Abstract
One facet of cytokine receptor signaling involves the activation of signal transducers and activators of transcription (STATs). STATs are rapidly activated via tyrosine phosphorylation by Janus kinase (JAK) family members and subsequently inactivated within a short period. We investigated the effect of proteasome inhibition on interleukin-3 (IL-3) activation of the JAK/STAT pathway following stimulation of Ba/F3 cells. Treatment of Ba/F3 cells with the proteasome inhibitor,N-acetyl-l-leucinyl-l-leucinyl-norleucinal (LLnL), led to stable tyrosine phosphorylation of the IL-3 receptor, beta common (βc), and STAT5 following stimulation. The effects of LLnL were not restricted to the JAK/STAT pathway, as Shc and mitogen-activated protein kinase (MAPK) phosphorylation were also prolonged in LLnL-treated cells. Further investigation showed these stable phosphorylation events were the result of prolonged activation of JAK2 and JAK1. These observations were confirmed using pharmacologic inhibitors. In the presence of LLnL, stable phosphorylation of STAT5 and βc was abrogated if the tyrosine kinase inhibitor, staurosporine, was added. The effect of staurosporine on STAT5 phosphorylation could be overcome if the phosphatase inhibitor, vanadate, was also added, suggesting phosphorylated STAT5 could be stabilized by phosphatase, but not by proteasome inhibition per se. These observations are consistent with the hypothesis that proteasome-mediated protein degradation can modulate the activity of the JAK/STAT pathway by regulating the deactivation of JAK.
THE ROLE(S) OF UBIQUITINATION and/or proteolytic degradation of proteins by the 20S and 26S proteasomes have received increased attention during recent years, and it is now apparent these two processes provide an additional point of regulation for many fundamental biologic functions.1,2Ubiquitin-dependent proteolysis has been shown to be integral in the following: the degradation of cyclins and cell-cycle progression3-7; the generation of peptides presented on the cell surface by major histocompatability complex (MHC) class I molecules8; and the modulation of several transcriptional regulators, including c-Jun9 and IκB,10-14 as well as the processing and activation of theRel family member, NF-κB.14 Recently, ubiquitination has also been shown to signal receptor-mediated endocytosis of the yeast G-protein receptor, Ste2p,15 and has been implicated in downmodulating c-kit receptor expression.16 Consequently, it is conceivable to expect many biochemical pathways to be affected by ubiquitin-dependent proteolysis, including the signaling cascades of cytokine receptors.
Interleukin-3 (IL-3) signaling pathways have been well characterized and constitute a useful model for growth factor signaling. One of the first signaling events, following IL-3 stimulation, is the increased tyrosine phosphorylation of and subsequent activation of the receptor-associated protein tyrosine kinase, Janus kinase-2 (JAK2).17-22 The newly activated JAK2 mediates the subsequent phosphorylation of tyrosine residues within the beta-chain of the IL-3 receptor.19,20,23-25 Activation of JAK1 by IL-3 has also been reported albeit to a lesser extent when compared with JAK2 activation.17 The phosphotyrosine residues of the beta subunit provide docking sites for signal transducers and activators of transcription (STATs).26,27 STATs bind to the activated receptors, via their SH2 domains, upon which they are phosphorylated on a single tyrosine residue C-terminal to its SH2 domain by JAK.28,29 Phosphorylated STATs dissociate from the receptor, homodimerize or heterodimerize, and translocate to the nucleus, where they bind specific DNA elements to activate transcription of target genes.30 IL-3 stimulation predominantly leads to the activation of STAT531-34 and induces the expression of several genes, including cytokine-inducible SH2-containing protein (CIS), pim-1, osm, and c-fos.32 Although the activation of STATs is well understood, this is not so for their inactivation. The most likely model is that STATs are negatively regulated by dephosphorylation, a process that probably occurs within the nucleus.35 The identification of the phosphatase(s) that catalyze this reaction has been actively pursued, but to date has remained elusive.
Recently, however, another model for the negative regulation of STATs has been proposed. Using specific inhibitors of the proteasome, active or phosphorylated STAT1 has been shown to be stabilized following interferon-gamma (IFN-γ) stimulation.36 Furthermore, this study identified ubiquitinated forms of phospho-STAT1, suggesting that active STAT1 was inactivated by ubiquitin-mediated proteolysis within the 26S proteasome. The proteasome has been shown to degrade several phosphorylated proteins, including IκB,10-13 cyclin G1,6,7 and SHP-1,37 via a ubiquitination-dependent pathway, and provides an attractive alternate mechanism by which the cell could downregulate STAT activity.
To determine which of these models play a role in STAT5 regulation, we investigated STAT5 inactivation in the IL-3–dependent hematopoietic progenitor cell line, Ba/F3.38 STAT5 is rapidly activated by IL-3 and accumulation of the activated protein reaches a maximum within 30 minutes of stimulation and then declines to baseline levels within 1 to 2 hours.31-34 The relatively short half-life of activated STAT5 indicates that the transcription factor's activity is tightly regulated and reduces the likelihood of the cell accumulating harmful levels of gene products. To examine the effect on IL-3–induced activation of the JAK/STAT5 pathway and whether STAT5 might also be proteolytically degraded, we treated Ba/F3cells with the proteasome-specific inhibitor,N-acetyl-l-leucinyl-l-leucinyl-norleucinal (LLnL),8 and investigated the effect of proteasome inhibition on JAK/STAT5 activation, as well as tyrosine phosphorylation of beta common (βc) following IL-3 stimulation. The results presented here show treatment of Ba/F3 cells with LLnL resulted in prolonged activation of the JAK/STAT5 pathway as a consequence of prolonged JAK phosphorylation/activation.
MATERIALS AND METHODS
Chemicals and antibodies.
Recombinant murine IL-3 was purchased from R&D (Minneapolis, MN). LLnL, staurosporine, and sodium orthovanadate were obtained from Sigma (St Louis, MO). LLnL and staurosporine were dissolved in dimethylsulfoxide (DMSO) and used at final concentrations of 50 μmol/L and 500 nMol/L, respectively. Sodium orthovanadate was prepared in phosphate-buffered saline (PBS) and used at a final concentration of 1 mmol/L. Sulfo-NHS-LC-Biotin was purchased from Pierce (Rockford, IL).
Antiphosphotyrosine-STAT5 sera was a generous gift from David Frank (Dana-Farber Cancer Institute, Boston, MA). Anti-STAT5b (sc-835) antibody was purchased from Santa Cruz (Santa Cruz, CA). The horseradish peroxidase (HRP)-conjugated antiphosphotyrosine (anti-ptyr) antibody, RC20, and anti-Shc polyclonal antibody were obtained from Transduction Laboratories (Lexington, KY). For immunoprecipitations of βc, an anti–βc C-terminal monoclonal antibody was obtained from Jan Tavernier (Flanders Interuniversity Institute of Biotechnology, Ghent, Belgium), while a different anti–βc C-terminal polyclonal antibody (sc-678; Santa Cruz) was used for Western blotting. Immunoprecipitations of JAK1 and JAK2 were performed with antibodies from UBI (Lake Placid, NY), while Western blotting was performed with antibodies from UBI and Santa Cruz (HR-758), respectively. p44/42 MAPK (#9102) and phosphospecific-p44/42 MAPK (#9101) were purchased from New England Biolabs (Beverly, MA).
Cell culture.
Ba/F3 cells were grown continuously in suspension culture in RPMI 1640 medium supplemented with 10% (vol/vol) fetal bovine serum (Hyclone, Logan, UT), penicillin G (50 U/mL), streptomycin (50 μg/mL), l-glutamine (2 mmol/L), and murine IL-3 (0.5 ng/mL) in a humidified atmosphere of 5% CO2 at 37°C.
Cell extracts, immunoprecipitations, and sodium dodecyl sulfate polyacrylamide electrophoresis.
Cells were washed three times with PBS and cultured for 11 hours in the absence of IL-3. Where appropriate, LLnL was added and the culture continued for a further 1 hour, unless otherwise indicated, before stimulation with IL-3 (0.5 ng/mL) for 0 to 2 hours. Cells were washed with PBS and both cytosolic and nuclear extracts were prepared as described previously.39
For βc immunoprecipitations, cells were resuspended in lysis buffer (1% Nonidet P-40 [NP-40], 50 mmol/L Tris.HCl, pH 7.5, 150 mmol/L NaCl, 0.5% sodium deoxycholate, 50 mmol/L NaF, 0.2 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L Na3VO4, 2 μg/mL aprotinin C, and 0.5 μg/mL leupeptin) and incubated on ice for 30 minutes. Extracts were centrifuged (4°C) for 15 minutes at 13,000 rpm and resulting supernatants were used for subsequent immunoprecipitations. Anti-βc C-terminal sera (1:300) was incubated overnight at 4°C in the presence of protein G-agarose (Santa Cruz).40 Immune complexes were washed twice with NP-40 lysis buffer and once with Tris-buffered saline (TBS) before addition of 2× Laemmli sample buffer. Bound proteins were eluted by boiling for 10 minutes and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). JAK1, JAK2, and Shc immunoprecipitations were performed as described earlier using protein A-Sepharose (Pharmacia, Piscataway, NJ) and the manufacturer's recommended dilution of JAK1 or JAK2 antisera and 1 μg of anti-Shc antibody per immunoprecipitation, respectively. For Western blots of STAT5 or phospho-STAT5, extracts were mixed with 2× sample buffer and resolved by SDS-PAGE.
Western blotting.
Electrophoresed proteins were transferred to Immobilon-P PVDF membrane (Millipore, Bedford, MA) and blocked with 3% bovine serum albumin (BSA) in TBST (TBS plus 0.05% Tween 20). Antiphospho-STAT5 or anti-STAT5b antisera were diluted 1:10,000 in 1% BSA/TBST and incubated for 1 hour at room temperature. Membranes were washed four times with TBST and incubated with a 1:5,000 dilution of HRP-conjugated protein A (Amersham, Arlington Heights, IL) in 1% BSA/TBST for 30 minutes at room temperature. After four washes with TBST, proteins were detected using enhanced chemiluminescence (ECL) reagent (Amersham, Arlington Heights, IL). Anti-βc, anti-Shc, antiphospho MAPK, and anti-MAPK blotting were similarly performed using 1:1,000 dilutions of antisera, while anti-JAK1 and anti-JAK2 blotting were performed with 1:5,000 dilutions of antisera. For RC20 blotting, membranes were incubated with a 1:5,000 dilution of antibody in 1% BSA/TBST, washed four times with TBST, and developed as described earlier. Where appropriate, membranes were stripped with a solution containing 2% SDS, 62.5 mmol/L Tris, and 0.7% β-mercaptoethanol for 30 minutes at 55°C, washed extensively with H2O and twice with TBST, and reblocked with 3% BSA/TBST before addition of primary antibody.
Surface biotinylation.
A total of 5 × 107 cells were washed three times in ice-cold PBS (pH 8.0) and resuspended in 1 mL of freshly prepared biotin/PBS (1 mg/mL). Cells were covered with foil and rotated for 40 minutes at 4°C. To stop biotinylation, cells were washed six times with ice-cold PBS (pH 8.0) supplemented with 0.15% (wt/vol) glycine. Cells were lysed in NP-40 lysis buffer and immunoprecipitations of βc performed as described earlier. Detection of biotinylated protein was achieved by Western blotting with streptavidin:HRP (Amersham) diluted 1:25,000 in 1% BSA/TBST.
Electrophoretic mobility shift assay.
Samples (5 μg) of nuclear extracts (described earlier) were used for electrophoretic mobility shift assay (EMSA). EMSA was performed with a STAT5 oligonucleotide probe from the β-casein promoter element (top strand—5′GTAGATTTCTAGGAATTCAAA3′) as described previously.34 After a 20-minute incubation on ice with32P-labeled probe, samples were electrophoresed on 6% nondenaturing polyacrylamide gels in 0.5× Tris/borate/EDTA (TBE) buffer. Gels were dried and subjected to autoradiography.
Phosphatase assay.
Nonradioactive tyrosine phosphatase assay kit, Cat. No. 1 534 513, was purchased from Mannheim Boehringer (Indianapolis, IN). Cells were washed once with PBS before NP-40 lysis (1% NP-40, 50 mmol/L Tris.HCl, pH 7.5, 150 mmol/L NaCl, 0.5% sodium deoxycholate, 50 mmol/L NaF, 0.2 mmol/L phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin C, and 0.5 μg/mL leupeptin). After 30 minutes, extracts were centrifuged (4°C) for 15 minutes at 13,000 rpm. Supernatants were diluted with lysis buffer to the equivalent of 105 cells in a 20-μL volume. Preliminary experiments determined this dilution to be optimal. Total phosphatase activity in 20-μL aliquots was assayed according to the manufacturer's protocol over 15-minute intervals at 37°C.
RESULTS
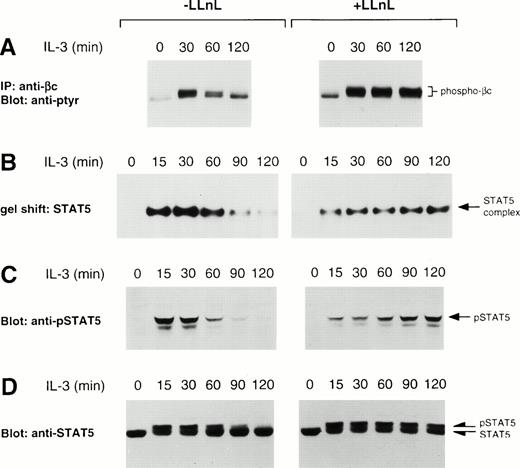
To examine the effect of proteasome inhibition on IL-3 signaling, the tyrosine phosphorylation pattern of the IL-3 receptor beta-subunit, βc, was determined following IL-3 stimulation of IL-3–depleted Ba/F3 cultures in both the presence and absence of LLnL. In the absence of LLnL, immunoprecipitations from unstimulated cells resulted in the detection of a single tyrosine-phosphorylated band, suggesting that βc was phosphorylated in the basal state (Fig1A). The addition of IL-3 induced the appearance of an upper band, indicating that βc was being converted into a highly phosphorylated form. This upper band was transiently induced by IL-3 and was maximal within 30 minutes of stimulation. In contrast, the presence of LLnL stabilized the transient nature of this upper band of phosphorylated βc. After stimulation with IL-3, the slower migrating species of βc was strongly induced and over the course of the experiment did not lose signal intensity. Furthermore, the results from several experiments showed that pretreatment with LLnL consistently resulted in a higher degree of tyrosine phosphorylation following IL-3 stimulation compared with untreated cells. Reblotting with an anti-βc antibody showed an equivalent amount of immunoreactive protein in all samples, ruling out the possibility that LLnL had affected the stability of βc (data not shown). Thus, one effect of LLnL is to stabilize the tyrosine phosphorylation of βc.
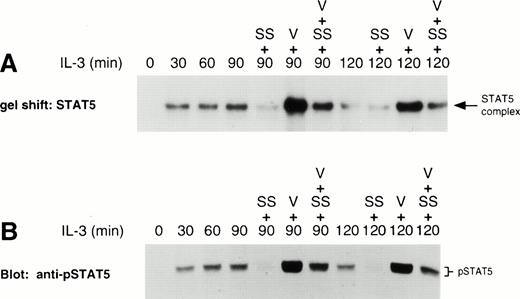
LLnL stabilizes tyrosine phosphorylation of βc and STAT5. IL-3–depleted Ba/F3 cells were treated with or without LLnL for 3 hours as indicated, then stimulated with IL-3 for 0 to 2 hours. (A) βc immune complexes (5 × 107 cells) were separated by SDS-PAGE (7% gel), transferred, and immunoblotted with RC20 (anti-ptyr) antibody. (B) Nuclear extracts from a separate experiment were prepared and the binding to 32P-labeled oligonucleotide STAT5 probe determined. (C) The same extracts as in B (75 μg) were separated by SDS-PAGE (7.5% gel), transferred, and immunoblotted with a specific anti-phosphotyrosine-STAT5 antisera. (D) NP-40 extracts (50 μg) from a similar experiment were separated by SDS-PAGE (6% gel), transferred, and immunoblotted with anti-STAT5b antibody. In panels A through D, the position of relevant bands are indicated.
LLnL stabilizes tyrosine phosphorylation of βc and STAT5. IL-3–depleted Ba/F3 cells were treated with or without LLnL for 3 hours as indicated, then stimulated with IL-3 for 0 to 2 hours. (A) βc immune complexes (5 × 107 cells) were separated by SDS-PAGE (7% gel), transferred, and immunoblotted with RC20 (anti-ptyr) antibody. (B) Nuclear extracts from a separate experiment were prepared and the binding to 32P-labeled oligonucleotide STAT5 probe determined. (C) The same extracts as in B (75 μg) were separated by SDS-PAGE (7.5% gel), transferred, and immunoblotted with a specific anti-phosphotyrosine-STAT5 antisera. (D) NP-40 extracts (50 μg) from a similar experiment were separated by SDS-PAGE (6% gel), transferred, and immunoblotted with anti-STAT5b antibody. In panels A through D, the position of relevant bands are indicated.
Because IL-3 is known to activate STAT5,31-34 we then examined the effect of LLnL on STAT5 activity. In the absence of proteasome inhibitor, IL-3 induced a rapid nuclear accumulation of STAT5 DNA-binding activity, which was maximal within 30 minutes of stimulation (Fig 1B). DNA-binding activity then declined to near baseline levels over the next 90 minutes. We have previously shown that the complex bound to this probe can be specifically competed with excess unlabeled oligonucleotide and supershifted with an anti-STAT5 antisera.34 In the presence of LLnL, IL-3 still induced a rapid accumulation in STAT5 DNA-binding activity. However, the subsequent decrease in DNA-binding activity was not observed and, if anything, DNA binding tended to increase over the 2-hour period (Fig1B).
The effects of LLnL on STAT5 regulation were confirmed by Western analysis of nuclear extracts using a specific antibody raised against the phosphotyrosine of STAT5.41 42 As seen in Fig 1C, the pattern of phospho-STAT5 immunoreactivity was consistent with that of DNA-binding activity. We also observed a slightly smaller immunoreactive protein that was coordinately regulated with the wild-type STAT5 protein and likewise was stabilized in the presence of LLnL. This smaller protein was not always observed in phospho-STAT5 blots and may represent the activation of a C-terminally truncated isoform of STAT5, as it was not immunoreactive with a C-terminal anti-STAT5 antibody (data not shown).
The pattern of total STAT5 immunoreactivity from NP-40 cellular extracts was also consistent with STAT5 DNA-binding activity and phosphotyrosine-STAT5 immunoreactivity (Fig 1D). In the absence of stimulation, both LLnL-treated and control cells gave rise to a single immunoreactive band, which represents nonphosphorylated STAT5 (data not shown).43 Upon stimulation with IL-3, there was induction of slower migrating band(s), most likely composed of both the tyrosine- and serine-phosphorylated forms of STAT5.43 In the absence of LLnL, the total STAT5 signal was split between the two bands. The upper band, representing phosphorylated STAT5, was maximally induced with IL-3 by 15 minutes. This band was then titrated back into the faster migrating species over the next 105 minutes. In the presence of LLnL, the accumulation of the upper band was not transient, but persisted over the entire time course. Importantly, under both conditions, the total STAT5 immunoreactivity remained constant over the time course of the experiment. Together, these results show that LLnL treatment also results in stabilization of IL-3 downstream signaling events, namely, STAT5 activation. Furthermore, these results argue in favor of a specific phosphatase inactivating STAT5, and suggest that dephosphorylation of STAT5 in the absence of LLnL is not the result of indiscriminate dephosphorylation upon cell lysis.
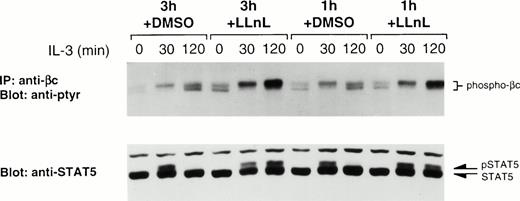
We considered the possibility that the influence of LLnL on IL-3 signaling could have been the result of an indirect effect due to the relatively long preincubation period or to the presence of carrier (DMSO) used in the previous experiments. The use of a shorter, 1-hour pretreatment with LLnL proved to be as effective as the longer 3-hour period in stabilizing the tyrosine phosphorylation of both βc and STAT5 (Fig 2) and had negligible effects on cell viability. Pretreatment with carrier alone resulted in normal phosphorylation kinetics (compare Figs 1 and 2). That LLnL is relatively fast-acting suggests its mechanism of action is specific and not due to general cell toxic effects.
Stable tyrosine phosphorylation of βc and STAT5 requires only a short treatment with LLnL. IL-3–depleted Ba/F3 cells were treated with LLnL or carrier (DMSO) for 1 or 3 hours as indicated, then stimulated with IL-3 for 0 to 2 hours. βc immune complexes (107 cells) were separated by SDS-PAGE (7% gel), transferred, and immunoblotted with RC20 antibody. Whole-cell extracts were also prepared by lysis in 0.1% SDS and 25-μg samples were separated by SDS-PAGE (6% gel), transferred, and immunoblotted with anti-STAT5b antibody. The position of phospho-βc, pSTAT5, and nonphosphorylated STAT5 is indicated.
Stable tyrosine phosphorylation of βc and STAT5 requires only a short treatment with LLnL. IL-3–depleted Ba/F3 cells were treated with LLnL or carrier (DMSO) for 1 or 3 hours as indicated, then stimulated with IL-3 for 0 to 2 hours. βc immune complexes (107 cells) were separated by SDS-PAGE (7% gel), transferred, and immunoblotted with RC20 antibody. Whole-cell extracts were also prepared by lysis in 0.1% SDS and 25-μg samples were separated by SDS-PAGE (6% gel), transferred, and immunoblotted with anti-STAT5b antibody. The position of phospho-βc, pSTAT5, and nonphosphorylated STAT5 is indicated.
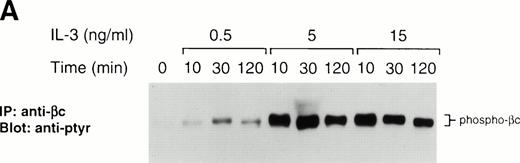
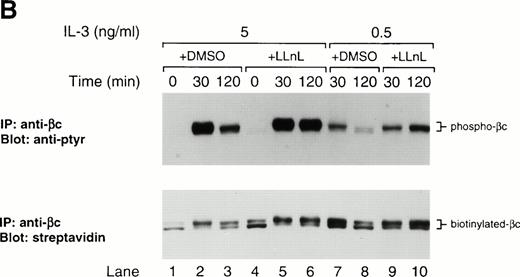
In yeast, the role of ubiquitination in the internalization of Ste2p following α-factor stimulation has been demonstrated.15Therefore, since ubiquitinated forms of growth hormone receptor44 and c-kit16 have been detected and postulated to be important in modulating signaling, we hypothesized that βc might also be ubiquitinated following IL-3 stimulation and that LLnL treatment may somehow prevent this modification or the subsequent internalization of βc, leading to a prolonged signal. We failed to detect laddering or smearing typical of multiubiquitinated proteins. However, close inspection of βc immunoblots following stimulation shows that in highly phosphorylated lanes, the slower migrating species has a shadow band, which might represent a further modified, possibly monoubiquitinated, receptor (Figs 1A and 3). The lack of a good anti-ubiquitin antibody prevented us from examining this possibility more directly. Nevertheless, we attempted cell-surface biotinylation experiments to see whether LLnL prevented βc internalization. Initial experiments failed to provide any evidence of receptor internalization in untreated cells. This may have been a consequence of using low concentrations of IL-3, which might only activate a small fraction of the surface receptor pool, thereby making it difficult to detect receptor internalization. An IL-3 dose-response experiment was performed and showed that maximal surface receptor activation, as evident by βc phosphorylation, was obtained at a concentration of 5 ng/mL (Fig 3A). Using this concentration of IL-3, we performed cell-surface biotinylation experiments. As shown in Fig 3B, the effect of LLnL on the stabilization of βc phosphorylation is more pronounced at a stimulating IL-3 concentration of 5 ng/mL (lanes 1 to 6) compared with 0.5 ng/mL (lanes 7 to 10). After stripping and reblotting with streptavidin, the biotinylation pattern of surface βc receptors did not appear to be significantly altered by LLnL treatment (Fig 3B). Furthermore, this result suggests that the receptor is not being degraded following stimulation, since under IL-3 concentrations that maximally activate the cell-surface receptor pool, no significant loss in signal was detected.
The effect of LLnL on surface biotinylation of βc. (A) Ba/F3 cells were depleted of IL-3 for 12 hours then stimulated with IL-3 (0.5 to 15 ng/mL) for 0 to 2 hours as indicated. βc immune complexes (107 cells) were separated by SDS-PAGE (7% gel), transferred, and immunoblotted with RC20 antibody. (B) IL-3–depleted Ba/F3 cells were treated with LLnL or carrier for 1 hour and stimulated with IL-3 for 0 to 2 hours before cell-surface biotinylation was performed. βc immune complexes (5 × 107 cells) were separated by SDS-PAGE (7% gel), transferred, and immunoblotted with RC20 antibody. After stripping, the membrane was reblotted with streptavidin:HRP. The position of phospho-βc and biotinylated-βc is indicated.
The effect of LLnL on surface biotinylation of βc. (A) Ba/F3 cells were depleted of IL-3 for 12 hours then stimulated with IL-3 (0.5 to 15 ng/mL) for 0 to 2 hours as indicated. βc immune complexes (107 cells) were separated by SDS-PAGE (7% gel), transferred, and immunoblotted with RC20 antibody. (B) IL-3–depleted Ba/F3 cells were treated with LLnL or carrier for 1 hour and stimulated with IL-3 for 0 to 2 hours before cell-surface biotinylation was performed. βc immune complexes (5 × 107 cells) were separated by SDS-PAGE (7% gel), transferred, and immunoblotted with RC20 antibody. After stripping, the membrane was reblotted with streptavidin:HRP. The position of phospho-βc and biotinylated-βc is indicated.
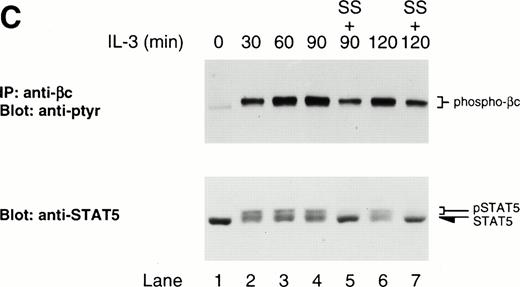
At this point, we turned our attention to the activity of JAKs. Using the pharmacologic inhibitors, staurosporine and orthovanadate, we tried to determine whether JAK was a target of LLnL. To this end, Ba/F3 cells were stimulated with IL-3 in the presence of LLnL. After 30 and 60 minutes of stimulation, either staurosporine, vanadate, or both were added to the culture and incubation continued for a further 60 minutes. Again, addition of LLnL stabilized the DNA-binding activity of STAT5 at both 90 and 120 minutes (Fig4A; data not shown). When staurosporine was added, it abolished the LLnL-induced stabilization of STAT5 DNA-binding activity at both 90 and 120 minutes. The addition of vanadate to the cells gave rise to an enhanced level of STAT5 DNA-binding activity compared with LLnL alone, while the addition of both vanadate and staurosporine gave rise to intermediate levels of DNA-binding activity compared with either agent alone. Identical results were obtained by Western analysis using an antiphosphotyrosine-STAT5 antibody (Fig 4B).
Staurosporine prevents LLnL-induced stabilization of STAT5 and βc phosphorylation. IL-3–depleted Ba/F3 cells were treated with LLnL for 3 hours then stimulated with IL-3 for 0 to 2 hours. (A) After 30 and 60 minutes stimulation, staurosporine and vanadate were added as indicated and the incubation continued for a further 60 minutes. Nuclear extracts were prepared and the binding to32P-labeled oligonucleotide STAT5 probe determined. (B) Nuclear extracts from A (25 μg) were separated by SDS-PAGE (6% gel), transferred, and immunoblotted with antiphosphotyrosine-STAT5 antibody. (C) In a separate experiment, after 30 and 60 minutes stimulation, staurosporine was added and the incubation continued for a further 60 minutes. βc immune complexes (107 cells) were separated by SDS-PAGE (7% gel), transferred, and immunoblotted with RC20 antibody. Whole-cell extracts were also prepared by lysis in 0.1% SDS and 50 μg samples were separated by SDS-PAGE (6% gel), transferred, and immunoblotted with anti-STAT5b antibody. In panels A through C, the position of relevant bands are indicated.
Staurosporine prevents LLnL-induced stabilization of STAT5 and βc phosphorylation. IL-3–depleted Ba/F3 cells were treated with LLnL for 3 hours then stimulated with IL-3 for 0 to 2 hours. (A) After 30 and 60 minutes stimulation, staurosporine and vanadate were added as indicated and the incubation continued for a further 60 minutes. Nuclear extracts were prepared and the binding to32P-labeled oligonucleotide STAT5 probe determined. (B) Nuclear extracts from A (25 μg) were separated by SDS-PAGE (6% gel), transferred, and immunoblotted with antiphosphotyrosine-STAT5 antibody. (C) In a separate experiment, after 30 and 60 minutes stimulation, staurosporine was added and the incubation continued for a further 60 minutes. βc immune complexes (107 cells) were separated by SDS-PAGE (7% gel), transferred, and immunoblotted with RC20 antibody. Whole-cell extracts were also prepared by lysis in 0.1% SDS and 50 μg samples were separated by SDS-PAGE (6% gel), transferred, and immunoblotted with anti-STAT5b antibody. In panels A through C, the position of relevant bands are indicated.
To eliminate the possibility that staurosporine was toxic, cytosolic extracts from the previous experiment were separated by SDS-PAGE and anti-ptyr and total STAT5 immunoreactivity determined. Total anti-ptyr immunoreactivity was considerably reduced in the staurosporine-treated sample, whereas the amount of immunoreactive STAT5 was comparable with untreated samples (data not shown). This result demonstrated that, over the indicated time course, the effect of staurosporine was due to the inhibition of tyrosine kinase activity and not to the accelerated loss of protein from the cell arising from toxic effects.
Since JAK also phosphorylates βc, we examined whether staurosporine could prevent the LLnL-induced stable phosphorylation of βc. As seen in Fig 4C, the presence of staurosporine diminished the phosphorylation signal compared with LLnL alone (compare lanes 4 with 5 and 6 with 7). Together, these experiments indicate that the stable phosphorylation of both βc and STAT5 in the presence of LLnL requires persistent kinase activity, suggesting the effect of LLnL is to prolong JAK activity. It is noteworthy that staurosporine only partially decreased βc phosphorylation, while it almost completely abolished the phosphorylation of STAT5 (Fig 4C), suggesting that the stabilization of βc phosphorylation by LLnL may not entirely be due to prolonged JAK activity.
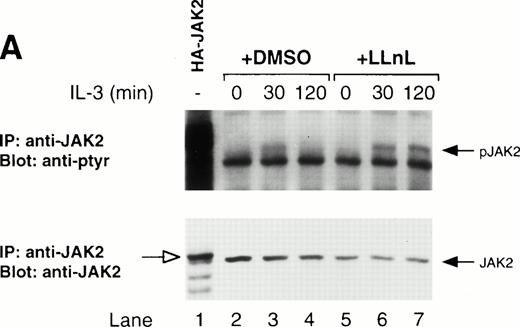
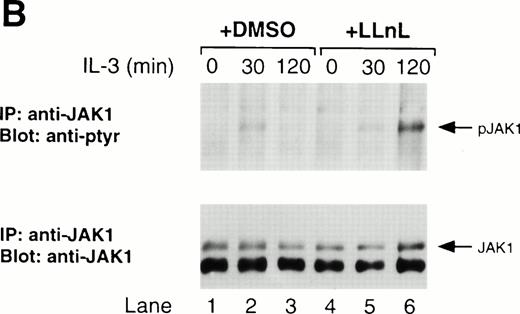
Both JAK1 and JAK2 have been reported to be activated by IL-3.17-22 In our hands, preliminary experiments showed that JAK2 was the principal kinase activated by IL-3, but some tyrosine phosphorylation of JAK1 was detected (data not shown; Fig5). The phosphorylation of both JAK2 and JAK1 was transient with both being induced within 30 minutes of stimulation and declining to basal levels by 120 minutes. In contrast, the treatment of cells with LLnL prevented the dephosphorylation of both JAK1 and JAK2. It was possible that LLnL affected the stability of JAK1 and JAK2; however, JAK1 and JAK2 immunoblots showed a similar amount of protein in all samples (Fig 5). The increased total JAK1 level at 120 minutes in the presence of LLnL (Fig 5B, lane 6) is due to an increased loading compared with other samples (data not shown). Together, these results show that the effect of LLnL on IL-3 signaling is to prolong the activity of JAK2 and JAK1, presumably through its stabilizing effect on JAK tyrosine phosphorylation.
LLnL stabilizes tyrosine phosphorylation of JAK2. IL-3–depleted Ba/F3 cells were treated with or without LLnL as indicated, and stimulated with IL-3 (1 ng/mL) for 0 to 2 hours. Extracts from 5 × 107 cells were immunoprecipitated with either anti-JAK2 (A) or anti-JAK1 (B) antibodies. JAK2 immune complexes were divided and electrophoresed on duplicate 7% SDS-PAGE gels, transferred, and immunoblotted with either anti-JAK2 or RC20 antibodies as indicated. JAK1 immune complexes were separated by SDS-PAGE (7% gel), transferred, and immunoblotted with RC20 antibody, stripped, and reblotted with anti-JAK1 antibody as indicated. The positions of JAK1, phosphorylated JAK1 (pJAK1), JAK2, and phosphorylated JAK2 (pJAK2) are indicated by solid arrows. The open arrow in A indicates the migration of hemagglutinin-tagged JAK2 (HA-JAK2) expressed in COS-7 cells.
LLnL stabilizes tyrosine phosphorylation of JAK2. IL-3–depleted Ba/F3 cells were treated with or without LLnL as indicated, and stimulated with IL-3 (1 ng/mL) for 0 to 2 hours. Extracts from 5 × 107 cells were immunoprecipitated with either anti-JAK2 (A) or anti-JAK1 (B) antibodies. JAK2 immune complexes were divided and electrophoresed on duplicate 7% SDS-PAGE gels, transferred, and immunoblotted with either anti-JAK2 or RC20 antibodies as indicated. JAK1 immune complexes were separated by SDS-PAGE (7% gel), transferred, and immunoblotted with RC20 antibody, stripped, and reblotted with anti-JAK1 antibody as indicated. The positions of JAK1, phosphorylated JAK1 (pJAK1), JAK2, and phosphorylated JAK2 (pJAK2) are indicated by solid arrows. The open arrow in A indicates the migration of hemagglutinin-tagged JAK2 (HA-JAK2) expressed in COS-7 cells.
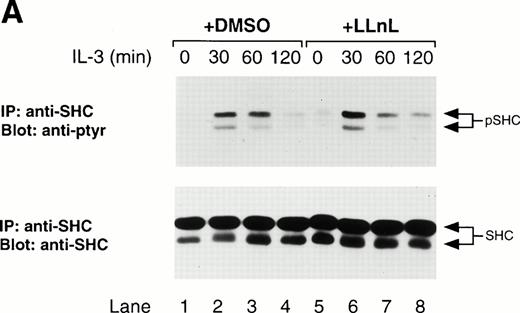
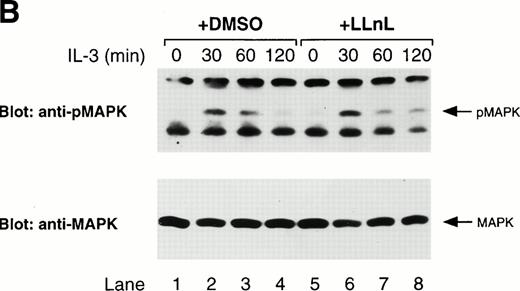
Since JAK activation is the initial event in activation of theRas/Raf-1/MAPK pathway, a prolonged activation of JAK should also affect components of this pathway. The adaptor molecule, Shc, which serves as a link between receptor phosphorylation and activation of Ras, can be phosphorylated by JAK2 in vitro45and is phosphorylated on tyrosine in response to IL-3 stimulation.46 Therefore, we examined the effect of LLnL treatment on Shc, as well as MAPK phosphorylation. As seen in Fig6, treatment of cells with LLnL resulted in a prolonged phosphorylation of both the 46- and 52-kD forms of Shc, as well as MAPK, compared with untreated cells. The differences in tyrosine phosphorylation were not attributable to differences in total Shc or MAPK levels (Fig 6). Together, these data support the conclusion that LLnL prolongs JAK activation.
LLnL stabilizes the phosphorylation of Shc and MAPK. IL-3–depleted Ba/F3 cells were treated with or without LLnL as indicated, and stimulated with IL-3 (1 ng/mL) for 0 to 2 hours. (A) Extracts from 107 cells were immunoprecipitated with an anti-Shc antibody and immune complexes were separated by SDS-PAGE (10% gel), transferred, and immunoblotted with RC20 antibody, stripped, and reblotted with anti-Shc antibody as indicated. (B) Whole-cell extracts from the same experiment were also prepared by lysis in 0.5% SDS and 50-μg samples were separated by SDS-PAGE (8% gel), transferred, and immunoblotted with phosphospecific MAPK antibody, stripped, and reblotted with MAPK antibody as indicated. The position of phosphorylated Shc (pShc), Shc, phosphorylated MAPK (pMAPK), and MAPK are indicated.
LLnL stabilizes the phosphorylation of Shc and MAPK. IL-3–depleted Ba/F3 cells were treated with or without LLnL as indicated, and stimulated with IL-3 (1 ng/mL) for 0 to 2 hours. (A) Extracts from 107 cells were immunoprecipitated with an anti-Shc antibody and immune complexes were separated by SDS-PAGE (10% gel), transferred, and immunoblotted with RC20 antibody, stripped, and reblotted with anti-Shc antibody as indicated. (B) Whole-cell extracts from the same experiment were also prepared by lysis in 0.5% SDS and 50-μg samples were separated by SDS-PAGE (8% gel), transferred, and immunoblotted with phosphospecific MAPK antibody, stripped, and reblotted with MAPK antibody as indicated. The position of phosphorylated Shc (pShc), Shc, phosphorylated MAPK (pMAPK), and MAPK are indicated.
To rule out the possibility that LLnL can directly inhibit overall tyrosine phosphatase activity, the following experiment was performed. Tyrosine phosphatase assays were performed in vitro on NP-40 extracts from untreated Ba/F3 cells. As illustrated in Fig7, neither DMSO nor LLnL affected total tyrosine phosphatase activity in NP-40 extracts. When orthovanadate was added to the lysate, only background levels of activity were detected. This result demonstrates that LLnL is not functioning directly as a tyrosine phosphatase inhibitor.
LLnL does not inhibit tyrosine phosphatase activity. Growing Ba/F3 cells were washed and lysed with NP-40 buffer in the absence of vanadate. Lysate from 105 cells was assayed for total tyrosine phosphatase activity. LLnL (▨) (50 μmol/L) or DMSO (□) as carrier were added to the reaction at a final concentration identical to that used when added to cell cultures. As a positive control, orthovanadate (▧) (1 mmol/L) was also added to inhibit phosphatase activity. Data were normalized to activity obtained from control reactions (no additions [▪]) and each bar represents the average of duplicate samples taken from a single experiment. Identical results were obtained from several experiments using different dilutions of lysate.
LLnL does not inhibit tyrosine phosphatase activity. Growing Ba/F3 cells were washed and lysed with NP-40 buffer in the absence of vanadate. Lysate from 105 cells was assayed for total tyrosine phosphatase activity. LLnL (▨) (50 μmol/L) or DMSO (□) as carrier were added to the reaction at a final concentration identical to that used when added to cell cultures. As a positive control, orthovanadate (▧) (1 mmol/L) was also added to inhibit phosphatase activity. Data were normalized to activity obtained from control reactions (no additions [▪]) and each bar represents the average of duplicate samples taken from a single experiment. Identical results were obtained from several experiments using different dilutions of lysate.
DISCUSSION
The results presented in this study revealed that pretreatment of Ba/F3 cells with the proteasome inhibitor, LLnL, resulted in the sustained tyrosine phosphorylation of both the IL-3 receptor subunit, βc, and STAT5 as opposed to their normal transient nature of induced phosphorylation following stimulation. Both JAK2 and to a lesser extent JAK1 were found to be activated by IL-3, and in the presence of LLnL resulted in persistent tyrosine phosphorylation. Because both βc and STAT5 are phosphorylated by JAK, we believe the sustained phosphorylation of both is most likely due to the prolonged activation of JAK (see later). LLnL required a relatively short preincubation period to elicit its full effects on βc and STAT5 phosphorylation, suggesting its effect is specific and unlikely to be due to general effects on cell viability.
Treatment of Ba/F3 cells with LLnL also resulted in a more prolonged activation of the Ras/Raf-1/MAPK pathway. This was evident by the prolonged tyrosine phosphorylation of the adaptor molecule, Shc, as well as MAPK. The effects of LLnL treatment were not as dramatic on components of the MAPK pathway compared with those of the JAK/STAT pathway. Despite the prolonged phosphorylation of Shc and MAPK compared with untreated cells, both were dephosphorylated to some extent in the presence of LLnL. This difference can be explained by the possibility that the rates of phosphorylation and dephosphorylation may be sufficiently different for components of the MAPK pathway such that a sustained activation loop is not as easily established and dramatic as it is for the JAK/STAT pathway. Nevertheless, the results obtained with Shc and MAPK are consistent with and support the conclusion that LLnL treatment results in prolonged activation of JAK.
It is worth noting that in the absence of LLnL, both βc and STAT5 appeared to have different rates of tyrosine dephosphorylation (Figs 1and 2). This was also evident when staurosporine was used to inhibit JAK activity (Fig 4C). The addition of staurosporine in the presence of LLnL almost completely abrogated the stabilizing effect of LLnL on STAT5 phosphorylation, while the effect on βc phosphorylation resulted in only an approximate 50% reduction in signal (Fig 4C). These observations suggest two possibilities. First, although JAK mediates both these events, it may be that the prolonged activation of JAK, in the presence of LLnL, accounts for the sustained phosphorylation of STAT5, but may not be entirely responsible for sustaining that of βc, suggesting that LLnL may induce other effects that contribute to prolonged receptor phosphorylation. In yeast, ubiquitination of the Ste2p receptor signals its endocytosis,15 and a recent study has demonstrated a similar role for ubiquitin in growth hormone receptor internalization.47 Although we found no significant effect of LLnL on βc surface biotinylation, it is possible that LLnL could also affect receptor internalization of βc and thus help prevent signal downmodulation. Future pulse-chase experiments using radiolabeled ligand will help to confirm or reject this possibility. Second, the phosphatase responsible for inactivating STAT5 has yet to be identified, whereas SHP-1 has been implicated to bind to and dephosphorylate βc.23 However, the different rates of dephosphorylation of STAT5 and βc suggest the two are likely substrates of different phosphatases.
It has been proposed that STATs may be inactivated by proteolytic degradation by the 26S proteasome and furthermore, ubiquitinated forms of phosphorylated STAT1 have been identified in response to IFNγ stimulation.36 However, we found no evidence to indicate that STAT5 may be inactivated via a degradative pathway involving the 26S proteasome. Preliminary experiments using an ectopically expressed tagged ubiquitin have failed to detect ubiquitinated STAT5 (C. Hilton, personal communication, March 1997). Although we and others35,36 48 have shown stabilization of activated STATs using proteasome inhibitors, this is the result of prolonged JAK activation. This conclusion was supported by data obtained using pharmacologic inhibitors.
LLnL failed to stabilize STAT5 activity in the presence of staurosporine. If, as proposed, LLnL was truly stabilizing phosphotyrosine-STAT5 by preventing its degradation, then inhibiting further tyrosine phosphorylation of STAT5 should not affect the ability of LLnL to stabilize that fraction of STAT5 already activated. Clearly, this was not the case (Fig 4). Rather, it appeared that LLnL induced its effects by preventing the signal for STAT5 phosphorylation from being downmodulated. As expected, the presence of orthovanadate alone resulted in an enhanced stabilization of STAT5 activity. Several points in the JAK/STAT pathway could be affected by phosphatase inhibition, leading to increased STAT5 activity, including the dephosphorylation of either the IL-3 receptor, JAK or STAT5. The presence of vanadate offset the effect of staurosporine, resulting in the persistence of STAT5 activity, albeit to a lesser extent than for vanadate alone (Fig 4 and data not shown). Thus, phosphorylated STAT5 could be stabilized by phosphatase inhibition, but not by proteasome inhibition per se. These results support the conclusion that the accumulation of active STAT5 in the presence of LLnL requires the persistent phosphorylation of STAT5 by JAK. By a similar argument, the LLnL-induced sustained phosphorylation of βc also requires persistent JAK activity, since staurosporine offset LLnL's effect on βc phosphorylation. The persistent phosphorylation of βc would allow STAT5 to continually dock onto the receptor and be phosphorylated by JAK and thus establish a persistent activation loop.
The transient nature of STAT5 activity observed in this study supports the model that its activity is upregulated by phosphorylation and downregulated principally by dephosphorylation (Fig 1D). Consistent with this model is the observation that naturally occurring dominant negative isoforms of STAT5 and C-terminally truncated mutants of STAT5 are stably phosphorylated in response to cytokine stimulation,49 50 implying that the C-terminus of STAT5 is crucial for dephosphorylation. Furthermore, the loss of tyrosine-phosphorylated STAT5 in the combined presence of staurosporine and LLnL suggests that the activity of the STAT5-specific phosphatase is unaffected by proteasome inhibitors. The identification of STAT-specific phosphatases still remains one of the outstanding questions in the field.
The normal inactivation of JAKs could be mediated by at least two possible mechanisms. First, dephosphorylation of JAK could lead to loss in activity. The SH2-containing protein tyrosine phosphatases, SHP-1 and SHP-2, have been implicated in the dephosphorylation of both JAK2 and JAK1.51-55 An attractive possibility could be that these candidate phosphatases may require proteasomal processing for activation or at least their activity may be modulated by proteasome function, perhaps by degrading an inhibitor complex akin to the degradation of IκB and subsequent activation of NF-κB.10-14 In support of this, SHP-1 has been shown to be degraded by ubiquitin-dependent proteolysis in mast cells expressing oncogenic c-kit,37 suggesting the proteasome regulates SHP-1 function. It is possible that LLnL could nonspecifically inhibit phosphatase activity, including that of SHP-1 and SHP-2; however, this is unlikely to be a general effect of LLnL, since STAT5 was still dephosphorylated in the combined presence of LLnL and staurosporine (Fig 4). Furthermore, the data presented in Fig 7argue against the possibility that LLnL functions as a tyrosine phosphatase inhibitor.
A second possibility involves the cytokine-induced expression of the newly identified, CIS-related, STAT-induced STAT-inhibitor (SSI) family of proteins.56-58 These proteins, once expressed, could negatively feedback and inhibit JAK activity by binding to and inactivating the kinase domain. Therefore, it is possible that LLnL's effect on JAK activity could be a combined result of modulation of SHP-1 or SHP-2 activity and inhibited expression of or function of SSI family proteins.
IL-3 has also been shown to induce the deubiquitinating enzyme, DUB-1.59 As we have shown that the proteasome can modulate JAK activity, it is possible that a deubiquitinating enzyme, such as DUB-1, might affect JAK activity. In view of our current lack of knowledge about DUB-1 substrate specificity, it is hard to evaluate its role in this process. Future studies on this family of proteins should help to address this question.
Recently, it has been shown that both JAK1 and JAK3 activities are stabilized by proteasome inhibition following IL-2 induction of T cells.48 The authors of that study attributed the effect on JAK to modulation of phosphatase activity by proteasome-mediated protein degradation. Similarly, we believe that the effect on JAK by LLnL is mediated by a similar mechanism in murine progenitor cells. In addition, the growing body of evidence derived from multiple cell lines activated by various cytokines suggests that the normal downregulation of the JAK/STAT pathway following cytokine activation requires functional proteasomes. How the proteasome modulates the deactivation of JAK is unknown and remains the focus of future studies.
ACKNOWLEDGMENT
We thank David Frank and Jan Tavernier for generously providing antibodies. We also thank Craig Hilton for his advice and for performing STAT5-ubiquitination studies, and Alan D. D'Andrea and Yongjui Jin for helpful discussion.
Supported in part by National Institutes of Health Grant No. P50 DK49216 (to B.M.-P.) and by the Genetics Institute, Cambridge, Boston, MA (B.M.-P.).
Address reprint requests to Bernard A. Callus, PhD, Dana-Farber Cancer Institute, Department of Pediatric Oncology, 44 Binney St, Boston, MA, 02115.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 7. LLnL does not inhibit tyrosine phosphatase activity. Growing Ba/F3 cells were washed and lysed with NP-40 buffer in the absence of vanadate. Lysate from 105 cells was assayed for total tyrosine phosphatase activity. LLnL (▨) (50 μmol/L) or DMSO (□) as carrier were added to the reaction at a final concentration identical to that used when added to cell cultures. As a positive control, orthovanadate (▧) (1 mmol/L) was also added to inhibit phosphatase activity. Data were normalized to activity obtained from control reactions (no additions [▪]) and each bar represents the average of duplicate samples taken from a single experiment. Identical results were obtained from several experiments using different dilutions of lysate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3182/3/m_blod40912007y.jpeg?Expires=1767848953&Signature=CFfFReOhoFCdcWSNoNho6-Y4E0pr4f2VMaJhJkoIfzknHQk6rQ23BSovdGe3UpOg06TpSmB1jhjZPlVxeLpm~sPHA7U6e6feMLFfVS5Sa4Ldd6lduUJHeXEWQbQUkipncDv8JnJLKxMGYJLCRm2KKK2fFWJr87oLS90BCk0NvodpykmtpnwUhVgf62EgxENOhuMBY~2nuOlLS9WVaLj6PH1JDhyBUk01ENGCRZi0XPZweu-bO9n8x6NeSzwwfCQemIawCROqou6Bpq1VfrNG3Rx795ojofOCNEvQsbcaW-fElp64qhIj4ET8jYISHBQb1JGfuCznyih1Lo8YlegWcw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal