Abstract
Activation of cyclic adenosine monophosphate (cAMP)-dependent protein kinase (A-kinase) promotes hemoglobin synthesis in several erythropoietin-dependent cell lines, whereas A-kinase–deficient murine erythroleukemia (MEL) cells show impaired hemoglobin production; A-kinase may regulate the erythroid transcription factor NF-E2 by directly phosphorylating its p45 subunit or by changing p45 interactions with other proteins. We have mapped the major A-kinase phosphorylation site of p45 to Ser169; Ala substitution for Ser169 resulted in a protein that was no longer phosphorylated by A-kinase in vitro or in vivo. The mutant protein formed NF-E2 complexes that bound to DNA with the same affinity as wild-type p45 and functioned normally to restore β-globin gene expression in a p45-deficient MEL cell line. Transactivation properties of the (Ser169 → Ala) mutant p45 were also indistinguishable from wild-type p45 when Gal4-p45 fusion constructs were tested with a Gal4-dependent reporter gene. Transactivation of the reporter by both mutant and wild-type p45 was significantly enhanced when A-kinase was activated by membrane-permeable cAMP analogs or when cells were cotransfected with the catalytic subunit of A-kinase. Stimulation of p45 transactivation by A-kinase required only the N-terminal transactivation domain of p45, suggesting that A-kinase regulates the interaction of p45 with downstream effectors.
NF-E2 IS A BASIC LEUCINE zipper (bzip) transcription factor consisting of a hematopoietic cell-specific subunit, p45, and a ubiquitously expressed subunit that is one of the small Maf proteins (p18/MafK or MafG).1-4 NF-E2 binds to the consensus sequence TGCTGA(G/C)TCA(T/C) found in several erythroid-specific promoters and in the α- and β-globin locus control regions (LCRs).1,5,6 The overall stimulatory activity of the LCRs depends on the presence of intact NF-E2 binding sites; an essential role for NF-E2 in remodeling of chromatin structure, disruption of nucleosomes, and transcriptional activation of globin genes has been recently demonstrated.6-9 Variant murine erythroleukemia (MEL) cells lacking p45 expression due to proviral integration in one allele and loss of the other allele show a marked reduction of globin gene expression, which is partially restored by reintroducing p45.9,10 Transgenic mice lacking p45 show surprisingly mild changes in erythropoiesis with normal globin switching, suggesting the presence of factors compensating for the lack of p45 in erythroid precursors; however, thrombopoiesis is severely impaired in these animals.11,12 Based on their ability to bind to the NF-E2 recognition sequence, several p45-related proteins have been identified, including Nrf1/LCR-F1 and Nrf2.13-15These proteins show significant homology with p45 in the bzip and surrounding region, but are otherwise distinct; they are strong transcriptional activators expressed in a wide variety of tissues, including erythroid cells.13-15 However, despite their ability to bind to NF-E2 recognition sites, LCR-F1 and Nrf2 cannot functionally replace p45 in MEL cells.10 16
In MEL cells, induction of erythroid differentiation is associated with transcriptional activation of erythroid-specific genes and increased DNA-binding activity of NF-E2.17-19 We found that expression of erythroid-specific genes, activity of the α-globin LCR and NF-E2/DNA complex formation are impaired in cyclic adenosine monophosphate (cAMP)-dependent protein kinase (A-kinase)–deficient MEL cells.18 Prolonged activation of A-kinase in normal MEL cells increases the amount of NF-E2/DNA complexes without significantly changing the expression of p45 or p18.18 The p45 NF-E2 subunit is efficiently phosphorylated by A-kinase in vitro18; thus, A-kinase could regulate NF-E2 through direct phosphorylation and/or through the regulation of other factors. The present work was undertaken to more clearly define the role of A-kinase in regulating p45 NF-E2. We generated a mutant form of p45 that was no longer phosphorylated by A-kinase; this mutant was indistinguishable from wild-type p45 with respect to DNA binding and transactivation properties. We demonstrated that A-kinase strongly enhanced the transactivation potential of wild-type or mutant p45, an effect that only required the N-terminal transactivation domain of p45. The transcriptional coactivator CREB-binding protein (CBP) and the TATA-binding protein-associated factor TAFII130 have been recently shown to bind to the N-terminus of p458,20; however, we found no significant effect of CBP on p45 transactivation. Our results suggest that A-kinase stimulates p45 transactivation by changing the interaction(s) of the p45 transactivation domain with other transcriptional regulatory protein(s). The regulation of p45 by A-kinase described in this work provides a possible mechanism whereby cAMP can potentiate erythropoietin-induced erythroid differentiation.21-23
MATERIALS AND METHODS
Plasmid constructs and site-directed mutagenesis.
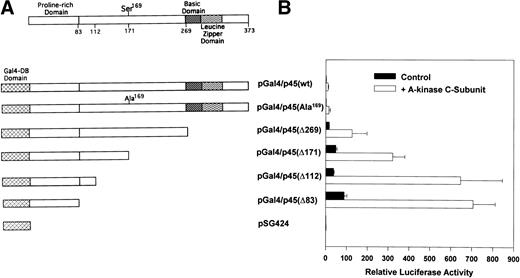
The complete murine p45 cDNA clone in pBluescriptKS was provided by N. Andrews.1 To replace Ser169 with Ala, we performed site-directed mutagenesis using polymerase chain reaction (PCR) and the principle of unique site elimination.24 The mutagenesis primer was 5′-GCGGAGGGCCGAGTACGTCGACATGTAC-3′ and a second silent mutation was included to introduce a new Sal I site; the selection primer was 5′-GCCGCTCTAGAACGCGTGGATCCCCC-3′, changing the unique Spe I site in the pBluescriptKS polylinker toMlu I. The mutant p45 plasmid was sequenced completely to confirm the presence of the desired mutation and to exclude the introduction of other unwanted mutations. To produce pMT2-p45(wt) and (mut), the wild-type or mutant p45 cDNAs were cloned downstream of the adenovirus major late promoter into the EcoRI site of the vector pMT2.25 For stable transfection of CB3 cells, the wild-type and mutant p45 cDNAs were cloned downstream of the chicken β-actin promoter of pRC/β-Act using HindIII and XbaI; pRC/β-Act contains a neor transcription unit to confer G418 resistance.26 To test the transactivation properties of p45 independently of its DNA binding properties, the N-terminus of wild-type and mutant p45 was fused in-frame to the DNA-binding domain of the yeast transcription factor Gal4 in the vector pSG424 (provided by M. Ptashne27). The resulting vectors pGal4/p45(wt) and pGal4/p45(mut) were sequenced across the Gal4-p45 fusion; Western blots developed with a p45-specific antibody (Santa Cruz Biotechnology, Santa Cruz, CA) detected full-length p45-Gal4 fusion proteins expressed in baby hamster kidney (BHK) cells (data not shown). Stepwise truncation of p45 from the C-terminus was performed by digesting pGal4/p45(wt) with Xba I plus Sal I, Stu I, or Sac I; the vector containing the remaining portion of p45 was blunted and religated, generating pGal4/p45 (Δ269), pGal4/p45(Δ112), and pGal4/p45(Δ83) (see Fig 5; the numbers in parentheses designate the number of N-terminal amino acids of p45 present in the construct). Digestion of pGal4/p45(mut) with Xba I plusSal I removed two C-terminal fragments because of the newSal I site at Ser169 introduced during site-directed mutagenesis; religation of the vector containing the remaining portion of p45 generated pGal4/p45(Δ171) (see Fig5). Partial digestion of Gal4/p45(wt) with Pst I was used to remove all but the first 108 nucleotides of the p45 coding sequence; religation of the 3.4-kb partial digestion product generated pGal4/p45(Δ36). Truncation of p45 in these vectors was confirmed by restriction analysis and partial DNA sequence analysis.
Transactivation properties of Gal4-fusion constructs containing truncated versions of p45: effect of A-kinase. The structure of p451 and the Gal4-fusion constructs containing variable amounts of N-terminal p45 sequences fused to the DNA binding domain of Gal4 are shown in (A); results of cotransfection experiments using these constructs in BHK cells are shown in (B). The indicated transactivator plasmid (5 ng) was cotransfected with the reporter pGAL4-Luc (100 ng), the control vector pRSV-βGal (50 ng), and either an expression vector for the C-subunit of A-kinase (pCMV-Cα, 50 ng, open bars) or empty vector (pRC/CMV, 50 ng, filled bars). Luciferase activity was normalized to β-galactosidase activity in each sample; the luciferase/β-galactosidase ratio obtained with the parent vector pSG424, which is lacking p45 sequences, was assigned a value of 1. Results represent the mean ± SD of three independent experiments.
Transactivation properties of Gal4-fusion constructs containing truncated versions of p45: effect of A-kinase. The structure of p451 and the Gal4-fusion constructs containing variable amounts of N-terminal p45 sequences fused to the DNA binding domain of Gal4 are shown in (A); results of cotransfection experiments using these constructs in BHK cells are shown in (B). The indicated transactivator plasmid (5 ng) was cotransfected with the reporter pGAL4-Luc (100 ng), the control vector pRSV-βGal (50 ng), and either an expression vector for the C-subunit of A-kinase (pCMV-Cα, 50 ng, open bars) or empty vector (pRC/CMV, 50 ng, filled bars). Luciferase activity was normalized to β-galactosidase activity in each sample; the luciferase/β-galactosidase ratio obtained with the parent vector pSG424, which is lacking p45 sequences, was assigned a value of 1. Results represent the mean ± SD of three independent experiments.
The p18 expression vector pMT2-p18 was from N. Andrews, the expression vector for the catalytic (C)-subunit of A-kinase, pCMV-Cα, was from S. Taylor, and the reporter plasmid pGAL4-Luc and the pGal4/Fos fusion vector were from M. Karin.3,28,29 An expression vector for full-length CBP was from M. Rosenfeld30; an expression vector for the p45-binding domain of CBP (amino acid residues 451 to 682) was constructed by PCR using previously described primers.20
Cell culture and transfections.
MEL cells (strain 745 A), A-kinase–deficient MEL cells,31and the p45-deficient MEL variant CB3 (provided by Y. Ben-David9) were cultured as previously described.18,31 To generate stable transfectants, 5 × 106 CB3 cells were transfected using 30 μg of Lipofectin (Life Technologies, Grand Island, NY) liposomes and 10 μg of pRC/βAct-p45(wt) and (mut) as described.31 After 2 weeks of growth in 1 mg/mL G418, individual colonies were expanded and tested for p45 expression by Western blot analysis and electrophoretic mobility shift assay (EMSA) as described later. For transient transfections of MEL cells, 1 × 106 cells were incubated with 6 μg of DMRIE liposomes (Life Technologies), 300 ng of the pGAL4-Luc reporter, 300 ng of pRSV-βGal (internal control), and 100 to 400 ng of the indicated pGal4 transactivator; control cultures received 100 to 400 ng of the pSG424 parent plasmid or no transactivator plasmid and the total amount of DNA was kept constant at 1.2 μg by adding carrier DNA. BHK cells were cultured and transfected using Lipofectamine (Life Technologies) as described.32 In some experiments, cells were treated with 1 mmol/L 8-Br-cAMP for 8 hours before harvesting or cells were cotransfected with 200 ng of the A-kinase C-subunit expression vector pCMV-Cα or pRC/CMV (empty vector). Cells were harvested 48 hours after transfection and luciferase and β-galactosidase activities were measured using luminometer-based assays as described.32
p45 phosphorylation studies.
BHK cells were transfected with 2 μg of pMT2-p45(wt) or pMT2-p45(mut). For in vitro phosphorylation studies, approximately 106 cells were harvested at 48 hours after transfection and cell lysates were subjected to immunoprecipitation using either p45-specific antibody or control rabbit serum as described.2,18 Washed immunoprecipitates were incubated with 50 μCi[γ-32PO4]adenosine triphosphate (ATP) (3,000 Ci/mmol) and 100 ng of purified C-subunit of A-kinase (provided by S. Taylor). For in vivo phosphorylation studies in BHK cells, approximately 106 transfected cells were transferred 32 hours posttransfection to phosphate-free medium containing 10% dialyzed serum and 100 μCi/mL of32PO4 and were harvested 16 hours later; 1 mmol/L 8-Br-cAMP was added to some cultures during the last hour of incubation. For in vivo phosphorylation studies in wild-type and A-kinase–deficient MEL cells (clone RImut/C331), the cells were incubated in phosphate-free medium containing 10% dialyzed serum and 300 μCi/mL of 32PO4 for 16 hours at a density of 2 × 106 cells/mL in 10 mL. Immunoprecipitation was performed with either p45-specific antibody or control rabbit serum as described.2,18 All immunoprecipitates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography.18
For phosphoamino acid analysis, immunoprecipitates were hydrolyzed in 6N HCl, lyophilized, and resuspended with phosphoserine, phosphothreonine, and phosphotyrosine markers. Samples were applied to cellulose thin-layer chromatography plates and analyzed by two-dimensional electrophoresis as described.33 The plates were exposed to x-ray films and the phosphoamino acid markers were visualized with ninhydrin.33
EMSAs.
Nuclear extracts were prepared and equal amounts of nuclear extract proteins were incubated with a radioactively labeled oligodeoxynucleotide (oligodNT) probe encoding the NF-E2 recognition sequence from the human porphobilinogen deaminase promoter as described previously.18 Quality and equal loading of nuclear extracts was tested by incubation with a probe for the ubiquitous transcription factor SP-1.18
Western and Northern blot analyses.
RESULTS
A-kinase phosphorylates p45 on Ser169 in vitro and in vivo.
Inspection of the amino acid sequence of p45 reveals a consensus sequence for A-kinase phosphorylation at residues 166 to 169 (Arg-Arg-Arg-Ser); we showed previously that A-kinase phosphorylates p45 in vitro.18 To determine whether Ser169 of p45 is the site phosphorylated by A-kinase in vitro, we replaced Ser169 by Ala using site-directed mutagenesis and transfected BHK cells, which do not express endogenous p45, with expression vectors encoding wild-type and mutant p45. Western blots demonstrated that the expression levels of wild-type and mutant proteins were the same (Fig1A). When p45 immunoprecipitates from cells transfected with wild-type and mutant p45 were incubated with [γ-32PO4]ATP and purified C-subunit of A-kinase, there was significant phosphorylation of the immunoprecipitates from the wild-type but not the mutant p45 transfected cells (Fig 1B).
A-kinase phosphorylates p45 on Ser169 in vitro and in vivo. (A) BHK cells were transfected with increasing amounts of expression vector encoding wild-type p45 [lanes 1 to 3, 0.3 μg, 0.6 μg, and 1.2 μg of pMT2-p45 (wt), respectively] or mutant (Ser169 → Ala)p45 [lanes 4 to 6, 0.3 μg, 0.6 μg, and 1.2 μg of pMT2-p45 (mut), respectively]. Cell extracts were subjected to Western blotting with a p45-specific antibody as described in Materials and Methods. The p45 doublet, which is thought to result from alternative usage of two translational start sites,50is indicated by a double arrow. (B) BHK cells were transfected with expression vectors encoding wild-type p45 [1.2 μg and 0.4 μg of pMT2-p45(wt), lanes 1 and 2, respectively] or mutant p45 [1.2 μg and 0.4 μg of pMT2-p45(mut Ser169 → Ala), lanes 3 and 4, respectively]; lane 5 shows mock-transfected cells. Cell extracts were subjected to immunoprecipitation with a p45-specific antibody; immunoprecipitates were incubated with [γ-32PO4]ATP and purified C-subunit of A-kinase and applied to SDS-PAGE/autoradiography as described in Materials and Methods. (C) BHK cells were transfected with expression vectors encoding wild-type p45 [1.2 μg of pMT2-p45(wt), lanes 1, 2, and 8] or mutant p45 [1.2 μg of pMT2-p45(mut Ser169 → Ala), lanes 4 and 5]; lanes 3 and 6 show cells transfected with 0.2 μg of wild-type or mutant p45 vector, respectively, and lane 7 shows mock-transfected cells. Cells were incubated with 32PO4 and some cultures were treated with 1 mmol/L 8-Br-cAMP (lanes 2, 3, 5, and 6); cell extracts were subjected to immunoprecipitation with p45-specific antibody (lanes 1 to 7) or control rabbit serum (lane 8) and immunoprecipitates were applied to SDS-PAGE/autoradiography as described in Materials and Methods. (D) Wild-type MEL cells (lanes 1 to 3) and A-kinase–deficient MEL cells (lanes 4 to 6) were incubated with32PO4 and some cultures were treated with 1 mmol/L 8-Br-cAMP (lanes 3 and 5); cell extracts were subjected to immunoprecipitation with p45-specific antibody (lanes 2 to 5) or control rabbit serum (lanes 1 and 6) as described in (C).
A-kinase phosphorylates p45 on Ser169 in vitro and in vivo. (A) BHK cells were transfected with increasing amounts of expression vector encoding wild-type p45 [lanes 1 to 3, 0.3 μg, 0.6 μg, and 1.2 μg of pMT2-p45 (wt), respectively] or mutant (Ser169 → Ala)p45 [lanes 4 to 6, 0.3 μg, 0.6 μg, and 1.2 μg of pMT2-p45 (mut), respectively]. Cell extracts were subjected to Western blotting with a p45-specific antibody as described in Materials and Methods. The p45 doublet, which is thought to result from alternative usage of two translational start sites,50is indicated by a double arrow. (B) BHK cells were transfected with expression vectors encoding wild-type p45 [1.2 μg and 0.4 μg of pMT2-p45(wt), lanes 1 and 2, respectively] or mutant p45 [1.2 μg and 0.4 μg of pMT2-p45(mut Ser169 → Ala), lanes 3 and 4, respectively]; lane 5 shows mock-transfected cells. Cell extracts were subjected to immunoprecipitation with a p45-specific antibody; immunoprecipitates were incubated with [γ-32PO4]ATP and purified C-subunit of A-kinase and applied to SDS-PAGE/autoradiography as described in Materials and Methods. (C) BHK cells were transfected with expression vectors encoding wild-type p45 [1.2 μg of pMT2-p45(wt), lanes 1, 2, and 8] or mutant p45 [1.2 μg of pMT2-p45(mut Ser169 → Ala), lanes 4 and 5]; lanes 3 and 6 show cells transfected with 0.2 μg of wild-type or mutant p45 vector, respectively, and lane 7 shows mock-transfected cells. Cells were incubated with 32PO4 and some cultures were treated with 1 mmol/L 8-Br-cAMP (lanes 2, 3, 5, and 6); cell extracts were subjected to immunoprecipitation with p45-specific antibody (lanes 1 to 7) or control rabbit serum (lane 8) and immunoprecipitates were applied to SDS-PAGE/autoradiography as described in Materials and Methods. (D) Wild-type MEL cells (lanes 1 to 3) and A-kinase–deficient MEL cells (lanes 4 to 6) were incubated with32PO4 and some cultures were treated with 1 mmol/L 8-Br-cAMP (lanes 3 and 5); cell extracts were subjected to immunoprecipitation with p45-specific antibody (lanes 2 to 5) or control rabbit serum (lanes 1 and 6) as described in (C).
DNA binding activity of wild-type and mutant (Ser169 → Ala) p45 in BHK cells. BHK cells were cotransfected with increasing amounts of expression vector encoding wild-type p45 [lanes 1 to 3, 0.3 μg, 0.6 μg, and 1.2 μg of pMT2-p45(wt), respectively] or mutant (Ser169 → Ala)p45 [lanes 4 to 6, 0.3 μg, 0.6 μg and 1.2 μg of pMT2-p45(mut), respectively] and equimolar amounts of p18 expression vector [pMT2-p18]; lanes 7 and 8 show mock-transfected cells. Ten micrograms of nuclear extract protein was incubated with 10 fmol of end-labeled oligodNT probe encoding a NF-E2 recognition site (A) or a SP-1 recognition site (B); lane 9 shows probe incubated in the absence of nuclear extract protein. EMSAs were performed as described in Materials and Methods. Nuclear extracts incubated with the NF-E2 oligodNT probe yielded 2 protein/DNA complexes (A, lanes 1 to 6); both were eliminated by adding a 50-fold excess of unlabeled oligodNT, but only the slower migrating complex was eliminated by adding excess unlabeled oligodNT containing a mutation that abolishes NF-E2 binding, but not AP-1 binding (data not shown).18 The arrow in (A) indicates the protein/DNA complex containing p45 and p18 NF-E2.
DNA binding activity of wild-type and mutant (Ser169 → Ala) p45 in BHK cells. BHK cells were cotransfected with increasing amounts of expression vector encoding wild-type p45 [lanes 1 to 3, 0.3 μg, 0.6 μg, and 1.2 μg of pMT2-p45(wt), respectively] or mutant (Ser169 → Ala)p45 [lanes 4 to 6, 0.3 μg, 0.6 μg and 1.2 μg of pMT2-p45(mut), respectively] and equimolar amounts of p18 expression vector [pMT2-p18]; lanes 7 and 8 show mock-transfected cells. Ten micrograms of nuclear extract protein was incubated with 10 fmol of end-labeled oligodNT probe encoding a NF-E2 recognition site (A) or a SP-1 recognition site (B); lane 9 shows probe incubated in the absence of nuclear extract protein. EMSAs were performed as described in Materials and Methods. Nuclear extracts incubated with the NF-E2 oligodNT probe yielded 2 protein/DNA complexes (A, lanes 1 to 6); both were eliminated by adding a 50-fold excess of unlabeled oligodNT, but only the slower migrating complex was eliminated by adding excess unlabeled oligodNT containing a mutation that abolishes NF-E2 binding, but not AP-1 binding (data not shown).18 The arrow in (A) indicates the protein/DNA complex containing p45 and p18 NF-E2.
To determine whether p45 is phosphorylated by A-kinase in vivo, we incubated the transfected BHK cells with 32PO4and treated them for 1 hour with 1 mmol/L 8-Br-cAMP to activate endogenous A-kinase. Immunoprecipitation of p45 from these cells demonstrated a significant amount of 32PO4incorporation into wild-type p45 in unstimulated cells. Treatment with 8-Br-cAMP induced a mobility shift on SDS-PAGE with a small increase in total 32PO4 incorporation (Fig 1C, compare lane 2, wild-type p45 from cells treated with 8-Br-cAMP, with lane 1, wild-type p45 from untreated cells). Similar changes in apparent molecular weight induced by phosphorylation of single amino acid residues have been observed in other proteins.34 35 Total32PO4 incorporation into p45 was determined by quantitating Cerenkov radiation of excised gel slices and increased 25% ± 7% in the presence of 8-Br-cAMP (result of three independent experiments). Basal phosphorylation of mutant p45 was considerably lower than that of wild-type p45 and neither the mobility nor the total32PO4 incorporation into mutant p45 was influenced by 8-Br-cAMP (Fig 1C, lanes 4 and 5 show mutant p45 from cells cultured in the absence and presence of 8-Br-cAMP, respectively). Similar results were obtained when p45 was immunoprecipitated from32PO4-labeled MEL cells: treatment with 8-Br-cAMP resulted in a mobility shift in p45 immunoprecipitated from wild-type MEL cells, but not from A-kinase–deficient MEL cells (Fig1D, compare lanes 2 and 3, wild-type MEL cells cultured in the absence or presence of 8-Br-cAMP, with lanes 4 and 5, A-kinase–deficient MEL cells cultured in the absence or presence of 8-Br-cAMP, respectively). Phosphoamino acid analysis of p45 from transfected BHK cells labeled with 32PO4 in vivo demonstrated32PO4 in p45 associated with phosphoserine, but not with phosphothreonine or phosphotyrosine.
The lower 32PO4 incorporation into mutant p45 compared with wild-type p45 suggests that the mutation at Ser169 may influence the phosphorylation of neighboring sites by other serine/threonine protein kinases; since the phosphorylation of Ser169 by A-kinase resulted in a mobility shift on SDS-PAGE, Ser169 does not appear to be targeted by other protein kinases in unstimulated cells. Thus, Ser169 is the only site in p45 that is phosphorylated in vitro and in vivo by A-kinase; p45 phosphorylation in vivo in the absence of 8-Br-cAMP can be attributed to other serine/threonine protein kinases.
DNA binding activity of mutant p45(Ser169 → Ala) in BHK cells.
To examine whether A-kinase phosphorylation of p45 Ser169influences NF-E2/DNA complex formation, we cotransfected BHK cells with increasing amounts of expression vectors for wild-type or mutant p45(Ser169 → Ala) and equimolar amounts of p18 expression vector. Equal amounts of nuclear extract proteins were incubated with a radioactively labeled oligodNT encoding the NF-E2 recognition site of the human porphobilinogen deaminase promoter and protein/DNA complexes were resolved on nondenaturing PAGE: the faster migrating protein/DNA complex contains the p45/p18 heterodimer and it is absent in mock transfected cells (Fig2A, compare lanes 1 through 6, transfected cells, with lanes 7 and 8, mock-transfected cells).10 18Mutant p45(Ser169 → Ala) formed the same amount of NF-E2/oligodNT complexes as wild-type p45 (Fig 2A, compare lanes 1 to 3, wild-type p45, with lanes 4 to 6, mutant p45). As a control for equal protein loading, we performed EMSAs with an oligodNT probe containing a SP-1 recognition site (Fig 2B). EMSAs performed with variable amounts of NF-E2 oligodNT probe demonstrated that the DNA-binding affinity of the NF-E2 complex containing mutant p45(Ser169 → Ala) was indistinguishable from that containing wild-type p45.
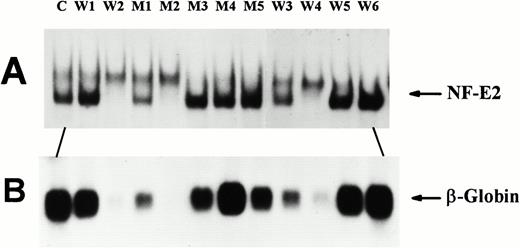
Function of (Ser169 → Ala) mutant p45 in the p45-deficient MEL cell variant CB3. P45-deficient CB3 cells were stably transfected with expression vectors encoding either wild-type (W) or (Ser169 → Ala) mutant (M) p45 and single G418-resistant colonies were selected as described in Materials and Methods. Cells were cultured for 72 hours in 4 mmol/L HMBA. (A) EMSAs were performed with 10 μg of nuclear extract protein and 10 fmol of NF-E2 oligodNT as described in Fig 2. The amount of NF-E2/oligodNT complexes formed correlated closely with the amount of p45 detected on Western blots (not shown). (B) Northern blots prepared with 8 μg of total cytoplasmic RNA were probed with a β-globin cDNA probe as described in Materials and Methods. Equal loading of the RNA was confirmed by ethidium fluorescence of the ribosomal RNA bands (not shown). Several representative clones expressing variable amounts of wild-type (W1-W6) or (Ser169 → Ala) mutant p45 (M1-M5) are shown; control MEL cells expressing endogenous p45 (C) are shown in the first lane for comparison.
Function of (Ser169 → Ala) mutant p45 in the p45-deficient MEL cell variant CB3. P45-deficient CB3 cells were stably transfected with expression vectors encoding either wild-type (W) or (Ser169 → Ala) mutant (M) p45 and single G418-resistant colonies were selected as described in Materials and Methods. Cells were cultured for 72 hours in 4 mmol/L HMBA. (A) EMSAs were performed with 10 μg of nuclear extract protein and 10 fmol of NF-E2 oligodNT as described in Fig 2. The amount of NF-E2/oligodNT complexes formed correlated closely with the amount of p45 detected on Western blots (not shown). (B) Northern blots prepared with 8 μg of total cytoplasmic RNA were probed with a β-globin cDNA probe as described in Materials and Methods. Equal loading of the RNA was confirmed by ethidium fluorescence of the ribosomal RNA bands (not shown). Several representative clones expressing variable amounts of wild-type (W1-W6) or (Ser169 → Ala) mutant p45 (M1-M5) are shown; control MEL cells expressing endogenous p45 (C) are shown in the first lane for comparison.
DNA binding and transactivation properties of mutant p45(Ser169 → Ala) in CB3 cells.
To examine the DNA-binding and transactivation properties of the mutant p45(Ser169 → Ala) in an erythroid background, we transfected the variant MEL cell line CB3 with expression vectors encoding either wild-type or mutant p45 (Ser169 → Ala). CB3 cells are completely deficient in p45 expression, and unlike wild-type MEL cells, they show no increase in globin gene expression when treated with differentiation-inducing agents like hexamethylene bisacetamide (HMBA).9,10 Single clones of stably transfected CB3 cells were tested for NF-E2 DNA-binding activity and β-globin mRNA expression in response to HMBA. Several clones showed no detectable NF-E2/DNA complexes and little β-globin mRNA expression (Fig 3,clones W2, M2, and W4; some globin mRNA was detectable in these clones on long exposures of the Northern blot). In clones that expressed p45, the amount of NF-E2/DNA complexes correlated with the amount of β-globin mRNA expression observed in both wild-type and mutant p45-transfected CB3 cells (Fig 3, eg, clones M1 and W3, which expressed low amounts of NF-E2/DNA complexes, expressed low amounts of β-globin mRNA). On Western blots, the amount of p45 expressed correlated closely with the amount of NF-E2/DNA complexes found by EMSA (data not shown). Thus, in p45-deficient CB3 cells, mutant p45(Ser169 → Ala) could restore NF-E2 DNA-binding activity and β-globin mRNA expression to the same degree as wild-type p45. Only levels of p45 expression that were higher than those found in wild-type MEL cells restored β-globin mRNA expression in the variant CB3 cells to the amount of β-globin mRNA detected in wild-type MEL cells; this finding is in agreement with previous reports.9 10
Transactivation properties of Gal4-fusion constructs containing full-length wild-type or (Ser169 → Ala) mutant p45: effect of 8-Br-cAMP. BHK cells were transfected with 100 ng of the reporter pGAL4-Luc, 50 ng of the β-galactosidase expression vector pRSV-βGal (internal control), and the indicated amounts of transactivator plasmid [pGal4/p45(wt), triangles; pGal4/p45(mut), squares; pSG424, diamonds]. The total amount of transfected DNA was kept constant by the addition of carrier DNA. Half of the cultures were treated with 1 mmol/L 8-Br-cAMP for 8 hours before harvesting to activate endogenous A-kinase (dashed lines and filled symbols). Reporter gene activities were determined as described in Materials and Methods; luciferase activity was normalized to β-galactosidase activity in each sample to correct for transfection efficiencies. Results represent the mean ± SD of three independent experiments.
Transactivation properties of Gal4-fusion constructs containing full-length wild-type or (Ser169 → Ala) mutant p45: effect of 8-Br-cAMP. BHK cells were transfected with 100 ng of the reporter pGAL4-Luc, 50 ng of the β-galactosidase expression vector pRSV-βGal (internal control), and the indicated amounts of transactivator plasmid [pGal4/p45(wt), triangles; pGal4/p45(mut), squares; pSG424, diamonds]. The total amount of transfected DNA was kept constant by the addition of carrier DNA. Half of the cultures were treated with 1 mmol/L 8-Br-cAMP for 8 hours before harvesting to activate endogenous A-kinase (dashed lines and filled symbols). Reporter gene activities were determined as described in Materials and Methods; luciferase activity was normalized to β-galactosidase activity in each sample to correct for transfection efficiencies. Results represent the mean ± SD of three independent experiments.
Effect of 8-Br-cAMP on the transactivation properties of p45.
The NF-E2 binding site is recognized by a number of different bzip proteins, including members of the AP-1 family, which are known to be regulated by A-kinase.36 To study the transactivation properties of p45 independently of its binding to the NF-E2 recognition site, we fused p45 sequences with the DNA-binding domain of the well-characterized yeast transcription factor Gal4; this allows testing of the fusion protein's activity on a test promoter bearing GAL4-binding sites.27 When we cotransfected BHK cells with the reporter pGAL4-Luc and increasing amounts of expression vector encoding Gal4 fusion proteins with either full-length wild-type (wt) or mutant (mut, Ser169 → Ala)p45, we observed the same modest degree of reporter gene transactivation by wild-type and mutant p45 (Fig 4, open squares and triangles). When cells were treated with 8-Br-cAMP to activate endogenous A-kinase, we observed a fivefold to sevenfold increase in the transactivation of pGAL4-Luc at each level of pGal4/p45 (wt) or (mut) expression (Fig 4, filled squares and triangles). When we transfected pGAL4-Luc with the parent vector pSG424, which contains only the Gal4 DNA-binding domain, luciferase expression was low and not significantly influenced by the activation of A-kinase (Fig 4, diamonds). When we cotransfected a Gal4-fusion protein containing the bzip transcription factor c-Fos, strong transactivation of pGAL4-Luc-1 was observed, which was not altered by 8-Br-cAMP (data not shown). Thus, the effect of A-kinase on p45 transactivation was specific for p45 sequences, but did not depend on the presence of the A-kinase phosphorylation site Ser169.
Effect of A-kinase on truncated versions of p45.
To determine which part of the p45 sequence was required for the stimulation of transactivation by A-kinase, we constructed a series of p45 deletions in the Gal4-fusion vectors (Fig 5A). We transfected the Gal4-fusion constructs containing N-terminal p45 sequences of decreasing length together with the reporter pGAL4-Luc into BHK cells and compared their activities with the activity of pSG424, the parent vector lacking p45 sequences (Fig 5B). In the absence of A-kinase, pGal4/p45 (wt) containing full-length p45 increased luciferase expression only about twofold over the level found in pSG424-transfected cells. Removing the bzip domain [pGal4/p45(Δ269)] increased luciferase activity approximately 10-fold, and removing all but the N-terminal 83 amino acids [pGal4/p45(Δ83)] increased luciferase activity approximately 50-fold when compared with the activity elicited by full-length p45 (Fig 5B, filled bars). Further deletion of the p45 coding sequence resulted in almost complete loss of transactivation: a construct containing only the N-terminal 36 amino acids of p45 fused to the Gal4 DNA-binding domain [pGal4/p45(Δ36)] increased luciferase activity only about 1.5-fold over the level found in pSG424-transfected cells (data not shown). The increase in transactivation observed with the removal of the bzip domain of p45 may be explained by inhibitory dimerization partners of p45 binding to the leucine zipper domain.4,37 The increase in transactivation seen with the deletion of sequences between amino acids 83 and 269 of p45 could be due to the removal of inhibitory protein interactions or to unmasking of the N-terminal transactivation domain by conformational changes. In agreement with our results, the main transactivation domain of p45 has been previously localized to the N-terminal 80 amino acids of the protein.8 38
When the C-subunit of A-kinase was cotransfected with the Gal4-p45 fusion constructs shown in Fig 5, there was a significant increase in the transactivation properties of each construct: the increase was approximately sevenfold to eightfold for most constructs, including full-length p45(wt) and (mut); the increase was 17-fold for the construct containing the N-terminal 112 amino acids (Fig 5B, open bars). These results suggest that A-kinase regulates protein(s) that interacts with the N-terminal 112 amino acids of p45; this regulation is obviously independent of Ser169 phosphorylation by A-kinase.
A similar effect of A-kinase on the activity of pGal4/p45(Δ83) was observed in MEL cells (Fig 6 shows the effect of cotransfected C-subunit of A-kinase; similar results were obtained when endogenous A-kinase was activated with 8-Br-cAMP). When we transfected pGal4/p45(Δ83) into severely A-kinase–deficient MEL cells (clones RImut/C1 and RImut/C3, described previously31), we found that the transactivation potential of pGal4/p45(Δ83) was reduced by 50% to 70% compared with the activity of this construct in control MEL cells with normal A-kinase activity (clones RIwt/Cl31 and parental MEL cells); gene expression from the cotransfected plasmid pRSV-βGal was similar in all clones. Thus, A-kinase appears to regulate p45 transactivation potential in erythroid as well as nonerythroid cells.
Regulation of the p45 transactivation domain by A-kinase in MEL cells. MEL cells were cotransfected with 300 ng of pGAL4-Luc, 300 ng of pRSV-βGal, the indicated amounts of transactivator plasmid containing the N-terminal 83 amino acids of p45 [pGal4/p45(Δ83)], and 200 ng of either A-kinase C-subunit expression vector (•) or empty vector (○) as described in Materials and Methods. Luciferase activity was normalized to β-galactosidase activity; results represent the mean ± SD of three independent experiments.
Regulation of the p45 transactivation domain by A-kinase in MEL cells. MEL cells were cotransfected with 300 ng of pGAL4-Luc, 300 ng of pRSV-βGal, the indicated amounts of transactivator plasmid containing the N-terminal 83 amino acids of p45 [pGal4/p45(Δ83)], and 200 ng of either A-kinase C-subunit expression vector (•) or empty vector (○) as described in Materials and Methods. Luciferase activity was normalized to β-galactosidase activity; results represent the mean ± SD of three independent experiments.
Effect of CBP on p45 transactivation.
Cheng et al20 recently demonstrated that the N-terminal transactivation domain of p45 binds the transcriptional coactivator CBP in vitro. Since CBP is known to be regulated by A-kinase phosphorylation, CBP would be an attractive candidate for the protein mediating the effect of A-kinase on p45 transactivation. If the interaction between p45 and CBP were responsible for the ability of A-kinase to stimulate p45 transactivation, then expression of the isolated p45-binding domain of CBP (amino acids 451 to 682) would be expected to dominantly inhibit this effect by competing with endogenous CBP for binding to p45.20 However, coexpression of CBP (451 to 682) had little or no effect on the transactivation potential of p45 in the presence or absence of A-kinase (data not shown). Moreover, when we cotransfected BHK cells with pGal4/p45(Δ83) and full-length CBP, transactivation of the Gal4-dependent reporter was stimulated twofold to threefold by CBP in the presence or absence of A-kinase; this effect on transactivation appeared to be nonspecific, since CBP also increased expression of the control plasmid pRSV-βGal twofold (data not shown). Thus, we could not demonstrate a convincing effect of CBP on p45 transactivation under our experimental conditions.
DISCUSSION
The importance of NF-E2 for the transcriptional activation of erythroid-specific genes and in particular for the activity of the α- and β-globin LCRs has been recognized, but little is known about the regulation of NF-E2 activity.5-7,10,39 NF-E2 is expressed in multipotential hematopoietic progenitor cells before their commitment to the erythroid lineage and the globin LCR NF-E2 recognition sites are occupied before globin gene expression is activated.6,39-41 NF-E2 activity may be regulated by posttranslational mechanisms, such as phosphorylation, or by interactions with ancillary proteins. Other mechanisms of regulation may include changes in NF-E2 complex composition through changes in the expression and activity of maf-related proteins or competition of other bzip proteins for binding at the NF-E2 recognition site.4,13-15,37 42
In this study, we found that A-kinase significantly stimulated the transactivation properties of p45 in erythroid and nonerythroid cells; in a previous study, we had shown impaired NF-E2/DNA complex formation and decreased α-LCR enhancer activity in A-kinase–deficient MEL cells.18 Since we could not detect any effect of p45 phosphorylation at the A-kinase recognition site Ser169 on NF-E2 DNA-binding or transactivation properties, our results suggest that A-kinase regulates NF-E2 indirectly, through changes in the activity of ancillary protein(s) present in erythroid and nonerythroid cells. Since modulation of p45 activity by A-kinase required only the N-terminal transactivation domain of p45, the effect of A-kinase is not likely to be mediated through modification of maf-related or other bzip proteins.
Although the activity of many transcription factors is modulated by phosphorylation, there are many examples of changes in transcription factor phosphorylation that have no detectable influence on DNA binding or transactivation.36,43 The erythroid transcription factor GATA-1 is a prime example, because careful mapping of seven major phosphorylation sites demonstrated that phosphorylation at an A-kinase consensus sequence increases during erythroid differentiation of MEL cells, but phosphorylation at this site or at the other sites does not measurably alter GATA-1 functions.43
The positive or negative regulation of transcription factors by changing protein/protein interactions has been described for several classes of bzip proteins. An example of negative regulation is the interaction of AP-1 with steroid hormone and retinoic acid receptors, which results in repression of AP-1 activity dependent on ligand binding to the steroid or retinoic acid receptors.30,44,45An example of positive regulation is the interaction of CREB with the transcriptional coactivator CBP, which requires several A-kinase–regulated events, including phosphorylation of CREB and CBP.46,47 Although CBP has been recently shown to bind to the transactivation domain of p45 in vitro and appears to mediate the potentiation of nuclear hormone receptor action by p45,20we were unable to demonstrate a significant effect of CBP on the transactivation potential of p45 under our experimental conditions. However, we cannot exclude that BHK cells express too much endogenous CBP to demonstrate a positive effect of transfected full-length CBP or a dominant negative effect of the isolated p45-binding domain of CBP.
A critical role for the N-terminal domain of p45 for globin gene expression in MEL cells has been shown previously.10,38 The main transactivation domain of p45 has been localized to the N-terminal 80 amino acids of the protein; this region is proline-rich and contains two PXXP motifs and a PPPSY motif that can mediate protein/protein interactions.8,38,48 We found that this N-terminal domain of p45 was sufficient to mediate the effect of A-kinase on p45 transactivation. Recently, direct interaction between this p45 domain and the TATA-binding protein-associated factor TAFII130, as well as other proteins, has been demonstrated.8 48 More work is necessary to determine whether the interaction of p45 with a component of the transcription initiation complex or other interactions between p45 and downstream effector molecules may be regulated by A-kinase.
The physiologic significance of A-kinase regulation of NF-E2 is supported by the effect of cAMP on the erythroid differentiation of various cell lines: stimulation of the cAMP signal transduction pathway promotes hemoglobin production in the erythropoietin-responsive cell lines SKT6, TSA8, and J2E.21-23 While binding of erythropoietin to its receptor does not change intracellular cAMP concentrations,22 the effect of cAMP on hemoglobin production in these cell lines is consistent with a role of A-kinase in the regulation of erythroid gene expression, possibly via changes in the activity of NF-E2. In MEL cells, we have demonstrated that A-kinase activity is necessary for erythroid gene expression,18,19although prolonged treatment of MEL cells with pharmacologic doses of cAMP analogs results in inhibition of erythroid differentiation.49 We have recently shown that this paradoxical effect is due to upregulation of c-myb, mediated at least in part by NF-κB (p50/relB), which is induced by prolonged activation of A-kinase.49 Thus, in MEL cells, A-kinase not only produces signals expected to promote differentiation (increased NF-E2 activity), but prolonged activation of the kinase can also produce signals that are incompatible with differentiation (upregulation of c-myb).
ACKNOWLEDGMENT
We thank Drs N. Andrews, M. Karin, M. Ptashne, and M. Rosenfeld for providing us with plasmids, Dr S. Taylor for the purified C-subunit of A-kinase, and Dr Y. Ben-David for the variant MEL cell line CB3.
Supported by National Science Foundation Grant No. MCB-9506327.
Address reprint requests to Renate B. Pilz, MD, Department of Medicine and Cancer Center, University of California, San Diego, Basic Science Building, 9500 Gilman Dr, La Jolla, CA 92093-0652.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. A-kinase phosphorylates p45 on Ser169 in vitro and in vivo. (A) BHK cells were transfected with increasing amounts of expression vector encoding wild-type p45 [lanes 1 to 3, 0.3 μg, 0.6 μg, and 1.2 μg of pMT2-p45 (wt), respectively] or mutant (Ser169 → Ala)p45 [lanes 4 to 6, 0.3 μg, 0.6 μg, and 1.2 μg of pMT2-p45 (mut), respectively]. Cell extracts were subjected to Western blotting with a p45-specific antibody as described in Materials and Methods. The p45 doublet, which is thought to result from alternative usage of two translational start sites,50is indicated by a double arrow. (B) BHK cells were transfected with expression vectors encoding wild-type p45 [1.2 μg and 0.4 μg of pMT2-p45(wt), lanes 1 and 2, respectively] or mutant p45 [1.2 μg and 0.4 μg of pMT2-p45(mut Ser169 → Ala), lanes 3 and 4, respectively]; lane 5 shows mock-transfected cells. Cell extracts were subjected to immunoprecipitation with a p45-specific antibody; immunoprecipitates were incubated with [γ-32PO4]ATP and purified C-subunit of A-kinase and applied to SDS-PAGE/autoradiography as described in Materials and Methods. (C) BHK cells were transfected with expression vectors encoding wild-type p45 [1.2 μg of pMT2-p45(wt), lanes 1, 2, and 8] or mutant p45 [1.2 μg of pMT2-p45(mut Ser169 → Ala), lanes 4 and 5]; lanes 3 and 6 show cells transfected with 0.2 μg of wild-type or mutant p45 vector, respectively, and lane 7 shows mock-transfected cells. Cells were incubated with 32PO4 and some cultures were treated with 1 mmol/L 8-Br-cAMP (lanes 2, 3, 5, and 6); cell extracts were subjected to immunoprecipitation with p45-specific antibody (lanes 1 to 7) or control rabbit serum (lane 8) and immunoprecipitates were applied to SDS-PAGE/autoradiography as described in Materials and Methods. (D) Wild-type MEL cells (lanes 1 to 3) and A-kinase–deficient MEL cells (lanes 4 to 6) were incubated with32PO4 and some cultures were treated with 1 mmol/L 8-Br-cAMP (lanes 3 and 5); cell extracts were subjected to immunoprecipitation with p45-specific antibody (lanes 2 to 5) or control rabbit serum (lanes 1 and 6) as described in (C).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3193/3/m_blod40916001w.jpeg?Expires=1767769497&Signature=qS5FljNsSJ~LTm70mHEmjAAENWMFnt6wa-ic2xaqTw4Qjv7-eJIXm5AQnFPa-hbhgfQv2D3SUH4be7txZAI0l9CT7x4pEdXHhLZTeND2zcDp6LPxtlbR-5hcISUSSo0T3GZR605PQZ~VzmH8~SY6PdUIVEyW-uj9fkomcpKgMbVRQ07PNcNPCQNZfl~TCiKB1okBiZyiwg-3O09S6-0yiGhp97hd2EomZf0c0aMABQd1ccd9m534M9g1y5DOsBxw~Nya7s0xAzF3Q2k-ZtdyQ8SJYFsP-9pwZJwMz25NsMI2aY7Xm1RKjzBLQ3haZLpLDhEiBdh4IamDJZIu7WuwFw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. DNA binding activity of wild-type and mutant (Ser169 → Ala) p45 in BHK cells. BHK cells were cotransfected with increasing amounts of expression vector encoding wild-type p45 [lanes 1 to 3, 0.3 μg, 0.6 μg, and 1.2 μg of pMT2-p45(wt), respectively] or mutant (Ser169 → Ala)p45 [lanes 4 to 6, 0.3 μg, 0.6 μg and 1.2 μg of pMT2-p45(mut), respectively] and equimolar amounts of p18 expression vector [pMT2-p18]; lanes 7 and 8 show mock-transfected cells. Ten micrograms of nuclear extract protein was incubated with 10 fmol of end-labeled oligodNT probe encoding a NF-E2 recognition site (A) or a SP-1 recognition site (B); lane 9 shows probe incubated in the absence of nuclear extract protein. EMSAs were performed as described in Materials and Methods. Nuclear extracts incubated with the NF-E2 oligodNT probe yielded 2 protein/DNA complexes (A, lanes 1 to 6); both were eliminated by adding a 50-fold excess of unlabeled oligodNT, but only the slower migrating complex was eliminated by adding excess unlabeled oligodNT containing a mutation that abolishes NF-E2 binding, but not AP-1 binding (data not shown).18 The arrow in (A) indicates the protein/DNA complex containing p45 and p18 NF-E2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3193/3/m_blod40916002y.jpeg?Expires=1767769497&Signature=0RO7E2Jv9p-SDh6rGfjBXSbRzKt~dfq-tMNq~8p4UVv3B0q7mvteKr0uu7DeB7217rzC4KT89haHVOTk~xnq8eK6-14p8WXzP5fsR8KtZ9hQbRSq0nEhrQPeQYo9OvOhjWTF0wm0Yd8OgYHc7nsln2VOoD~TpbIoZfcGDnnVldQLJun8-hPoJnO~FkSjvhOI7Ev0uFCk1Tc67QTLdH7V1wPrUmm0NDRzmeiGn913vWZCX-xdxYwJsykvvDrxG7pCgyyDyk6OfjBEiigomwHHXiJM3SlVqXe~Oj4M7yUODVuIWPTICS-FqszQneNPjo3dAOK0puFN5htm0wn942MqHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Transactivation properties of Gal4-fusion constructs containing full-length wild-type or (Ser169 → Ala) mutant p45: effect of 8-Br-cAMP. BHK cells were transfected with 100 ng of the reporter pGAL4-Luc, 50 ng of the β-galactosidase expression vector pRSV-βGal (internal control), and the indicated amounts of transactivator plasmid [pGal4/p45(wt), triangles; pGal4/p45(mut), squares; pSG424, diamonds]. The total amount of transfected DNA was kept constant by the addition of carrier DNA. Half of the cultures were treated with 1 mmol/L 8-Br-cAMP for 8 hours before harvesting to activate endogenous A-kinase (dashed lines and filled symbols). Reporter gene activities were determined as described in Materials and Methods; luciferase activity was normalized to β-galactosidase activity in each sample to correct for transfection efficiencies. Results represent the mean ± SD of three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3193/3/m_blod40916004x.jpeg?Expires=1767769497&Signature=qCBY7~Z3PJMej67lm4t0C7mEtXOsxEh6eHbyqi-bmzacXEsfJh3RPS14BFn2Oyb~5rpsXhmra-vxEu8n7Diajo-PXB7hIKKVDe8ipfGUyu95Y88i50P-jYSSnRb5ZMCk6tiSNEQjbBHdWSf2vsKVKcO~2Jlv-quDFUnjp2mYHksjDBbwemOlp42pQpXCyTfyS7fJqSsOqHfXFxdxAUV0yxXxCHIGFtLmmNh8Xi41IkGrbDeNDzuk3SR8i2BwRVIh5r38MH0Z2-GAAbagV3vp-fqhZmVBOTodWOXW4rD5H-df~W2hh8fqmUgz7SNSlYKW6CtNlIYLDdcIH-Q6qsLlHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Regulation of the p45 transactivation domain by A-kinase in MEL cells. MEL cells were cotransfected with 300 ng of pGAL4-Luc, 300 ng of pRSV-βGal, the indicated amounts of transactivator plasmid containing the N-terminal 83 amino acids of p45 [pGal4/p45(Δ83)], and 200 ng of either A-kinase C-subunit expression vector (•) or empty vector (○) as described in Materials and Methods. Luciferase activity was normalized to β-galactosidase activity; results represent the mean ± SD of three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3193/3/m_blod40916006x.jpeg?Expires=1767769497&Signature=m-6wHdYaPRbOmksOf4B0Ea~ZGWtwhjIy1srBQPKSIDCjMkbeOxQEKzBK5ZajwRmcdYu832lH~yEl4ZGsSOLE5DQngf-y~Irx9FkNqgKRlmCm0lK0d3F7OxK61lqFk4q5GtHSHxb6jqb~7J~ULPZDyz4FRRFon4lVE7DrtOiO6ub6PcILvU6mYSxLegTCZ1X~T7HWPQasHa6mU0NRv2PTOi4QtrsVP3JzlK5dcB6-JVR8~idREhBmMpIph57zW~oGESXW2X27rMApy3G3IPcYXNXtSPKBXZPNaxoGnl6HnwEvhTkEZAu0ptxfVO~BtHjxUg70QJh6jUn0l43lrvJH4w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal