Fas ligand (FasL) is a membrane protein that is expressed in activated T cells and natural killer cells. FasL binds to Fas on target cells and induces apoptosis. There exists a soluble form of FasL (sFasL), and sFasL also induces apoptosis of Fas-bearing cells. The serum sFasL concentrations were reported to be elevated in patients with large granular lymphocytic leukemia and natural killer cell lymphoma. In this study, we have measured serum sFasL concentrations in other hematological disorders, including severe aplastic anemia (SAA), hemophagocytic lymphohistiocytosis (HLH), and Diamond-Blackfan anemia (DBA). The serum sFasL concentration of age-matched healthy controls was 0.16 ± 0.11 ng/mL (mean ± SD, n = 22). The serum sFasL levels in the patients with HLH and DBA were 3.75 ± 3.82 (n = 19;P < .0001, HLH v control) and 2.76 ± 2.43 ng/mL (n = 6; P = .012, DBA v control), respectively. Serum interferon-γ concentration was elevated in the patients with HLH (1.61 ± 2.62 ng/mL) but not in those with DBA (below the detectable level). These results suggest that the Fas-FasL system plays a role, at least in part, in the pathophysiology of HLH and DBA.
Fas (ALSO KNOWN AS APO-1 or CD95) is a member of the tumor necrosis factor (TNF) receptor/nerve growth factor receptor family and transduces an apoptotic signal by activating a cascade of interleukin-1β–converting enzyme (ICE)-like cysteine proteases (caspases).1 Fas is constitutively expressed in a variety of tissues, including thymus, liver, heart, and kidney. Fas is barely detectable in bone marrow CD34+ cells isolated from healthy volunteers. Treatment of these cells with interferon-γ (IFN-γ) or TNF-α increases the expression of Fas and these cells become susceptible to anti-Fas antibody-induced apoptosis.2,3 The ligand for Fas (FasL) is a type II membrane protein that is expressed in the cells having cytotoxic activity, including activated T cells and natural killer (NK) cells, and in the cells in immune-privileged sites such as the testes, the brain, and the anterior chamber of the eye.4,5 Mice carrying an abnormality in either the Fas (lpr) or FasL (gld) gene show marked lymphoadenopathy and autoimmune disease.6,7 Moreover, an abnormality of the Fas gene was shown in human children suffering from autoimmune lymphoproliferative syndrome.8,9 These results indicate that the Fas/FasL system plays an important role in the clonal selection of T cells. The role of the Fas/FasL system has also been implicated in cell-mediated cytotoxicity during virus infections, autoimmune diseases, and tumor immunity.1 It has been recently reported that the Fas/FasL system is involved in the pathogenesis of Hashimoto's thyroiditis and hepatitis.10 11
Cleavage of membrane-bound FasL by a metalloproteinase-like enzyme results in the formation of soluble FasL (sFasL).12 sFasL binds to Fas antigen and transduces an apoptotic signal in Fas-bearing cells.13,14 Therefore, cytotoxic cells can kill the target cells either via direct contact or by secreting sFasL. Tanaka et al12 reported that serum sFasL concentrations are elevated in the patients with large granular lymphocytic (LGL) leukemia and NK lymphoma. They suggested that neutropenia and liver dysfunction, both of which are common complications of these diseases, can be attributed to the elevated levels of sFasL.
In this study, we have measured serum sFasL concentrations in the patients with hematological disorders in which immunological mechanisms are suspected to play a role in the pathophysiology, including severe aplastic anemia (SAA), hemophagocytic lymphohistiocytosis (HLH), and Diamond-Blackfan anemia (DBA), to clarify the role of the Fas/FasL system in these disorders.
MATERIALS AND METHODS
Patients.
Eleven patients with SAA, 6 patients with DBA, 19 patients with HLH, and 5 patients with infectious mononucleosis (IM) were studied. The diagnosis of HLH was based on clinical findings (fever and hepatosplenomegaly), laboratory findings (cytopenia affecting at least 2 lineages in the peripheral blood, hypertriglyceridemia, and/or hypofibrinogenemia), and histopathologic findings (hemophagocytosis in the bone marrow, spleen, or lymph nodes without evidence of malignancy).15 The diagnosis of familial hemophagocytic lymphoproliferative syndrome (FHL) was made with a positive family history. SAA was defined as pancytopenia with at least two of the following abnormalities: an absolute neutrophil count of less than 500/μL, a platelet count of less than 20,000/μL, and a reticulocyte count of less than 60,000/μL, in association with a bone marrow cellularity of less than 30%. Patients were diagnosed as having posthepatitis aplastic anemia when they developed the disease within 3 months after documented hepatitis.16 The diagnosis of DBA was based on the following criteria: (1) normochromic anemia in early infancy, (2) reticulocytopenia, (3) normocellular bone marrow with selective deficiency of red blood cell precursors, (4) normal or slightly decreased leukocyte counts, and (5) normal or often increased platelet counts.17
Serological diagnosis of primary Epstein-Barr virus (EBV) infection was made by the detection of either an increase in EBV viral capsid antigen (VCA)-specific IgG antibody in the absence of antibody against EBV nuclear antigen or an increase in VCA-specific IgM antibody titers. Detection of the EBV genome in the patients' peripheral blood was performed with the polymerase chain reaction as described by Saito et al.18
Enzyme-linked immunosorbent assay (ELISA) for sFasL and IFN-γ.
The serum sFasL levels of age-matched healthy controls and patients with hematological disorders were measured with ELISA essentially as described by Tanaka et al12 using monoclonal anti-FasL antibodies and recombinant human FasL. The limit of detection was 5 pg/mL. The serum IFN-γ level was determined with a commercially available kit obtained from Diaclone Research (Besançon, France). The limit of detection was 5 pg/mL.
Statistical analysis.
The differences in the sFasL levels among controls and SAA, HLH, DBA, and IM patients were analyzed with Mann-Whitney's U-test using the computer program DAStat (developed by O. Nagata), whereas that in the sFasL levels between the active phase and remission phase of HLH patients were analyzed with Students' t-test. The values below detectable level in the sFasL level were treated as 5 pg/mL in the statistical analysis. The differences in the IFN-γ level among controls and HLH, DBA, IM, and AA patients were also analyzed with Mann-Whitney's U-test. The values below detectable level in the IFN-γ level were treated as 5 pg/mL in the statistical analysis. The difference was considered to be significant when the P value was less than .05.
Flow cytometric analysis.
Quantitative analysis of fluorescence was performed using a Coulter Epics Flow Cytometry System (Coulter, Hialeah, FL). Phycoerythrin (PE)-conjugated mouse anti-Fas monoclonal antibody (clone UB2; MBL, Nagoya, Japan) and fluorescein isothiocyanate (FITC)-conjugated mouse anti-glycophorin A (GPA) monoclonal antibody (clone D2.10; MBL) were used to determine the Fas expression on bone marrow erythroid lineage cells. Appropriate isotypic controls were used in all of the experiments.
RESULTS
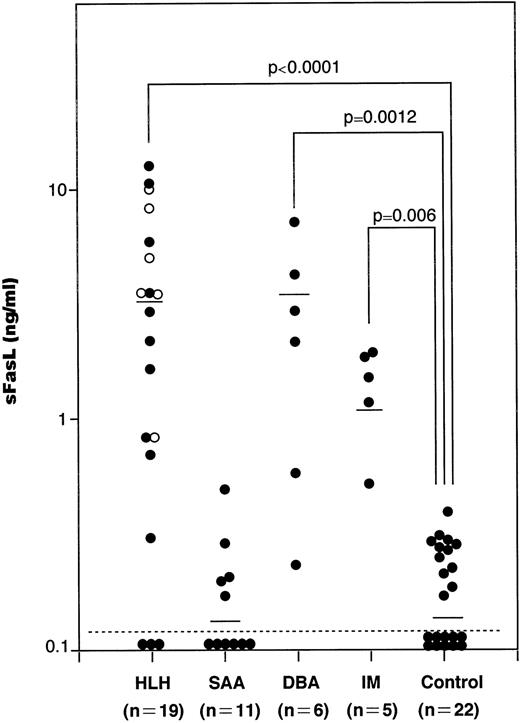
We have measured serum sFasL concentrations in a total of 41 patients with hematological disorders, including 11 SAA, 19 HLH, 6 DBA, and 5 IM patients and 22 age-matched healthy controls. The values in each group were plotted and shown in Fig 1. The serum samples from SAA, HLH, DBA, and IM patients were taken upon diagnosis and kept at −80°C until analysis. The serum sFasL concentration in healthy controls was 0.16 ± 0.11 ng/mL (mean ± SD; range, <0.005 to 0.37 ng/mL). We tentatively set the upper normal limit of serum sFasL at 0.5 ng/mL. IM patients showed a moderate increase in their serum sFasL levels (1.39 ± 0.56 ng/mL; mean ± SD). These IM patients showed mild liver dysfunction with a moderate increase in serum aminotransferases and leukocytosis with atypical lymphocytes. None of the IM patients analyzed showed anemia or thrombocytopenia.
The serum sFasL concentrations in patients with hematologic disorders. Sera from patients and age-matched healthy controls were assayed by ELISA for sFasL as described before.12 The mean values are shown by horizontal bars. (○) Familial cases of HLH (FHL). Statistical analysis was performed as described in the Materials and Methods and the P value is presented.
The serum sFasL concentrations in patients with hematologic disorders. Sera from patients and age-matched healthy controls were assayed by ELISA for sFasL as described before.12 The mean values are shown by horizontal bars. (○) Familial cases of HLH (FHL). Statistical analysis was performed as described in the Materials and Methods and the P value is presented.
The serum sFasL concentration exceeded 0.5 ng/mL in 15 of 19 HLH patients. The mean ± SD value was 3.75 ± 3.82 ng/mL (range, <0.005 to 12.25 ng/mL), and this was significantly higher than the controls (P < .0001). The clinical profiles of the HLH patients are shown in Table 1. Three patients underwent bone marrow transplantation and 2 patients died. The other patients were treated with steroids with or without other immunosuppressive and cytotoxic agents, including cyclosporin A, FK506, OKT3, and etoposide; 4 of these patients died from the disease.
Clinical Characteristics of the Patients With HLH
| Case No. . | Age/Sex . | Family History . | Etiology . | Fever . | Hepatomegaly . | Splenomegaly . | DIC . | Pancytopenia . | Liver Dysfunction . | Hyperferritinemia . | Hypertriglydemia . | BMT . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1/F | − | Unknown | + | + | − | + | + | + | + | ND | Alive | |
| 2 | 3/F | − | Unknown | + | − | − | − | + | + | + | − | Alive | |
| 3 | 3/F | − | EBV | + | + | + | + | − | + | + | ND | Alive | |
| 4 | 13/F | − | Ki-lymphoma | + | − | − | + | + | + | + | ND | Alive | |
| 5 | 4/F | + | FHL | + | + | + | + | + | + | + | + | Allogeneic | Dead |
| 6 | 1/M | + | FHL | + | + | − | + | + | + | + | ND | Allogeneic | Dead |
| 7 | 0/M | + | FHL | + | + | + | + | + | + | + | + | Alive | |
| 8 | 1/F | + | FHL | + | + | + | + | + | + | + | + | Alive | |
| 9 | 1/M | + | FHL/EBV | + | + | + | + | + | + | + | + | Dead | |
| 10 | 1/F | + | FHL/EBV | + | + | + | + | + | + | + | + | Dead | |
| 11 | 2/F | − | EBV | + | + | + | + | + | + | + | + | Alive | |
| 12 | 0/M | − | EBV | + | + | + | + | + | + | + | + | Dead | |
| 13 | 1/F | − | Influenza AH3 | + | + | − | + | + | + | + | − | Alive | |
| 14 | 1/F | − | EBV | + | + | + | + | + | + | − | − | Alive | |
| 15 | 5/M | − | Unknown | + | + | + | + | + | + | + | + | Alive | |
| 16 | 10/F | − | Unknown | + | + | + | + | + | + | + | + | Alive | |
| 17 | 2/M | − | Unknown | + | + | + | − | + | + | + | − | Alive | |
| 18 | 7/F | − | EBV | + | + | − | + | + | + | + | − | Syngeneic | Alive |
| 19 | 4/F | − | EBV | + | + | − | + | + | + | + | − | Dead |
| Case No. . | Age/Sex . | Family History . | Etiology . | Fever . | Hepatomegaly . | Splenomegaly . | DIC . | Pancytopenia . | Liver Dysfunction . | Hyperferritinemia . | Hypertriglydemia . | BMT . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1/F | − | Unknown | + | + | − | + | + | + | + | ND | Alive | |
| 2 | 3/F | − | Unknown | + | − | − | − | + | + | + | − | Alive | |
| 3 | 3/F | − | EBV | + | + | + | + | − | + | + | ND | Alive | |
| 4 | 13/F | − | Ki-lymphoma | + | − | − | + | + | + | + | ND | Alive | |
| 5 | 4/F | + | FHL | + | + | + | + | + | + | + | + | Allogeneic | Dead |
| 6 | 1/M | + | FHL | + | + | − | + | + | + | + | ND | Allogeneic | Dead |
| 7 | 0/M | + | FHL | + | + | + | + | + | + | + | + | Alive | |
| 8 | 1/F | + | FHL | + | + | + | + | + | + | + | + | Alive | |
| 9 | 1/M | + | FHL/EBV | + | + | + | + | + | + | + | + | Dead | |
| 10 | 1/F | + | FHL/EBV | + | + | + | + | + | + | + | + | Dead | |
| 11 | 2/F | − | EBV | + | + | + | + | + | + | + | + | Alive | |
| 12 | 0/M | − | EBV | + | + | + | + | + | + | + | + | Dead | |
| 13 | 1/F | − | Influenza AH3 | + | + | − | + | + | + | + | − | Alive | |
| 14 | 1/F | − | EBV | + | + | + | + | + | + | − | − | Alive | |
| 15 | 5/M | − | Unknown | + | + | + | + | + | + | + | + | Alive | |
| 16 | 10/F | − | Unknown | + | + | + | + | + | + | + | + | Alive | |
| 17 | 2/M | − | Unknown | + | + | + | − | + | + | + | − | Alive | |
| 18 | 7/F | − | EBV | + | + | − | + | + | + | + | − | Syngeneic | Alive |
| 19 | 4/F | − | EBV | + | + | − | + | + | + | + | − | Dead |
Abbreviations: BMT, bone marrow transplantation; ND, not determined.
The serum sFasL levels exceeded 0.5 ng/mL in 5 of the 6 DBA patients. Three patients were steroid-dependent and the others were transfusion-dependent. The serum sFasL concentration was 2.75 ± 2.42 ng/mL (mean ± SD; range, 0.23 to 6.78 ng/mL). There was a significant difference between the controls and DBA patients (P= .0012).
We have measured the serum sFasL concentrations in 11 SAA patients, including 3 with posthepatitis SAA. Although the involvement of CTL has been implicated in SAA,19 none of the patients analyzed exceeded 0.5 ng/mL. The mean ± SD was 0.15 ± 0.14 ng/mL. There was no significant difference between the controls and SAA patients (P = .41).
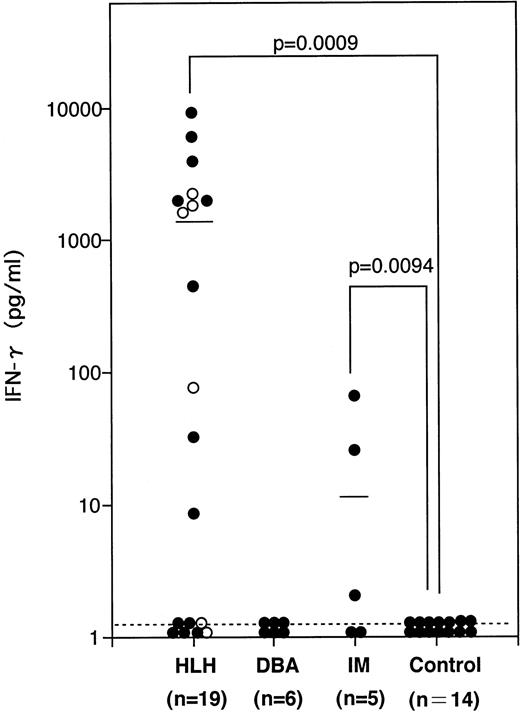
Administration of agonistic anti-Fas antibodies to mice causes liver damages in vivo.20 The same antibodies decrease hematopoietic colony formation2,3 and induce apoptosis in a variety of cell types, including hepatocytes and leukemic cells in vitro. Therefore, it is conceivable that a high level of sFasL produced by circulating and/or tissue-infiltrating cytotoxic cells causes cell damage in patients with HLH or DBA. However, DBA patients do not show liver dysfunction and pancytopenia, both of which are commonly observed in HLH. Therefore, the elevation of serum sFasL per se may not be enough to cause cell damage in the liver and bone marrow. It is known that IFN-γ upregulates the Fas expression and makes target cells susceptible to Fas-mediated apoptosis in a variety of cell types.21,22 High serum levels of IFN-γ are reported to be a common feature in HLH patients.23 We then measured the serum IFN-γ concentrations in our HLH and DBA patients. As shown in Fig 2, most of the HLH patients showed high serum IFN-γ levels (mean ± SD; 1.61 ± 2.62 ng/mL), whereas those in all of the DBA patients were below the detectable level. IM patients showed a moderate increase in their serum IFN-γ concentrations. These results raise the possibility that IFN-γ upregulates Fas in the target cells in HLH and makes them susceptible to FasL-mediated cell death.
The serum IFN-γ concentrations in patients with hematologic disorders. The mean values are shown by horizontal bars. (○) FHL cases. Statistical analysis was performed as described in the Materials and Methods and the P value is presented.
The serum IFN-γ concentrations in patients with hematologic disorders. The mean values are shown by horizontal bars. (○) FHL cases. Statistical analysis was performed as described in the Materials and Methods and the P value is presented.
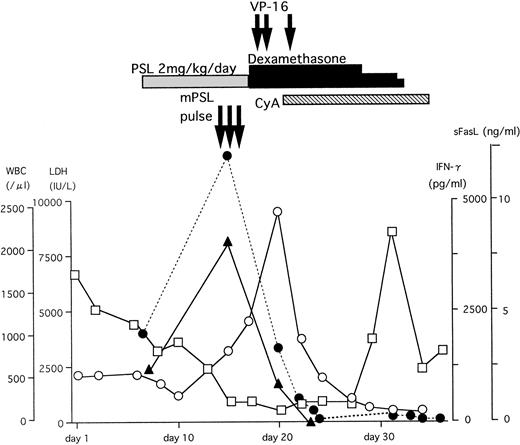
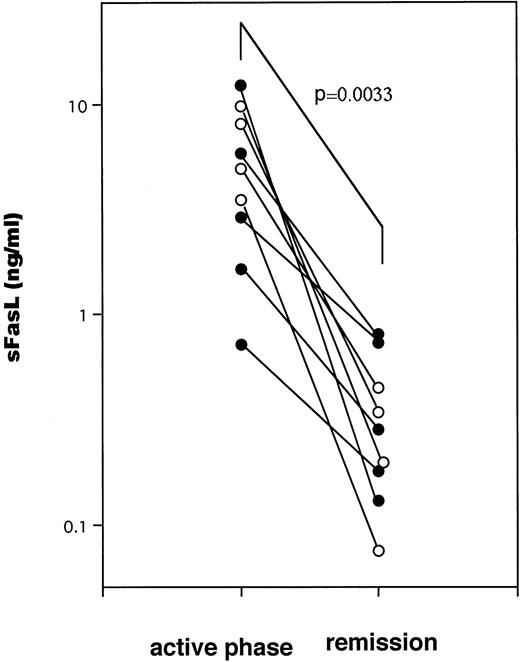
A typical example of the changes in the serum sFasL and IFN-γ levels and the clinical course of one HLH patient is shown in Fig 3. The peak of serum sFasL and IFN-γ levels paralleled with the degree of leukocytopenia and preceded the peak of the serum lactate dehydrogenase (LDH) level in this patient. These values decreased after chemotherapy with steroids and etoposide. This patient underwent syngeneic bone marrow transplantation using an identical twin sister as the donor. She is alive and has been well for 9 months after the bone marrow transplantation. We have compared the serum sFasL levels during the acute phase and remission state in 9 HLH patients who showed elevated serum sFasL levels during the acute phase. As shown Fig 4, the serum sFasL level decreased with remission in all of the patients analyzed.
The clinical course and serum sFasL and IFN-γ concentrations in an HLH patient. γ-glb, γ-globulin; PSL, prednisolone; mPSL, methylprednisolone; CyA, cyclosporine A. (□) WBC; (○) LDH; (•) sFasL; (▴) IFN-γ.
The clinical course and serum sFasL and IFN-γ concentrations in an HLH patient. γ-glb, γ-globulin; PSL, prednisolone; mPSL, methylprednisolone; CyA, cyclosporine A. (□) WBC; (○) LDH; (•) sFasL; (▴) IFN-γ.
The serum sFasL concentrations during active phase and remission state in 9 HLH patients. (○) FHL cases.
The serum sFasL concentrations during active phase and remission state in 9 HLH patients. (○) FHL cases.
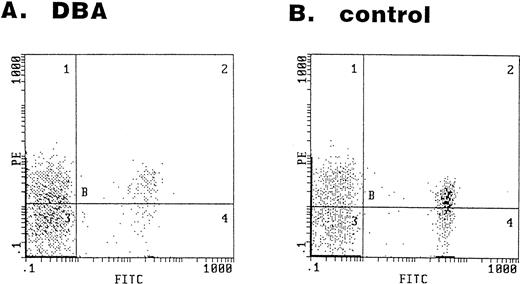
The serum sFasL level was high in most of the DBA patients (Fig 1). Given that cytotoxic cells are involved in the DBA, these cells might recognize some foreign or self antigen on the cell surface of erythroid progenitors and cause apoptosis via the Fas/FasL and/or perforin/granzyme pathways. We have examined the bone marrow cells obtained from 2 DBA patients and 4 healthy volunteers for the expression of the Fas antigen on the erythroid lineage (GPA+) cells. Representative results of the flow cytometry analysis are shown in Fig 5. In the bone marrow cells obtained from the DBA patients, 63% and 79% of GPA+ cells were Fas+ (Fig 5A). The expression of Fas antigen on erythroid lineage cells is not restricted to DBA patients. In bone marrow cells obtained from healthy volunteers, 87%, 46%, 54%, and 65% of GPA+ cells were Fas+(Fig 5B).
Expression of the Fas antigen in erythroid lineage cells in bone marrow. Bone marrow cells obtained from a DBA patient (A) and a healthy volunteer (B) were stained with the FITC-conjugated anti-GPA and PE-conjugated anti-Fas antibodies. The panel shows the fluorescence intensity of GPA (x-axis) and Fas (y-axis).
Expression of the Fas antigen in erythroid lineage cells in bone marrow. Bone marrow cells obtained from a DBA patient (A) and a healthy volunteer (B) were stained with the FITC-conjugated anti-GPA and PE-conjugated anti-Fas antibodies. The panel shows the fluorescence intensity of GPA (x-axis) and Fas (y-axis).
DISCUSSION
FasL is a 40-kD glycoprotein that is expressed on the cell membrane of cytotoxic cells, including CD4+ and CD8+ T cells and NK cells.4 FasL is cleaved by a metalloproteinase-like enzyme to release a 26-kD glycoprotein, sFasL.12 Therefore, serum sFasL levels can be a marker for activation of cytotoxic cells in a variety of human diseases. A moderate increase in serum sFasL was observed in IM patients (Fig 1). These results might reflect the facts that CD8+ T cells are activated in IM and that these cells play a major role in host defense against EBV.24
HLH is a term applied to a life-threatening disease characterized by a generalized histiocytic proliferation with marked hemophagocytosis.25 HLH occurs in several disorders, including an autosomal recessive inherited disease called FHL and virus- or infection-associated hemophagocytic syndrome (VAHS or IAHS). The distinction between FHL and VAHS/IAHS is not always easy without a positive family history, because some FHL cases seem to be triggered by an infection of viral nature. Therefore, the Histiocyte Society has proposed to call these disorders HLH.15 The patients have intermittent fever, lymph node enlargement, and hepatosplenomegaly.26 The most common virus implicated in this syndrome is EBV. IM is a benign, self-limiting disease associated with a primary EBV infection. Most of the IM patients show lymphocytosis with atypical lymphocytes, liver enzyme abnormalities, neutropenia, and mild thrombocytopenia. In rare cases, patients develop severe or fatal IM with clinical characteristics that resemble those of HLH. Laboratory studies of HLH patients demonstrate severe cytopenia and abnormal liver function tests with elevated levels of serum transaminases and LDH. Most of the patients show elevated serum levels of soluble interleukin-2 (IL-2) receptor and cytokines, including IFN-γ and TNF-α, suggesting that the pathogenesis of this syndrome is attributable to cytotoxic damage mediated by abnormally activated T and/or NK cells.23 However, the precise mechanism causing this syndrome is not known. The serum sFasL concentration is elevated in most of the HLH patients and its level is closely related to the disease status (Figs 1, 3, and 4). These results indicate that cytotoxic cells are activated in HLH. It is well established that sFasL or agonistic anti-Fas antibody causes apoptosis of Fas-bearing cells, including hepatocytes, both in vivo and in vitro.11,14,27As little as 1 ng/mL of sFasL kills 90% of a mouse T-cell line expressing Fas in 15 hours and 3.2 ng/mL of sFasL kills 40% of a mouse fibroblast cell line expressing Fas in 7 hours.13 The mean serum sFasL concentration in HLH patients was 3.75 ng/mL (Fig 1), and 2 patients exceeded 10 ng/mL. These values were high enough to cause cell damage to these cells in vitro. Hepatocytes naturally express Fas and can be a target for FasL-triggered cell death. However, Fas surface expression does not necessarily render cells susceptible to Fas ligand-induced cell death.1 Tanaka et al14showed that injection of recombinant sFasL alone does not kill the mice. Simultaneous administration of killed bacteria,Propionibacterium acnes, renders them susceptible to sFasL, and the treated mice die of hepatic failure within 24 hours after intravenous injection of 30 μg of sFasL. Because the administration of killed P acnes stimulates the production of a variety of cytokines, including IFN-γ, TNF, and IL-1, it is conceivable that these cytokines make the animals sensitive to sFasL. Tamura et al28 reported that IFN-γ upregulates the ICE gene in the U937 monocytic cell line. Because both IFN-γ and sFasL are elevated in HLH patients (Figs 1 and 2), these cytokines may act together to cause hepatic damage in these patients.
IFN-γ is a potent inhibitor of myeloid colony formation in vitro.29 On the other hand, IFN-γ has been used to treat chronic granulomatous disease patients without serious side effects.30 Transgenic mice expressing high levels of IFN-γ in their bone marrow did not show decreased hematopoiesis despite the decreased number of hematopoietic progenitor cells.31 Therefore, IFN-γ alone may not cause myelosuppression in animals. It has been reported that IFN-γ induces Fas receptor expression on CD34+ human bone marrow cells and agonistic anti-Fas antibody enhances IFN-γ–mediated suppression of hematopoietic colony formation.2,3 Neutrophils are highly susceptible to agonistic anti-Fas antibody-mediated apoptosis.32 Therefore, it is possible that sFasL is involved in the cytopenia observed at least in some of the HLH patients. However, whether sFasL and IFN-γ suppress hematopoiesis in vivo should be studied further.
Immunosuppressive therapy consisting of antilymphocyte globulin and cyclosporin A is effective in the majority of patients with SAA,33 suggesting that T-cell–mediated cytotoxicity is involved in the pathogenesis of SAA. The bone marrow cells obtained from the patients with SAA show markedly increased percentages of Fas-expressing CD34+ cells, which correlated with their increased sensitivity to anti-Fas antibody-mediated inhibition of colony formation in culture.19 Aberrant IFN-γ gene expression and elevated numbers of activated suppressor T cells are present in the bone marrow of the SAA patients.34,35 These results suggest that Fas-mediated cell death plays a role in the pathogenesis of SAA. However, our results showed that serum sFasL is not elevated in SAA patients, including primary and posthepatitis diseases (Fig 1). These results do not exclude the possibility that FasL-expressing cytotoxic cells are present in the patients' bone marrow and play a role in the pathogenesis of SAA. However, if FasL-expressing cytotoxic cells actively cause the apoptosis of CD34+ cells, Fas+-CD34+ cells might not be able to survive in the bone marrow. The presence of Fas+-CD34+ cells might reflect the high IFN-γ levels in the patients' bone marrow and does not necessarily indicate that Fas-mediated cell death occurs in SAA. Nakao et al36established a CD4+-CTL clone from an SAA patient. The cytolytic activity of this clone is EGTA-sensitive, indicating that this clone uses perforin/granzyme pathway to kill the target cells.37 The involvement of the Fas/FasL system in SAA should be re-evaluated in future studies.
DBA is a severe congenital abnormality of erythropoiesis developing early in childhood. The bone marrows of affected individuals have markedly reduced or absent red blood cell precursors with normal marrow cellularity and preservation of other hematopoietic cell lineages. Although congenital genetic abnormalities have been suspected in this disorder, the analysis of erythropoietin,17 erythropoietin receptor,38 c-kit, and c-kit ligand39,40 genes has failed to detect any abnormalities in DBA patients. Some of DBA patients respond to corticosteroids, suggesting that an immunological abnormality underlies this disease. However, no clear explanation has been given for its defective erythropoiesis. We showed here that the serum sFasL concentration is high in most of the DBA patients (Fig 1), indicating that activation of cytotoxic cells occurs in DBA. The origin of increased serum sFasL is not clear at present. Lymphocyte-mediated inhibition of erythroid colony formation was implicated in some DBA cases.41,42 Therefore, it might be possible that certain subsets of lymphocytes overproduce sFasL in DBA patients. This should be clarified in future study. We have clearly shown that erythroid lineage (GPA+) cells in BM express Fas antigen (Fig 5). Bouscary et al43 examined Fas expression on BM cells obtained from normal subjects and myelodysplastic syndrome patients. They reported that GPA+ cells did not express Fas antigen to a detectable extent by their FACS analysis using FITC-labeled UB2 anti-Fas MoAb. The exact reason for the discrepancy between our results (Fig 5) and theirs is not certain. However, it could be due to a different labeling of the UB2 MoAb. We used PE-labeled UB2, which might give brighter fluorescence than the FITC-labeled one. Based on our findings shown in Fig 5, it is possible that erythroid lineage cells are killed by cytotoxic cells via the Fas-mediated pathway in certain conditions. However, an analysis of large samples is required to establish the role of the Fas/FasL system in DBA.
ACKNOWLEDGMENT
The authors thank Dr Osamu Mabuchi (Department of Hemato-Oncology, Hyogo Prefecture Children's Hospital, Hyogo, Japan) and Dr Masuji Yamamoto (Department of Pediatrics, Hyogo Medical College, Hyogo, Japan) for providing us with the serum samples of DBA patients.
Supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture (1996-1997), a grant from The Ryoichi Naito Foundation for Medical Research (1996), and a grant from The Shinryoku Foundation (1997).
Address reprint requests to Kimihiko Sano, MD, PhD, Department of Pediatrics, Kobe University School of Medicine, 7-5-1 Kusunoki-cho, Chuo-ku, Kobe 650, Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal