ADHESION IS REQUIRED for cell growth, differentiation, survival, and function. Nowhere is this more evident than in the response to tissue injury, where vascular damage triggers reparative processes, such as hemostasis, inflammation, and wound healing. These processes depend on a coordinated series of cell adhesion and migration events by platelets, leukocytes, and vascular cells for their successful execution.1 Cell adhesion is mediated by a structurally diverse group of plasma membrane receptors, each exhibiting specialized ligand-binding properties that are needed for specific tasks in the injury response. For example, when blood flows through a damaged blood vessel, leukocytes slow down and roll on the endothelial surface as a consequence of the interaction of appropriate sialyl Lewis X-rich membrane glycoproteins on the leukocytes with selectins on the endothelial cells.2,3Platelets also roll under conditions of high shear on perturbed endothelium4 as well as on denuded vascular surfaces, in the latter case through interactions of the platelet glycoprotein (GP) Ib-V-IX complex with von Willebrand factor (vWF) in the subendothelial matrix.5 Once the rolling process has slowed down these blood cells, they come to an abrupt stop at the right place through regulated interactions between integrin adhesion receptors and either counter-receptors on endothelial cells or adhesive proteins in the matrix.2,5 Integrins also mediate responses necessary for eventual completion of the injury response, including leukocyte transmigration and platelet aggregation.2 6
Although adhesion receptors rightfully deserve this moniker, any implication that they are simply cellular velcro is incorrect. Most, if not all, adhesion receptors engage in a dialogue with the extracellular and intracellular milieus. Integrins are a case in point. Cells often regulate ligand binding to integrins through a process known as inside-out signaling or integrin activation. Furthermore, once integrins have become occupied and clustered by their ligands, they can transmit information into cells. These outside-in signals collaborate with signals originating from growth factor receptors and other plasma membrane receptors to regulate a host of anchorage-dependent cellular functions. One of the best studied cases of integrin signaling involves αIIbβ3, an integrin of particular significance to hematologists because it is required for aggregation and adhesive spreading of platelets during hemostasis (Fig 1). The purpose of this review is to describe the platelet paradigm of integrin signaling and to emphasize the advances and gaps in our understanding of this process and place it into clinical perspective. We have tried to cite authoritative reviews whenever possible to provide interested readers with additional sources of primary references. Several excellent general reviews of integrins7-12 and platelet biochemistry13 14are available.
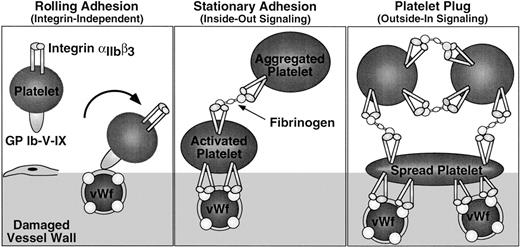
Integrin signaling in hemostasis. Platelet adhesion to the damaged vessel wall is initiated by platelet rolling, an integrin-independent event mediated by binding of GP Ib-V-X to vWF (left panel). Subsequent stationary adhesion and primary platelet aggregation require inside-out signaling through and ligand binding to αIIbβ3 (center panel). Full platelet spreading, aggregation, and effective hemostatic plug formation also require outside-in signaling through αIIbβ3 (right panel).
Integrin signaling in hemostasis. Platelet adhesion to the damaged vessel wall is initiated by platelet rolling, an integrin-independent event mediated by binding of GP Ib-V-X to vWF (left panel). Subsequent stationary adhesion and primary platelet aggregation require inside-out signaling through and ligand binding to αIIbβ3 (center panel). Full platelet spreading, aggregation, and effective hemostatic plug formation also require outside-in signaling through αIIbβ3 (right panel).
WHAT IS INTEGRIN SIGNALING?
αIIbβ3 consists of a two-chain α subunit bound noncovalently to a single-chain β subunit. Each subunit spans the platelet membrane once. The N-terminus and most of the remainder of each subunit are extracellular, and the membrane-spanning domain is connected to a short C-terminal cytoplasmic tail consisting of 20 amino acid residues in αIIb and 47 residues in β3. Electron microscopy of heterodimers shows an N-terminal globular head connected to two C-terminal stalks.15,16 Although the atomic structure of αIIbβ3 is not known, biochemical, genetic, and molecular modeling studies indicate that ligand binding is primarily a function of the globular heads.17 Because ligand binding is regulated by signals from within the platelet and also triggers platelet responses, mechanisms must exist to propagate information back and forth between the cytoplasmic tails and the globular heads. This overall process is referred to as integrin signaling.
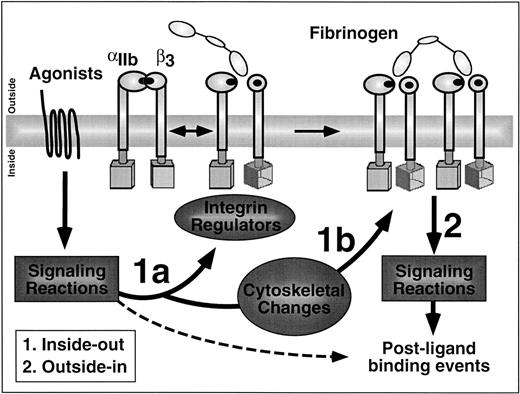
A didactic distinction is often made between inside-out and outside-in signaling. Inside-out signaling denotes those reactions initiated by the binding of one or more agonists to their plasma membrane receptors, leading to the conversion of αIIbβ3 from a low-affinity/avidity receptor to a high-affinity/avidity receptor. This conversion has profound consequences in that it determines whether αIIbβ3 can engage soluble adhesive ligands, such as fibrinogen and vWF, which contain a classical integrin recognition sequence, Arg-Gly-Asp. These multivalent ligands can function as bridges between receptors on adjacent platelets, thus allowing platelet aggregation to proceed.18 Because αIIbβ3 can diffuse laterally within the plasma membrane, inside-out signaling can have two distinct components that are often difficult to distinguish in practice: (1) affinity modulation, which implies a structural change intrinsic to the heterodimer that results in a greater strength of ligand binding; and (2) avidity modulation, which implies a change in the functional affinity of the interaction between receptor and ligand due to chelate or rebinding effects.19 One plausible way that the latter could occur is through integrin clustering within the plane of the plasma membrane (Fig 2).
What is integrin signaling? In this cartoon of the platelet membrane interface, arrows labeled 1a and 1b denote inside-out signaling pathways and arrow 2 denotes outside-in signaling pathways. Inside-out signaling increases the affinity (1a) and avidity (1b) of αIIbβ3 for ligands such as fibrinogen. Affinity modulation is depicted hypothetically here as a signal-induced rotation of the β3 subunit to generate and unmask fibrinogen binding sites in the extracellular domains of αIIbβ3. Outside-in signaling triggers a number of postligand binding events and these require cooperative signaling between αIIbβ3 and agonist receptors (hashed arrow).
What is integrin signaling? In this cartoon of the platelet membrane interface, arrows labeled 1a and 1b denote inside-out signaling pathways and arrow 2 denotes outside-in signaling pathways. Inside-out signaling increases the affinity (1a) and avidity (1b) of αIIbβ3 for ligands such as fibrinogen. Affinity modulation is depicted hypothetically here as a signal-induced rotation of the β3 subunit to generate and unmask fibrinogen binding sites in the extracellular domains of αIIbβ3. Outside-in signaling triggers a number of postligand binding events and these require cooperative signaling between αIIbβ3 and agonist receptors (hashed arrow).
Outside-in signaling denotes reactions initiated by integrin ligation and clustering, and these must be coordinated with signals emanating from other plasma membrane receptors (eg, growth factor, cytokine, and G-protein–linked receptors).10,20,21 Integrin signals help to regulate a host of postligand binding events, the particular pattern varying with the cell and the integrin. Postligand binding events regulated by αIIbβ3 in platelets include the stabilization of large platelet aggregates, platelet spreading, granule secretion, clot retraction, and possibly platelet procoagulant activity.22
INSIDE-OUT SIGNALING IN PLATELETS
Resting platelets contain about 80,000 surface copies of αIIbβ3, with additional pools of αIIbβ3 in the membranes of α-storage granules and the open-canalicular system.23 The binding of soluble ligands to αIIbβ3 can be detected within seconds of platelet activation, and it reaches a steady-state within minutes.18,24 Although ligand binding is at first reversible, it becomes progressively irreversible.22Purified αIIbβ3 can bind fibrinogen with a stoichiometry up to a 1:1, but the stoichiometry may be lower in platelets. Although ligand-binding to surface-expressed αIIbβ3 is essential for initial, primary platelet aggregation, the internal pools of αIIbβ3 can become exposed after cell activation and participate in the secondary phase in which larger platelet aggregates are formed. In fact, the α-granule membrane pool of αIIbβ3 may already be complexed with fibrinogen stored within these granules.25 Should the surface pool of receptors on resting platelets become unavailable to bind ligand, as for example after infusion of a function-blocking antibody,26 the α-granule pool may be able to support platelet aggregation.27
Affinity versus avidity modulation.
Platelets and other cells use a conformational switch mechanism (affinity modulation) and receptor clustering (avidity modulation) to regulate ligand binding to integrins, and the relative contribution of each varies with the integrin and the cell type.28,29Ligand binding studies alone cannot usually distinguish between these two mechanisms. Available evidence indicates that the initial, reversible phase of ligand binding to αIIbβ3 is due to affinity modulation, whereas the irreversible phase may be due to several factors, including (1) ligand-induced changes intrinsic to the receptor (perhaps analogous to those responsible for induced fit between an antibody and antigen)30,31; (2) receptor clustering32-35; and (3) receptor internalization.36 In addition, thrombospondin and other substances released from α-granules during secretion may bind to fibrinogen and/or αIIbβ3 and stabilize the ligand-receptor interaction.37 An initial conformational switch mechanism is consistent with the rapid and selective binding of a monovalent, ligand-mimetic antibody Fab fragment to αIIbβ3 after platelet activation.38 Moreover, fluorescence resonance energy transfer studies using monoclonal antibodies bound to extracellular domains of αIIb and β3 show that platelet activation is associated with a change in the relative orientation of the subunits.39 Electron micrographs of purified αIIbβ3 have shown that fibrinogen binding to the globular head of the integrin can be triggered by interaction of a monoclonal antibody with the membrane-proximal stalk of β3.40 This proves that a long-range conformational change can be propagated along the integrin, a possible requirement for affinity modulation. It is logical to assume that αIIbβ3 also clusters into multimers in response to cytoskeletal changes during platelet activation. A subpopulation of αIIbβ3 already is linked to the membrane skeleton in resting platelets, and there is a wholesale redistribution of this integrin to the F-actin core cytoskeleton during platelet activation.14 However, major cytoskeletal rearrangements do not seem necessary for initial high-affinity ligand binding to αIIbβ3, because inhibitors of actin polymerization have a minimal effect on reversible ligand binding, although they do have a more substantial effect on irreversible binding.34
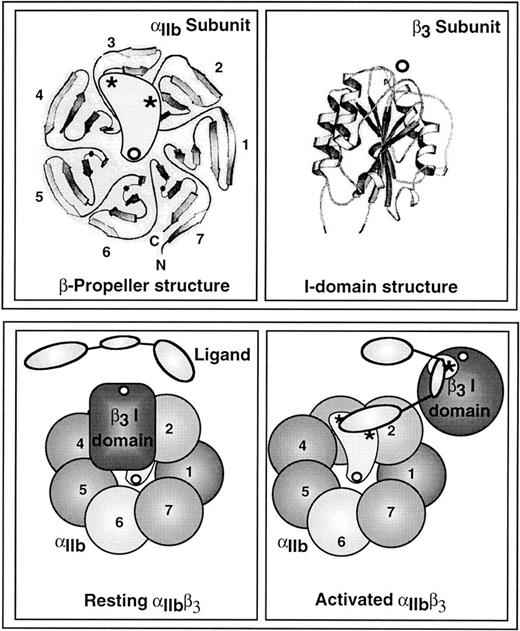
The structural changes in αIIbβ3responsible for interconversion between low- and high-affinity states are not known. One model posits that the signaling reactions triggered by platelet agonists cause some modification of the integrin cytoplasmic tails which is then propagated to the extracellular domains to effect ligand binding (Fig 2). Recent progress has been made in understanding the kinds of structural changes in the globular head of αIIbβ3 that may be required. Based on model building and the functional effects of mutations, Springer41 has proposed that the N-terminal region of αIIb (and other integrin α subunits) conforms to the shape of a β-propeller with seven blades oriented radially and pseudosymmetrically around a central axis and parallel to the plasma membrane. The ligand binding interface would lie on the top surface of the propeller (Fig 3). Tozer et al42 have proposed that a second ligand binding site is located in an N-terminal region of β3 that bears homology with an I-domain, which is, ironically, a ligand-binding module of approximately 190 amino acids inserted within certain α subunits (but not αIIb). Crystallographic analyses of I domains from αL and αM show an α/β fold consisting of seven α-helices packed against a six-stranded β-sheet. At one end of the β-sheet is a cation binding MIDAS motif implicated in ligand binding (Fig 3).43 44
A model depicting the potential changes in the extracellular domains of αIIbβ3 that are required for high-affinity ligand binding. The top left panel shows an overhead view of the proposed β-propeller domain within the N-terminal segment of αIIb,41 and the top right panel shows the crystal structure of an I-domain,43 a homologue of which appears to be present in the N-terminal segment of β3.42 Open circles denote divalent cations and asterisks denote regions presumed to be directly involved in ligand binding. Thick ribbons are strands of β-sheet, and coiled ribbons are α-helices (adapted from Chothia and Jones172 with permission, from the Annual Review of Biochemistry, Volume 66, ©1997, by Annual Reviews Inc). The bottom panels illustrate potential changes in these domains as αIIbβ3 is converted from a resting state (left panel) to an activated state (right panel). (Adapted from Loftus and Liddington.45 Adapted and reproduced from The Journal of Clinical Investigation, 1997, Vol. 99, pp. 2302, by copyright permission of The American Society for Clinical Investigation.)
A model depicting the potential changes in the extracellular domains of αIIbβ3 that are required for high-affinity ligand binding. The top left panel shows an overhead view of the proposed β-propeller domain within the N-terminal segment of αIIb,41 and the top right panel shows the crystal structure of an I-domain,43 a homologue of which appears to be present in the N-terminal segment of β3.42 Open circles denote divalent cations and asterisks denote regions presumed to be directly involved in ligand binding. Thick ribbons are strands of β-sheet, and coiled ribbons are α-helices (adapted from Chothia and Jones172 with permission, from the Annual Review of Biochemistry, Volume 66, ©1997, by Annual Reviews Inc). The bottom panels illustrate potential changes in these domains as αIIbβ3 is converted from a resting state (left panel) to an activated state (right panel). (Adapted from Loftus and Liddington.45 Adapted and reproduced from The Journal of Clinical Investigation, 1997, Vol. 99, pp. 2302, by copyright permission of The American Society for Clinical Investigation.)
Based on this information, Loftus and Liddington45 have proposed a model for the conformational switch in αIIbβ3 that provides a good framework for further studies. It predicts that, in resting platelets, the I-domain–like region in β3 is incapable of binding ligand, but it occludes the ligand binding site in αIIb.45 Platelet activation would then induce ligand binding by (1) causing a conformational change in the β3 I domain to expose its ligand binding site and (2) changing the orientation of the subunits to unmask the ligand binding site in αIIb (Fig 3). This implies that the receptor may engage discontinuous regions of the ligand, consistent with the fact that each fibrinogen monomer is multivalent with respect to αIIbβ3. For example, the C-terminus of the fibrinogen γ chain is essential for initial binding of the soluble ligand to platelet αIIbβ3,46,47but one or both of the Arg-Gly-Asp sites in the Aα chain may provide secondary points of attachment needed for tighter binding. These Aα sites may also assume importance when the fibrinogen is immobilized on a surface or converted to fibrin.48 49
Reactions that initiate and propagate inside-out signaling.
Inside-out signaling involves reactions that (1) initiate and propagate the flow of information from agonist or antagonist receptors to integrin proximal effectors and (2) directly effect integrin activation or deactivation. Currently, only a broad outline of these reactions can be provided.
Inside-out signaling is triggered by many excitatory agonists, some of which, including thrombin, ADP, epinephrine, and thromboxane A2, bind to heptahelical receptors coupled to heterotrimeric (αβγ) G proteins.13,50-52 In the case of some of these agonists, one consequence important for inside-out signaling is activation of phospholipase Cβ by the activated α subunit of Gq, resulting in hydrolysis of phosphatidylinositol and production of the second messengers, diacylglycerol and IP3. Mouse platelets that have been rendered null for Gq undergo shape change but fail to aggregate in response to thrombin, ADP, or a thromboxane A2receptor agonist, and the mice exhibit prolonged tail bleeding times.53 Occupancy of many G-protein–coupled platelet receptors also leads to rapid activation of nonreceptor protein tyrosine kinases, including Src, Syk, and Pyk2 (also known as RAFTK or CAKβ).54,55 Although the mechanism by which G proteins couple to tyrosine kinase cascades in platelets has not been characterized, the net result is tyrosine phosphorylation of a number of proteins, including phospholipase Cγ,56Vav (a guanine nucleotide exchange factor for the Rac GTP-ase), and cortactin (a cortical actin-binding protein).54,57,58 A role for tyrosine phosphorylation-dephosphorylation in integrin activation is suggested by observations that tyrosine kinase inhibitors partially block fibrinogen binding and platelet aggregation, whereas inhibitors of protein tyrosine phosphatases trigger platelet activation.14,54,59 Furthermore, mouse platelets that have been rendered null for Syk show a modest reduction in fibrinogen binding in response to ADP and epinephrine.60 Additional support for a tyrosine phosphorylation-integrin activation connection comes from studies of three agonist receptors that are not known to be coupled to G proteins.
The Fc receptor, FcγRIIA, contains an immune receptor tyrosine activation motif (ITAM) in its cytoplasmic tail. When the receptor is clustered by aggregated Igs, two tyrosines in the ITAM are phosphorylated by a Src family kinase, enabling Syk, which contains tandem SH2 domains, and possibly other proteins with SH2 domains to bind. This leads to Syk activation and, eventually, platelet aggregation.61,62 Surprisingly, a similar scheme may underlie platelet aggregation by collagen. Collagen supports platelet adhesion indirectly by helping to retain vWF in the vessel wall.63,64 It also supports adhesion directly through interactions with integrin α2β1 and GP IV (CD36).65,66 However, none of these interactions is sufficient to trigger platelet activation and recent evidence implicates a 62-kD membrane protein, GP VI, in this process.67 GP VI exists in a complex with FcRγ, a 14-kD ITAM-containing signaling subunit.68,69 Collagen or suitable triple helical collagen-like peptides bind to GP VI, stimulating tyrosine phosphorylation of FcRγ, activation of Syk, and tyrosine phosphorylation and activation of phospholipase Cγ2.70,71 A similar chain of events is observed if platelets are incubated with convulxin, a snake venom protein specific for GP VI,72 or if GP VI is cross-linked by an antibody.67 Collagen-induced platelet aggregation is absent in patients deficient in GP VI as well as in mice null for Fcγ or Syk.67,73 Interestingly, activation of Syk and αIIbβ3 is also triggered by platelet adhesion to vWF, despite the fact that the relevant adhesion receptor, GP Ib-V-IX, does not possess ITAMs.74
Thus, one common feature of most agonists that activate αIIbβ3 is their ability to induce (poly)phosphoinositide hydrolysis and formation of IP3 and diacylglycerol, either through Gq and phospholipase Cβ or through tyrosine kinases and phospholipase Cγ.51 IP3 stimulates an increase in cytoplasmic free Ca2+, but this alone is not sufficient to activate αIIbβ3.75 A Na+/Ca2+ exchanger may change the sensitivity of αIIbβ3 to agonists, but it is not clear how.76 Activation of conventional PKC isoforms by diacylglycerol (or by phorbol myristate acetate) leads to activation of αIIbβ3, a response blocked by PKC inhibitors.77 A prominent PKC substrate in platelets is pleckstrin, a protein with two PH domains,78 but no functional link between pleckstrin and αIIbβ3 has been established. Parenthetically, MARCKS proteins are prominent PKC substrates in some cells, and they have been implicated in integrin-dependent spreading of macrophages.79
Another signaling molecule that has been implicated in integrin function is phosphatidylinositol 3-kinase (PI 3-kinase), which converts PtdIns(4)P and PtdIns(4,5)P2 to the 3-phosphorylated phosphoinositides, PtdIns(3,4)P2and PtdIns(3,4,5)P3, respectively.80,81 Two isoforms of this enzyme have been described in platelets, p85/p110 and p110γ.80,82 The catalytic activity and subcellular localization of the p110 subunit of p85/p110 are regulated through protein-protein interactions of p85, which contains a Bcr homology domain, SH3 domain, two SH2 domains, and proline-rich sequences. Accordingly, this isoform would be expected to be regulated by proteins that become tyrosine phosphorylated in response to platelet agonists. Consistent with this idea, PI 3-kinase can be coprecipitated with Src and Syk from lysates of activated platelets.83,84 The catalytic activity of p110γ, which exists in a complex with a 101-kD protein, is regulated by G protein βγ subunits.80 82
PtdIns(3,4)P2 and PtdIns(3,4,5)P3 are membrane-embedded and transduce signals, at least in part, by binding proteins via their specific PH or SH2 domains and recruiting them to the membrane. Examples include the PH domain-containing proteins, Akt, a serine-threonine kinase, and TIAM-1, a Rho family guanine nucleotide exchange protein; or the SH2 domain-containing proteins, phospholipase Cγ and Src.85 In platelets, thrombin stimulates a rapid and transient increase in PtdIns(3,4,5)P3 and a later increase in PtdIns(3,4)P2. Whereas inhibitors of PI 3-kinase partially block agonist-induced activation of αIIbβ3and platelet aggregation,80 it has been suggested that 3-phosphorylated phosphoinositides function more to stabilize fibrinogen binding than to initiate it.86 Furthermore, accumulation of PtdIns(3,4)P2 is dependent on fibrinogen binding to αIIbβ3,80 more consistent with a role for this particular lipid in outside-in signaling. Indeed, PtdIns(3,4)P2 has been implicated in mediating actin assembly within filopodia and in stimulating a late phase of pleckstrin phosphorylation in activated platelets.80,87,88 One possible link between PI 3-kinase, PKC, and affinity modulation of αIIbβ3 is the observation that 3-phosphorylated phosphoinositides can activate certain atypical and novel isoforms of PKC, some of which are present in platelets.14 85
Members of the Ras superfamily of GTP-ases have also been implicated in integrin function. Platelets contain several members of the Ras (H-Ras, Rap1a) and Rho (cdc42, Rac1, RhoA) families and proteins that regulate their GDP/GTP contents: guanine nucleotide exchange factors, guanine nucleotide dissociation inhibitors, and GTP-ase activating proteins.14 Rac1 regulates thrombin-induced actin polymerization in platelets.89 It has been suggested that RhoA regulates platelet aggregation based on the observation that C3 exoenzyme, an inhibitor of Rho, blocks aggregation responses to thrombin.90 However, C3 exoenzyme has no effect on affinity modulation of αIIbβ3 or primary platelet aggregation, although it does block the formation of focal adhesions and stress fibers during platelet spreading on fibrinogen.91 Thus, one function of Rho A may be to regulate cytoskeletal organization and integrin clustering rather than integrin affinity.92 Expression of activated R-Ras increases integrin-mediated adhesion in some cells,93 but its presence in platelets has not been demonstrated. Thrombin stimulation of platelets causes GTP loading of H-Ras in a PKC-dependent manner and of Rap1 in a Ca2+-dependent manner.94,95 Platelets contain several potential H-Ras effectors, including PI 3-kinase and Raf-1, and thrombin induces activation of MAP (ERK2) kinase in platelets, possibly through the classical H-Ras pathway.96 When overexpressed in CHO cells, activated H-Ras or Raf-1 can suppress integrin activation.97 In platelets, the converse is true: the ERK2 response to thrombin is dampened by fibrinogen binding and aggregation.98
Pathways that inhibit αIIbβ3 are just as important as those that activate it. Prostaglandin I2produced by endothelial cells is a potent platelet activation and aggregation inhibitor that binds to a specific Gs-coupled heptahelical receptor, thereby activating adenylyl cyclase and cyclic AMP-dependent protein kinase (PKA). Platelet aggregation is also inhibited by nitric oxide, which is synthesized by both endothelial cells and platelets and activates soluble guanylyl cyclase (PKG).99 The importance of the nitric oxide inhibitory pathway in vivo is shown by two brothers with a defect in the bioavailability of nitric oxide, heightened platelet reactivity to agonists, and a history of cerebrovascular events.100 One common substrate of PKA and PKG is VASP, a 50-kD protein that localizes to focal adhesions and regulates actin dynamics.101 The phosphorylation of VASP on specific serine residues by agents that activate PKA or PKG correlates with inhibition of platelet aggregation.102 However, PKA and PKG are likely to exert their inhibitory effects on αIIbβ3at several levels of stimulus-response coupling, implying that more than one effector of these serine-threonine kinases is involved.13,102,103 CD39 is an ecto-ADPase on endothelial cells that may be an important regulator of platelet responses to ADP.104 Platelets express PDGF α-receptors and store PDGF in their α-granules. Incubation of platelets with PDGF dampens subsequent aggregation responses to excitatory agonists.105
Reactions that effect inside-out signaling.
The conformational switch necessary for ligand binding to αIIbβ3 could be regulated by intracellular molecules that bind to the cytoplasmic tails of the integrin or by integrin-associated membrane proteins. Evidence directly implicating the cytoplasmic tails in affinity modulation comes from studies of naturally occurring and experimental integrin mutations, from analyses of αIIbβ3 function in heterologous expression systems, and from identification of integrin tail-binding proteins. In addition, several membrane proteins have been reported to form complexes with αIIbβ3 or other integrins.
The sequences of the cytoplasmic tails of αIIb and β3 are shown in Fig 4. Two patients with genetic abnormalities in the β3 cytoplasmic tail provide living examples of the importance of this tail in integrin signaling. Both exhibit bleeding disorders of mild to moderate severity due to variant thrombasthenia: despite near-normal levels of αIIbβ3, their activated platelets bind neither fibrinogen nor aggregate. One of these individuals exhibits a point mutation in β3 (β3S752P),106 the other exhibits a deletion of the 39 C-terminal residues from the β3 tail (β3 Δ 724).107 In each case, the profound defect in activation of αIIbβ3 can be recapitulated by expressing the recombinant mutant in CHO cells.107,108 Transfection studies in CHO cells and in a B-lymphocyte cell line have shown that other mutations in the cytoplasmic tails also affect αIIbβ3affinity.109-113 These results can be summarized as follows. Wild-type αIIbβ3 exists in a default low-affinity state in these cells, but two different classes of tail alterations lead to a constitutive high-affinity state. One involves deletions or mutations of specific membrane-proximal residues in the αIIb or β3 tails, causing the receptor to remain in a high-affinity state even if cellular ATP is depleted. The other class involves replacement of the αIIb tail with certain other α tails (eg, α5 or α6), but in this case the receptor reverts to a low-affinity state upon depletion of ATP. This class of energy-dependent, high-affinity mutants can also be inhibited by overexpression of isolated β3 cytoplasmic tail chimeras, suggesting that integrin affinity is being regulated by titratable intracellular factors.114 This idea is supported by the observation that αIIbβ3-dependent adhesion of a megakaryocytic cell line is inhibited by cellular incorporation of peptides derived from the membrane-distal region of the β3 tail.115
Amino acid sequences of the cytoplasmic tails of αIIb and β3. The space inserted into each sequence arbitrarily separates the N-terminal membrane-proximal and C-terminal membrane-distal regions, the significance of which is discussed further in the text. Numbered residues are as in the full-length integrin subunit.
Amino acid sequences of the cytoplasmic tails of αIIb and β3. The space inserted into each sequence arbitrarily separates the N-terminal membrane-proximal and C-terminal membrane-distal regions, the significance of which is discussed further in the text. Numbered residues are as in the full-length integrin subunit.
These results suggest a working model in which the membrane-proximal portions of the αIIb and β3 tails normally interact, possibly in part through a salt bridge, to form a hinge through which signals impacting on membrane distal tail residues are propagated across the membrane to modulate receptor affinity. Certain membrane-proximal mutations or deletions break this hinge, leaving the receptor in a permanent high-affinity state. Membrane-distal tail residues might regulate receptor affinity in several ways: In unstimulated cells, the αIIb tail might bind a negative regulator or interact with the β3 tail in such a way as to prevent the action of a positive regulator. In stimulated cells, a change in these relationships would either relieve the negative constraint or trigger the function of the positive regulator. This model predicts close but dynamic interactions between the αIIb and β3 tails. In fact, synthetic peptides derived from these tails do interact in vitro.116 117
A number of proteins have been shown to bind directly to integrin cytoplasmic tails, at least in vitro (Table1), but there is no evidence yet that the endogenous forms of any of these proteins modulate integrin affinity in cells. One such protein, β3-endonexin, binds selectively to the β3tail and is present in platelets.118,119 Overexpression of a β3-endonexin fusion protein in CHO cells increases the affinity state of αIIbβ3 and causes fibrinogen-dependent cell aggregation.120 Although some of the other proteins listed in Table 1 are present in platelets, it is not known if they influence αIIbβ3affinity. Two proteins listed in the table may not be relevant to αIIbβ3, but they provide potential novel links between integrins and cellular signaling pathways. Cytohesin-1 binds selectively to the β2 integrin tail and when overexpressed in T lymphocytes, it increases cell adhesion through αLβ2.121 Cytohesin-1 contains a PH domain, which binds 3-phosphorylated phosphoinositides, and a sec 7 domain, which binds the β2 tail and possesses guanine nucleotide exchange activity for a small GTP-ase, ARF.122,123 One serine-threonine kinase, p59ILKhas been shown to bind to integrin β tails, to inhibit β1-mediated cell adhesion, and to promote anchorage-independent cell cycle progression and growth of epithelial cells.124 These studies suggest that, in some cases, integrins may be direct targets of protein kinases, phosphatases, or GTPases. In this regard, the β3 cytoplasmic tail does become phosphorylated on serine, threonine, and tyrosine residues in thrombin-stimulated platelets.125-127 However, the stoichiometry and functional significance of these events are not clear. Furthermore, the tyrosine phosphorylation of β3 is dependent on platelet aggregation; therefore, it is more likely to play some role in outside-in signaling.
Integrin Tail-Binding Proteins
| Protein . | Integrin Tail Partner . | Notable Features . | Reference . |
|---|---|---|---|
| Calreticulin | α* | Expression correlates with integrin-mediated cell adhesion; present in many subcellular locations. | 173-176 |
| F-actin | α2 only | Structural cytoskeletal protein | 177 |
| Calcium- and integrin-binding protein (CIB) | αIIb only | Sequence homology to calcineurin B; contains 2 EF-hand motifs | 178 |
| Talin | αIIb; β | Structural cytoskeletal protein | 179, 180 |
| α-Actinin | β | Structural cytoskeletal protein | 181 |
| Skelemin | β | A myosin and intermediate filament-associated protein | 182 |
| pp125FAK (focal adhesion kinase) | β | Protein tyrosine kinase localized to focal adhesions | 183 |
| p59ILK (integrin-linked kinase) | β | Contains ankyrin repeats and serine threonine kinase domain; overexpression inhibits cell adhesion and induces anchorage-independent growth | 184 |
| Paxillin | β1 | Adapter with SH2 and SH3 binding motifs and LIM domains | 185 |
| ICAP-1 | β1 only | Cell adhesion via β1 modulates phosphorylation state of ICAP-1 | 186 |
| Filamin | β2 | Structural cytoskeletal protein | 187 |
| Cytohesin-1 | β2 only | Contains Sec7 and PH domains; guanine nucleotide exchange activity for ADP-ribosylation factor; overexpression increases αLβ2-mediated adhesion | 121, 123 |
| β3-endonexin | β3 only | Overexpression increases αIIbβ3affinity and adhesive function | 118, 120 |
| p27BBP | β4 | May link β4 to the intermediate filament cytoskeleton in epithelial cells | 188 |
| Rack 1 | β1; β2; β5 | Binds to the β tails via its 5-7th WD repeats in response to cell stimulation with phorbol ester | 202 |
| Protein . | Integrin Tail Partner . | Notable Features . | Reference . |
|---|---|---|---|
| Calreticulin | α* | Expression correlates with integrin-mediated cell adhesion; present in many subcellular locations. | 173-176 |
| F-actin | α2 only | Structural cytoskeletal protein | 177 |
| Calcium- and integrin-binding protein (CIB) | αIIb only | Sequence homology to calcineurin B; contains 2 EF-hand motifs | 178 |
| Talin | αIIb; β | Structural cytoskeletal protein | 179, 180 |
| α-Actinin | β | Structural cytoskeletal protein | 181 |
| Skelemin | β | A myosin and intermediate filament-associated protein | 182 |
| pp125FAK (focal adhesion kinase) | β | Protein tyrosine kinase localized to focal adhesions | 183 |
| p59ILK (integrin-linked kinase) | β | Contains ankyrin repeats and serine threonine kinase domain; overexpression inhibits cell adhesion and induces anchorage-independent growth | 184 |
| Paxillin | β1 | Adapter with SH2 and SH3 binding motifs and LIM domains | 185 |
| ICAP-1 | β1 only | Cell adhesion via β1 modulates phosphorylation state of ICAP-1 | 186 |
| Filamin | β2 | Structural cytoskeletal protein | 187 |
| Cytohesin-1 | β2 only | Contains Sec7 and PH domains; guanine nucleotide exchange activity for ADP-ribosylation factor; overexpression increases αLβ2-mediated adhesion | 121, 123 |
| β3-endonexin | β3 only | Overexpression increases αIIbβ3affinity and adhesive function | 118, 120 |
| p27BBP | β4 | May link β4 to the intermediate filament cytoskeleton in epithelial cells | 188 |
| Rack 1 | β1; β2; β5 | Binds to the β tails via its 5-7th WD repeats in response to cell stimulation with phorbol ester | 202 |
*Unless specified otherwise, the integrin-binding protein has been shown to bind to more than type of α or β subunit.
Adapted and reprinted with permission.189 Reproduced from The Journal of Clinical Investigation, 1997, Vol. 100, pp. 1, by copyright permission of The American Society for Clinical Investigation.
Several transmembrane or GPI-linked membrane proteins have been shown to either coimmunoprecipitate with integrins or colocalize with them by fluorescence microscopy (Table 2). These associations may be direct or indirect, and several are relevant to αIIbβ3. CD47, also known as integrin-associated protein, spans the platelet plasma membrane five times and coimmunoprecipitates with β3integrins.128 So far, no direct role for CD47 in αIIbβ3 function has been demonstrated, either in platelets or in the CHO cell model system.129However, CD47 may function as a costimulatory agonist receptor in platelets because binding of thrombospondin to CD47 leads to activation of αIIbβ3 in a Gi-dependent manner.130,131 CD98, a type II transmembrane protein implicated in neutral amino acid transport and viral syncytia formation, was recently identified in a genetic screen by its ability to complement dominant suppression of αIIbβ3 activation in CHO cells, but its abundance in platelets is not known.132 CD9, a member of the tetraspanin family of transmembrane proteins, colocalizes with αIIbβ3 in platelet α-granule membranes and filopodia.133 Antibodies to CD9 can stimulate platelet aggregation in an Fc receptor-independent manner.134However, tetraspanins may exist in multimolecular complexes, and the interaction of CD9 with αIIbβ3 may not be direct. There is similar uncertainty in interpreting the reported associations of integrins with caveolin or other proteins, such as Src family kinases, that may become part of large complexes within lipid-rich membrane microdomains.135 136 Thus, the functional and physical relationships between αIIbβ3 and other proteins remain a fertile area for further investigation.
Membrane Proteins Associated With Integrins
| Protein . | Associated Integrin . | Reference . |
|---|---|---|
| CD47 (integrin-associated protein) | αIIbβ3; αVβ3; leukocyteresponse integrin | 128, 190 |
| CD98 | αIIbβ3 | 132 |
| Tetraspanins | 133, 191-195 | |
| CD9 | αIIbβ3; α3β1; α4β1; α5β1; α6β1 | |
| CD63 | α3β1 | |
| CD81; NAG-2 | α3β1; α6β1 | |
| CD151 | α5β1 | |
| EMMPRIN | α3β1; α6β1 | 196 |
| Caveolin | α1β1 | 135 |
| CD87 (urokinase plasminogen activator receptor) | αMβ2; αVβ3; αVβ5; α3β1; α5β1; α6β1 | 197-199 |
| CD16 (FcγRIIIB) | αMβ2 | 198 |
| Protein . | Associated Integrin . | Reference . |
|---|---|---|
| CD47 (integrin-associated protein) | αIIbβ3; αVβ3; leukocyteresponse integrin | 128, 190 |
| CD98 | αIIbβ3 | 132 |
| Tetraspanins | 133, 191-195 | |
| CD9 | αIIbβ3; α3β1; α4β1; α5β1; α6β1 | |
| CD63 | α3β1 | |
| CD81; NAG-2 | α3β1; α6β1 | |
| CD151 | α5β1 | |
| EMMPRIN | α3β1; α6β1 | 196 |
| Caveolin | α1β1 | 135 |
| CD87 (urokinase plasminogen activator receptor) | αMβ2; αVβ3; αVβ5; α3β1; α5β1; α6β1 | 197-199 |
| CD16 (FcγRIIIB) | αMβ2 | 198 |
OUTSIDE-IN SIGNALING IN PLATELETS
Platelet functions regulated by outside-in signaling.
Signaling through αIIbβ3 determines the extent to which platelets spread on a vascular matrix containing vWF or fibrinogen and their resistance to detachment from the matrix.5,137 Similarly, outside-in signals triggered during platelet aggregation or spreading promote granule secretion and secondary aggregation. Consequently, outside-in signals are a determinant of the ultimate size of a hemostatic plug or a pathological thrombus (Fig 1). The retraction of a fibrin clot also involves outside-in signals because it represents the interaction of both fibrin and the actin cytoskeleton with αIIbβ3 and the contraction of actin-myosin.138-140 Under certain conditions, even the development of platelet procoagulant activity due to scrambling of membrane phospholipids is dependent, in part, on events subsequent to platelet aggregation.141
The temporal and spatial hierarchy of outside-in signaling.
Outside-in signaling is initiated at localized regions of cell matrix and cell-cell contact. In the platelet, it is triggered by ligand-induced oligomerization of αIIbβ3, because only multivalent ligands are capable of inducing the signal.38 142 Signaling is propagated by interactions between integrin cytoplasmic tails, signaling molecules, and structural cytoskeletal proteins, including vinculin, talin, and α-actinin. The initial signaling reactions foster continued assembly of the complex by promoting protein-protein interactions, actin polymerization, and cytoskeletal reorganization. Complex assembly continues until the supply of new components is exhausted or a set of inhibitory signaling reactions takes over, at which time the complex may even disassemble.
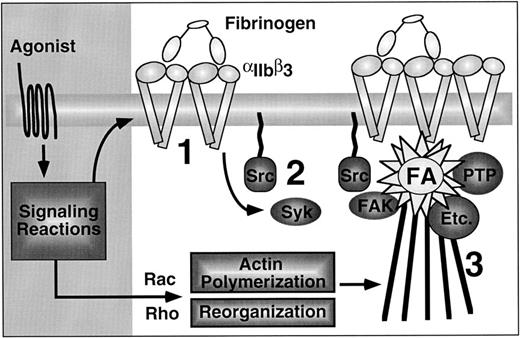
The platelet has provided a good model system to study outside-in signaling and cytoskeletal reorganization in the absence of nuclear signaling (Fig 5).143-145Within seconds of binding soluble or immobilized fibrinogen or vWF, platelets extend filopodia coincident with activation of Syk and tyrosine phosphorylation of substrates of 50 to 68 kD and 140 kD.87,143,145,146 Shortly thereafter, the platelets begin to flatten out or form microscopic aggregates. At this intermediate stage, there is detectable activation of pp60Src, and clusters of αIIbβ3 are discernible by immunofluorescence microscopy on the basal surfaces of the adherent cells. Recent studies in CHO cell transfectants indicate that fibrinogen binding can trigger activation of Syk in a manner that is independent of ITAMs and actin polymerization, due to a combination of autophosphorylation and phosphorylation by Src.147
Outside-in signaling through αIIbβ3 in platelets, emphasizing the sequential nature of the process. First, agonists induce affinity modulation and ligand binding promotes integrin clustering (1). Second, the ligated and clustered integrins trigger early outside-in signaling events, such as activation of Syk and Src (2). Although not shown, this may be associated with filopodial extension. Finally, activation and/or cytoskeletal translocation of FAK, protein tyrosine phosphatases (PTP), and many other important enzymes (Etc.) occurs, coincident with their assembly into mature focal adhesions that are connected to actin stress fibers (3).
Outside-in signaling through αIIbβ3 in platelets, emphasizing the sequential nature of the process. First, agonists induce affinity modulation and ligand binding promotes integrin clustering (1). Second, the ligated and clustered integrins trigger early outside-in signaling events, such as activation of Syk and Src (2). Although not shown, this may be associated with filopodial extension. Finally, activation and/or cytoskeletal translocation of FAK, protein tyrosine phosphatases (PTP), and many other important enzymes (Etc.) occurs, coincident with their assembly into mature focal adhesions that are connected to actin stress fibers (3).
Platelet spreading on fibrinogen or vWF reaches a maximum after several minutes, during which time the platelets display microscopic vinculin clusters connected to F-actin cables, the platelet equivalent of focal adhesions.91,146,148 Full spreading or aggregation is associated with activation of the tyrosine kinase, pp125FAK, and tyrosine phosphorylation of additional substrates, including proteins of 101 and 105 kD, Tec (a tyrosine kinase that contains a PH domain), and SHIP, an SH2 domain-containing inositol 5-phosphatase.9,145,149,200 None of these changes occur if spreading or aggregation is blocked. Eventually there is a decrease in tyrosine phosphorylation of many substrates, due to cytoskeletal recruitment and activation of protein tyrosine phosphatases (eg, PTP-1B and SHP-1), and cleavage of protein tyrosine kinases by calpain.54,146 150-152
FAK provides a well-studied example of how integrin-associated signaling complexes may assemble.9,12,153 It contains a central catalytic domain flanked by N- and C-terminal domains. Subcellular localization of FAK is dictated by a focal adhesion targeting region in the C-terminal domain and possibly by a binding site for integrin β tails in the N-terminal domain. FAK undergoes autophosphorylation at Y397 in response to cell adhesion, providing a docking site for the SH2 domain of Src and possibly PI-3-kinase. Src phosphorylates FAK at additional tyrosine residues, creating sites for interaction of the adapter, Grb2. FAK also complexes through proline-rich motifs in the C-terminal domain with the SH3 domains of the adaptors, p130cas and paxillin, and a Rho GTPase-activating protein, GRAF. Indeed, p130cas and paxillin are phosphorylated by the FAK/Src complex, enabling the recruitment of even more proteins. FAK-null mice die in fetal life and, ex vivo, their fibroblasts form focal adhesions but migrate poorly.154 In platelets, activation of FAK requires both αIIbβ3 ligation and agonist receptor occupancy, the latter being required to provide costimulatory signals through Ca2+ and PKC.155
Studies of naturally occurring and experimentally induced mutations and deletions in αIIbβ3 provide strong evidence for involvement of the αIIb and β3cytoplasmic tails in outside-in signaling.107,108,156However, many fundamental questions remain. What is the role of tyrosine phosphorylation of the β3 tail in response to platelet aggregation?127 Can certain protein or lipid kinases and phosphatases couple directly to αIIbβ3? What are the effectors of Syk, Src, and FAK? Whereas tyrosine phosphorylation is an early event in outside-in signaling, there is an impressive and growing list of other protein and lipid kinases, phosphatases, phospholipases, and GTP-ases that redistribute to the αIIbβ3-rich core cytoskeleton or become activated during platelet aggregation and spreading.89,144,157-161 201 How are the functions of so many proteins and their effectors integrated into the highly coordinated response to platelet adhesion?
PERSPECTIVE
What are the practical implications of integrin signaling? Integrin cytoplasmic tail mutations in patients with variant thrombasthenia prove that integrin signaling is required for hemostasis, but these patients are very rare. However, other individuals with unexplained platelet aggregation defects are encountered more frequently. Some of these suffer from inherited defects, others from acquired disorders that affect platelet function. Once an aggregation defect has been established in the clinical laboratory, further evaluation can be facilitated by conducting flow cytometry analyses of platelets, even in whole blood. Fluorophore-conjugated reagents are available to quantitate platelet surface antigens, including activation-specific antigens (eg, P-selectin) and epitopes (eg, activated or ligand-occupied αIIbβ3).162This allows facile categorization of the abnormality as either an αIIbβ3 activation defect or a postligand binding defect.163 In the case of an activation defect, the subsequent work-up can focus on specific agonist receptors and biochemical pathways responsible for inside-out signaling.164-166 In the case of a postligand binding defect, the work-up can focus on the possibility of storage pool disease or an abnormality in pathways triggered by integrin ligation.107,167 168 We speculate that the spectrum of clinical abnormalities in integrin signaling might even include inappropriate increases in αIIbβ3 function. For example, several dominant mutations introduced experimentally into the αIIb or β3 cytoplasmic tails result in constitutive activation of the receptor, as discussed above. If such mutations were to occur naturally, they might be responsible for some cases of unexplained, chronic thrombocytopenia or even represent a risk factor for arterial thrombosis.
Interest in αIIbβ3 has expanded beyond the realm of the hematologist because of the development of pharmacological inhibitors of ligand binding to αIIbβ3 for prophylaxis and therapy of arterial thrombosis.169 170Abciximab, a chimeric mouse-human antibody that blocks ligand binding to αIIbβ3, is already licensed for use as adjunctive therapy in patients undergoing coronary angioplasty, and additional parenteral and orally active compounds are now in clinical trials. It is too early to predict the full range of indications for these agents or the degree of efficacy and risk of long-term use, but it is satisfying that platelet research has yielded the first integrin-based therapeutics. In this context, the orally active antiplatelet agents currently available in developed countries are, in one way or another, inhibitors of inside-out integrin signaling: aspirin inhibits cyclooxygenase-1 and, ultimately, the production of thromboxane A2; ticlopidine and clopidogrel inhibit signaling through the ADP receptor171; and phosphodiesterase inhibitors decrease catabolism of cyclic AMP, a suppressor of platelet activation. If the intracellular events responsible for αIIbβ3 signaling can be better defined, it may be possible to identify new integrin-proximal signaling proteins as drug targets.
ACKNOWLEDGMENT
The authors are grateful to our collaborators, past and present, and in particular Joan Brugge and Mark Ginsberg, for many of the concepts summarized herein, and to Mark Ginsberg and Martin Schwartz for critical review of the manuscript. Cited work from the authors' laboratory was supported by National Institutes of Health Grants No. HL56595 and HL57900 and from Cor Therapeutics, Inc.
Address reprint requests to Sanford J. Shattil, MD, Department of Vascular Biology, The Scripps Research Institute, 10550 N Torrey Pines Rd, VB-5, La Jolla, CA 92037.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal