likely correspond to those generated from human cord blood CD34+ HPCs as described by Caux et al.14,15 The phenotype of CD11b−/dullCD11c+ precursor-derived DCs appears identical to that of CD14−c-fms−CD1a+precursor-derived DCs. CD11b+hiCD11c+precursor-derived DC populations appear similar to CD14+c-fms+CD1a−precursor-derived DCs.14,15 Since it has been shown that CD14−c-fms−CD1a+ and CD14+c-fms+CD1a−precursor cell–derived mature DCs play in vitro differential roles in regulating cellular and humoral immune responses, respectively,14 15 our findings might be helpful for elucidating their individual biological function in vivo by using various murine models.
Furthermore, it remains unclear whether the DC populations described here correspond to those reported by Maraskovsky et al16and Pulendran et al,17 who characterized several in vivo DC subsets including CD11b−CD11c+, CD11bdullCD11c+, and CD11b+hiCD11c+ cell populations in the spleen, but only CD11b−CD11c+ DC subset in the thymus, of Flt3L-treated mice.16,17 It is noted that in Flt3L-treated mice CD11b+hiCD11c+-derived DCs, which have been considered to be of myeloid precursor origin, cannot be induced to differentiate into CD11b−CD11c+and CD11bdullCD11c+ cells in vitro by overnight culture.17 CD11b+hiCD11c+ DC precursors in our cultures can consistently not differentiate into the same mature DCs with CD11b−/dullCD11c+ DC precursor-derived mature DCs in the expression of E-cadherin, c-fms, and nonspecific esterase activity; this substantially supports the findings observed by Maraskovsky et al and Pulendran et al that various DC subsets may develop in vivo along distinct differentiation pathways.16,17 However, the DC and DC precursor subsets described here were generated in the culture system in vitro, which are apparently different from the DC subpopulations in Flt3L-treated mice in vivo.16,17 It will be difficult to directly compare at this time the DC and DC precursor subsets generated in vitro with those DC subsets developed in vivo in Flt3L-treated mice. Moreover, previous investigations have shown that CD4low thymic precursor cells can differentiate in vivo into CD8α+ lymphoid mature DCs in mice. In contrast, CD8α antigen cannot be detected on mature DCs derived in vitro from the same CD4low thymic precursor cells stimulated with various combinations of cytokines,36 37 indicating that some other unknown factor might control DC differentiation and its phenotype in vivo. It will be necessary to directly show the differentiation pathways of DC subsets from Lin−c-kit+ HPCs in vivo by taking the advantage of animal models and/or by establishing in vitro a culture system for generating lymphoid mature DCs, which may help to elucidate the relationship of DCs and their precursor subsets generated in vitro and those in vivo.
Several lines of evidence indicate that murine DCs bearing CD8α antigen are of lymphoid-precursor origin, whereas DCs expressing high levels of CD11b antigen are derived from myeloid-precursors.3-6,36,37 Furthermore, early murine thymic precursors can only differentiate into lymphoid cells and DCs, but not myeloid cells,3,36,37 suggesting that the topographic organization of heterogeneous DC may have been already determined, at least in part, at the progenitor levels.14 38-46 Therefore, further characterization of the phenotype of murine DC precursor or DC-committed progenitor cells, which account for the generation of CD11b−/dullCD11c+ and CD11b+hiCD11c+ precursors, respectively, will prove to be valuable for investigating the cellular and molecular mechanisms of DC differentiation from HPCs in vivo and in vitro.
ACKNOWLEDGMENT
We express our sincere gratitude to Dr R.M. Steinman (The Rockefeller University, New York, NY) for his kind gift of MoAbs to DEC-205 (NLDC145) and 33D1; and to Dr T. Sudo (Basic Research Institute of Toray Co, Kanagawa, Japan) for his generous gift of anti–c-kitMoAb, GM-CSF, and SCF. We also highly appreciate Dr J.J. Oppenheim (NCI-FCRDC, Frederick, MD) for his critical review of the manuscript.
Supported in part by Grants-in-Aid from the Ministry of Education, Culture, Science, and Sports of the Japanese Government.
Address reprint requests to Kouji Matsushima, MD, PhD, Department of Molecular Preventive Medicine, School of Medicine, The University of Tokyo, 7-3-1, Hongo, Bunkyoku, Tokyo 113, Japan; e-mail:koujim@m.u-tokyo.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal