The influence of graft-versus-host (GVH) reaction on the host hematopoietic cells clinically manifests itself both as adverse reactions in transfusion-associated GVH disease (GVHD) and as a therapeutic graft-versus-leukemia (GVL) effect in either donor lymphocytes transfusion (DLT) or allogeneic bone marrow (BM) transplantation. We examined the effect of GVH reaction on the host hematopoiesis in the murine parent-into-F1 (P1 → F1) model of GVHD. The systemic transfer of 5 × 107 of C57BL/6 (B6) splenocytes into (B6xDBA/2)F1 mice (BDF1), which results in acute GVHD, reduced the peripheral blood cell counts, the number of BM cells, and colony-forming unit–granulocyte macrophage (CFU-GM), whereas the injection of 108 of DBA/2 cells into BDF1, which results in chronic GVHD, did not affect hematopoiesis 2 weeks after the transfer. To clarify the mechanism of such myelosuppression, we examined the Fas expression in both hematopoietic progenitor cells as well as whole BM cells. The Fas expressions in each fraction significantly increased in BDF1 mice 2 weeks after the induction of acute GVHD, whereas no such effects were observed in the BDF1 mice with chronic GVHD. Furthermore, when such BM cells were incubated with anti-Fas antibody (Jo2), which induces apoptosis through Fas, the fraction of apoptotic cells increased and the number of CFU-GM decreased significantly. The in vivo administration of neutralizing anti-FasL antibody into BDF1 mice receiving with B6 spleen cells thus protected the host mice from BM failure. These results indicate that the functional expression of Fas on hematopoietic cells plays an essential role in the myelosuppressive effect of GVHD.
IMMUNOLOGICALLY competent cells contained in a graft can result in the immunologic recognition of the host major histocompatability complex (MHC) in the opposite direction and thus initiate a graft-versus-host (GVH) reaction.1-3 A GVH reaction to the host hematopoietic cells manifests itself as either an adverse effect or a therapeutic effect: the potential susceptibility of the host hematopoietic system to immune attack by donor lymphocytes is dramatically illustrated in the lethal marrow aplasia seen in transfusion-associated GVH disease (TA-GVHD)4-6 and the efficacy of donor lymphocyte transfusions (DLT) in irradicating large leukemic cell loads when chronic myelocytic leukemia (CML) relapse follows allogeneic bone marrow transplantation (BMT).7 Therefore, a clear understanding of the molecular mechanism of the effect of the GVH reaction on the host hematopoietic cells is of profound clinical importance. A better understanding and improved management of this GVH reaction should allow for the improved prevention and treatment of GVHD and the development of new strategies to enhance the graft-versus-leukemia (GVL) effect8 9 and to also decrease the incidence of leukemic relapse after transplantation.
The murine parent-into-F1 (P → F1) GVHD, which results from the injection of parental splenic lymphocytes into unirradiated, immune competent F1 adult hosts, has been extensively used as an experimental model of TA-GVHD.1 In murine models, the use of inbred strains of defined genetic background has allowed for the development of systems capable of reproducibly generating either acute or chronic GVHD. In the case of (C57BL/6xDBA/2)F1 mice (BDF1), the transfer of parental C57BL/6 (B6)(H-2b) spleen cells results in an acute, life-threatening GVHD,10 whereas the transfer of parental DBA/2 (D2)(H-2d) spleen cells results in a chronic GVHD syndrome with symptoms similar to those seen in systemic lupus erythematosus.11,12 Although the precise kinetics of Th1 and Th2 cytokine production during the early course of GVHD have been analysed in this model, these effects on the hematopoietic cells have not yet been fully studied.13 14
We herein examined the effect of GVHD on host hematopoiesis in this P1 → F1 model. The systemic transfer of 5 × 107 of B6 splenocytes into BDF1 mice reduced the peripheral blood (PB) cell counts, the number of BM cells, and colony-forming unit–granulocyte macrophage (CFU-GM), whereas the injection of 108 of D2 cells into BDF1 did not affect hematopoiesis 2 weeks after the transfer. To clarify the mechanism of such myelosuppression, we examined the Fas expression in both hematopoietic progenitor cells as well as in whole BM cells. The Fas expressions in each fraction significantly increased in the BDF1 mice 2 weeks after the induction of acute GVHD, whereas no such effect was observed in the BDF1 mice with chronic GVHD. Furthermore, when such BM cells were incubated with anti-Fas antibody (Jo2), which is known to induce apoptosis through Fas, the fraction of apoptotic cells increased and the number of CFU-GM decreased significantly. Both the in vivo administration of neutralizing anti-FasL antibody into BDF1 mice receiving B6 spleen cells and the transfer of spleen cells from FasL-defective (gld) B6 mice into BDF1 mice protected the host mice from BM failure. Therefore, these results indicate that the functional expression of Fas on hematopoietic cells thus plays an essential role in the myelosuppressive effects of GVHD.
MATERIALS AND METHODS
Mice.
The female mice of an inbred C57BL/6 (B6) (H-2b), DBA/2 (D2) (H-2d), and B6 x DBA F1 (BDF1) strain were obtained from Nippon Clea Inc (Tokyo, Japan). B6-gld (generalized lymphoproliferative disease) (H-2b) mice were purchased from Jackson Laboratory (Bar Harbor, ME). These mice were maintained in specific pathogen-free conditions. Six- to 8-week-old mice were used for all experiments. All animals were handled in accordance with the guidelines established by the Animal Experimentation Committee of Tokai University School of Medicine. Four or five mice per group were used for each experiment.
Induction of GVHD.
Single-cell suspensions were prepared in an RPMI 1640 medium (GIBCO-BRL, Grand Island, NY) from the spleens of B6 and D2 mice. The cell suspensions were passed through sterile nylon mesh, then were washed and diluted to a cell concentration of 25 × 107 and 50 × 107/mL for acute and chronic GVHD induction, respectively. Acute and chronic GVHD were induced by injecting 5 × 107 B6 spleen cells and 1 × 108 DBA spleen cells into the BDF1 mice.13 14Age-matched uninjected BDF1 mice were used as control mice.
PB cell counts.
Blood samples were obtained by means of an intracardiac puncture after anesthetization with ether. Samples were analyzed by an Automated Hematology Analyzer (NE8000; Toa Medical Electronics Corp, Kobe, Japan).
BM cell collection.
Suspensions of BM cells were obtained by flushing both the femurs and tibias with phosphate-buffered saline (PBS).15 The suspensions were passed through nylon mesh and then were treated with NH4Cl to lyse red blood cells. The cells were resuspended with PBS and the cell viability was assessed by trypan blue exclusion. All samples showed more than a 90% cell viability.
In vitro colony assay.
A CFU-GM assay was performed in a methylcellulose culture using the METHOCULT M3430 kit (Stem Cell Technologies, Vancouver, Canada). Briefly, 1 mL culture medium contained 105 of BM cells, 0.9% methylcellulose, 1% bovine serum albumin, 30% fetal bovine serum, 0.1 mmol/L 2-mercaptoethanol, 2 mmol/L L-glutamine, 3 U erythropoietin, and 2% pokeweed mitogen-stimulated spleen cell–conditioned medium. The culture medium was prepared in a 35-mm petri dish (Becton Dickinson, San Jose, CA) and incubated at 37°C in a 5% CO2 atmosphere. The number of colonies was counted after 14 days of culture using an inverted microscope.
Cell staining for flow cytometry.
Briefly, BM cells were suspended with PBS containing 2% fetal calf serum (FCS; GIBCO-BRL) and 0.1% NaN3, and incubation for cell staining was performed on ice for 30 minutes. Regarding the detection of lineage markers, rat monoclonal antibodies (MoAbs) recognizing Mac-1 (M1/70), Gr-1 (RB6-8C5), B220 (RA3-6B2), and an erythroid marker (TER119) and fluorescein isothiocyanate (FITC)-conjugated MoAb for murine CD3 (145-2C11, hamster IgG) were used. Antibodies for Mac-1, Gr-1, and B220 were purchased from Pharmingen (San Diego, CA), and TER119 was kindly provided by Dr Tatsuo Kina (Kyoto University, Kyoto, Japan). FITC-conjugated anti-rat IgG polyclonal antibody (Jackson Immuno Research Laboratories Inc, West Grove, PA) was used as a secondary antibody for the detection of lineage markers. To assess the expression of Fas, hamster anti-Fas MoAb (Jo2; Pharmingen, San Diego, CA) conjugated with phycoerythrin (PE) was used. To detect hematopoietic progenitor cells, biotinylated anti–c-kit (2B8; Pharmingen) was used. To assess the expression of Fas on lineage marker negative (Lin−) c-kit positive (c-kit+) cells, the cells stained with anti-lineage markers were further stained with a biotinylated anti–c-kitand PE-conjugated anti-Fas antibodies followed by the incubation with streptavidin-RED670 (GIBCO-BRL). After the Lin−population was gated, the expressions of c-kit and Fas were observed by detecting RED670 and PE, respectively.
Staining for the detection of apoptosis.
To assess the cells undergoing apoptosis, the relative DNA content was observed as previously described, with some modifications.15 After permeabilization with 0.1% NP-40 (Sigma, St Louis, MO), the cells were treated with 0.05 mg/mL RNaseA (Sigma) for 30 minutes at 37°C. After washing, the cells were suspended in 0.5 mL of propidium iodide (PI) solution (50 mg/mL of PI with 0.1% Na citrate) and then were incubated for 30 minutes at 4°C. The cells in a discrete subpopulation of signals under the G0/G1 cell cycle region (subdiploid cells) were designated as cells undergoing apoptosis.
Flow cytometry analysis.
Flow cytometry was performed using a FACScan (Becton Dickinson) equipped with three filters including a 530-nm band-pass (for FITC), a 585-nm band-pass (for PE and PI), and a 650-nm long-pass filter (for RED670). The data were analyzed on the LYSIS II program (Becton Dickinson). To analyze the cells stained with PI, the coefficient variance of PI-stained thymocytes from an uninfected BDF1 mouse was confirmed to be under 2% on a linear scale before the experiments.
Treatment of BM cells in vitro with anti-Fas MoAb.
BM cells were suspended in an RPMI 1640 medium (GIBCO-BRL) supplemented with 30% FCS in a 12-well plate (Iwaki Glass, Tokyo, Japan) at a density of 1 × 106 cells/mL (2 mL/well) and incubated for 18 hours with hamster anti-mouse Fas antigen MoAb (Jo2; Pharmingen) at a concentration of 1 μg/mL.16 Incubation was performed under a humidified 5% CO2 atmosphere at 37°C. The ratio of the cells undergoing apoptosis was then determined by flow cytometry as described above.
Ultrastructural studies.
BM cells were fixed with 2.5% glutaraldehyde in sodium phosphate buffer, pH 7.2, for 1 hour, postfixed in 1% osmium tetroxide, dehydrated, and embedded in Quetol-512 (Nisshin EM, Tokyo, Japan) according to routine techniques. Thin sections were mounted on copper grids and examined by transmission electron microscope (JEOL 1200 EX; JEOL, Tokyo, Japan) after staining with uranyl acetate and lead solution.
In vivo administration of anti-Fas ligand antibodies.
Intraperitoneally, five mice received 500 μg/body of MoAb against Fas ligand (MFL1) on day 0, 1, 4, 7, and 10 after spleen cell transfer.17 As a control, the same amounts of rat IgG1 (Pharmingen) were intraperitoneally injected into mice.
Enzyme-linked immunosorbent assay (ELISA).
Mouse tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) in the sera were evaluated with commercial ELISA (Amersham Life Science, Arlington Heights, IL) according to the manufacturer's instruction. The results from four mice were expressed as the mean ± SEM.
Statistical analysis.
A comparison of the mean values of the parameters was done using the two-group paired t-test.
RESULTS
PB cell counts, BM cellularity, and CFU-GM after GVHD induction.
On day 14 after acute GVHD induction by B6 spleen cells, peripheral blood cells significantly decreased (Table1). The reduction of blood cells was not obvious on day 7 (data not shown). In contrast, the induction of chronic GVHD by D2 spleen cells had no such a pancytopenic effect on day 14. BM cellularity also decreased on day 14 in the BDF1 mice receiving B6 spleen cells but not D2 spleen cells. To further assess the status of functional progenitor cells, we did a CFU-GM assay using BM cells on day 14 and found the reduction of CFU-GM in BDF1 mice receiving B6 spleen cells but not D2 spleen cells. This reduction of BM cellularity and the number of GFU-GM was not obvious on day 7 (data not shown). These findings indicated that the myelosuppression evolved in the second week of acute GVHD but not chronic GVHD in this model.
Effects of GVHD on the PB Cells, BM Cellularity, and CFU-GM
| . | PB Cell Counts . | Total No. of BM Cells (×106) . | CFU-GM/105 . | ||
|---|---|---|---|---|---|
| Total Leukocyte (/μL) . | Hematocrit (%) . | Platelet (×104/μL) . | |||
| BDF1 | 3,150 ± 45 | 54.1 ± 3.0 | 69.2 ± 7.4 | 34.1 ± 1.7 | 53.8 ± 10.3 |
| B6/BDF1 | 2,160 ± 307-150 | 37.6 ± 0.4-150 | 23.4 ± 8.7-150 | 6.2 ± 1.8-150 | 29.7 ± 7.2-150 |
| D2/BDF1 | 3,433 ± 553 | 51.0 ± 6.2 | 62.3 ± 5.7 | 36.0 ± 7.4 | 54.6 ± 6.4 |
| . | PB Cell Counts . | Total No. of BM Cells (×106) . | CFU-GM/105 . | ||
|---|---|---|---|---|---|
| Total Leukocyte (/μL) . | Hematocrit (%) . | Platelet (×104/μL) . | |||
| BDF1 | 3,150 ± 45 | 54.1 ± 3.0 | 69.2 ± 7.4 | 34.1 ± 1.7 | 53.8 ± 10.3 |
| B6/BDF1 | 2,160 ± 307-150 | 37.6 ± 0.4-150 | 23.4 ± 8.7-150 | 6.2 ± 1.8-150 | 29.7 ± 7.2-150 |
| D2/BDF1 | 3,433 ± 553 | 51.0 ± 6.2 | 62.3 ± 5.7 | 36.0 ± 7.4 | 54.6 ± 6.4 |
The BDF1 mice were injected with either 5 × 107 of B6 spleen cells (B6/BDF1) or 9 × 107 of D2 cells (D2/BDF1). The PB cell counts, BM cellularity, and CFU-GM were examined on day 14 after transfer. The results from three to five mice were expressed as the mean ± SEM.
Significant decrease versus the uninjected control BDF1 mice (P < .01).
Induction of apoptosis and Fas expression on the BM cells by GVHD.
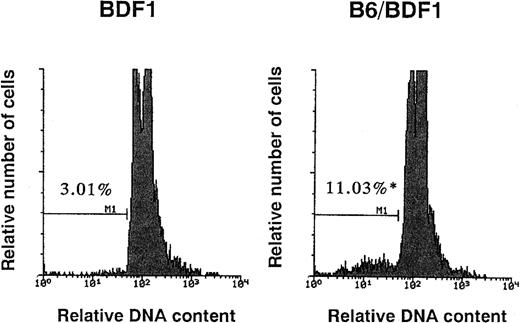
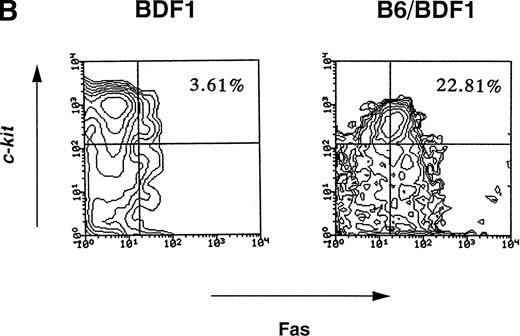
Fas-mediated apoptosis has been reported to be associated with myelosuppression in various pathological conditions.15,18-20 Apoptosis was examined by staining with PI and the percentage of cells undergoing apoptosis significantly increased in the BM cells from mice with acute GVHD, from 3.21% ± 1.02% to 10.26% ± 2.02% (P < .05, Fig 1). The expression of Fas on hematopoietic progenitor cells as well as whole BM cells and cells of each lineages were thus examined on days 7, 10, and 14. The representative patterns of Fas expression on day 14 are shown in Fig 2. The Fas expressions on whole BM cells significantly increased by acute GVHD, whereas no such effects were observed in chronic GVHD (Fig 2A). The Fas expressions on Lin−c-kit+ fraction of BM cells, which represent hematopoietic progenitor cells,21 was also upregulated by acute GVHD (Fig 2B). As summarized in Table 2, upregulation of Fas expression on hematopoietic progenitor cells was significant on days 10 and 14. Induction of Fas expression was accompanied with the reduction of the total number of hematopoietic progenitor cells. Both upregulation of Fas and the reduction of progenitor cells were not significant on day 7 (data not shown). The Fas expressions in Gr-1+, Mac-1+, B220+, and Ter-119+fractions were also increased by acute GVHD (data not shown). These data suggest that the Fas-mediated apoptosis of hematopoietic cells including progenitor cells was thus involved in the myelosuppression induced by acute GVHD.
The detection of cells undergoing apoptosis in BM. The mice were injected with 5 × 107 of B6 spleen cells. On day 14 after transfer, the mice were killed to remove their BM cells. Using flow cytometry, the signals of PI, which represent the DNA contents, were examined. The G0/G1 peak was adjusted at channel 100 and subdiploid cells were designated as cells undergoing apoptosis. The numbers in the figure represent the percentage of cells undergoing apoptosis. Four mice were examined in each group and the representative data were shown. *Significant increases versus uninjected control (P < .05).
The detection of cells undergoing apoptosis in BM. The mice were injected with 5 × 107 of B6 spleen cells. On day 14 after transfer, the mice were killed to remove their BM cells. Using flow cytometry, the signals of PI, which represent the DNA contents, were examined. The G0/G1 peak was adjusted at channel 100 and subdiploid cells were designated as cells undergoing apoptosis. The numbers in the figure represent the percentage of cells undergoing apoptosis. Four mice were examined in each group and the representative data were shown. *Significant increases versus uninjected control (P < .05).
(A) The expression level of Fas antigen on whole BM cells. The mice were injected with 5 × 107 of B6 spleen cells (B6/BDF1) or 1 × 108 of D2 cells (D2/BDF1). On day 14 after transfer, the mice were killed to remove their BM cells. Using flow cytometry, the expression level of Fas on these cells was examined. BDF1 represents the uninjected control mice. Four mice were examined in each group and the representative data were shown. (B) The expression level of Fas on hematopoietic progenitor cells. The expression level of c-kit and Fas on the BM cells obtained on day 14 after the transfer of B6 cells (B6/BDF1) was examined after the Lin− populations were gated. The numbers in the figure represent the percentage of c-kit+Fas+ cells in the Lin− cell populations. BDF1 represents the uninjected control mice. Twelve mice were examined in both groups and the representative data were shown.
(A) The expression level of Fas antigen on whole BM cells. The mice were injected with 5 × 107 of B6 spleen cells (B6/BDF1) or 1 × 108 of D2 cells (D2/BDF1). On day 14 after transfer, the mice were killed to remove their BM cells. Using flow cytometry, the expression level of Fas on these cells was examined. BDF1 represents the uninjected control mice. Four mice were examined in each group and the representative data were shown. (B) The expression level of Fas on hematopoietic progenitor cells. The expression level of c-kit and Fas on the BM cells obtained on day 14 after the transfer of B6 cells (B6/BDF1) was examined after the Lin− populations were gated. The numbers in the figure represent the percentage of c-kit+Fas+ cells in the Lin− cell populations. BDF1 represents the uninjected control mice. Twelve mice were examined in both groups and the representative data were shown.
Effects of GVHD on the Number of Hematopoietic Progenitor Cells and Fas Expression
| . | n . | Days After Transfer . | No. of Lin−c-kit+ Cells . | c-kit+Fas+ in Lin− . | ||
|---|---|---|---|---|---|---|
| ×104 . | P Value . | % . | P Value . | |||
| Exp 1 | ||||||
| BDF1 | 4 | — | 52.8 ± 4.5 | — | 3.1 ± 0.4 | — |
| B6/BDF1 | 4 | 14 | 12.5 ± 1.2 | .0009 | 20.8 ± 4.0 | .0035 |
| Exp 2 | ||||||
| BDF1 | 4 | — | 53.7 ± 2.0 | — | 2.6 ± 0.3 | — |
| B6/BDF1 | 4 | 14 | 12.6 ± 2.3 | .0048 | 24.2 ± 2.5 | .0005 |
| Exp 3 | ||||||
| BDF1 | 4 | — | 56.1 ± 3.3 | — | 2.8 ± 0.2 | — |
| B6/BDF1 | 4 | 14 | 7.5 ± 1.5 | .0001 | 20.8 ± 2.7 | .0015 |
| D2/BDF1 | 4 | 14 | 57.9 ± 5.7 | .4248 | 1.9 ± 0.6 | .1159 |
| Exp 4 | ||||||
| BDF1 | 5 | — | 55.2 ± 0.8 | — | 2.5 ± 0.2 | — |
| B6/BDF1 | 5 | 10 | 33.8 ± 2.9 | .0029 | 7.9 ± 1.7 | .0026 |
| . | n . | Days After Transfer . | No. of Lin−c-kit+ Cells . | c-kit+Fas+ in Lin− . | ||
|---|---|---|---|---|---|---|
| ×104 . | P Value . | % . | P Value . | |||
| Exp 1 | ||||||
| BDF1 | 4 | — | 52.8 ± 4.5 | — | 3.1 ± 0.4 | — |
| B6/BDF1 | 4 | 14 | 12.5 ± 1.2 | .0009 | 20.8 ± 4.0 | .0035 |
| Exp 2 | ||||||
| BDF1 | 4 | — | 53.7 ± 2.0 | — | 2.6 ± 0.3 | — |
| B6/BDF1 | 4 | 14 | 12.6 ± 2.3 | .0048 | 24.2 ± 2.5 | .0005 |
| Exp 3 | ||||||
| BDF1 | 4 | — | 56.1 ± 3.3 | — | 2.8 ± 0.2 | — |
| B6/BDF1 | 4 | 14 | 7.5 ± 1.5 | .0001 | 20.8 ± 2.7 | .0015 |
| D2/BDF1 | 4 | 14 | 57.9 ± 5.7 | .4248 | 1.9 ± 0.6 | .1159 |
| Exp 4 | ||||||
| BDF1 | 5 | — | 55.2 ± 0.8 | — | 2.5 ± 0.2 | — |
| B6/BDF1 | 5 | 10 | 33.8 ± 2.9 | .0029 | 7.9 ± 1.7 | .0026 |
The BDF1 mice were injected with 5 × 107 of B6 spleen cells (B6/BDF1) or 1 × 108 of D2 cells (D2/BDF1). The number of Lin−c-kit+ cells and c-kit+Fas+ fraction of Lin− cells were examined on day 10 or 14 after transfer of spleen cells. The results from four to five mice were expressed as the mean ± SEM. P value were calculated when compared with uninjected control BDF1 mice.
Induction of apoptosis by anti-Fas MoAb in vitro.
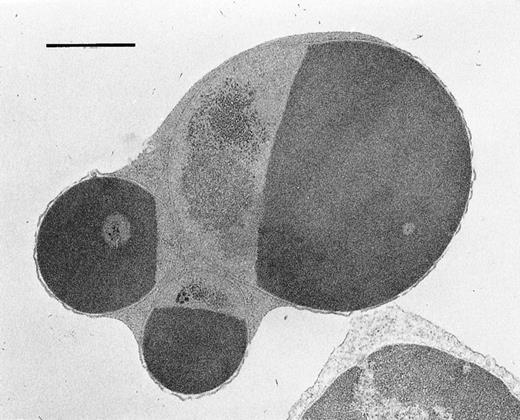
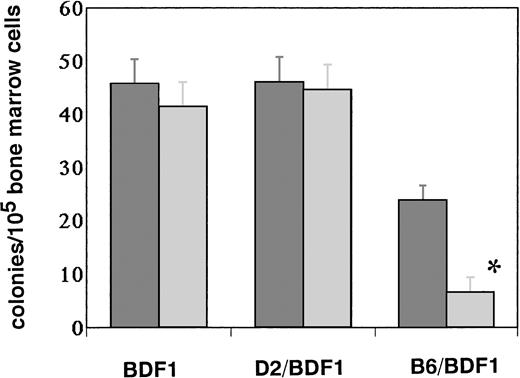
To confirm whether the expressed Fas on hematopoietic progenitor cells is functional or not, we examined the effects of apoptosis-inducing anti-Fas antibody (Jo2) on BM cells in vitro.16 On day 14 after GVHD induction, BM cells were obtained and incubated with 1 μg/mL of Jo2 in vitro. After 18 hours of incubation, the percentage of cells undergoing apoptosis significantly increased in the BM cells from BDF1 mice receiving B6 spleen cells, from 7.25% ± 0.63% to 14.83% ± 2.22% (P < .05) . In contrast, no such an increase was observed in the BM cells from the normal control mice (data not shown). Apoptosis was further confirmed by the electron microscopic feature of cells with chromatin condensation in the presence of plasma membrane integrity (Fig3). To assess the number of functional progenitor cells, a CFU-GM assay was performed using BM cells incubated with Jo2 for 18 hours in vitro (Fig 4). CFU-GM significantly decreased after treatment with Jo2 in the mice with acute GVHD, but no such effect was observed in the control BDF1 mice or mice with chronic GVHD. These data confirmed that the induced expression of Fas in the hematopoietic cells in acute GVHD was functional in vitro.
Electron microscopic appearance of BM cells after treatment with anti-Fas antibody (Jo2) . Cells were cultured for 18 hours in the presence of Jo2. (Original magnification × 13,500.)
Electron microscopic appearance of BM cells after treatment with anti-Fas antibody (Jo2) . Cells were cultured for 18 hours in the presence of Jo2. (Original magnification × 13,500.)
The effects of anti-Fas antibody (Jo2) treatment on CFU-GM. The mice were injected with 5 × 107 of B6 spleen cells (B6/BDF1) or 1 × 108 of D2 cells (D2/BDF1). On day 14 after transfer, the mice were killed to remove their BM cells. The cells were treated with 1 mg/mL of Jo2 antibody for 18 hours. A CFU-GM assay was performed in methylcellulose culture containing 105 BM cells. The number of colonies was then counted after 14 days of culture. The results from four mice were expressed as the mean ± SEM. (▧), αFas(−); (▧), αFas(+). *Significant decrease versus the cells incubated without anti-Fas antibody (P < .01).
The effects of anti-Fas antibody (Jo2) treatment on CFU-GM. The mice were injected with 5 × 107 of B6 spleen cells (B6/BDF1) or 1 × 108 of D2 cells (D2/BDF1). On day 14 after transfer, the mice were killed to remove their BM cells. The cells were treated with 1 mg/mL of Jo2 antibody for 18 hours. A CFU-GM assay was performed in methylcellulose culture containing 105 BM cells. The number of colonies was then counted after 14 days of culture. The results from four mice were expressed as the mean ± SEM. (▧), αFas(−); (▧), αFas(+). *Significant decrease versus the cells incubated without anti-Fas antibody (P < .01).
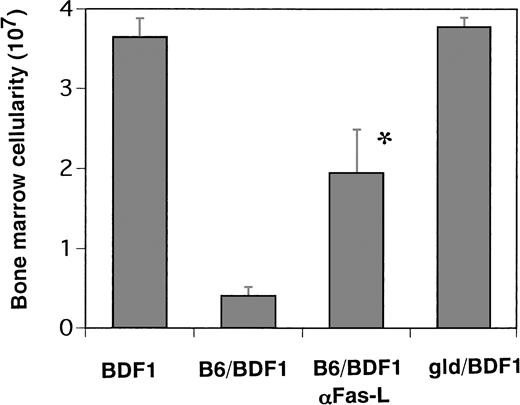
In vivo administration of anti-FasL antibody or transfer of spleen cells from FasL-defective (gld) B6 mice protect the recipients from myelosuppression induced by GVHD.
To further confirm the role of Fas-mediated apoptosis in vivo, we examined the effects of the administration of the neutralizing anti-FasL antibody (MFL1) on the myelosuppression induced by GVHD. On day 14 after acute GVHD induction, the total number of BM cells in the mice treated with MFL1 was significantly higher than that in the control IgG-treated mice with GVHD, although the number of cells significantly decreased in comparison to the normal control mice (Fig 5). Furthermore, spleen cells from FasL-defective (gld) B6 mice of 5 week of age, which induced severe GVHD-associated cachexia but only minimal signs of hepatic and cutaneous GVHD pathology in recipient mice,22 were transferred into the BDF1 mice and myelosuppression was found to be completely protected in these mice (Fig 5). Thus, these data confirmed the essential role of Fas-mediated apoptosis in BM aplasia associated with GVHD.
The effects of anti-Fas ligand antibody administration in vivo and the transfer of Fas ligand defective B6 spleen cells. The mice were received 500 μg/body of MoAb against Fas ligand on days 0, 1, 4, 7, and 10 after B6 spleen cell transfer (B6/BDF1 αFas-L). The mice were injected with 5 × 107 of FasL-defective (gld) B6 spleen cells (gld/BDF1) or B6 cells (B6/BDF1). On day 14 after transfer, the mice were killed to remove their BM cells. The total cell numbers from five mice were expressed as the mean ± SEM. BDF1 represents the control mice injected with the same amounts of rat IgG1 intraperitoneally on the same schedule as mentioned above. *Significant increase versus B6/BDF1 (P < .01).
The effects of anti-Fas ligand antibody administration in vivo and the transfer of Fas ligand defective B6 spleen cells. The mice were received 500 μg/body of MoAb against Fas ligand on days 0, 1, 4, 7, and 10 after B6 spleen cell transfer (B6/BDF1 αFas-L). The mice were injected with 5 × 107 of FasL-defective (gld) B6 spleen cells (gld/BDF1) or B6 cells (B6/BDF1). On day 14 after transfer, the mice were killed to remove their BM cells. The total cell numbers from five mice were expressed as the mean ± SEM. BDF1 represents the control mice injected with the same amounts of rat IgG1 intraperitoneally on the same schedule as mentioned above. *Significant increase versus B6/BDF1 (P < .01).
Serum levels of IFN-γ and TNF-α.
Recently, the Fas expression on hematopoietic progenitor cells was reported to be induced by cytokines such as IFN-γ and TNF-α.18,19 Both cytokines have also been shown to play pivotal roles in the pathogenesis of acute GVHD.13,14 23In this study, elevated levels of both IFN-γ(23.0 ± 1.5 ng/mL) and TNF-α (66.0 ± 10.3 pg/mL) were observed in the sera from acute GVHD mice on day 10. In contrast, serum IFN-γ and TNF-α were not detectable (<10 pg/mL) in control mice. These data suggested that IFN-γ or TNF-α might mediate the Fas expression on hematopoietic progenitor cells in acute GVHD.
DISCUSSION
We showed myelosuppression characterized by pancytopenia and decreased BM cellurarity and CFU-GM in the BDF1 mice receiving B6 spleen cells but not those of D2 at 2 weeks after parental cell transfer. The injection of B6 lymphocytes into immunocompetent BDF1 hosts results in acute GVHD characterized by anti-host cytotoxic T-lymphocyte (CTL) development.1,10 In contrast, the injection of lymphocytes from the opposite parent, D2, into the same F1 host results in chronic GVHD characterized by B-cell hyperactivity, autoantibody formation, and a lupuslike disease.11,12Therefore, the myelosuppression was characteristic feature of acute GVHD in this model. Although acute and chronic GVHD are strikingly different entities at 2 weeks of disease, both forms of GVHD evolve from a common starting point, ie, the donor CD4+ T-cell recognition of host alloantigen and interleukin-2 (IL-2) production.1 A precise analysis of the kinetics of cytokine production shows that both forms of GVHD are initially characterized by increased T-helper 2 (Th2) cytokines (IL-4 and IL-10) production and the earliest distinguishing features of acute GVHD are detectable at days 5 through 7 of the disease at which time no myelosuppression was yet detected.13 14 Therefore, the development of myelosuppression closely paralleled the evolution of acute GVHD.
The recipient hematopoietic system is reported to be the most sensitive target of GVHD in that it is affected by spleen cell doses that do not apparently damage other target organs in the P → F1 GVHD model with class I and II disparities.24-26 In particular, Sprent et al27 reported that the transfer of small doses of purified unprimed CD4+ cells from a donor was able to cause marrow aplasia in lightly irradiated (600 cGy) recipients expressing major histocompatibility complex class II differences. When class II–deficient knockout mice were used as recipients in this experiment, no atrophy of the host marrow occurred, thus demonstrating the class II-mediated inhibition of hematopoiesis.27 Therefore, the allospecific CD4+ CTL induced from the B6 graft but not from the D2 graft would undoubtedly be the prime effector attacking the BM cells in the recipient BDF1 mice. Apoptosis was reported to be involved in class II–mediated inhibition of hematopoiesis in long-term marrow cultures.28 In addition to these, however, we also showed that the induced expression of functional Fas on the hematopoietic cells was also associated with the effect of GVH reaction on hematopoiesis.
The role of the Fas-FasL pathway to induce apoptosis is established in the primary target organs of acute GVHD—skin and liver—where Fas is constitutively expressed: T cells from FasL-defective (gld) and perforin-deficient donor mice were transplanted into lethally irradiated MHC-mismatched allogeneic recipients mice to determine the role of these cytotoxic pathways in acute GVHD, and Fas-mediated cytotoxicity was thus shown to play an essential role in the pathophysiology of hepatic and cutaneous GVHD.22,29Both liver and skin tissue is known to express Fas constitutively,30 and mice injected with the anti-Fas MoAb Jo2 rapidly develop fulminant lethal hepatitis.16 But hematopoietic progenitor cells do not express Fas constitutively and are not sensitive to FasL mimicking stimulation by anti-Fas antibody, Jo2, as shown in Fig 4.31 Therefore, the induced expression of Fas on the hematopoietic cells including progenitor cells is considered to play an essential role in the GVH-associated myelosuppression in this model.
Recently, the Fas expression on hematopoietic progenitor cells and its role in apoptosis in vitro has been reported.18,19 We already reported the significance of Fas-mediated apoptosis of hematopoietic progenitor cells in myelosuppression induced by a murine CMV (MCMV) infection in vivo.15 We showed herein that the induction of Fas on the hematopoietic progenitors is also required in the effect of GVHD on hematopoiesis.
The precise mechanism for the induction of Fas on hematopoietic progenitor cells still needs to be determined, whereas we showed elevated levels of IFN-γ and TNF-α in mice with acute GVHD. The predominant cytokines produced in acute GVHD are Th1 cytokines, IL-2 and IFN-γ, in response to host antigens, whereas those in chronic GVHD are Th2.13,14,32 IFN-γ production was reported to increase in the B6 → BDF1 mice but not in the D2 → BDF1 mice.13,14 On the other hand, we reported that D2 → BDF1 mice with the systemic administration of IL-12 developed acute GVHD with the generation of antihost CTL.33 We examined the effects of the systemic administration of anti–IL-12 or anti–IFN-γ antibody into BDF1 mice with acute GVHD (manuscript in preparation). Both antibodies showed a decreased expression of Fas on hematopoietic cells and also protect the suppression of hematopoiesis. These results indicate that IL-12 and IFN-γ were associated with the functional induction of Fas on hematopoietic cells in acute GVHD. TNF-α is another candidate for the mediater of Fas induction because it has been implicated in the pathogenesis of GVHD.23 Further studies including in vivo administration of anti–TNF-α neutralizing antibody are needed to determine the precise mechanism for the induction of Fas on hematopoietic progenitor cells.
These findings might have important clinical implications, especially regarding the treatment of CML. IFN-α was shown to enhance the expression of Fas on CML progenitor cells and also rendered them more susceptible to apoptosis induced by the agonistic anti-Fas antibody.34 On the other hand, CML is the most susceptible type of hematologic neoplasm when donor lymphocytes are administered.7 Further studies to elucidate the mechanism of inducing Fas in hematopoietic cells and its difference between normal cells and leukemic cells is thus expected to produce tremendous benefits in the field of leukemia and lymphoma therapy.
ACKNOWLEDGMENT
We thank Drs A. Akatsuka and M. Tokunaga for expert techinical assistance in ultrastructural studies and A. Kanemura for preparation of mice.
Supported in part by Grant No. JSPS-RFTF97I00201 from the Japan Society for the Promotion of Science and the Science Frontier Program of MESSC.
Address reprint requests to Kiyoshi Ando, MD, Division of Hematology, Department of Internal Medicine, Tokai University School of Medicine, Bohseidai, Isehara, Kanagawa, 259-1193 Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal