Abstract
Activation and recruitment of eosinophils in allergic inflammation is in part mediated by chemoattractants and T-helper 2 (Th2)-derived cytokines. However, little is known concerning the signal transduction mechanisms by which this activation occurs. We have investigated tyrosine kinase-mediated activation of phosphatidylinositol 3-kinase (PI3K) and compared this with the activation of the p21ras-ERK signaling pathway in human eosinophils. The related cytokines interleukin-3 (IL-3), IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF), all induced PI3K activity detected in antiphosphotyrosine immunoprecipitates. Furthermore, the chemoattractants platelet-activating factor (PAF), RANTES, and C5a were also able to induce phosphotyrosine-associated PI3K activity. Protein kinase B (PKB) is a downstream target of PI3K activation by growth factors. Induction of PKB phosphorylation in human eosinophils was transiently induced on activation with the cytokines IL-4 and IL-5, as well as the chemoattractants PAF, C5a, and RANTES showing a broad activation profile. Surprisingly, analysis of the activation of the mitogen-activated protein (MAP) kinases p44ERK1 and p42ERK2, showed that ERK2, but not ERK1, was transiently activated in human eosinophils after stimulation with IL-5 or PAF. Activation kinetics correlated with activation of p21ras by both cytokines and chemoattractants as measured by a novel assay for guanosine triphosphate (GTP)-loading. Finally, using specific inhibitors of both the p21ras-ERK and PI3K signaling pathways, a role was demonstrated for PI3K, but not p21ras-ERK, in activation of the serum-treated zymosan (STZ)-mediated respiratory burst in IL-5 and PAF-primed eosinophils. In summary, these data show that in human eosinophils, Th2-derived cytokines differentially activate both PI3K and MAP kinase signal transduction pathways with distinct functional consequences showing complex regulation of eosinophil effector functions.
ALLERGIC INFLAMMATION in bronchial tissue in asthma is characterized by the presence of T-helper 2 (Th2) lymphocytes and eosinophilic granulocytes.1,2 Th2 lymphocytes are characterized by production of a distinct cytokine pattern in vitro, which includes interleukin-5 (IL-5), IL-4, and IL-13.3 These cytokines play an important role in the propagation of the allergic inflammatory response and in the activation and recruitment of eosinophils.2,4 Furthermore, elevated levels of the cytokines IL-5 and granulocyte-macrophage colony-stimulating factor (GM-CSF) have been detected in sera from allergic asthmatic patients.5,6 In vivo, IL-5 is the terminal differentiation factor for eosinophils and an important modulator of eosinophil functions.7 In vitro, IL-5 induces chemokinesis and priming of eosinophil functions such as migration, respiratory burst activation, and platelet-activating factor (PAF) release.8-11 Transmembrane receptors for these cytokines are tyrosine kinase-linked and associate with members of the JAK family and agonist binding results in intracellular increases in tyrosine phosphorylation.12 The second major effector molecules regulating both eosinophil chemotaxis and priming are chemoattractants. These proinflammatory molecules include PAF, fMLP, C5a, as well as chemokines such as IL-8 and RANTES.13,14 In contrast to cytokine receptors, chemoattractant receptors are serpentine G-protein coupled receptors. The mechanisms by which these receptors induce tyrosine phosphorylation and regulate intracellular signal transduction are poorly defined. Furthermore, there is significant cross-talk between the tyrosine-kinase linked and G-protein coupled receptor signal transduction systems, as observed during priming of granulocytes in vitro and in vivo.15
Recently, much attention has been paid to the activation of the lipid kinase phosphatidylinositol 3-kinase (PI3K) in the context of cytokine signaling. At least three families of PI3K have been described in mammalian cells.16 One is activated by tyrosine kinases and consists of a regulatory subunit of 85 kD (p85) and a catalytic subunit of 110 kD (p110).17,18 The second family consists of a 110-kD protein (p110γ), which is activated by the βγ subunit of heterotrimeric G-proteins.19,20 The third consists of a phosphatidylinositol (PI)-specific PI3K.21Activated PI3K phosphorylates the hydroxyl group on the D-3 position of the inositol ring of the lipids PI, PI 4-phosphate (PI4-P), and PI 4,5-biphosphate (PI4,5-P2), which results in formation of PI3-P, PI3,4-P2, and PI3,4,5-P3. These products are potential second messengers and have been implicated in the activation of several members of the protein kinase C (PKC) family.22-25 The small guanosine triphosphate (GTP)-binding protein Rac has also been proposed to be a target of PI3,4,5-P3.26 Furthermore, phosphoinositide products of PI3K-activity have also been shown to bind to pleckstrin homology (PH) domain-containing proteins.27-29 We and others have shown that the PH domain-containing protein kinase B/c-Akt (PKB) is a downstream target for PI3K and has been shown to bind and be activated by 3-phosphorylated lipids in vitro.30,31 Recent data has indicated that constitutive activation of PI3K in a monoblastic phagocyte cell line (GM-1) results in constitutive activation of PKB and furthermore phosphorylation of p47phox suggesting a potential role in regulating the respiratory burst.32
Although activation of p21ras and the mitogen-activated protein (MAP) kinase p44ERK1 has been recently demonstrated in human eosinophils, this work has only investigated the cytokine IL-5.33 The potential for activation of p21ras-ERK signal transduction in eosinophils by chemokines or other Th2-derived cytokines, such as IL-4, have not been investigated. Activation of MAP kinases have been postulated to play an important role in regulating granulocyte effector functions such as superoxide production, although no direct evidence has been provided linking p21ras-ERK signaling to these events in either neutrophils or eosinophils.34-36
Here we show that PI3K-PKB and p21ras-ERK signal transduction pathways are activated in human eosinophils after stimulation with Th2-derived cytokines. We show that while activation of PI3K is critical for both IL-5 and PAF-primed STZ-mediated respiratory burst, activation of p21ras-ERK is not. These data show that in human eosinophils, Th2-derived cytokines differentially activate both PI3K and MAP kinase signal transduction pathways with distinct functional consequences.
MATERIALS AND METHODS
Reagents.
PAF(1-0-hexadecyl-2-acetyl-sn-glycero-3-phosphoryl-choline), C5a, L-α–phosphatidylinositol (PI), and bovine serum albumin (BSA) were purchased from Sigma (St Louis, MO). Human serum albumin (HSA) was from the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service (Amsterdam, The Netherlands). RANTES was purchased from Pepro Tech (Rocky Hill, NJ). Recombinant human (rh) GM-CSF (2.5 × 108 U/mg) was from Genzyme (Boston, MA). rhIL-4 was a kind gift from Dr F. Kalthoff (Sandoz Forschungsinstitut, Vienna, Austria). rhIL-5 was a kind gift from Dr D. Fattah (GlaxoWellcome, Stevenage, UK). Ficoll-Hypaque and Percoll were obtained from Pharmacia (Uppsala, Sweden). Wortmannin and PD098059 were purchased from Biomol (Plymouth Meeting, PA). All other materials were reagent grade. Experiments were performed in incubation buffer, containing 132 mmol/L NaCl, 6.0 mmol/L KCL, 1.0 mmol/L CaCl2, 1.0 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 20 mmol/L HEPES, 5 mmol/L glucose, and 1.0% HSA (wt/vol).
The murine antiphosphotyrosine monoclonal antibody (MoAb) (4G10, IgG2bk) and anti-p85 rabbit antiserum were obtained from UBI (Lake Placid, NY), the peroxidase-conjugated rabbit antimouse (P 260) and swine antirabbit antibodies were from Dakopatts (Glostrup, Denmark). Monoclonal anti–IL-4 (clone 1-41-1) was a kind gift from Dr F. Kalthoff (Sandoz Forschungsinstitut, Vienna, Austria). Rabbit polyclonal antisera for ERK1 (C-16) and ERK2 (C-14) were from Santa Cruz Biotechnology Inc (Santa Cruz, CA). PKB antisera were from UBI (Lake Placid, NY). All antibodies were stored at 4°C.
Isolation of human eosinophils.
Blood was obtained from healthy volunteers from the Blood Bank, Utrecht, The Netherlands. Mixed granulocytes were isolated from the buffy coat of 500 mL of blood anticoagulated with 0.4% (wt/vol) trisodium citrate (pH 7.4) as described previously.37 To reduce the number of neutrophils in this mixed granulocyte population, the cells were subjected to discontinuous Percoll gradient (1.084 to 1.1 g/mL) centrifugation (20 minutes, 1,000g, room temperature). Eosinophils were subsequently isolated by the method described by Hansel et al.38 This isolation method is based on the fact that, in marked contrast to neutrophils, eosinophils lack the epitope on FcγRIII recognized by the MoAb CLB-FcR-gran 1 (CD16) directed against FcγRIII.39 As a result, highly purified eosinophils can be isolated by removing neutrophils coated with CLB-FcR-gran 1 with immunomagnetic dynabeads (Dynal, Oslo, Norway). Briefly, neutrophils were coated with a MoAb against FcγRIII (CLB-FcR-gran 1, CD16, 2 μg/107 cells/mL) during 30 minutes at 4°C. The cells were washed twice and subsequently incubated head over head in a rotator with dynabeads at a ratio of 1:2 (cells/beads) for 20 minutes at 4°C. Neutrophils were subsequently removed by a magnetic particle concentrator (MPC-1, Dynal). Eosinophil purity was always more than 95%.
Phosphatidylinositol 3(OH)-kinase assays.
Eosinophils were resuspended in incubation buffer and preincubated for 30 minutes at 37°C. Thereafter, the cells were stimulated with different agonists before being stopped by the addition of 2 volumes of ice-cold incubation buffer containing 2 mmol/L Na3VO4. Subsequently the eosinophils were pelleted by centrifugation at 4°C. Thereafter, the cells were resuspended in lysis buffer (1% Triton X-100, 20 mmol/L Tris/HCl, 100 mmol/L NaCl, 10 mmol/L Na4P2O7, 2 mmol/L EDTA, 50 mmol/L NaF, 10% glycerol, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL soybean tryptase inhibitor, 1 mmol/L phenylmethyl sulfonyl fluoride (PMSF), and 1 mmol/L Na3VO4, pH 8.0) for 30 minutes on ice. After 30 minutes, detergent-insoluble material was removed by centrifugation for 10 minutes at 14,000 rpm at 4°C.
Lysates were treated for 1 hour at 4°C with the antiphosphotyrosine MoAb 4G10 (2.5 μg/mL). Thereafter, protein A-sepharose (Pharmacia, Uppsala, Sweden) was added for another hour and subsequently, the protein sepharose beads were washed three times with lysis buffer and two times with 10 mmol/L Tris-HCl (pH 7.4) containing 1 mmol/L Na3VO4. PI3K activity was measured by adding 100 μg of sonicated PI, and 20 μCi of (γ-32P) adenosine triphosphate (ATP) (ICN, Plainview, NY) in the presence of 200 μmol/L adenosine to inhibit PI4K activity, 30 mmol/L MgCl2 and 35 μmol/L ATP in a volume of 60 μL. Reactions were performed for 20 minutes at room temperature and stopped by addition of 100 μL 1 mol/L HCl and 200 μL chloroform:methanol (1:1 vol/vol). After centrifugation and taking away the upper layer, 80 μL methanol/HCl was added. After centrifugation, lipids were separated on thin-layer chromatography (TLC) plates (Merck, Darmstadt, Germany) using a solvent system of chloroform:methanol:ammoniumhydroxide (45:35:10 vol/vol/vol). TLC plates were exposed to x-ray film at −80°C. The identity of the labeled phosphatidylinositol phosphate (PIP) spot was verified by comigration with a PIP standard, which was visualized in a iodine chamber (results not shown). Immunoprecipitation with polyclonal anti-p85 antibody was used as positive control for PI3K activity (results not shown). In Figs 1 and2, x-ray films were scanned on a densitometer (Molecular Dynamics, Sunnyvale, CA) analyzed with Image Quant software (Molecular Dynamics). These results are expressed as fold induction compared with the unstimulated control (see legends).
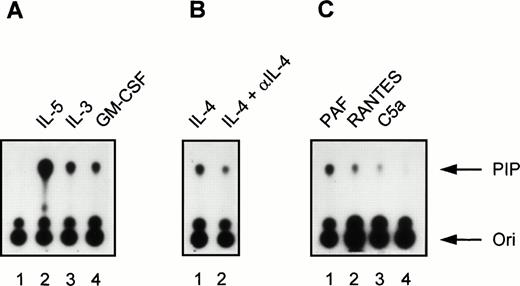
Induction of PI3K activity by cytokines and chemoattractants in human eosinophils. (A) Eosinophils (2 × 106) were left unstimulated for 5 minutes at 37°C (lane 1) or stimulated with the cytokines IL-5 (10−9 mol/L), IL-3 (10−9 mol/L), and GM-CSF (6 × 10−8mol/L) for 5 minutes at 37°C (lanes 2 to 4). Densitometric analysis showed that the fold induction compared with the unstimulated control (lane 1) was 61.7 (lane 2), 20.4 (lane 3), and 14.4 (lane 4). (B) Eosinophils (2 × 106) were stimulated with IL-4 (10−8 mol/L, lane 1) or with IL-4 (10−8mol/L) in combination with a MoAb directed against IL-4 (lane 2). The fold induction compared with the unstimulated control (lane 1) was 14.7 (lane 2) and 6.2 (lane 3). (C) In lanes 1 to 3, eosinophils (2 ×106) were stimulated for 1 minute at 37°C with PAF (10−6 mol/L), RANTES (10−6 mol/L), C5a (10−8 mol/L), respectively. Lane 4 represents an unstimulated control sample that was kept at 37°C for 1 minute. The fold induction compared with the unstimulated control (lane 4) was 15.3 (lane 1), 6.3 (lane 2), and 3.0 (lane 3). The experiment shown is representative of three other experiments.
Induction of PI3K activity by cytokines and chemoattractants in human eosinophils. (A) Eosinophils (2 × 106) were left unstimulated for 5 minutes at 37°C (lane 1) or stimulated with the cytokines IL-5 (10−9 mol/L), IL-3 (10−9 mol/L), and GM-CSF (6 × 10−8mol/L) for 5 minutes at 37°C (lanes 2 to 4). Densitometric analysis showed that the fold induction compared with the unstimulated control (lane 1) was 61.7 (lane 2), 20.4 (lane 3), and 14.4 (lane 4). (B) Eosinophils (2 × 106) were stimulated with IL-4 (10−8 mol/L, lane 1) or with IL-4 (10−8mol/L) in combination with a MoAb directed against IL-4 (lane 2). The fold induction compared with the unstimulated control (lane 1) was 14.7 (lane 2) and 6.2 (lane 3). (C) In lanes 1 to 3, eosinophils (2 ×106) were stimulated for 1 minute at 37°C with PAF (10−6 mol/L), RANTES (10−6 mol/L), C5a (10−8 mol/L), respectively. Lane 4 represents an unstimulated control sample that was kept at 37°C for 1 minute. The fold induction compared with the unstimulated control (lane 4) was 15.3 (lane 1), 6.3 (lane 2), and 3.0 (lane 3). The experiment shown is representative of three other experiments.
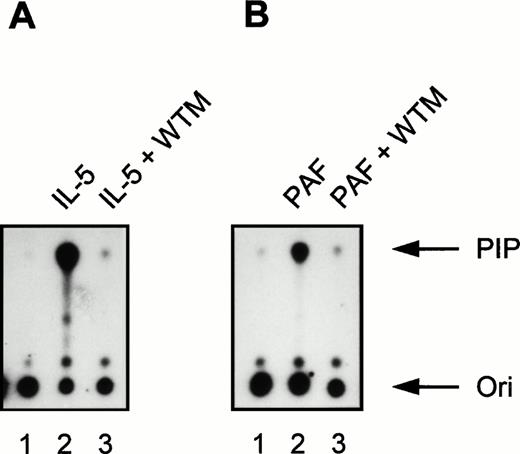
Effect of the PI3K inhibitor wortmannin on IL-5 and PAF-induced PI3K activity in human eosinophils. All samples (2 × 106 cells) were pretreated for 15 minutes at 37°C in the presence or absence of 30 nmol/L wortmannin (WTM). (A) Eosinophils were stimulated with IL-5 (10−9 mol/L) for 5 minutes at 37°C (lanes 2 and 3). Lane 1 represents PI3K activity in unstimulated eosinophils not treated with WTM and incubated at 37°C for 5 minutes. Densitometric analysis showed that the fold induction compared with the unstimulated control (lane 1) was 49.8 (lane 2) and 1.1 (lane 3). (B) Eosinophils were left untreated (lane 1, 1 minute at 37°C) or stimulated with PAF (10−6 mol/L) for 1 minute at 37°C (lanes 2 and 3). The fold induction compared with the unstimulated control (lane 1) was 24.3 (lane 2) and 1.2 (lane 3). The experiment shown is representative of three other experiments (n = 3).
Effect of the PI3K inhibitor wortmannin on IL-5 and PAF-induced PI3K activity in human eosinophils. All samples (2 × 106 cells) were pretreated for 15 minutes at 37°C in the presence or absence of 30 nmol/L wortmannin (WTM). (A) Eosinophils were stimulated with IL-5 (10−9 mol/L) for 5 minutes at 37°C (lanes 2 and 3). Lane 1 represents PI3K activity in unstimulated eosinophils not treated with WTM and incubated at 37°C for 5 minutes. Densitometric analysis showed that the fold induction compared with the unstimulated control (lane 1) was 49.8 (lane 2) and 1.1 (lane 3). (B) Eosinophils were left untreated (lane 1, 1 minute at 37°C) or stimulated with PAF (10−6 mol/L) for 1 minute at 37°C (lanes 2 and 3). The fold induction compared with the unstimulated control (lane 1) was 24.3 (lane 2) and 1.2 (lane 3). The experiment shown is representative of three other experiments (n = 3).
p21ras Activation assay.
GST-Ras-binding domain (RBD) (aa51-131 of Raf1) fusion protein was constructed and isolated as previously described.40 The desired amount of crude GST-RBD was incubated with glutathione-agarose beads at 4°C for 1 hour. The beads were isolated by centrifugation and washed five times with lysis buffer (50 mmol/L Tris-Cl pH 7.4, 150 mmol/L NaCl, 1% NP-40, 10% glycerol, 0.1 μmol/L aprotinin, 1 μmol/L leupeptin, and 1 mmol/L PMSF). After stimulation, eosinophils (7.5 × 106 cells) or neutrophils (107cells) were lysed in 1 mL lysis buffer at 4°C and centrifuged to remove nuclei. Precoupled GST-RBD beads were added and the lysates incubated on a rotating wheel for 30 minutes at 4°C. Beads were pelleted by centrifugation and washed three times with lysis buffer before being resuspended on Laemmli sample buffer. Protein samples were separated on a 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to polyvinylidene fluoride (PVDF)-membrane. Western blots were probed with anti-Ras MoAb Y13-259 (16 hours at 4°C) followed by horseradish peroxidase (HRP)-coupled goat antirat antiserum (Santa Cruz; 2 hours). Blots were developed by enhanced chemiluminescence (ECL; Amersham, Amersham, UK).
MAP kinase assays.
Eosinophils and neutrophils were isolated as described above and incubated at 37°C for 30 minutes. After stimulation, the cells were washed twice in ice-cold phosphate-buffered saline (PBS) and lysed in 50 mmol/L Tris-HCl (pH 7.5), 100 mmol/L NaCl, 50 mmol/L NaF, 5 mmol/L EDTA, 40 mmol/L β-glycerophosphate, 1 mmol/L Na3VO4, 1% Triton X-100, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mmol/L PMSF. Lysates were precleared for 30 minutes at 4°C with protein A-sepharose. MAP kinase was immunoprecipitated with 1 μg of either ERK1 or ERK2 polyclonal antisera for 1 hour at 4°C on a rotating wheel. Protein A-sepharose was then added for an additional 1 hour at 4°C. After washing twice with lysis buffer, samples were washed twice with kinase buffer without ATP. Precipitates were then incubated in 25 μL kinase buffer consisting of 30 mmol/L Tris-HCL (pH 8.0), 20 mmol/L MgCl2, 2 mmol/L MnCl2, 10 μmol/L rATP, 10 μg myelin basic protein (MBP), and 0.3 μCi of (γ-32P) ATP for 20 minutes at 30°C. Reactions were stopped by the addition of 5× Laemmli sample buffer. Samples were separated by electrophoresis on 15% SDS-polyacrylamide gels. MBP phosphorylation was detected by autoradiography.
Analysis of ERK and PKB phosphorylation.
For detection of phosphorylation of ERK1, ERK2, and PKB, cells were washed twice in ice-cold PBS after stimulation and immediately lysed in Laemmli buffer. After being heated for 5 minutes at 95°C, cell lysates were run on 12.5% SDS-PAGE (173:1). After gel electrophoresis, proteins were transferred to Immobilon-P and blocked with PBS-Tween (0.3%) containing 4% low-fat milk powder for 1 hour at room temperature. Blots were then probed in PBS-Tween containing either ERK1, ERK2, or PKB antisera (1:2,000) for 1 hour at room temperature as previously described.41 An additional incubation for 1 hour with goat antirabbit peroxidase-conjugated antibody was performed. Proteins were visualized by luminol-enhanced chemiluminescence (ECL) using a standard kit (RPN 2109, Amersham, UK).
Measurement of oxygen consumption.
Oxygen consumption was measured at 37°C with an oxygen electrode as described previously.42 In short, eosinophils were resuspended (1.6 × 106 per mL) in incubation buffer. After 5 minutes of incubation at 37°C, either IL-5 (10−10 mol/L, 20 minutes) or PAF (1 μmol/L, 2 minutes) or solvent was added. For some experiments, wortmannin (100 nmol/L) or PD098059 (25 μmol/L) were preincubated for 5 or 15 minutes, respectively. Oxygen consumption was measured for 2 minutes before and continually after addition of STZ (1 mg/mL). The maximal rate of oxygen consumption is depicted ± standard error (SE).
RESULTS
Activation of phosphotyrosine-associated PI3K in human eosinophils by cytokines and chemoattractants.
The lipid kinase, PI3K, has been shown to be activated by mediators that induce intracellular protein tyrosine phosphorylation.16 In eosinophils, both IL-5 and PAF are capable of inducing protein tyrosine phosphorylation11 (and unpublished observations) and are potent primers of eosinophil functions. Here we studied activation of PI3K by cytokines and chemoattractants in antiphosphotyrosine immunoprecipitates derived from lysates prepared from normal human eosinophils.
Incubation for 5 minutes at 37°C with the cytokines IL-5 (10−9 mol/L), IL-3 (10−9 mol/L), and GM-CSF (6 × 10−10 mol/L) induced PI3K activity in human eosinophils (Fig 1A; lane 1 represents the unstimulated control). Importantly, IL-4 (10−8 mol/L) clearly also induced PI3K activity in eosinophils (Fig 1B, lane 1). Control experiments were performed with 10−8 mol/L IL-4 in combination with a blocking MoAb against IL-4, to show the specificity of the IL-4–induced activation, as the presence of this receptor on eosinophils has not been widely reported.43 In the presence of anti–IL-4, PI3K activity was reduced (Fig 1B, lane 2). This is the first example of activation of specific downstream signaling events in human eosinophils stimulated by IL-4. In addition to cytokines, incubation for 1 minute at 37°C with the chemoattractants PAF (10−6 mol/L), RANTES (10−6 mol/L), and C5a (10−8 mol/L) also induced PI3K activity in human eosinophils (Fig 1C). This is somewhat surprising, as previous reports have linked these G-protein coupled receptor agonists to the nonphosphotyrosine-associated p110γ isoform of PI3K.16,19,20 This shows for the first time activation of PI3K in eosinophils and furthermore that G-protein coupled receptors can activate phosphotyrosine-associated PI3K isoforms in these cells. Control experiments were performed using the potent PI3K inhibitor wortmannin.44 Incubation of eosinophils with 30 nmol/L wortmannin, a relatively specific PI3K inhibitor, completely prevented PI3K activation in eosinophils stimulated by IL-5 and PAF (Fig 2).
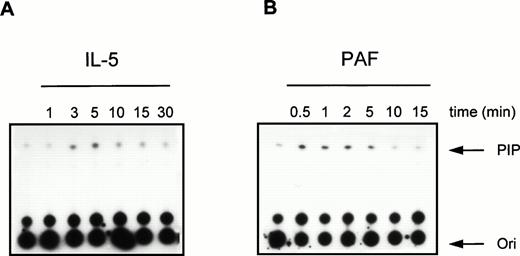
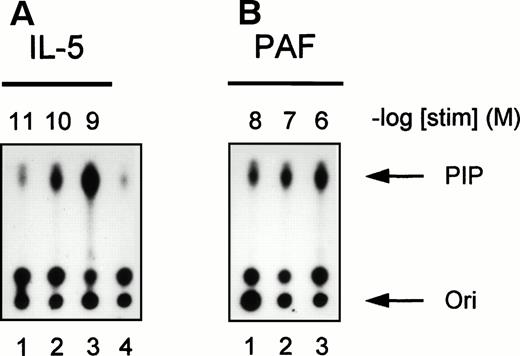
To further investigate the activation of PI3K in human eosinophils, we studied the time- and dose-dependency of phosphotyrosine-associated PI3K activity induced by IL-5 and PAF. In Fig 3, a time-dependent activation of PI3K in antiphosphotyrosine immunoprecipitates from IL-5–(10−9 mol/L) and PAF (10−6mol/L)-stimulated eosinophils is shown. Both compounds induced a transient activation of PI3K with maximal activation occurring at 5 minutes after addition of IL-5 (Fig 3A) and within 1 minute after addition of PAF (Fig 3B). This correlates with the more rapid responses of G-protein coupled receptors versus tyrosine kinase-linked receptors with respect to other signaling events (see below). Figure 4 shows PI3K activity in human eosinophils induced by different concentrations of IL-5 and PAF at the optimal time points. PI3K activation in eosinophils was found to increase to 10−9 mol/L IL-5 (Fig 4A, lane 3) and 10−6 mol/L PAF (Fig 4B, lane 3). While higher concentrations yielded essentially the same result, concentrations of PAF above 10−6 mol/L are toxic, and the mechanism of PI3K-activation may involve alternative stress-related mechanisms.
Time-course of PI3K activation in human eosinophils treated with IL-5 and PAF. Eosinophils (1.5 × 106) were treated with (A) IL-5 (10−9 mol/L) or (B) PAF (10−6 mol/L) at 37°C for the indicated time intervals. After cell lysis and immunoprecipitation, PI3K activity was detected as previously described.
Time-course of PI3K activation in human eosinophils treated with IL-5 and PAF. Eosinophils (1.5 × 106) were treated with (A) IL-5 (10−9 mol/L) or (B) PAF (10−6 mol/L) at 37°C for the indicated time intervals. After cell lysis and immunoprecipitation, PI3K activity was detected as previously described.
Concentration-dependence of IL-5 and PAF on PI3K activation in human eosinophils. PI3K activation was detected in human eosinophils (2 × 106) after stimulation with (A) IL-5 (10−11 to 10−9 mol/L) for 5 minutes or (B) PAF (10−8 to 10−6 mol/L) for 1 minute at 37°C. Lane 4 represents the unstimulated control for both experiments. The experiment shown is representative of three other experiments.
Concentration-dependence of IL-5 and PAF on PI3K activation in human eosinophils. PI3K activation was detected in human eosinophils (2 × 106) after stimulation with (A) IL-5 (10−11 to 10−9 mol/L) for 5 minutes or (B) PAF (10−8 to 10−6 mol/L) for 1 minute at 37°C. Lane 4 represents the unstimulated control for both experiments. The experiment shown is representative of three other experiments.
Phosphorylation of PKB/c-Akt in human eosinophils by cytokines and chemoattractants.
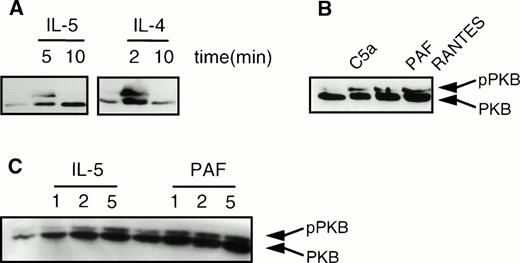
Recently, we and others have shown that phosphorylation and activation of PKB/c-Akt, a serine/threonine kinase, was mediated by PI3K.30,31 We analyzed induction of PKB phosphorylation in human eosinophils, as measured by the previously described lower mobility of phosphorylated PKB in SDS-PAGE gels.30,31 As shown in Fig 5A, phosphorylation of PKB (pPKB) was transiently induced in human eosinophils on activation with the cytokines IL-5 and IL-4. Furthermore, it can be seen that the chemoattractants C5a (10−8 mol/L), PAF (10−6 mol/L), and RANTES (10−6mol/L) also induced phosphorylation of PKB (Fig 5B). Finally, in Fig5C, a time course of IL-5 (10−9 mol/L) and PAF (10−6 mol/L)-induced phosphorylation of PKB is shown, demonstrating that PKB phosphorylation coincides with PI3K activation, as previously described.30 31 Again, while IL-5 produces a relatively slow phosphorylation of PKB, that of PAF is much more rapid.
Cytokines and chemoattractants stimulate phosphorylation of PKB in human eosinophils. Phosphorylation was measured by an SDS-PAGE mobility shift as described in Materials and Methods. (A) Eosinophils (2 × 106) were stimulated with the cytokines IL-5 (10−9 mol/L), and IL-4 (10−8 mol/L) at 37°C for the indicated time intervals. (B) Eosinophils (2 × 106) were stimulated with the chemoattractants C5a (10−8 mol/L), PAF (10−6 mol/L), and RANTES (10−6) for 2 minutes at 37°C. (C) Eosinophils (2 × 106) were treated with IL-5 (10−9 mol/L) and PAF (10−6 mol/L) at 37°C for the indicated time intervals.
Cytokines and chemoattractants stimulate phosphorylation of PKB in human eosinophils. Phosphorylation was measured by an SDS-PAGE mobility shift as described in Materials and Methods. (A) Eosinophils (2 × 106) were stimulated with the cytokines IL-5 (10−9 mol/L), and IL-4 (10−8 mol/L) at 37°C for the indicated time intervals. (B) Eosinophils (2 × 106) were stimulated with the chemoattractants C5a (10−8 mol/L), PAF (10−6 mol/L), and RANTES (10−6) for 2 minutes at 37°C. (C) Eosinophils (2 × 106) were treated with IL-5 (10−9 mol/L) and PAF (10−6 mol/L) at 37°C for the indicated time intervals.
ERK2, but not ERK1, is activated in human eosinophils after stimulation with IL-5 and PAF.
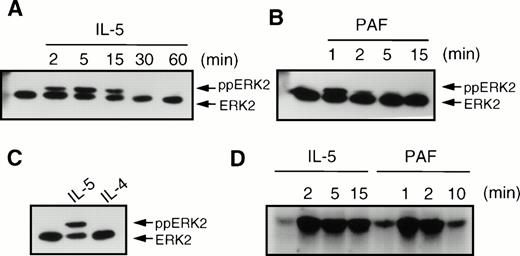
We also analyzed the phosphorylation and activation of the MAP kinases ERK1 and ERK2, which are both downstream targets of p21ras signal transduction. IL-5 (10−10 mol/L) induced a transient induction of phosphorylation of ERK2 (ppERK2), which was optimal at 5 minutes (Fig 6A) and declined to basal levels within 30 minutes. The chemoattractant PAF (10−6 mol/L) induced a very rapid and transient phosphorylation of ERK2, which was maximal within 1 minute and declined to basal levels within 5 minutes (Fig 6B). However, in contrast to IL-5 and PAF, IL-4 (10−8 mol/L) did not induce phosphorylation of ERK2 in human eosinophils (Fig 6C). IL-4 also failed to induce ERK2 phosphorylation at other time points (data not shown). While ERK phosphorylation generally correlates with enzyme activity, it is itself not a direct measure of kinase activity. We thus analyzed the activation of ERK2 after IL-5 (10−10 mol/L) or PAF (10−6 mol/L) stimulation by specific immunoprecipitation and kinase assay as described in Materials and Methods. As seen in Fig 6D, IL-5 induces relatively slow and sustained activation of ERK2 kinase activity, lasting for more than 15 minutes. PAF, however, stimulates a very rapid, but short-lived, activation of ERK2 activity, returning to basal levels after several minutes.
Effect of cytokines and chemoattractants on the phosphorylation of ERK2 in human eosinophils. After stimulation, the eosinophils (2 × 106) were immediately lysed in Laemmli buffer. Phosphorylation of ERK2 (ppERK2) was detected as described in Materials and Methods. (A) Time-course of IL-5 (5 × 10−10 mol/L) induced ERK2 phosphorylation. (B) Time course of phosphorylation of ERK2 by PAF (10−6 mol/L). (C) IL-5 (5 × 10−10 mol/L, 5 minutes), but not IL-4 (10−8 mol/L, 5 minutes) induced ERK2 phosphorylation. (D) Time course of ERK2 activation by IL-5 (10−10 mol/L) and PAF (10−6 mol/L) as measured by MBP phosphorylation. Kinase assays with 7.5 × 106 eosinophils were performed as described in Materials and Methods.
Effect of cytokines and chemoattractants on the phosphorylation of ERK2 in human eosinophils. After stimulation, the eosinophils (2 × 106) were immediately lysed in Laemmli buffer. Phosphorylation of ERK2 (ppERK2) was detected as described in Materials and Methods. (A) Time-course of IL-5 (5 × 10−10 mol/L) induced ERK2 phosphorylation. (B) Time course of phosphorylation of ERK2 by PAF (10−6 mol/L). (C) IL-5 (5 × 10−10 mol/L, 5 minutes), but not IL-4 (10−8 mol/L, 5 minutes) induced ERK2 phosphorylation. (D) Time course of ERK2 activation by IL-5 (10−10 mol/L) and PAF (10−6 mol/L) as measured by MBP phosphorylation. Kinase assays with 7.5 × 106 eosinophils were performed as described in Materials and Methods.
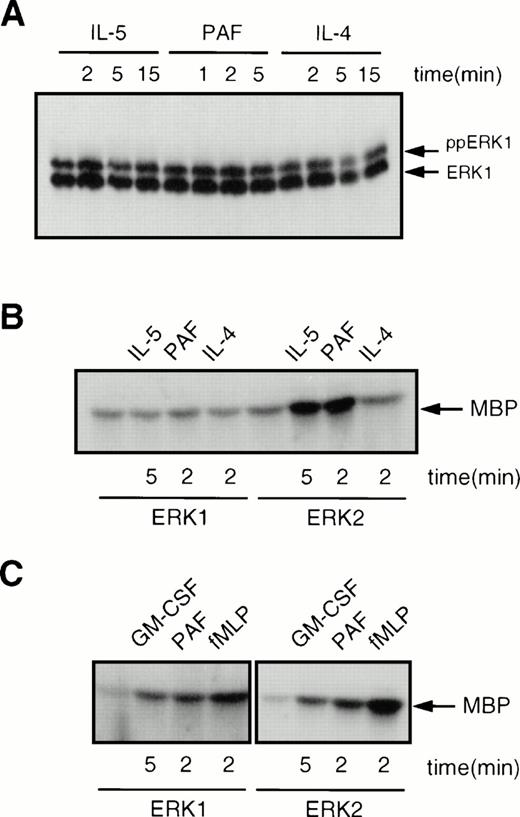
A recent study has suggested that ERK1, but not ERK2, can be activated in human eosinophils.33 However, this study only analyzed ERK phosphorylation and not ERK activity. To determine whether ERK1, as well as ERK2, was activated in human eosinophils, we analyzed ERK1 phosphorylation as described above. Surprisingly, as shown in Fig 7A, ERK1 was constitutively phosphorylated in human eosinophils, and this phosphorylation was not modified by addition of IL-5, PAF, or IL-4. As mentioned previously, ERK phosphorylation is not a direct measure of ERK activity. Thus, we performed immune-complex kinase assays on both ERK1 and ERK2 immunoprecipitated from human eosinophils. As shown in Fig 7B, ERK1 kinase activity was not modified by addition of IL-5, PAF, or IL-4. The activity of ERK2, however, was induced by both IL-5 and PAF, but not by IL-4, in agreement with the data presented in Fig 6. As a control, we analyzed the activation of both ERK1 and ERK2 in human neutrophils isolated from the same donor at the same time as the eosinophils. Stimulation of neutrophils with either GM-CSF, PAF, or fMLP resulted in activation of both ERK1 and ERK2 as shown in Fig 7C. This difference between neutrophils and eosinophils was consistently observed with all donors tested. In contrast to previous data,33 this clearly shows that while both ERK1 and ERK2 activity can be modulated in human neutrophils, only ERK2 activity seems to be inducible in human eosinophils.
Comparison of the activation of ERK1 and ERK2 in human eosinophils and neutrophils. (A) ERK1 is constitutively phosphorylated in human eosinophils. Cells (2 × 106) were treated with either IL-5 (10−10 mol/L), PAF (10−6 mol/L) or IL-4 (10−8 mol/L) for the times indicated before lysis and SDS-PAGE analysis. (B) ERK1 is constitutively active, while ERK2 is inducible in human eosinophils. Cells (7.5 × 106) were stimulated with either IL-5 (10−10mol/L), PAF (10−6 mol/L), or IL-4 (10−8mol/L) for the times indicated. Samples were lysed and immunoprecipitated with either ERK1 or ERK2 specific antibodies. Kinase activity was analyzed as described in Materials and Methods. (C) ERK1 and ERK2 activity can both be induced in human neutrophils. Cells (107) were stimulated with either GM-CSF (10−10 mol/L), PAF (10−6 mol/L), or fMLP (10−6 mol/L) for the times indicated. Kinase activity was analyzed as described above.
Comparison of the activation of ERK1 and ERK2 in human eosinophils and neutrophils. (A) ERK1 is constitutively phosphorylated in human eosinophils. Cells (2 × 106) were treated with either IL-5 (10−10 mol/L), PAF (10−6 mol/L) or IL-4 (10−8 mol/L) for the times indicated before lysis and SDS-PAGE analysis. (B) ERK1 is constitutively active, while ERK2 is inducible in human eosinophils. Cells (7.5 × 106) were stimulated with either IL-5 (10−10mol/L), PAF (10−6 mol/L), or IL-4 (10−8mol/L) for the times indicated. Samples were lysed and immunoprecipitated with either ERK1 or ERK2 specific antibodies. Kinase activity was analyzed as described in Materials and Methods. (C) ERK1 and ERK2 activity can both be induced in human neutrophils. Cells (107) were stimulated with either GM-CSF (10−10 mol/L), PAF (10−6 mol/L), or fMLP (10−6 mol/L) for the times indicated. Kinase activity was analyzed as described above.
Activation of p21ras correlates with ERK2 activation by IL-5 and PAF.
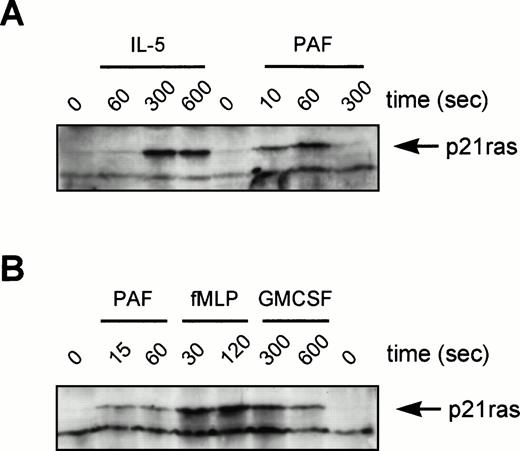
Although the activation of ERKs by tyrosine kinase-linked receptors is p21ras-dependent, this correlation has not been widely established for other receptor families. Original studies in neutrophils have suggested that while fMLP can activate GTP-loading of p21ras,45 p21ras activation is not observed for PAF in Chinese hamster ovary (CHO) cells.46 We have used a Raf1-RBD as an activation-specific probe for p21ras GTP-loading in eosinophils after stimulation by IL-5 and PAF. This technique uses the principle that Raf1 only interacts with active GTP-bound p21ras. Thus, a GST-fusion protein containing the minimal Ras-Binding Domain (RBD) of Raf1 (aa51-131) is used to “pull-down” GTP-bound p21ras.40 While not quantitative, this methodology provides data concerning the ability and kinetics of activation. Eosinophils were stimulated as indicated and p21ras precipitated with GST-RBD bound to glutathione-agarose beads and identified by Western blotting using a MoAb against p21ras (Fig 8). IL-5, which resulted in a relatively slow activation of ERK2 in eosinophils, also produced an optimal activation of p21ras only after several minutes (Fig 8A). In contrast, the G-protein receptor agonist PAF, resulted in a very rapid and short-lived p21ras-activation (Fig 8A), correlating with the kinetics of ERK2 activation (Figs 6 and 7). Activation by PAF is in contrast to previous studies in cell lines transfected with the PAF receptor showing the cell-type specificity of this response.46 The activation of p21ras in human neutrophils was also analyzed (Fig 8B). Activation of p21ras by PAF/fMLP is very rapid, while GM-CSF is much slower. This is similar to the activation profiles observed in eosinophils from the same donor.
Activation of p21ras by cytokines and chemoattractants in human eosinophils and neutrophils. In (A) eosinophils (6 × 106) or (B) neutrophils (107) were stimulated as indicated (IL-5, 10−10 mol/L; PAF, 10−6mol/L; fMLP, 10−6 mol/L; GM-CSF, 10−10mol/L) before lysis and incubation with GST-Raf1(RBD) to bind GTP-bound p21ras as described in Materials and Methods. Samples were analyzed by SDS-PAGE and immunoblotting with a MoAb against p21ras. The position of GTP-bound p21ras is marked by an arrowhead. This is representative of three individual experiments.
Activation of p21ras by cytokines and chemoattractants in human eosinophils and neutrophils. In (A) eosinophils (6 × 106) or (B) neutrophils (107) were stimulated as indicated (IL-5, 10−10 mol/L; PAF, 10−6mol/L; fMLP, 10−6 mol/L; GM-CSF, 10−10mol/L) before lysis and incubation with GST-Raf1(RBD) to bind GTP-bound p21ras as described in Materials and Methods. Samples were analyzed by SDS-PAGE and immunoblotting with a MoAb against p21ras. The position of GTP-bound p21ras is marked by an arrowhead. This is representative of three individual experiments.
Activation of the respiratory burst in human eosinophils by opsonized particles is inhibited by wortmannin, but not PD098059.
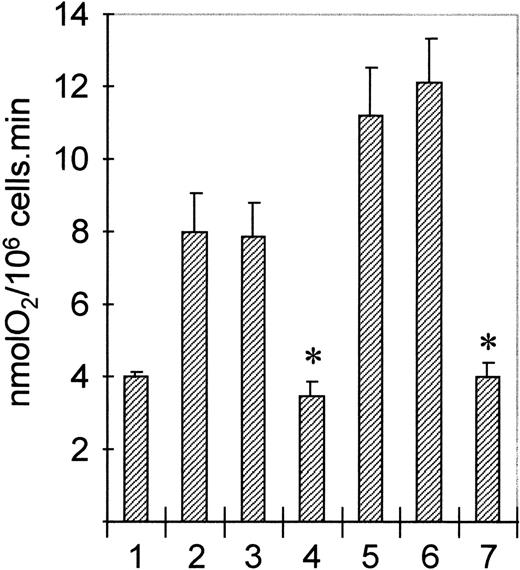
To determine the functional consequences of PI3K or p21ras-ERK activation by cytokines and chemoattractants in human eosinophils, we used specific pharmacologic inhibitors of both signal transduction pathways. Wortmannin is a specific inhibitor of PI3K at nanomolar concentrations,44 while PD098059 has been recently described as a specific inhibitor of MEK, a kinase mediating activation of ERK1/ERK2 by p21ras.47,48 Addition of opsonized particles (serum-treated zymosan, STZ) to eosinophils results in activation of the respiratory burst. Unlike human neutrophils, this response is very sensitive for priming by both IL-5 and PAF.49 Addition of wortmannin to eosinophils blocks activation of PI3K by both IL-5 and PAF (Fig 2). Furthermore, addition of PD098059 blocks both IL-5 and PAF-mediated ERK activation (data not shown). To determine the effect of inhibiting PI3K and p21ras-ERK signal transduction on this process, we preincubated cells with or without either wortmannin or PD098059. Subsequently, cells were primed by treatment with IL-5 (10−10 mol/L, 30 minutes) or PAF (1 μmol/L, 2 minutes) before stimulation with STZ (1 mg/mL). As shown in Fig 9, both IL-5 and PAF prime STZ-mediated respiratory burst (Fig 9, lanes 2 and 5). This primed respiratory burst is profoundly inhibited by preincubation with wortmannin (Fig 9, lanes 4 and 7). Inhibition of ERK activation in eosinophils by preincubation with PD098059, however, had no effect (Fig9, lanes 3 and 6). These data show that while activation of PI3K appears to play a critical role in superoxide generation, a major effector function of eosinophils, the activation of ERK is not necessary contrary to previous suggestions for neutrophilic granulocytes.34-36
Effect of PI3K- and MEK-inhibitors on STZ-induced eosinophil respiratory burst. Eosinophils (1.6 × 106/mL) were preincubated with wortmannin (100 nm, 5 minutes, lanes 4 and 7) or PD098059 (50 μmol/L, 15 minutes, lanes 3 and 6). Cells were then primed with IL-5 (10−10 mol/L, 20 minutes, lanes 2 to 4) or PAF (10−6 mol/L, 2 minutes, lanes 5 to 7) before addition of STZ (1 mg/mL). Oxygen uptake was continually measured and results represent the maximal rate (nmolO2/106cells/min) of four experiments ± SE (P < .05, Student's t-test).
Effect of PI3K- and MEK-inhibitors on STZ-induced eosinophil respiratory burst. Eosinophils (1.6 × 106/mL) were preincubated with wortmannin (100 nm, 5 minutes, lanes 4 and 7) or PD098059 (50 μmol/L, 15 minutes, lanes 3 and 6). Cells were then primed with IL-5 (10−10 mol/L, 20 minutes, lanes 2 to 4) or PAF (10−6 mol/L, 2 minutes, lanes 5 to 7) before addition of STZ (1 mg/mL). Oxygen uptake was continually measured and results represent the maximal rate (nmolO2/106cells/min) of four experiments ± SE (P < .05, Student's t-test).
DISCUSSION
Eosinophil functioning in vivo is modulated by cytokines and chemoattractants. Despite these well-documented effects, relatively little is known about the downstream signaling events in human eosinophils initiated on receptor activation. Recently, much attention has been paid to the activation of tyrosine kinases in human eosinophils.50 Here, we describe the activation of phosphotyrosine-associated PI3K and phosphorylation of PKB/c-Akt and activation of p21ras and p42ERK2, induced by both cytokine and serpentine receptors.
The regulation of PI3K by tyrosine kinases has been the subject of many recent studies. The p85 regulatory subunit, of which several forms have been cloned, contains two SH2 domains. These subunits contain a proline rich sequence and one SH3 domain, which accounts for the adaptor function of p85.16,17 Activation of the regulatory subunit is essential for the activation of the p110 catalytic subunit. Recently, a novel PI3K was cloned, which is activated by βγ-subunits of heterotrimeric G-proteins and thus stimulated primarily by serpentine receptor agonists.19,20 This PI3K lacks a p85 regulatory domain and consists of only a p110γ subunit. In our experiments, tyrosine-phosphorylated proteins were immunoprecipitated and PI3K activity was measured in vitro. In this way, tyrosine phosphorylation or recruitment of PI3K(s) to signaling complexes containing tyrosine-phosphorylated proteins is measured. It is likely that this PI3K belongs to the p85/p110 class, as we observed that wortmannin inhibits PI3K activity in eosinophils at relatively low concentrations (30 nmol/L, see Fig 2B), whereas it has previously been shown in neutrophils that wortmannin inhibits p110γ only at higher concentrations.19 Thus, it appears that G-protein–coupled receptor agonists are capable of activating phosphotyrosine-associated PI3K isoforms in human eosinophils. Further work will be necessary to determine the mechanisms by which these receptors, which all lack a consensus SH2-binding sequence for p85, are able to activate PI3K.51,52 The mechanisms by which IL-5 and IL-4 can activate PI3K are also unclear. Recently, an 80-kD protein (p80) has been described to directly link activated receptors for IL-3, IL-5, and GM-CSF to PI3K signaling.53 Furthermore, the involvement of different Src-type family protein tyrosine kinases has been described to mediate the activation of PI3K.54,55 The IL-4 receptor, which belongs to the cytokine receptor superfamily that makes use of a cytokine specific α-chain and a γc-chain, has recently been shown to engage IRS-1 and 4PS (IRS-2) adaptor proteins.56 These tyrosine-phosphorylated adaptor proteins contain motifs, which could also potentially bind proteins such as p85 and Grb2.
We and others have recently identified the protein serine/threonine kinase PKB/c-Akt as being a downstream target of PI3K activation.30,31 These studies focused on signal transduction pathways induced only by growth factors. The present study clearly shows that PKB activation is a widespread phenomenon occurring not only through intrinsic tyrosine kinase receptors, eg, the platelet-derived growth factor (PDGF) receptor, but also through associated-tyrosine kinase receptors, eg, the IL-5 and the IL-4 receptor, and G-protein–coupled serpentine receptors, eg, the PAF receptor. Thus, the activation of PKB is probably a primary target function for PI3K activity, while its function still remains elusive. We have previously shown that the rapamycin-sensitive p70 S6 kinase can be activated by an oncogenic form of PKB, while PI3K inhibitors have been shown to inhibit p70 S6 kinase activation.31,57 Interestingly, rapamycin has been described to partially reverse the IL-5–mediated eosinophil survival in vitro.58 It is, therefore, possible that p70 S6 kinase is activated in human eosinophils in response to cytokine stimulation and that the PI3K-PKB pathway could play a role in cytokine-mediated eosinophil survival. Preliminary data suggests that PI3K inhibitors block IL-5–mediated rescue of eosinophil apoptosis (P.J.C., unpublished data).
We have analyzed the activation of p21ras using a novel assay relying on the specificity of p21ras-GTP, but not p21ras-GDP binding to Raf1.40 This assay has many advantages over traditional methods of analyzing small G-protein activation including no requirement for phosphate labeling of cells and the ability to measure very rapid time points (<10 seconds). Activation of p21ras in human eosinophils occurs not only by tyrosine kinase–linked receptors, eg, IL-5, but also by serpentine receptors such as PAF. Previous studies in CHO cell lines have failed to measure an activation of p21ras by PAF,46 while this study clearly shows activation of p21ras in both eosinophils and neutrophils (Fig 8). This demonstrates, as was originally shown for fMLP in neutrophils, that serpentine receptors use similar signaling components in the activation of MAP kinases as tyrosine kinase-linked receptors. Most striking is the dramatic difference in kinetics of activation between IL-5 and PAF in eosinophils. While activation by IL-5 occurs between 1 to 5 minutes and lasts longer than 10 minutes, PAF-stimulated p21ras GTP-loading occurs within 10 seconds and decreases to basal levels after several minutes (Fig 8). This activation correlates with the activation of MAP kinases for all stimuli in both neutrophils and eosinophils, suggesting either p21ras must be GTP-bound to maintain ERK activation or that these signals are concomitantly downregulated. The precise mechanism by which these receptors stimulate GTP- for GDP-exchange on p21ras remains to be identified.
It has been previously decribed that PI3K can be activated in a p21ras-dependent mechanism.59,60 Therefore, we wished to analyze the activation of p21ras-ERK in human eosinophils by cytokines and chemoattractants. In human eosinophils, IL-5 has recently been shown to activate several MAP kinases including a 44-kD (ERK1) and 45-kD MAP kinase.33,61 We have analyzed the activation of p44ERK1 and p42ERK2, two members of the MAP kinase family, in terms of phosphorylation and in vitro kinase assays (Figs 6 and 7). Our data show that in human eosinophils, ERK2 phosphorylation and activity, but surprisingly not ERK1, is stimulated by both cytokines and chemoattractants. In human neutrophils, however, both ERK1 and ERK2 activities are inducible (Fig 7C). IL-4 signaling in eosinophils did not induce activation of ERK2 (Fig 6C), indicating important differences in signaling between the IL-4 and IL-5 receptors. It has been reported in other cell systems that the IL-4 receptor failed to initiate the activation of the p21ras-ERK pathway.62,63 Our data are in marked contrast to that recently reported by Pazdrak et al33 in which activation of ERK1, but not ERK2, in human eosinophils was reported. However, in this report, no specific antibodies to ERK-isoforms were used. Furthermore, tyrosine phosphorylation of ERK isoforms was measured rather than ERK-activity per se. Although tyrosine phosphorylation of MAP kinases correlates with activity, it is only through immune-complex kinase assays with specific antibodies that ERK activation can truly be measured. Pazdrak et al use a MoAb that recognizes both p44ERK1 and p42ERK2 thus not allowing the distinction of which isoform is activated.
Our data indicate that there appears to be differential regulation of the p44ERK1 and p42ERK2 MAP kinases in human eosinophils (Figs 6 and 7). Although unusual, this differential regulation is not unprecedented. For example, stimulation of mouse macrophages with CSF-1 results in activation of only ERK164; and tumor necrosis factor (TNF)-α has been reported to only activate ERK2 in mouse macrophages and B-cells.64,65 The implications of these responses are as yet unclear, as the specific downstream targets of MAP kinases have not yet been identified. However, it has been reported using overexpression of MAP kinase constructs in cell lines that ERK1 results in the activation of the transcription factor Elk-1, while ERK2 activates c-Myc, but not Elk-1.66 This suggests that there may be a role for differential activation of MAP kinase isoforms in the regulation of specific eosinophil functions.
To determine whether activation of PI3K or p21ras-ERK signal transduction may regulate eosinophil effector function, we used specific inhibitors of these pathways. Activation of the respiratory burst is an event whereby phagocytes generate the antimicrobial oxidants via a multicomponent NADPH oxidase system. Association with oxidase activation is the phosphorylation of cytosolic components of this complex, eg, p47phox.34,67 Both MAP kinases and PI3K have been implicated in this process. Indeed, introduction of a constitutively activated form of PI3K into a monoblastic phagocyte line (GM-1) caused activation of PKB and furthermore phosphorylation of p47phox.32 Using wortmannin to inhibit PI3K and PD098059 to inhibit MEK and thus ERK activation, we show that indeed activation of PI3K is required for STZ-mediated respiratory burst in human eosinophils (Fig 9). Inhibition of MEK-ERK with high concentrations of PD098059 (50 μmol/L) had no effect on oxygen consumption and thus suggests that p21ras-ERK signal transduction is not necessary for phagocytic killing by eosinophils.
The cytokines IL-4 and IL-5 are both potent primers of eosinophil migration in vitro.43,68 However, because ERK2 is not activated by IL-4, the signaling pathway involving phosphorylation of ERK2 is not essential for priming by IL-4. It is tempting to speculate that PI3K and PKB are involved in priming of eosinophils in vivo, as until now, no consensus has been reached on the signals involved in this process.68 In fact, all primers of eosinophils we have used are activators of the PI3K-PKB signaling pathway in vitro (Fig 1A and B).
In summary, this is the first report on the activation and function of PI3K-PKB and p21ras-ERK signal transduction pathways in human eosinophils by Th2-derived cytokines and chemoattractants. These data suggest that both cytokines and chemokines can use both similar and/or distinct mechanisms for transducing an extracellular signal into an effector response. The role of these pathways, particularly p21ras-ERK, requires further work. However, it appears that PI3K plays a critical role in the activation of human phagocytes mediating host defense.
ACKNOWLEDGMENT
Many thanks to Prof. Hans Bos and Laura M'Rabet for the kind gift of Raf1 GST-RBD and helpful discussions.
Supported by research grants from the Dutch Asthma Foundation (Grant No. 91-36) and GlaxoWellcome bv, The Netherlands.
P.J.C. and R.C.S. contributed equally to this study.
Address reprint requests to Leo Koenderman, MD, Department of Pulmonary Diseases, Room F 02.333, University Hospital Utrecht, PO Box 85500, 3508 GA Utrecht, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal