Most patients with follicular lymphoma (FL) achieve a complete response (CR) after treatment, but eventually most of them, particularly those with stage IV, relapse due to minimal residual disease (MRD). The t(14;18) gives rise to a rearrangement of the bcl-2 oncogene that constitutes an excellent target for detection of MRD by polymerase chain reaction (PCR). One hundred ninety-four previously untreated patients with indolent FL and detectable bcl-2 rearrangement were studied. The PCR assay was used to detect bcl-2–rearranged cells in blood and marrow before and after treatment. Molecular response rate was 37%, 53%, 56%, and 66% at 3 to 5, 6 to 8, 9 to 14, and 15 to 18 months from the start of therapy, respectively. Although molecular response was higher among clinical CRs, one third of partial responders at 3 to 5 months also achieved a molecular response. Patients who achieved a molecular response during the first year of treatment had a significantly longer failure-free survival (FFS) than those who did not (4-year FFS: 76% v 38%, respectively; P < .001). Similar results were also observed in the subset of patients in clinical CR 1 year after treatment. By multivariate analysis, β2-microglobulin (β2-M; P < .01), and molecular response (P < .001) were the most important variables associated with outcome. When we combined β2-M and molecular response, three prognostic groups emerged: (1) low β2-M and molecular responders, (2) low β2-M and nonresponders or high β2-M and responders, and (3) high β2-M and nonresponders. The 4-year FFS of these 3 groups were 86%, 65%, and 23%, respectively. Finally, patients who achieved molecular response and sustained it had better FFS than those who either reverted back to PCR-positive or who never achieved molecular response. Serial PCR analysis to determine the molecular response in FL correlates well with outcome especially when combined with pretreatment β2-M.
ALTHOUGH PATIENTS with follicular lymphoma (FL) often achieve a clinical complete response (CR), the majority of them, in particular those with advanced presentations, eventually develop relapse.1,2 This probably happens because residual lymphoma persists below the detection threshold of standard procedures. FL are characterized by the t(14;18) translocation, present in up to 85% of the cases, resulting in rearrangement of the bcl-2 oncogene.3-5 The translocation occurs most frequently in one of two molecular sites: the major breakpoint cluster region or MBR (∼70% of the cases)6,7 and the minor cluster region or mcr (10% to 15% of the cases).8 Potentially, the most important practical application is the use of bcl-2/IgH rearrangement as a marker to detect minimal residual disease (MRD).9 In 1987, we developed a polymerase chain reaction (PCR) assay to detect as little as one cell carrying the bcl-2 rearrangement among several hundred thousand normal cells and postulated that this could be used to detect MRD.9 Using this method, we and others were able to demonstrate persistent t(14;18) cells after therapy.9-14 To better understand the meaning of a molecular response and also to evaluate the significance of such response at various time points during therapy, we decided to analyze serial post-therapy PCR results in a large cohort of patients. A similar study has been published on patients with relapsed FL submitted to high-dose chemotherapy and bone marrow transplantation15 16 but not in previously untreated patients receiving conventional dose regimens.
Using the PCR assay, we serially tested during and after treatment the peripheral blood and, when possible, the bone marrow of 194 patients who had either MBR or mcr bcl-2 rearrangements at diagnosis. The major aim was to correlate the presence of minimal residual disease with failure-free survival (FFS) in de novo indolent FL treated with conventional dose therapy.
PATIENTS AND METHODS
Patients.
We analyzed by means of the PCR assay the pretreatment peripheral blood of 236 patients with FL for the presence of bcl-2 gene rearrangements. A total of 194 cases (82.2%) had a bcl-2/IgH rearrangement detectable: 167 (70.8%) had the rearrangement within the major breakpoint region (MBR) and other 27 patients (11.4%) in the minor cluster region (mcr), whereas the remaining 42 cases (17.8%) were germline for both MBR and mcr. The 194 cases with assessable bcl-2 rearrangement were the patients included for the longitudinal follow-up in the present study.
Staging evaluation included physical examination, measurement of serum lactate dehydrogenase (LDH) and β2-microglobulin (β2-M) levels, chest x-ray, computer tomography (CT) scans of abdomen and pelvis, and bilateral bone marrow biopsies. The median age was 52 years (range, 18 to 84 years); 92 (47%) were men and 102 (53%) were women. The histologic distribution was follicular center cell lymphoma grade I (follicular small cleaved cell; Working Formulation) in 126 cases (65%), follicular center cell lymphoma grade II (mixed small and large cell) in 62 cases (32%), and follicular center cell grade III (large cell) in 6 cases (3%). Only 6 patients whose tumor predominantly contained cleaved cells were considered indolent and admitted into the study as grade III histology. Forty-three patients (22%) presented with stage I-II, 28 (14.5%) with stage III, and 123 (63.5%) with stage IV.
All patients were informed of the investigational nature of this study and informed consent was obtained from each patient in accordance with institutional guidelines.
Treatment regimens and evaluation of clinical response.
Patients with stage IV were treated with the investigational anthracycline containing protocols available at the time of their presentation. Therapy was alternating triple therapy (ATT)17 in 87 cases, Fludarabine, Mitoxantrone (Novantrone), and Dexamethasone (FND) in 24, and CHOP in 12 patients who refused investigational treatments. Interferon maintenance was used after completion of chemotherapy in all those cases. Patients with stage III were treated with ATT in 13 cases or with CHOP plus radiotherapy in 15. Patients with early stages (I or II) received radiotherapy (6 cases) or COP-bleo plus involved field radiotherapy (35 cases).
Follow-up consisted of physical examination, routine blood tests, and CT scans. If positive at diagnosis, bilateral bone marrow biopsies were repeated after every 3 to 4 cycles during the first year and every 4 to 6 months thereafter.
CR was defined as the disappearance of all signs and symptoms of disease as determined by clinical, radiographic, and laboratory parameters. Partial remission (PR) was defined as a reduction of 50% or more in measurable disease for at least 1 month. Any other responses, including mixed response, stable disease, progressive disease, early death, or death from toxicity, were considered treatment failures.
PCR methods for detecting bcl-2 rearrangements.
Samples from both peripheral blood and/or bone marrow aspirates were collected before starting treatment and, whenever possible, every 3 to 4 months for the first 2 years after starting therapy, and then every 6 months until relapse or progression of the lymphoma.
DNA was isolated from blood and/or bone marrow using conventional methods. PCR amplification was used to detect both MBRand mcr breakpoints by subjecting one microgram of genomic DNA to 45 cycles of amplification.9,18 The primersMBR(+), mcr(+), and JH(−) have been previously described.8,9,18 Twenty percent of PCR products were size-fractionated in a 2% NuSieve gel and then transferred to a nylon membrane. Membranes were hybridized with 5′ end radiolabeled oligonucleotide probes MBR and mcr at 63°C (MBR) or 42°C (mcr) overnight. Washing was performed in 2 SSPE/0.1% sodium dodecyl sulfate at 55°C (MBR) or at room temperature (mcr) for 1 hour. Autoradiography was performed against a double intensifying screen at −70°C for 72 hours.9
To ensure the reliability of the PCR assay, we routinely included a weak positive control (100 pg of positive DNA), a negative control from normal DNA, a reagent control, and an internal control. These controls helped to detect contamination, avoid false negativity due to suboptimal PCR efficiency, and standardize the variation in PCR efficiency. The sensitivity of the PCR technique was estimated by a dilutional method in 1/105 to 1/106.
Molecular response.
Molecular response was defined as the disappearance of t(14;18) amplicons in peripheral blood at any given point during or after therapy in a patient with a known baseline bcl-2 rearrangement. Molecular response during the first year was considered as achievement of a PCR-negative status at any point during the first year of treatment. In analyses involving the molecular response during the first year, patients were omitted who did not have at least two of the first three PCR determinations or who were censored before 1 year.
Statistical analysis.
FFS was measured from the start of therapy until relapse or toxic death. Patients not relapsing were censored at the last follow-up. Actuarial probability of FFS was estimated according to the method of Kaplan-Meier,19 and curves compared using the log-rank test.20 Categorical data were compared using χ2 or Fisher's exact tests.
Although the intent was to obtain peripheral blood and bone marrow samples for PCR analysis every 3 to 4 months during the first 2 years, this was not possible for every patient. Because the exact timing of specimens was determined by clinic visits, the first posttreatment PCR determination may have occurred within 3 to 5 months after the start of treatment, the second determination within 6 to 8 months posttreatment start, and the third determination within 9 to 14 months. In tabulating molecular response status within these time intervals, all patients with PCR determinations within that time were included.
The association of molecular response to FFS was evaluated by two approaches: (1) the landmark method, comparing the FFS subsequent to 1 year according to molecular response at that time,21 and (2) the use of a proportional hazards regression model.22In the landmark method, patients who failed or were censored before 12 months were excluded, and molecular response was determined based on samples taken before 12 months. In the proportional hazards model, an indicator variable took the value zero for each patient until the time a molecular response was detected and a value of one for each patient thereafter. A test for statistical significance of the coefficient for this term was, therefore, a test for whether conversion to PCR negativity was associated with subsequent risk of treatment failure.
RESULTS
Patients and clinical response.
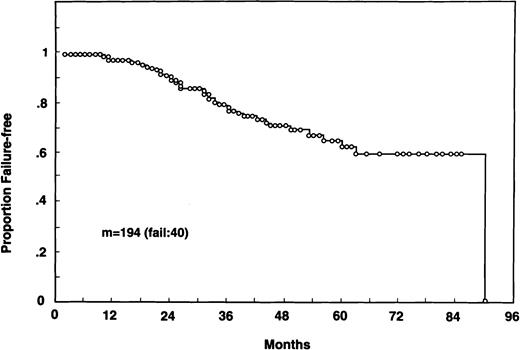
Patients were seen between July 1988 and August 1995. The median follow-up is 29 months (range, 6 to 91 months). Clinical CR was attained in 173 patients (90.6%), PR was attained in 17 (8.9%), and 1 (0.5%) did not respond (in 3 cases the response was not evaluable). Forty patients have so far developed relapse, with an actuarial FFS of 89.1% (95% confidence interval [CI], 83.8% to 94%) and 62.5% (95% CI, 51.5% to 73.5%) at 2 and 5 years from diagnosis, respectively (Fig 1).
Overall FFS of 194 patients with indolent FL and detectable bcl-2 rearrangement before therapy.
Overall FFS of 194 patients with indolent FL and detectable bcl-2 rearrangement before therapy.
Correlation of clinical and molecular response.
Clinical and molecular response assessed in blood at different time-points are detailed in Table 1. The clinical response refers to that observed in all sites where there was disease present at baseline. The molecular response rates progressively increased from 37% at 3 to 5 months to 66% at 15 to 19 months. On the other hand, the proportion of patients in clinical PR that presented with molecular response, as assessed in blood, was 37% (31/84), 31% (10/32), and 35% (5/14) at 3 to 5, 6 to 8, and 9 to 14 months, respectively. At the same times, the proportion of patients in CR who achieved molecular response were 38%, 67%, and 60%, respectively. Although molecular CRs were more frequent in patients with clinical CR, the molecular response was not completely dependent on the clinical response. Of note, all the patients that were in clinical PR but in molecular CR at 3 to 5 months, and with follow-up greater than 1 year, eventually reached clinical CR.
Clinical and Molecular Response Assessed in Peripheral Blood in 194 Patients With FL at Different Time Points From the Beginning of Treatment
| Time Points . | No. of Patients . | Clinical CR Rate (%) . | Molecular Response Rate (%) . |
|---|---|---|---|
| 3-5 mo | 118 | 29 | 37 |
| 6-8 mo | 86 | 63 | 53 |
| 9-14 mo | 101 | 86 | 56 |
| 15-19 mo | 74 | 97 | 66 |
| Time Points . | No. of Patients . | Clinical CR Rate (%) . | Molecular Response Rate (%) . |
|---|---|---|---|
| 3-5 mo | 118 | 29 | 37 |
| 6-8 mo | 86 | 63 | 53 |
| 9-14 mo | 101 | 86 | 56 |
| 15-19 mo | 74 | 97 | 66 |
The number of patients varies at different time points because occasional patients did not have clinical or molecular assessment of response at a given time point.
Pretreatment variables and molecular response.
Pretreatment variables, including histology, Ann Arbor stage, bulky disease, bone marrow infiltration, and serum levels of LDH and β2-M, did not predict the achievement of a molecular response within the first year of follow-up. No correlation was observed between treatment regimen and molecular response. Although stage IV patients receiving the FND fludarabine combination presented a higher molecular response rate at 6 to 8 months than those treated with ATT (82% v 49%, respectively; P = .024), such difference disappeared at later time points.
Molecular response and FFS.
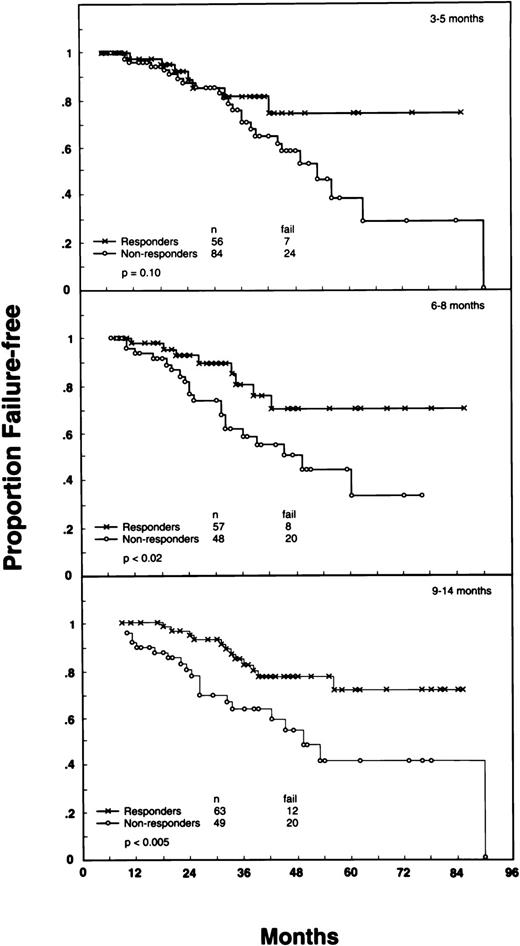
FFS results correlated with achievement of molecular response at each of the first three PCR determinations (3 to 5, 6 to 8, and 9 to 14 months; Fig 2). In each set of curves, patients observed for less than the landmark time were omitted. Patients who were PCR negative at the respective intervals tended to have longer FFS than nonresponders (PCR positive) at the same times. The differences in FFS were more pronounced at the later time points. These results suggest that determination of molecular response may be more clinically meaningful after the first 5 months of therapy.
FFS of patients with FL according to molecular response (Responders: PCR-negative status; Nonresponders: PCR-positive status), assessed in peripheral blood at different time points from the onset of therapy: (A) 3 to 5 months (P = .1), (B) 6 to 8 months (P < .02), and (C) 9 to 14 months (P < .005). In each time point, patients observed for less than that time or who relapsed before were omitted.
FFS of patients with FL according to molecular response (Responders: PCR-negative status; Nonresponders: PCR-positive status), assessed in peripheral blood at different time points from the onset of therapy: (A) 3 to 5 months (P = .1), (B) 6 to 8 months (P < .02), and (C) 9 to 14 months (P < .005). In each time point, patients observed for less than that time or who relapsed before were omitted.
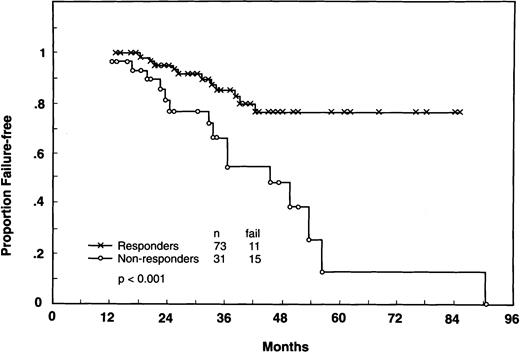
In Fig 3, FFS is compared from 1 year after the start of therapy according to molecular response status during the first year. Only patients who achieved a molecular response at any point during the first year were considered as molecular responders. There was a substantial failure-free advantage for patients with evidence of molecular response within the first year of therapy (4-year FFS: 76% v 38%; P < .001). Moreover, late failures occurred more frequently in the molecular nonresponders.
FFS from 1 year after the start of treatment according to the molecular response status within the 1st year (Responders: PCR-negative; Nonresponders: PCR-positive status). Landmark: 12 months:P < .001. Only 116 patients with two or more PCR determinations during the first year were included; in addition, 4 patients who failed and 8 censored before 1 year were omitted. According to the landmark rules, patients who achieved a late molecular response (ie, after 1 year) were included in the nonresponders group.
FFS from 1 year after the start of treatment according to the molecular response status within the 1st year (Responders: PCR-negative; Nonresponders: PCR-positive status). Landmark: 12 months:P < .001. Only 116 patients with two or more PCR determinations during the first year were included; in addition, 4 patients who failed and 8 censored before 1 year were omitted. According to the landmark rules, patients who achieved a late molecular response (ie, after 1 year) were included in the nonresponders group.
The same landmark analysis was performed only on patients who achieved clinical CR during the first year and whose follow-up exceeded 1 year (88 of 116 cases). The substantial difference in FFS between molecular responders and nonresponders was preserved (P < .02), suggesting a further prognostic role for molecular response beyond that of clinical response.
In a separate analysis of the association between pretreatment patient characteristics and FFS (including age, sex, histologic subtype, performance status, B-symptoms, bulky disease, extranodal involvement, bone marrow infiltration, Ann Arbor stage, serum LDH, serum β2-M, and treatment; results not presented), it was determined that serum LDH and β2-M values were the two factors most closely associated with FFS outcomes. The second approach to analyzing the association between molecular response and FFS involved fitting a proportional hazards model with a time-dependent covariate indicating whether a molecular response had been achieved. Terms identifying abnormal levels for pretreatment values of LDH and β2-M were also included because of their recognized prognostic importance. Results indicated a highly statistically significant association between attaining a molecular response and risk of failure, with the direction of effect for the reduced risk of failure when molecular response was achieved (P< .001). In addition, β2-M retained independent prognostic value (P < .01).
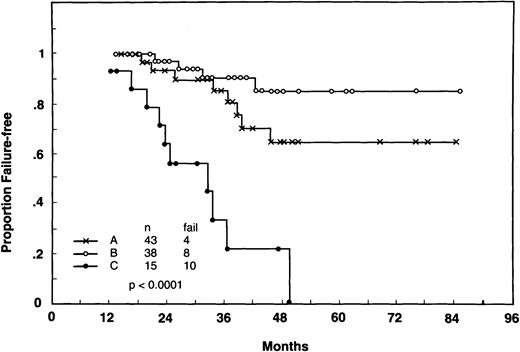
As a further demonstration of the independent association of β2-M and molecular response with FFS, the landmark analysis of Fig 3 was repeated with patients in the molecular responder and nonresponder groups divided according to whether their pretreatment levels of β2-M were abnormal. Figure 4 depicts the FFS curves according to the various combinations of serum β2-M levels and molecular response within the first year. By combining patients into these categories, they can be divided into three groups: normal β2-M and molecular CR (45% of all cases), either normal β2-M with no molecular CR or high β2-M with molecular CR (40%), and high β2-M without molecular CR during the first year (15%). The 4-year FFS for these three groups were 86%, 65%, and 23%, respectively (P< .001).
FFS from 1 year after the onset of treatment according to the different combinations of β2-M at presentation (normal vhigh) and molecular response within the first year (Respondersv Nonresponders). Patients included were the same as in Fig 3. Three possible combinations were plotted: (A) normal β2-M and molecular responders, (B) either high β2-M and molecular responders or normal β2-M and molecular nonresponders, and (C) high β2-M and molecular nonresponders. Landmark: 12 months; P < .0001.
FFS from 1 year after the onset of treatment according to the different combinations of β2-M at presentation (normal vhigh) and molecular response within the first year (Respondersv Nonresponders). Patients included were the same as in Fig 3. Three possible combinations were plotted: (A) normal β2-M and molecular responders, (B) either high β2-M and molecular responders or normal β2-M and molecular nonresponders, and (C) high β2-M and molecular nonresponders. Landmark: 12 months; P < .0001.
Molecular response pattern and FFS.
Because not only the achievement of a molecular response, but also the maintenance of such response could be important for outcome, we analyzed the molecular response pattern for the first four determinations (up to 18 months). Patients were excluded if they developed clinical relapse before 18 months (4 cases), if there was less than 18 months of follow-up (19 cases), or if fewer than 3 PCR determinations were made during that time (18 cases). In the remaining 75 patients, three patterns of PCR response could be distinguished: (1) 35 achieved molecular response during the first year of therapy and sustained it at least for the 18 months period; (2) 25 achieved molecular response but reverted back to PCR-positive status; and (3) 15 never achieved molecular response or did so after 1 year. Median FFS has not been reached yet for the sustained response group; for the mixed responders and for the nonresponder groups, estimated medians were 50 and 31 months, respectively (P < .05; Fig 5).
FFS survival from 18 months after the onset of treatment according to the pattern of molecular response. Seventy-five patients with at least three PCR determinations in this period were included. Three patterns could be distinguished: (A) 35 patients achieved molecular response and sustained it at least for 18 months, (B) 25 patients achieved molecular response but reverted back to PCR-positive status, and (C) 15 patients did not achieve molecular response. Patients who relapsed before 18 months were omitted from the analysis. Landmark: 18 months; P < .05.
FFS survival from 18 months after the onset of treatment according to the pattern of molecular response. Seventy-five patients with at least three PCR determinations in this period were included. Three patterns could be distinguished: (A) 35 patients achieved molecular response and sustained it at least for 18 months, (B) 25 patients achieved molecular response but reverted back to PCR-positive status, and (C) 15 patients did not achieve molecular response. Patients who relapsed before 18 months were omitted from the analysis. Landmark: 18 months; P < .05.
In the overall series, 43 patients that had previously achieved molecular response reverted back to positive during the follow-up. The risk of clinical relapse was of 41% (95% CI, 23% to 59%) and 52% (95% CI, 32% to 72%) at 2 and 3 years from the first PCR-positive status, respectively.
PCR results before and after clinical relapse.
Finally, we studied the subset of 40 patients who developed relapse at any point during or after treatment. Four molecular response patterns in peripheral blood emerged in this group: 15 patients (37.5%) were always PCR positive; 13 (32.5%) had achieved a PCR negative state but had reverted back to a positive PCR before or at clinical relapse; 9 (22.5%) attained a molecular CR, reverted back to a positive PCR, and then fluctuated between positive and negative; and 3 (7.5%) had been always PCR negative during the follow-up after achieving a molecular CR.
Molecular response assessed in bone marrow samples.
The degree of agreement between the molecular response assessed in bone marrow and in peripheral blood analyzed for each time point is detailed in Table 2. In addition to the correlation observed between peripheral blood PCR results and FFS, there was also a similar trend for a correlation between bone marrow molecular response and FFS at each time point considered, but the number of samples was not large enough to allow in-depth statistical analysis (data not shown).
Agreement Between the Molecular Response Assessed in Peripheral Blood or in Bone Marrow at Different Time Points
| . | 3-5 mo (n = 57) . | 6-8 mo (n = 41) . | 9-14 mo (n = 35) . |
|---|---|---|---|
| PB(+)/BM(+) or PB(−)/BM(−) | 70 | 66 | 77 |
| PB(+)/BM(−) | 13 | 5 | 9 |
| PB(−)/BM(+) | 17 | 24 | 14 |
| . | 3-5 mo (n = 57) . | 6-8 mo (n = 41) . | 9-14 mo (n = 35) . |
|---|---|---|---|
| PB(+)/BM(+) or PB(−)/BM(−) | 70 | 66 | 77 |
| PB(+)/BM(−) | 13 | 5 | 9 |
| PB(−)/BM(+) | 17 | 24 | 14 |
Values are the percentage of patients.
Abbreviations: PB, peripheral blood; BM, bone marrow.
DISCUSSION
The t(14;18) translocation provides an excellent target that can be applied to the detection of minimal residual disease in peripheral blood and bone marrow. Using the PCR technique, we detected the t(14;18) in blood and/or bone marrow of more than 80% of these cases. We have also shown that (1) molecular remissions can be achieved with standard-dose chemotherapy and (2) molecular remissions correlate with durable clinical remissions.
During the first year of treatment, more than 60% of patients became PCR negative in blood and bone marrow. Agreement in the molecular response rate assessed in simultaneous blood and marrow specimens was observed in 70% of cases (Table 2). In the 30% of cases in which the results were different, the marrow aspirate tended to be positive more frequently than blood, but in a substantial number of cases the blood was positive when the marrow was negative. Good concordance between blood and bone marrow PCR results at diagnosis has been previously observed by different groups.23-25 Gribben et al26,27 were able to detect bcl-2–positive cells in both blood and marrow before and after high-dose chemotherapy and bone marrow transplantation, although in their experience bone marrow biopsy samples were more predictive of outcome.26 In our experience, peripheral blood PCR seems to be almost as sensitive as bone marrow for detecting minimal residual disease; and from the practical standpoint, it is easier to obtain.
The achievement of molecular response in peripheral blood was to a certain extent independent of the clinical response. At 6 months from the beginning of treatment, the proportion of molecular responders was higher in clinical CRs than in PRs. Approximately one third of clinical CRs failed to attain a molecular response at that point; on the other hand, one third of PRs achieved a molecular response. Patients in clinical CR with positive PCR probably represent those with MRD not assessable by conventional methods. Conversely, the patients in PR with a negative PCR in peripheral blood likely are those with residual fibrotic masses or with nonmalignant marrow lymphocytic aggregates. In fact, all the clinical PRs whose molecular response was a CR eventually reached clinical CR with longer follow-up.
The most important conclusion of this study was that the molecular response at different time-points during the first year of therapy strongly correlates with FFS (Figs 2 and 3). More than three fourths of the patients who achieved a PCR-negative state at any of the tested time points (3 to 5, 6 to 8, and 9 to 14 months) were expected to be alive and in clinical CR 5 years after starting therapy. An interesting observation is that, irrespective of the PCR results, there was very little difference in the FFS during the first 2 years of follow-up. Thus, PCR only seems to predict the outcome several years after therapy (Figs 2 and 3). Although our patient population differs from that of Gribben et al13,15 27 in various aspects, the results resemble those reported by them.
We also found that both the molecular response within the first year and the pretreatment serum β2-M were independent and important prognostic factors for FFS. With the combination of β2-M and molecular response within 1 year it is possible to identify those cases whose prognosis is excellent, with more than 80% of them projected to be alive and in CR 5 years later (Fig 4). This group with excellent prognosis represents approximately one half of all the FL cases in this study. Longer follow-up will be necessary to determine if a significant proportion of those patients are cured. On the other hand, a small subset of patients consisting of approximately 15% of the population are at high risk of early relapse. These cases could in the future be considered candidates for investigational therapy.
The serial pattern of the PCR results is also important in its correlation with clinical outcome. Those who never became PCR negative showed a high risk of relapse, in contrast with those that were persistently negative after treatment. Patients who attained a molecular response but later on experienced a molecular relapse also had a higher risk of clinical relapse (Fig 5).
One potential criticism of these data is that the bcl-2–rearranged cells we detected might not represent malignant cells. Healthy donors can show evidence of bcl-2–rearranged cells in the peripheral blood.28-30 However, when only 1 μg of DNA is loaded for the PCR assay, the proportion of normal individuals who had detectable bcl-2–rearranged cells by PCR was only 6.6%.28 Moreover, when unsorted peripheral blood white blood cells are examined, as in the present study, the proportion would be expected to be much lower. Therefore, although we cannot discard the possibility that circulating bcl-2–rearranged cells in a small proportion of our patients represent instances of nonmalignant cells, this is expected to be unusual. Furthermore, if the bcl-2–rearranged cells we detected in this study do not represent the malignant clone, it would be unlikely that the clinical outcome would correlate so well with the molecular response.
In conclusion, the use of serial PCR analysis to determine the molecular response is a very useful tool, especially when combined with other prognostic factors such as β2-M.
Supported in part by National Cancer Institute Grant No. CA62518 and by the Herschel and Hilda Rich Fund for Lymphoma Research. A.L.-G. was supported by the Hospital Clı́nic of Barcelona and the Spanish Ministry of Education (EX-43390399/95).
Address reprint requests to Fernando Cabanillas, MD, Lymphoma Department, MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal