Abstract
BCR-ABL antisense oligodeoxynucleotides (ODN) have provided evidence of antileukemia effect when tested in vitro against Philadelphia-positive (Ph-pos) cells and in vivo when injected into leukemic mice. On the basis of the results obtained in vitro at diagnosis, eight patients with chronic myelogenous leukemia (CML) were selected and submitted to autologous bone marrow transplantation (ABMT) with bone marrow (BM) cells purged in vitro with junction-specific (J-sp) BCR-ABL antisense ODN at the time of transformation in accelerated phase or during second chronic phase. Mononuclear BM cells were treated in vitro for 24 or 72 hours with 150 μg/mL of antisense ODN yielding a median recovery of 47.6% mononuclear cells, 48.8% CD34+ cells, and 20.3% clonogenic cells. After a conditioning regimen including busulphan and etoposide, the reinfused treated cells allowed engraftment and hematologic reconstitution in all patients. Evaluation of the antileukemic effect by standard cytogenetic analysis and fluorescence in situ hybridization showed a complete karyotypic response in two cases and a minimal or no response in the other six. The patient autografted in second chronic phase died in blast crisis 7 months after ABMT; of the seven patients autografted in transformation, three developed blast crisis 21 to 39 months after reinfusion, one died from unrelated BMT complications 30 months after ABMT, and three are in persistent second chronic phase 14 to 26 months after autograft. The low toxicity of the protocol and the hemopoietic reconstitution observed in all patients make this approach feasible; the marked karyotypic response observed in some patients and the duration of the second chronic phase show that ODN-mediated BM purging and autograft is a promising treatment for this high-risk group of CML.
CHRONIC MYELOGENOUS leukemia is ideally suited for experimental therapeutic approaches with antisense ODN1 because the Philadelphia chromosome translocation results in the generation of BCR-ABL fusion gene2,3 that encodes for leukemia-specific transcripts. Furthermore, CML remains a fatal disease that might benefit from experimental approaches. A number of studies have shown that BCR-ABL ODN have antileukemia effects in vitro and in mice,4,5 suggesting that suppression of Philadelphia-positive (Ph-pos) cell growth may be due, at least in part, to a specific mechanism. One of the problems associated with the systemic administration of ODN in patients with CML may relate to the nonspecific effects of these compounds and to the possible toxicity toward normal marrow progenitors that could limit their use as in vitro purging agents and as drugs for systemic therapy. The application of antisense ODN in bone marrow (BM) purging before autologous BM transplantation (ABMT) has been recently reported6,7; in these studies, however, the c-myb gene was the target for antisense ODN therapy using CD34+-enriched cells that were reinfused in patients subjected to high-dose chemotherapy. We have recently reported that in vitro purging of CML mononuclear cells with BCR-ABL antisense ODN does not prevent hematologic reconstitution after ABMT in a patient in accelerated phase.8 In that study, cells were incubated for 24 hours with the 26-mer phosphorothioate B2A2 antisense ODN, and the patient showed marrow engraftment and hematologic reconstitution after a period of time comparable to that necessary for engraftment using unpurged ABMT.
We now report the results obtained in eight patients with CML autografted in an advanced phase of the disease. Patients were selected for purged ABMT on the basis of the clinical status and in vitro tests performed at diagnosis, which identified as “responders” 62% of the patients tested.9 In the first three autografts, cells were incubated with ODN for 24 hours; in the subsequent five patients the incubation time was prolonged to 72 hours, based on the evidence that a more pronounced inhibitory effect is obtained following a more prolonged incubation with ODN and in view of the stable engraftment observed in the first three patients after a preincubation of 24 hours. The evaluation of Ph-pos cells before and after ABMT by standard cytogenetics and fluorescence in situ hybridization (FISH) in interphase nuclei together with the clinical follow-up have shown the feasibility and potential benefits of this combined procedure for patients with CML in advanced phase of the disease.
PATIENTS AND METHODS
Patients.
From a group of 35 patients with Ph-pos CML tested in vitro at diagnosis for inhibition of methylcellulose colony formation with 26-mer BCR-ABL antisense ODN, 8 patients were selected for in vitro purging with junction-specific antisense ODN followed by ABMT. Selection was based on the in vitro sensitivity to antisense ODN, which discriminated between “responders” (cases showing a significative inhibition of clonogenic Ph-pos cells by the specific antisense ODN as compared with untreated cells) and “nonresponders” (cases in which the inhibition of colony formation was not significant), and on the clinical status (age less than 55 years; good performance status; no cardiac, renal, or hepatic alterations). Clinical and hematological data of the patients at diagnosis and at the time of ABMT are reported in Table 1. Polymerase chain reaction (PCR) analysis revealed the presence of the B2A2junction in five cases and of the B3A2 junction in three. All patients received interferon-α (IFN-α) as standard treatment after diagnosis, obtaining a 24-months (range, 3 to 52) median duration of the first chronic phase. ABMT was performed in accelerated phase in seven cases and in second chronic phase in one; in all cases, BM harvest and in vitro purging were performed immediately before starting the conditioning regimen.
Characteristics at Diagnosis and at ABMT
| Patients . | Characteristics at Diagnosis . | Characteristics at ABMT . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age/ Sex . | Disease Phase . | Breakpoint . | Hb g/dL . | WBC ×109/L . | PLTS ×109/L . | Liver Size . | Spleen Size . | Therapy . | Duration 1 CP (mo) . | Disease Phase . | % BM Blast Cells . | |
| 1 | 30/M | CP | B2A2 | 13 | 130 | 300 | Not palpable | Not palpable | IFN-α/HU | 3 | AP | 10 |
| 2 | 20/M | CP | B3A2 | 12 | 380 | 500 | Not palpable | Enlarged | IFN-α | 22 | AP | 7 |
| 3 | 39/M | CP | B3A2 | 14 | 64 | 294 | Not palpable | Not palpable | IFN-α | 26-150 | 2nd CP | — |
| 4 | 21/F | CP | B2A2 | 14 | 31 | 800 | Enlarged | Enlarged | IFN-α | 18 | AP | 10 |
| 5 | 41/F | CP | B3A2 | 10 | 127 | 900 | Not palpable | Massive | IFN-α | 35 | AP | 15 |
| 6 | 29/F | CP | B2A2 | 7 | 300 | 500 | Enlarged | Enlarged | IFN-α | 21 | AP | 10 |
| 7 | 47/M | CP | B2A2 | 13 | 130 | 300 | Enlarged | Not palpable | IFN-α | 33 | AP | 8 |
| 8 | 39/M | CP | B2A2 | 12 | 17 | 260 | Not palpable | Not palpable | IFN-α | 52 | AP | 15 |
| Patients . | Characteristics at Diagnosis . | Characteristics at ABMT . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age/ Sex . | Disease Phase . | Breakpoint . | Hb g/dL . | WBC ×109/L . | PLTS ×109/L . | Liver Size . | Spleen Size . | Therapy . | Duration 1 CP (mo) . | Disease Phase . | % BM Blast Cells . | |
| 1 | 30/M | CP | B2A2 | 13 | 130 | 300 | Not palpable | Not palpable | IFN-α/HU | 3 | AP | 10 |
| 2 | 20/M | CP | B3A2 | 12 | 380 | 500 | Not palpable | Enlarged | IFN-α | 22 | AP | 7 |
| 3 | 39/M | CP | B3A2 | 14 | 64 | 294 | Not palpable | Not palpable | IFN-α | 26-150 | 2nd CP | — |
| 4 | 21/F | CP | B2A2 | 14 | 31 | 800 | Enlarged | Enlarged | IFN-α | 18 | AP | 10 |
| 5 | 41/F | CP | B3A2 | 10 | 127 | 900 | Not palpable | Massive | IFN-α | 35 | AP | 15 |
| 6 | 29/F | CP | B2A2 | 7 | 300 | 500 | Enlarged | Enlarged | IFN-α | 21 | AP | 10 |
| 7 | 47/M | CP | B2A2 | 13 | 130 | 300 | Enlarged | Not palpable | IFN-α | 33 | AP | 8 |
| 8 | 39/M | CP | B2A2 | 12 | 17 | 260 | Not palpable | Not palpable | IFN-α | 52 | AP | 15 |
Abbreviations: Hb, hemoglobin; WBC, white blood cell; PLTS, platelets; CP, chronic phase; AP, accelerated phase.
This patient developed lymphoid blast crisis with extramedullary localization and was treated with high-dose ARA-C + etoposide + adryamycin.
Oligodeoxynucleotides.
Phosphorothioate ODN ([S]ODN) were synthesized on a Lynx-made DNA synthesizer (Lynx Therapeutics, Hayward, CA) by means of β-cyanoethilphosphoramidite chemistry. The sequences of the apyrogenic sterile 26-mer B2A2 and B3A2 antisense ODN used in these studies were as follows: 5′-CGC TGA AGG GCT TCT TCC TTA TTG AT-3′ (B2A2) and 5′-CGC TGA AGG GCT TTT GAA CTG TGC TT-3′ (B3A2). Controls included the antisense not corresponding to the patient m-RNA junction, sense sequences, and a 12-mer ODN containing a two-base mismatched and the TAT motif (5′-CTC TTT CCT TAT-3′), postulated to nonspecifically inhibit proliferation of Ph-pos cells.
In vitro purging and colony assay.
BM (10 to 15 mL/kg body weight) was aspirated from the pelvis under general anesthesia and collected in transfer packs (Baxter, Deerfield, IL) containing heparinized medium. After removing plasma and the majority of red cells by centrifugation, mononuclear cells were separated on a Ficoll density gradient (Nycomed, Oslo, Norway) and resuspended at a concentration of 1 to 1.5 × 107/mL into 175-cm2 vented tissue culture flasks (Falcon; Becton Dickinson, Mississauga, Ontario, Canada), each containing 100 mL of Iscove's medium supplemented with 10% autologous serum and 150 μg/mL of B2A2 or B3A2 antisense ODN. Flasks were incubated for 24 or 72 hours at 37°C in a humidified atmosphere of 5% CO2 in air. At the end of the incubation period, adherent and nonadherent cells were poured into 50-mL conical tubes, centrifuged, washed, and cryopreserved in 40% autologous serum using standard methods.
Control samples (6 × 105 mononuclear cells/mL), either from the eight CML patients or from normal BM donors, were seeded into 24-well cell culture plates in the same conditioned medium and for the same incubation period as the marrow used for reinfusion and were incubated in the absence of ODN or in the presence of sense ODN, antisense ODN not corresponding to the patient m-RNA junction (J-nonsp AS) and a mismatched ODN. After incubation, cells were plated in methylcellulose in the presence of interleukin-3 (IL-3; 20 ng/mL), granulocyte-macrophage colony-stimulating factor (GM-CSF; 5 ng/mL), and fetal calf serum (30%). After 12 days, plates were scanned with an inverted-phase microscope and granulocyte colony-forming unit (CFU-GM)–derived colonies were counted.
Detection of p210 protein.
For the detection of the BCR-ABL protein, untreated or B2A2 and B3A2 antisense ODN–treated cells were lysed and supernatants collected after centrifugation. Electroblotting to nitrocellulose, probing with the anti-abl antibody, and protein detection were as described.9
Conditioning regimen and supportive care.
Conditioning regimen consisted of busulfan (4 mg/kg/d over 4 days) and VP-16 (20 mg/kg/d over 2 days) followed 48 hours later by reinfusion of cryopreserved purged cells. Patients were settled in double rooms in a protected environment and treated with antibiotics, amphotericin, irradiated blood products, and intravenous nutrition as indicated. Those seropositive for herpes simplex virus received prophylactic acyclovir.
Standard cytogenetics.
Cytogenetic evaluation by standard methods was performed on BM cells by direct technique and short-term culture (24 hours) followed by GTG banding. The karyotype was expressed according to standard nomenclature (ISCN 1991).
FISH.
The proportion of Ph-pos cells in the marrow during the follow-up of the autografted patients was investigated on interphase cells with FISH, using a mixture of digoxigenin and biotin-labeled cosmid DNA probes specific for the BCR and ABL genes, respectively (Oncor, Gaithesburg, MD), according to Arnoldus et al,10 with slight modifications based on the manufacturer's instructions. A minimum of 500 evaluable cells in interphase were scored for each sample. The mean percentage of interphase cells with an apparent BCR-ABL fusion signal, evaluated in 15 selected specimens (BM donors and patients in remission for hematologic diseases other than CML) was 3.5% ± 0.5 and was interpreted due to coincidental overlapping of the BCR and ABL signal. A BCR-ABL fusion in the test specimens was, therefore, diagnosed if more than 5.0% (mean ± 3 SD of the controls).
Criteria for BM engraftment and response.
Peripheral blood and marrow samples were taken at regular intervals after autografting for morphologic, cytogenetic, and FISH analyses. Hematologic recovery was defined as the number of days necessary to reach 0.5 × 109/L neutrophils and 50 × 109/L platelets. Cytogenetic and FISH were comparatively evaluated at 30, 90, 180, 365, and 730 days after ABMT or until a second blastic transformation was documented.
RESULTS
Effect of antisense ODN on mononuclear, CD34+, and clonogenic CML cells.
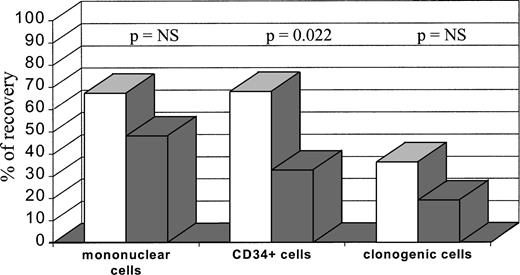
Table 2 shows the number of mononuclear, CD34+, and clonogenic cells obtained and incubated with antisense ODN, together with the number of cells recovered after exposure to conditioned medium without ODN and to junction-specific (J-sp) antisense ODN for 24 or 72 hours. A median of 6.1 × 109 mononuclear cells (range 1.8 to 18), containing a median of 3.6% CD34+ cells (0.7 to 13) and a median of 4.5 × 106 clonogenic cells (range 0.4 to 136), were treated in vitro with antisense ODN, yielding a median recovery of 47.6% (range 30 to 89) mononuclear cells, 48.8% (range 11 to 85) CD34+ cells, and 20.3% (range 0 to 70) clonogenic cells. Incubation of cells in culture medium that did not contain ODN produced a recovery of CD34+ and clonogenic cells similar to the values found before purging. In some cases, incubation with the conditioned medium without ODN caused a slight increase of mononuclear cells (Table 2). As compared with the 24-hour incubation, the 72-hour incubation produced a further reduction of cell recovery, which was statistically significant for CD34+ cells (P = .022) but not for mononuclear and clonogenic cells (Fig1).
Mononuclear Cells, CD34+ Cells and Clonogenic Cells Before and After ODN BM Purging
| Patients . | Incubation Time (h) . | Mononuclear Cells × 109 . | CD34+ Cells × 107 . | Clonogenic Cells × 106 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Purging . | Incubation With Culture Medium . | Incubation With J-sp-AS . | Before Purging . | Incubation With Culture Medium . | Incubation With J-sp-AS . | Before Purging . | Incubation With Culture Medium . | Incubation With J-sp-AS . | ||
| 1 | 24 | 6.7 | 7.2 | 5.5 | 16.7 | 16.9 | 9.9 | 1.0 | 0.9 | 0.7 |
| 2 | 24 | 5.4 | 5.8 | 2.6 | 21.6 | 20.5 | 18.3 | 6.3 | 6.2 | 1.3 |
| 3 | 24 | 1.8 | 1.6 | 1.3 | 1.3 | 1.5 | 0.8 | 0.5 | 0.4 | 0.1 |
| 4 | 72 | 1.9 | 2.7 | 0.9 | 6.3 | 6.3 | 1.4 | 0.4 | 0.4 | 0.2 |
| 5 | 72 | 3.5 | 3.6 | 1.6 | 45.5 | 43.8 | 19.6 | 26.0 | 23.9 | 9.7 |
| 6 | 72 | 14.0 | 15.4 | 4.9 | 32.2 | 32.4 | 3.7 | 2.8 | 3.1 | 0 |
| 7 | 72 | 13.7 | 14.5 | 4.1 | 64.0 | 62.3 | 20.9 | 25.0 | 20.0 | 1.9 |
| 8 | 72 | 18.0 | 21.1 | 16.0 | 111.0 | 104.5 | 60.8 | 136.0 | 119.6 | 6.5 |
| Median | 6.1 | 6.5 | 3.4 | 26.9 | 26.4 | 14.1 | 4.5 | 4.6 | 1.0 | |
| Patients . | Incubation Time (h) . | Mononuclear Cells × 109 . | CD34+ Cells × 107 . | Clonogenic Cells × 106 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Purging . | Incubation With Culture Medium . | Incubation With J-sp-AS . | Before Purging . | Incubation With Culture Medium . | Incubation With J-sp-AS . | Before Purging . | Incubation With Culture Medium . | Incubation With J-sp-AS . | ||
| 1 | 24 | 6.7 | 7.2 | 5.5 | 16.7 | 16.9 | 9.9 | 1.0 | 0.9 | 0.7 |
| 2 | 24 | 5.4 | 5.8 | 2.6 | 21.6 | 20.5 | 18.3 | 6.3 | 6.2 | 1.3 |
| 3 | 24 | 1.8 | 1.6 | 1.3 | 1.3 | 1.5 | 0.8 | 0.5 | 0.4 | 0.1 |
| 4 | 72 | 1.9 | 2.7 | 0.9 | 6.3 | 6.3 | 1.4 | 0.4 | 0.4 | 0.2 |
| 5 | 72 | 3.5 | 3.6 | 1.6 | 45.5 | 43.8 | 19.6 | 26.0 | 23.9 | 9.7 |
| 6 | 72 | 14.0 | 15.4 | 4.9 | 32.2 | 32.4 | 3.7 | 2.8 | 3.1 | 0 |
| 7 | 72 | 13.7 | 14.5 | 4.1 | 64.0 | 62.3 | 20.9 | 25.0 | 20.0 | 1.9 |
| 8 | 72 | 18.0 | 21.1 | 16.0 | 111.0 | 104.5 | 60.8 | 136.0 | 119.6 | 6.5 |
| Median | 6.1 | 6.5 | 3.4 | 26.9 | 26.4 | 14.1 | 4.5 | 4.6 | 1.0 | |
Mononuclear cells, CD34+ cells, and clonogenic cells recovered after bone marrow purging. Bone marrow cells were incubated for 24 hours (□) (n = 3) or 72 hours (▧) (n = 5). The recovery of different cell fractions was calculated as the proportion of the pretreatment count.
Mononuclear cells, CD34+ cells, and clonogenic cells recovered after bone marrow purging. Bone marrow cells were incubated for 24 hours (□) (n = 3) or 72 hours (▧) (n = 5). The recovery of different cell fractions was calculated as the proportion of the pretreatment count.
All patients were also tested for inhibition of methylcellulose colony formation with several control ODN. Table 3reports the proportion of clonogenic cells (CFU-GM) of the untreated samples counted after incubation with the J-sp antisense ODN, J-nonsp antisense ODN, sense sequences, and a mismatched TAT containing sequence ODN. As expected, neither sense nor the mismatched sequences caused a significant inhibition of CML clonogenic cells. Incubation with J-nonsp antisense ODN produced a variable recovery of clonogenic cells, greater in all cases than the recovery found after treatment with the J-sp antisense. Moreover, mononuclear cells from five normal BM samples were incubated with the above ODN under the same conditions used for CML cells. As previously reported,9 11 no toxic effect on clonogenic cell growth was found after treatment of normal BM for 72 hours with B2A2 and B3A2 antisense ODN, sense sequence ODN, and the mismatched ODN (data not shown).
Recovery of Clonogenic Cells (% of Untreated Cells) After Incubation of Mononuclear Cells With the J-sp Antisense or Different Control ODN
| Patients . | J-sp Antisense . | J-nonsp Antisense . | Sense . | Mismatched-TAT Sequence . |
|---|---|---|---|---|
| 1 | 70 | 85 | 95 | 90 |
| 2 | 21 | 35 | 90 | 94 |
| 3 | 20 | 68 | 92 | 90 |
| 4 | 50 | 80 | 96 | 95 |
| 5 | 37 | 58 | 102 | 96 |
| 6 | 0 | 70 | 100 | 100 |
| 7 | 8 | 56 | 90 | 94 |
| 8 | 5 | 60 | 88 | 92 |
| Patients . | J-sp Antisense . | J-nonsp Antisense . | Sense . | Mismatched-TAT Sequence . |
|---|---|---|---|---|
| 1 | 70 | 85 | 95 | 90 |
| 2 | 21 | 35 | 90 | 94 |
| 3 | 20 | 68 | 92 | 90 |
| 4 | 50 | 80 | 96 | 95 |
| 5 | 37 | 58 | 102 | 96 |
| 6 | 0 | 70 | 100 | 100 |
| 7 | 8 | 56 | 90 | 94 |
| 8 | 5 | 60 | 88 | 92 |
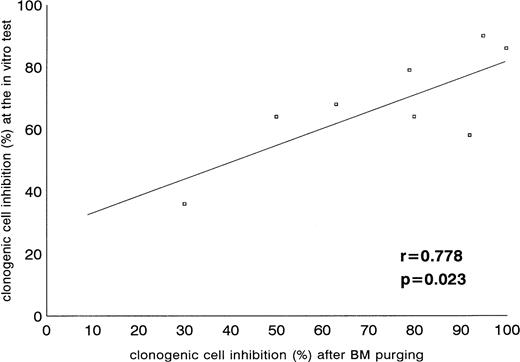
As mentioned previously, Ph-pos cells from all cases were tested at diagnosis for in vitro inhibition of colony formation by the J-sp antisense ODN. Table 4 reports the number of colonies/105 mononuclear cells after exposure to J-sp antisense ODN, J-nonsp antisense ODN, and culture medium without ODN found at the screening test of each CML patient. All cases showed a significant inhibition (P < .05) of colony formation compared with untreated samples and were, therefore, admitted to the ABMT program. However, incubation with the J-nonsp antisense ODN showed a number of colonies significantly different in three cases (patient nos. 4, 6, and 8) and not different (P = not significant) in five cases, compared with the J-sp antisense treatment, confirming that a certain degree of nonspecific effect on BCR-ABL–positive CML cells might be seen using both B2A2 and B3A2 antisense ODN. The incubation with culture medium without ODN showed, on the contrary, no inhibition in all eight samples. Finally, when the results of the screening test were compared with those of BM purging, a significant correlation was found, validating the screening test performed during the chronic phase (Fig2).
Clonogenic Cells After In Vitro Incubation of Ph-pos Cells With J-sp Antisense, J-nonsp Antisense, or Culture Medium at the Time of Diagnosis
| Patients . | Untreated Cells . | Culture Medium Without ODN . | J-sp Antisense . | J-nonsp Antisense . | ||
|---|---|---|---|---|---|---|
| Colonies . | P Value3-150 . | Colonies . | P Value3-151 . | |||
| 1 | 1363-152 | 145 | 93 | .002 | 100 | NS |
| 2 | 90 | 94 | 20 | .005 | 32 | NS |
| 3 | 148 | 162 | 59 | .001 | 66 | NS |
| 4 | 142 | 116 | 42 | .015 | 102 | .034 |
| 5 | 83 | 90 | 29 | .001 | 42 | NS |
| 6 | 140 | 135 | 20 | .016 | 125 | .015 |
| 7 | 183 | 198 | 85 | .006 | 126 | NS |
| 8 | 136 | 124 | 13 | .017 | 106 | .028 |
| Patients . | Untreated Cells . | Culture Medium Without ODN . | J-sp Antisense . | J-nonsp Antisense . | ||
|---|---|---|---|---|---|---|
| Colonies . | P Value3-150 . | Colonies . | P Value3-151 . | |||
| 1 | 1363-152 | 145 | 93 | .002 | 100 | NS |
| 2 | 90 | 94 | 20 | .005 | 32 | NS |
| 3 | 148 | 162 | 59 | .001 | 66 | NS |
| 4 | 142 | 116 | 42 | .015 | 102 | .034 |
| 5 | 83 | 90 | 29 | .001 | 42 | NS |
| 6 | 140 | 135 | 20 | .016 | 125 | .015 |
| 7 | 183 | 198 | 85 | .006 | 126 | NS |
| 8 | 136 | 124 | 13 | .017 | 106 | .028 |
Abbreviation: NS, not significant.
Difference between the number of colonies grown after incubation of Ph-pos cells with the J-sp antisense ODN and culture medium without ODN.
Difference between the number of colonies grown after incubation of Ph-pos cells with the J-nonsp antisense and the J-sp antisense ODN.
Values are the mean of the number of colonies/105 cells grown in triplicate after 120 hours of incubation.
Correlation of clonogenic cell inhibition after the screening test at diagnosis and after the large-scale bone marrow purging.
Correlation of clonogenic cell inhibition after the screening test at diagnosis and after the large-scale bone marrow purging.
BCR-ABL protein expression after treatment with J-sp and J-nonsp antisense ODN.
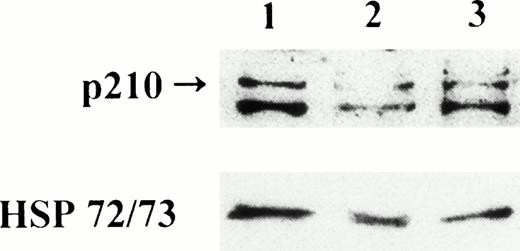
Western blot analysis has been performed on untreated and antisense ODN–treated samples from all patients. Figure3 shows blots of patient no. 4 who had a marked decrease in the BCR-ABL protein after treatment with B2A2 antisense ODN, subsequently confirmed by the cytogenetic results obtained in samples taken after autograft. Of the seven remaining cases, one showed a degree of p210 protein downregulation comparable with the in vitro inhibition of Ph-pos clonogenic cells (no. 6), two had a modest (nos. 7 and 8), and the remaining four showed an absence of effect by J-sp and J-nonsp antisense ODN, including the three cases incubated in vitro for 24 hours, consistent with the relatively long (>48 hours) half life of the protein.12
Effect of BCR-ABL antisense ODN treatment on p210 levels in cells from patient no. 4. Lane 1, mononuclear cells incubated for 72 hours with culture medium without ODN (control); lane 2, mononuclear cells incubated for 72 hours with the J-sp B2A2antisense ODN; lane 3, mononuclear cells incubated for 72 hours with the J-nonsp B3A2 antisense ODN.
Effect of BCR-ABL antisense ODN treatment on p210 levels in cells from patient no. 4. Lane 1, mononuclear cells incubated for 72 hours with culture medium without ODN (control); lane 2, mononuclear cells incubated for 72 hours with the J-sp B2A2antisense ODN; lane 3, mononuclear cells incubated for 72 hours with the J-nonsp B3A2 antisense ODN.
Toxicity.
The conditioning regimen was well tolerated in all patients. A mild degree of nausea and sporadic episodes of vomiting were observed during the administration of chemotherapy. Two patients suffered from severe mucositis during aplasia, requiring local or systemic treatment. All patients developed fever; seven were responsive to broad spectrum antibiotics. In one patient an enterococcus sepsis was documented; one patient developed severe pneumonitis with blood culture positivity for Pseudomonas aeruginosa, Serratia odorifera, and enterococcus at the same time. No side effects related to the reinfusion of residual amounts of antisense ODN or long-term toxicities were observed in this group of patients.
Hematopoietic reconstitution.
The eight patients reached aplasia (<0.1 × 109/L neutrophils) within 7 days from BM reinfusion. All of them had BM engraftment, reaching a number of neutrophils >0.5 × 109/L after a median of 26.5 days (range 19 to 56), and seven patients showed a platelet recovery >50 × 109/L after a median of 45 days (range 21 to 105). One patient developed blastic crisis and died before platelet reconstitution after 7 months from ABMT; one patient, who showed no signs of engraftment after 35 days from ABMT, received the reinfusion of back-up peripheral blood stem cells cryopreserved at diagnosis because of a severe pulmonary infection and sepsis as described above. When we compared the time required for hematologic recovery with the duration of the in vitro incubation (24 hours v 72 hours), no differences were found in the two groups of patients with regard to both neutrophil and platelet reconstitution (data not shown).
Cytogenetic and FISH evaluation.
Table 5 summarizes the cytogenetic and FISH data investigated side by side at various times before and after ABMT. By cytogenetic analysis, two patients showed complete disappearance of Ph-pos cells within 90 days from ABMT, although at the subsequent control (day 180) the leukemic clone reemerged in both of them. One patient died after 7 months from ABMT in second lymphoid blast transformation, and one showed a progressive increase in the proportion of Ph-pos cells, still remaining in second chronic phase with a proportion of BM ph-negative cells at 2 years of follow-up. The other six patients showed no significant decrease in the proportion of Ph-pos cells after ABMT (5% to 15% being the range of Ph-negative metaphases found at various controls), not influenced by the starting of maintenance therapy after ABMT.
Proportion of Ph-pos Cells by FISH/Cytogenetics Before ABMT and During Follow-up
| Patients . | Before ABMT . | Day 30 . | Day 90 . | Day 180 . | Day 365 . | Day 730 . |
|---|---|---|---|---|---|---|
| 1 | 944-150/1004-151 | 92/88 | 94/100 | 90/90 | 84/100 | BC |
| 2 | 90/88 | 90/100 | 85/95 | 87/100 | NE/100 | 95/100 |
| 3 | NE/100 | 10/5 | 30/0 | 88/20 | death | |
| 4 | NE/88 | 23/0 | 10/0 | 13/9 | 90/79 | 92/75 |
| 5 | 90/100 | 80/100 | 80/85 | 95/90 | 96/100 | BC |
| 6 | 95/100 | 91/NM | 75/87 | 91/100 | 90/100 | 92/100 |
| 7 | 92/100 | 90/100 | 88/100 | 92/100 | 94/100 | |
| 8 | 95/100 | 92/100 | 86/100 | 95/100 | 90/100 |
| Patients . | Before ABMT . | Day 30 . | Day 90 . | Day 180 . | Day 365 . | Day 730 . |
|---|---|---|---|---|---|---|
| 1 | 944-150/1004-151 | 92/88 | 94/100 | 90/90 | 84/100 | BC |
| 2 | 90/88 | 90/100 | 85/95 | 87/100 | NE/100 | 95/100 |
| 3 | NE/100 | 10/5 | 30/0 | 88/20 | death | |
| 4 | NE/88 | 23/0 | 10/0 | 13/9 | 90/79 | 92/75 |
| 5 | 90/100 | 80/100 | 80/85 | 95/90 | 96/100 | BC |
| 6 | 95/100 | 91/NM | 75/87 | 91/100 | 90/100 | 92/100 |
| 7 | 92/100 | 90/100 | 88/100 | 92/100 | 94/100 | |
| 8 | 95/100 | 92/100 | 86/100 | 95/100 | 90/100 |
Abbreviations: NE, not evaluable; NM, no analyzable metaphases; BC, blast crisis.
Proportion of rearranged cells on 500 interphase nuclei evaluated.
Proportion of Ph-pos cells on greater than 25 analyzable metaphases.
FISH analysis showed a proportion of rearranged cells comparable with that found by standard cytogenetics. It is interesting to note, however, that samples with 100% Ph-pos metaphases at cytogenetics displayed a proportion of nonrearranged cells (4% to 20% of interphase cells) by FISH; conversely, when no Ph-pos metaphases were found at cytogenetics, a proportion of 10% to 30% rearranged cells was found among the 500 cells evaluated in interphase. As already reported, FISH analysis appears to be more sensitive than cytogenetics for monitoring of residual disease in CML.
Follow-up.
Table 6 shows the present clinical situation of the eight patients after a median follow-up of 27.5 months from ABMT. The patient autografted in second chronic phase died from second blast transformation 7 months after reinfusion; three patients developed blast crisis 21, 21, and 39 months after ABMT, and one patient died from interstitial pnumonitis after unrelated BMT 30 months after ABMT, while in second chronic phase. The other three cases are still in second chronic phase 14, 20, and 26 months after autograft. Four of the eight patients are long survivors after 48, 50, 54, and 86 months from diagnosis. In four patients, the duration of the chronic phase after autograft has exceeded that of the first chronic phase.
Clinical Status and Follow-up
| Patients . | Duration of Chronic Phase After ABMT (mo) . | Duration of Survival After ABMT (mo) . | Therapy After ABMT . | Disease Phase . | Total Survival From Diagnosis (mo) . |
|---|---|---|---|---|---|
| 1 | 21 | 44+ | None after ABMT high-dose therapy for second BC | Third BC | 50+ |
| 2 | 39 | 39 | IFN-α/HU | death | 72 |
| 3 | 7 | 7 | — | death | 42 |
| 4 | 30+ | 30 | — | death in second CP | 48 |
| 5 | 21 | 29 | HU | death | 74 |
| 6 | 26+ | 26+ | IFN-α | second CP | 48+ |
| 7 | 20+ | 20+ | IFN-α | second CP | 54+ |
| 8 | 14+ | 14+ | IFN-α | second CP | 86+ |
| Patients . | Duration of Chronic Phase After ABMT (mo) . | Duration of Survival After ABMT (mo) . | Therapy After ABMT . | Disease Phase . | Total Survival From Diagnosis (mo) . |
|---|---|---|---|---|---|
| 1 | 21 | 44+ | None after ABMT high-dose therapy for second BC | Third BC | 50+ |
| 2 | 39 | 39 | IFN-α/HU | death | 72 |
| 3 | 7 | 7 | — | death | 42 |
| 4 | 30+ | 30 | — | death in second CP | 48 |
| 5 | 21 | 29 | HU | death | 74 |
| 6 | 26+ | 26+ | IFN-α | second CP | 48+ |
| 7 | 20+ | 20+ | IFN-α | second CP | 54+ |
| 8 | 14+ | 14+ | IFN-α | second CP | 86+ |
Abbreviations: BC, blast crisis; HU, hydroxyurea; CP, chronic phase.
DISCUSSION
Antisense ODN directed to leukemia-specific transcripts have the potential to specifically target genes with a pathogenetic role in leukemogenesis while sparing normal hematopoietic cells. Animal studies have confirmed the effectiveness of the in vivo treatment of malignant and nonmalignant disease processes with antisense ODN.5 13-15 However, for the majority of tumors, including CML, problems of toxicity, uptake, and specificity still need to be completely solved. The use of ODN for in vitro purging before autograft partly overcomes these problems. Compared with the dosages necessary for systemic therapy, the total amount of ODN required for BM purging is relatively small, the excess of antisense ODN can be washed out following the in vitro incubation period, and the effects on malignant and normal clonogenic cells can be monitored with in vitro tests. An exact quantification of the antileukemia effect exerted by antisense ODN is, however, complicated by the concomitant effect of the conditioning regimen.
ABMT has been successfully used in recent years for the treatment of CML, and both single center and multicenter studies have shown the feasibility of ABMT in chronic phase CML, although a long-term advantage for patients receiving an autograft is still to be fully shown.16-18 Evaluation of the role of autograft in blast-phase CML is more difficult; very few studies have been reported in this phase of the disease19 at a time when patients are often in very critical conditions. In an earlier study,20we treated five patients with CML in blast phase with unpurged autograft; in all cases, a very short second chronic phase was obtained and all patients died within 6 months from the autograft. The validity of purging has been investigated mainly in acute leukemias21; the lack of CML-specific antigens on Ph-pos cells and the involvement of the CD34+ cell compartment in the disease have hampered the development of immunologic strategies for ex vivo purging in CML. The use of BCR-ABL antisense ODN may be considered one of the newest approaches for the treatment of CML, and the in vitro treatment before autograft appears, at the moment, the clinical application more likely to produce therapeutic results.22 We and others have reported that BCR-ABL ODN inhibit CML colony formation both in blast and in chronic phase.4,9,11,23 Moreover, BCR-ABL ODN are capable of suppressing the growth of Ph-pos cells when injected into leukemic mice.5,13 However, nonspecific effects of ODN on chronic-phase cells and on normal cells have been reported,24,25 raising concerns on the feasibility of BM purging before autograft. In the present study we describe the biological and clinical results obtained in eight patients with CML in advanced phase autografted with BM cells treated in vitro with BCR-ABL antisense ODN. Patients were selected on the basis of the in vitro sensitivity of CML cells to BCR-ABL antisense ODN evaluated at diagnosis9 and on the basis of clinical criteria. Twenty-two of the 35 patients tested in vitro showed a significant reduction of Ph-pos clonogenic cells after treatment with BCR-ABL J-sp antisense ODN. The validity and reproducibility of the in vitro test was confirmed by the significant correlation found with the large-scale BM purging in terms of clonogenic cell inhibition. When compared with the 24-hour incubation time, the 72-hour incubation of BM cells with antisense ODN induced a further decrease in the number of residual leukemic cells and allowed in some cases a downregulation of the p210 protein. In spite of the small number of cases in the two treatment groups, the difference was statistically significant for the CD34+ cell recovery, suggesting that a more prolonged incubation with BCR-ABL antisense ODN is needed to obtain a marked inhibition of CML clonogenic cells. These observations are consistent with the relatively long half-life of the p210 protein.
In general, the overall procedure of ABMT was well tolerated in all patients. No side effects related to the reinfusion of residual amounts of antisense ODN were observed, and only one patient developed a severe pulmonary infection with sepsis that required antibacterial and antifungal therapy. Although the patient's BM showed initial signs of engraftment, we decided to reinfuse the untreated mononuclear back-up cells because of the delay in hematologic reconstitution. All patients showed neutrophil reconstitution. In one patient, platelet recovery was not observed because of a second blast transformation developed 7 months after ABMT. A wide range of variation in the number of days to neutrophil and platelet reconstitution was noted within the eight patients; however, the intensity of the conditioning regimen currently used not only for autologous but also for allogeneic BMT makes it highly likely that BM engraftment was due to the reinfused cells and that the incubation with the 26-mer antisense ODN, therefore, was not toxic for the pluripotent stem-cell compartment.
The impact of antisense ODN as in vitro purging agents on the reduction of Ph-pos cells evaluated after ABMT by standard cytogenetics and by FISH is of difficult interpretation. Autografts performed in blast transformation rarely allow a karyotypic response, although sporadic cases of partial or complete disappearance of Ph-pos cells have been reported.19 In our study, two patients showed a complete karyotypic response between 30 and 90 days after ABMT. This result, however, did not correlate with a better clinical outcome; one patient (autografted in second chronic phase) in fact developed a second lymphoid blast transformation and died within 7 months from ABMT, while the other showed the reappearance of leukemic cells at day 180 from ABMT. In general, the impact of the purged autograft on the duration of second chronic phase and survival after autograft is encouraging (median duration of chronic phase 21 months and survival 27.5 months).
In a recent study,6 CD34+ selected cells or mononuclear cells were in vitro treated for 24 or 72 hours with antisense ODN to the c-myb gene and reinfused in patients with chronic phase or accelerated phase. Despite the very efficient purge, engraftment in some patients was poor. In our study, all patients showed hematologic reconstitution and no procedure-related deaths were observed. Although the rationale for using the c-myb antisense ODN for in vitro purging has been well shown,26 it cannot be excluded that prolonged treatment with antisense ODN might partly affect normal stem/progenitor cells, thus interfering with subsequent BM engraftment. The selection and in vitro treatment of enriched CD34+ cells may also reduce the stem-cell compartment. CD34+ cell loss due to selection of CD34+ cells and the greater antisense ODN uptake compared with the mononuclear cell population9 may produce a greater inhibition of CD34+ cells. Elimination of a greater number of Ph-pos cells is probably achievable through the in vitro incubation of CD34+ cells with c-myb antisense ODN,7but the effects on normal cells need to be further investigated. We have used an antisense ODN specific for the BCR-ABL junction using the mononuclear cell compartment as the target of the in vitro incubation. In addition, after BM reinfusion patients were not treated with growth factors to accelerate BM engraftment or hematologic recovery. Although the antileukemia effects might be improved, the aim of our procedure was to minimize the nonspecific toxicity of antisense ODN and avoid manipulations that might prolong the time necessary to engraftment of BM cells. The data on hematopoietic reconstitution after purging may serve as a reference for future investigations of antisense ODN in patients with advanced CML and also assess the nonspecific toxicity of antisense ODN that might be associated with modifications of the procedure.
The overall antileukemia effect of the protocol used can probably be improved. Patients in chronic phase still responding to conventional therapy may represent a more appropriate target both for a greater inhibition of Ph-pos cells in vitro by antisense ODN and for a better activity of the conditioning regimen in vivo. The development of more specific and active antisense ODN and the optimization of in vitro and in vivo treatment conditions may produce better results in autografted CML patients.
ACKNOWLEDGMENT
We thank Prof R Foà for his helpful comments and for revising the manuscript.
Supported in part by a grant from Consiglio Nazionale delle Ricerche (CNR), Special project “Applicazioni Cliniche della Ricerca Oncologica” (ACRO), Roma, Italy.
Address reprint requests to Paolo de Fabritiis, MD, Institute of Hematology, Via Benevento, 6, 00161 Rome, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal