Abstract
Cytotoxic T lymphocytes (CTL) specific for human minor histocompatibility (H) antigens can be isolated from the blood of major histocompatibility complex (MHC)-matched allogeneic bone marrow transplant (BMT) recipients and may play a prominent role in the graft-versus-host (GVH) and graft-versus-leukemia (GVL) reactions (Tsoi et al, J Immunol 125:2258, 1980; Tsoi et al, Transplant Proc 15:1484, 1983; Goulmy et al, Nature 302:159, 1983; Irle et al, Transplantation 40:329, 1985; and Niederwieser et al, Blood 81:2200, 1993). The identification of minor H antigens that are expressed in hematopoietic cells, including leukemic cells, but not in fibroblasts and other tissue types has suggested that such tissue-restricted antigens could potentially serve as targets for T-cell immunotherapy to enhance GVL activity without inducing GVH disease (de Bueger et al, J Immunol 149:1788, 1992; van der Harst et al, Blood 83:1060, 1994; and Dolstra et al, J Immunol 158:560, 1997). To explore the feasibility of this strategy, donor CD3+CD8+ CTL clones specific for recipient minor H antigens were isolated and characterized from allogeneic BMT recipients. CTL clones were obtained from the majority of donor/recipient pairs. Seventeen distinct minor H antigens distinguishable by their MHC-restricting allele, population frequency, and/or distribution of tissue expression were defined by 56 CD3+CD8+ CTL clones isolated from these patients. The MHC-restricting alleles for these CTL clones included HLA-A2 and HLA-B7, which had previously been shown to present minor H antigens to CTL, as well as HLA-A3, -A11, -B8, -B53, and -Cw7, which had not previously been described to present minor H antigens to CTL. Estimated phenotype frequencies for these 17 distinct minor H antigens range from 0.17 to 0.92. In vitro cytotoxicity assays using hematopoietic cells and fibroblasts as target cells showed that 5 of the 17 minor H antigens were expressed in both hematopoietic cells and fibroblasts. However, 12 were presented for CTL recognition only by hematopoietic cells and not by dermal fibroblasts derived from the same donors. These results significantly extend the spectrum of CTL-defined human minor H antigens that could potentially serve as target antigens for cellular immunotherapy to promote GVL activity after allogeneic BMT.
THE USE OF T-CELL–depleted bone marrow (BM) for major histocompatibility complex (MHC)-matched allogeneic BM transplantation (BMT) confers a reduced incidence of graft-versus-host disease (GVHD) but a higher probability of leukemic relapse compared with the use of unmodified BM.1-7 This observation and the results of experimental studies in animal models have established a critical role for donor T lymphocytes specific for recipient minor histocompatibility (H) antigens in mediating the GVH and graft-versus-leukemia (GVL) reactions that occur after allogeneic BMT and have suggested that infusions of donor T cells may be useful therapeutically in individuals at high risk of developing leukemic relapse after BMT.8 The adoptive transfer of donor peripheral blood mononuclear cells (PBMC) containing large numbers of CD3+ T cells to patients with documented leukemic relapse after allogeneic BMT has induced complete remissions in most patients with relapse of chronic myelogenous leukemia (CML) and some patients with relapse of acute myelogenous leukemia (AML).9-22 Unfortunately, the administration of unselected polyclonal donor lymphocytes has also resulted in acute and/or chronic GVHD in the majority of patients leading to significant morbidity and mortality.9-22
A potential strategy to treat leukemic relapse without inducing GVHD would be to isolate donor T-cell clones specific for recipient minor H antigens and to administer to the recipient only those clones that recognize hematopoietic cells, including leukemic blasts, but not nonhematopoietic tissues. The feasibility of using T-cell clones has been suggested by studies demonstrating that cytomegalovirus (CMV)-specific T-cell immunity can be successfully reconstituted in allogeneic BMT recipients without causing GVHD by the adoptive transfer of donor T-cell clones selected for reactivity with CMV-infected but not uninfected recipient cells.23,24However, CMV seropositive donors maintain a high frequency of CMV-reactive T cells in the PB, and T-cell clones specific for CMV antigens can be readily isolated and expanded ex vivo.24 In contrast, T cells reactive with minor H antigens are present in low frequency in the blood of unprimed donors and the isolation of minor H antigen-specific T-cell clones from donor PBMC samples is difficult.25,26 Goulmy et al27-29 overcame this obstacle and generated polyclonal T-cell lines and several T-cell clones that recognized recipient minor H antigens by obtaining PBMC from the recipient after BMT, and stimulating these cells in vitro with γ-irradiated PBMC cryopreserved from the recipient pretransplant.
These prior studies suggest the potential for adoptive T-cell immunotherapy with minor H antigen-specific T-cell clones to augment GVL reactivity after allogeneic BMT without causing GVHD. However, only four CD8+ cytotoxic T lymphocyte (CTL)-defined minor H antigens that appear to be selectively expressed by hematopoietic cells have been described: HA-1, HA-2, and HA-5, which are all presented for T-cell recognition by HLA-A2, and HB-1, which is presented by HLA-B44.28,30 Because 50% of BMT donor/recipient pairs do not express HLA-A2 and 80% do not express HLA-B44, most recipients would not be eligible for therapy targeting any of these four minor H antigens.31 Moreover, even for donor/recipient pairs expressing HLA-A2, the clinical use of HA-2 and HA-5 as targets for GVL therapy is limited because HA-2 and HA-5 are expressed in an estimated 95% and 7% of the population, respectively.29 Thus, less than 10% of HLA-A2+ donor/recipient pairs would be appropriately discordant for the expression of either of these antigens. HA-1 and HB-1 are expressed in 69% and 28% of the population, respectively, and recipients who express one of these antigens and who have a donor that is discordant should be identified more frequently.29,30 CTL clones specific for HA-1 appear to recognize leukemias of both myeloid and lymphoid lineages.30a However, HB-1–specific CTL recognize only transformed B-lymphoid cells and show no cytolytic activity against either monocytes or phytohemagglutinin (PHA)-stimulated T cells, suggesting that expression of HB-1 is restricted to the B-cell lineage.30 Thus, adoptive immunotherapy with CD8+ CTL specific for minor H antigens as a general strategy to induce GVL activity after allogeneic BMT will require the identification of additional CD8+ CTL-defined antigens that (1) exhibit restricted or preferential expression in hematopoietic cells including myeloid and lymphoid leukemias and (2) are presented by class I MHC molecules other than HLA-A2 and -B44.
To identify novel human minor H antigens that might be potential targets for GVL therapy, we generated minor H antigen-specific T cells from 10 allogeneic BMT donor/recipient pairs. T-cell lines with recipient-specific reactivity were obtained from 8 of the 10 cultures, and a panel of 56 CD3+ CD8+ CTL clones were isolated from 6 of these 8 T-cell lines. Seventeen distinct minor H antigen specificities restricted by 7 different class I MHC alleles were identified using this panel of CD8+ CTL clones. CD8+ CTL specific for 12 of these minor H antigens lysed hematopoietic cells but not fibroblasts derived from the same donors, and CTL specific for 6 of these tissue-restricted antigens lysed leukemic blasts. These results show that T cells potentially capable of mediating GVL activity without causing GVHD can be isolated for use in adoptive immunotherapy from a significant proportion of allogeneic BMT recipients.
MATERIALS AND METHODS
Donor/recipient pairs.
Ten patients with hematologic malignancies undergoing allogeneic BMT and their HLA-matched related donors were enrolled on this study. Characteristics of the 10 donor/recipient pairs, including sex, HLA type, recipient's diagnosis, source of hematopoietic stem cells, GVHD prophylaxis, and GVHD status, are shown in Table 1. Nine of the 10 donor/recipient pairs were full siblings. Eight of these nine sibling pairs (nos. 2, 3, 4, 5, 6, 8, 9, and 10 in Table 1) were HLA-A-, -B-, -Cw-, -DR-, and -DQ-genotypically identical as demonstrated by serologic and DRB1 DNA sequence-based typing of the siblings, the parents, and/or other siblings in each family. One pair (no. 7 in Table 1) was HLA-A-, -B-, -Cw-, and -DQ-identical by serology but mismatched for one DRB1 allele (1501 v 1601). The remaining donor/recipient pair (no. 1 in Table 1) was a mother/son combination who were HLA-A- and -B-identical by serology but matched for only one DR allele (DR 7, 6v DR 7, 8).
Characteristics of the 10 Allogeneic BMT Donor/Recipient Pairs Recruited for This Study, Including Gender of Recipient and Donor, Recipient's Class I MHC Typing, Recipient's Diagnosis, Source of Donor Hematopoietic Stem Cells, Regimen Used for GVHD Prophylaxis, and Acute GVHD Status of the Recipient
| Pair No. . | Recipient . | Donor . | HLA-A . | HLA-C . | HLA-B . | Diagnosis . | Source of Donor Stem Cells . | GVHD Prophylaxis . | Acute GVHD, Grade . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | Female | 2, 11 | w6, w3 | 37, 62 | ALL | BM | ATG/MTX/CsA | III |
| 2 | Female | Male | 11, 24 | w3 | 51, 44 | AML | BM | MTX/CsA | II |
| 3 | Male | Male | 1, 32 | w7, w1 | 8, 27 | MDS/AML | BM | MTX/CsA | III |
| 4 | Male | Female | 29, 30 | w7, w6 | 8, 13 | MDS/AML | G-PBSC | MTX/CsA | I |
| 5 | Male | Male | 3 | w7 | 7 | MM | G-PBSC | MTX/CsA | III |
| 6 | Male | Male | 2, 3 | w5, w7 | 44, 35 | ALL | G-PBSC | MTX/CsA | II |
| 7 | Male | Male | 23, 2 | w7 | 58, 7 | ALL | BM | ATG/MTX/CsA | III |
| 8 | Female | Male | 3, 30 | w3, w4 | 70, 53 | MDS/AML | G-PBSC | MTX/CsA | I |
| 9 | Female | Female | 3, 24 | w2 | 51, 38 | AML | G-PBSC | MTX/CsA | I |
| 10 | Male | Female | 2, 3 | w4, w7 | 35, 7 | AML | G-PBSC | MTX/CsA | II |
| Pair No. . | Recipient . | Donor . | HLA-A . | HLA-C . | HLA-B . | Diagnosis . | Source of Donor Stem Cells . | GVHD Prophylaxis . | Acute GVHD, Grade . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | Female | 2, 11 | w6, w3 | 37, 62 | ALL | BM | ATG/MTX/CsA | III |
| 2 | Female | Male | 11, 24 | w3 | 51, 44 | AML | BM | MTX/CsA | II |
| 3 | Male | Male | 1, 32 | w7, w1 | 8, 27 | MDS/AML | BM | MTX/CsA | III |
| 4 | Male | Female | 29, 30 | w7, w6 | 8, 13 | MDS/AML | G-PBSC | MTX/CsA | I |
| 5 | Male | Male | 3 | w7 | 7 | MM | G-PBSC | MTX/CsA | III |
| 6 | Male | Male | 2, 3 | w5, w7 | 44, 35 | ALL | G-PBSC | MTX/CsA | II |
| 7 | Male | Male | 23, 2 | w7 | 58, 7 | ALL | BM | ATG/MTX/CsA | III |
| 8 | Female | Male | 3, 30 | w3, w4 | 70, 53 | MDS/AML | G-PBSC | MTX/CsA | I |
| 9 | Female | Female | 3, 24 | w2 | 51, 38 | AML | G-PBSC | MTX/CsA | I |
| 10 | Male | Female | 2, 3 | w4, w7 | 35, 7 | AML | G-PBSC | MTX/CsA | II |
Abbreviations: MDS/AML, AML arising in the setting of an antecedent myelodysplastic syndrome; MM, multiple myeloma; G-PBSC, granulocyte colony-stimulating factor–mobilized PBSC; ATG, antithymocyte globulin; MTX, methotrexate; CsA, cyclosporine A.
Generation of Epstein-Barr virus (EBV)-transformed B-cell lines, PHA blasts, and primary fibroblast lines.
PB was obtained pretransplant from each donor and recipient to generate EBV-transformed B-cell lines, and aliquots of PBMC were cryopreserved for later preparation of PHA blasts. EBV-transformed B-cell lines (EBV-LCL) were generated and cultured as described.32 Our laboratory has compiled a cell bank containing a large number of EBV-LCL lines generated from individuals of known HLA type, and these were used in experiments to define the MHC-restricting allele and the population frequency for each of the minor H antigens. PHA blasts were generated by culturing PBMC for 72 hours in CTL media containing 3 μg/mL PHA (Sigma, St Louis, MO), washed and resuspended in CTL medium supplemented with 25 U/mL recombinant human IL-2 (Chiron, Emeryville, CA), and used as target cells in cytotoxicity assays within 7 days. Primary fibroblast lines were grown from explants of skin biopsy specimens as described.33
Generation and characterization of minor histocompatibility antigen-specific T-cell lines.
T-cell lines and clones were cultured in RPMI-HEPES supplemented with 10% pooled, heat-inactivated human serum, 2 mmol/L L-glutamine, and 1% penicillin/streptomycin (termed CTL medium). Donor T cells with reactivity for recipient minor H antigens were generated in 24-well plates by stimulating in each well 1 to 4 × 106responder PBMC obtained from the recipient posttransplant with 1 to 4 × 106 γ-irradiated (35 Gy) PBMC obtained from the recipient pretransplant. The cell lines were restimulated with γ-irradiated recipient PBMC at 7 and 14 days after the initial stimulation and the media was supplemented with interleukin-2 (10 to 15 U/mL) after each restimulation. The resulting T-cell lines were expanded by restimulation at weekly intervals with irradiated EBV-LCL derived from the recipient pretransplant. After 4 to 6 weeks, the cultures were tested for cytolytic activity against donor- and recipient-derived EBV-LCL and/or PHA blast targets.
Cytotoxicity assays and blocking studies.
Aliquots of 1 to 2 × 106 target cells were labeled with 50 μCi of 51Cr overnight, washed twice, dispensed at 5 × 103 cells/well into triplicate cultures in 96-well round-bottom plates, and incubated for 4 hours with effector cells at various effector to target ratios in a total volume of 200 μL. Some assays were performed by preincubating the target cells for 30 minutes at room temperature in the presence and absence of 25 μg/mL of the anti-pan class I MHC monoclonal antibody W6/32 (a generous gift of Dr Daniel Geraghty, Fred Hutchinson Cancer Research Center, Seattle, WA). The percentage of specific lysis was calculated using the standard formula.33
Isolation of minor H antigen-specific CD8+ and CD4+ T-cell clones.
T-cell lines exhibiting recipient-specific cytolytic activity were cloned by limiting dilution in 96-well round-bottom plates. Each well received 200 μL of a cell suspension containing 5 × 104/mL γ-irradiated (65 Gy) recipient-derived EBV-LCL as antigen-presenting cells (APC), 2.5 × 105/mL γ-irradiated (35 Gy) PBMC as feeder cells, and 2 cells/mL (0.4 cells/well) of responder T cells. In some experiments, CD4+T cells were depleted from the T-cell lines before cloning by adherence to tissue culture flasks coated with anti-CD4 monoclonal antibody (Applied Immune Sciences, Santa Clara, CA). After 13 to 14 days, wells exhibiting T-cell growth were identified by microscopy and aliquots of the well were screened for lytic activity against donor- and recipient-derived EBV-LCL or PHA blast targets. T-cell clones with lytic activity against only recipient-derived targets were expanded in vitro for further analysis of phenotype and function.
Collection and processing of leukemic samples.
Samples of PB and/or BM were obtained from patients with acute myelogenous leukemia (n = 10), acute lymphoblastic leukemia (ALL; n = 2), or chronic lymphocytic leukemia (CLL; n = 2), all of whom had either primary refractory disease or relapse after conventional chemotherapy or allogeneic BMT. All leukemic samples (PB and/or BM) contained greater than 90% malignant cells as judged by morphologic criteria on Wright-Giemsa–stained specimens. Leukemic cells were isolated by Ficoll-Hypaque density gradient centrifugation. When not used immediately after isolation, the cells were cryopreserved in RPMI-HEPES with 20% human serum and 10% dimethyl sulfoxide for subsequent use. PHA blast preparations from leukemic patients were prepared as described above. Flow cytometry on these cell populations before use showed that they consisted of greater than 90% CD3+ cells.
Flow cytometric analysis of CTL clones and leukemic cells.
T-lymphocyte lines and clones were analyzed by two-color flow cytometry for expression of CD3, CD4, and CD8 using fluorescein isothiocyanate (FITC)-conjugated anti-CD3 and either phycoerythrin (PE)-conjugated anti-CD4 or anti-CD8 (all from Becton Dickinson, Mountain View, CA). Samples of leukemic blasts were analyzed for expression of class I MHC by staining with the anti-pan class I MHC monoclonal antibody W6/32 followed by FITC-conjugated goat-antimouse Ig (Becton Dickinson). AML samples were also stained with PE-conjugated anti-CD13 or anti-CD33 (Becton Dickinson) and ALL and CLL samples were stained with FITC-conjugated anti-CD19 (Caltag, San Francisco, CA) or FITC-conjugated anti-CD20 (Becton Dickinson). Analysis was performed on a FACScalibur flow cytometer with CellQuest software (Becton Dickinson).
RESULTS
Cytotoxic minor H antigen-specific T-cell lines can be generated from a majority of HLA-matched donor/recipient pairs.
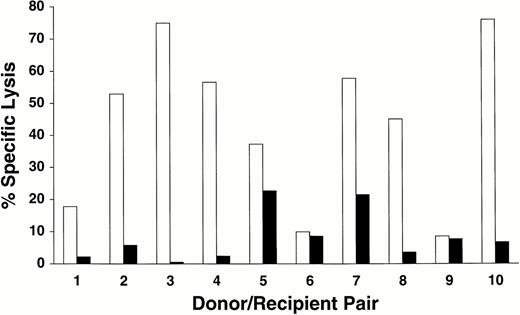
To generate donor T cells reactive with recipient minor H antigens, responder PBMC were isolated between 14 and 156 days after transplant from the PB of 10 transplant recipients with donor engraftment, and cultured as described in the Materials and Methods. Lines from 8 of the 10 donor/recipient pairs exhibited preferential lytic activity against recipient EBV-LCL compared with donor EBV-LCL (Fig 1). Three cycles of stimulation with irradiated recipient PBMC were generally required to first detect significant cytotoxicity against recipient targets, and the cytotoxic activity of these polyclonal T-cell lines was increased by restimulating the lines with irradiated recipient-derived EBV-LCL for 2 or 3 additional cycles (data not shown). In this small series of patients, the ability to isolate recipient-specific cytolytic T cells did not appear to correlate with the development of clinically significant GVHD. Recipient-specific cytolytic T-cell lines were generated from 6 of the 7 patients with acute GVHD of grade II or greater and from 2 of the 3 patients with mild (grade I) GVHD.
Cytolytic activity of T-cell lines generated from 10 donor/recipient pairs against recipient- and donor-derived EBV-LCL targets. Lines were tested 4 to 6 weeks after initial stimulation for lytic activity against recipient-derived (□) or donor-derived (▪) EBV-LCL targets in a standard 4-hour 51Cr release assay at an effector to target (E:T) ratio of 20:1.
Cytolytic activity of T-cell lines generated from 10 donor/recipient pairs against recipient- and donor-derived EBV-LCL targets. Lines were tested 4 to 6 weeks after initial stimulation for lytic activity against recipient-derived (□) or donor-derived (▪) EBV-LCL targets in a standard 4-hour 51Cr release assay at an effector to target (E:T) ratio of 20:1.
Characterization of cell surface phenotype and isolation of minor H antigen-specific cytotoxic T-cell clones.
Analysis of the cell surface phenotype of the 8 T-cell lines with preferential lytic activity against recipient but not donor EBV-LCL showed a mixed population of CD3+CD4+CD8− and CD3+CD4−CD8+ cells in all cases (data not shown). To determine if CD8+ T cells contributed to the cytolytic activity against recipient target cells and whether multiple minor H antigen specificities were being recognized by each line, T-cell clones were isolated in limiting dilution cultures from the 8 T-cell lines using recipient EBV-LCL as APC. In 5 of the cloning experiments (donor/recipient pairs no. 1, 2, 3, 4, and 5; Table 1) the T cells were plated without prior selection for CD4+ or CD8+ T cells. A total of 527 T-cell clones were isolated from 4 of these 5 T-cell lines. Fifty-four of the T-cell clones exhibited cytolytic activity for recipient but not donor EBV-LCL; 25 of these T-cell clones were CD3+CD4+CD8− and 29 were CD3+CD4−CD8+. Fourteen of the 25 cytolytic CD3+CD4+CD8−clones were derived from donor/recipient pair no. 1, between whom a major mismatch at DR (DR6 v DR8) was present, suggesting that these clones may recognize MHC determinants rather than minor H determinants. Because the focus of our study was to identify minor H antigens presented by class I MHC molecules, further characterization of the cytolytic CD3+CD4+CD8−clones was not performed. Of the 29 CD3+CD4−CD8+ CTL clones isolated in these initial experiments, 1 was obtained from donor/recipient pair no. 1, 2 from pair no. 2, 23 from pair no. 4, and 3 from pair no. 5.
The small number of CD3+CD4−CD8+ minor H antigen-specific CTL clones obtained from 3 of these 5 T-cell lines with recipient-specific cytolytic activity suggested that enrichment of CD8+ T cells before cloning may be necessary to improve the efficiency with which CD3+CD8+CD4− T-cell clones reactive with recipient minor H antigens are isolated. Thus, 3 subsequent T-cell lines derived from donor/recipient pairs no. 7, 8, and 10 (Table 1), respectively, were depleted of CD4+ T cells and the remaining cells plated at limiting dilution. A total of 357 T-cell clones were isolated from 2 of these T-cell lines (pairs no. 7 and 8) and screened for cytolytic activity against recipient- but not donor-derived EBV-transformed B-cell targets. Nine clones from donor recipient/pair no. 7 and 18 clones from donor/recipient pair no. 8 exhibited cytolytic activity against recipient-but not donor-derived target cells. These 27 minor H antigen-specific clones were all CD3+CD4−CD8+.
Determination of class I MHC restriction for CD3+CD8+CTL-defined human minor H antigens.
Minor H antigens recognized by CD8+ CTL are presumed to be encoded by allelic forms of polymorphic genes that differ in nucleotide sequence between the donor and recipient and give rise to unique antigenic peptide epitopes. Such CTL-defined minor H antigens have previously been characterized by determining the class I MHC-restricting allele and the frequency of the minor H antigen in a population of individuals expressing this class I MHC allele.29 34 To determine whether the minor H antigens recognized by the CD8+ CTL clones generated in our study correspond to previously described minor antigens or represent distinct specificities, the MHC-restricting elements for each of the 56 CD8+ CTL clones were identified by assessing the lytic activity of the T-cell clones against a panel of EBV-transformed B-cell lines derived from unrelated individuals, each of whom shared only one class I MHC allele with the donor and recipient. Seven different class I MHC alleles were identified to present minor H antigens to the CTL clones isolated in this study. These included HLA-A2 and HLA-B7, which were described in earlier studies as restricting elements for minor H antigen-specific CTL, as well as HLA-A3, -A11, -B8, -B53, and -Cw7, which have not previously been described as restricting alleles for minor H antigen-specific CTL (Table 2). Representative data identifying the class I MHC-restricting alleles for four of the CTL clones are shown in Fig 2.
Summary of the 17 Minor H Antigens Defined by the CD8+ CTL Clones Described in This Report
| Minor H Antigen . | Representative Clone . | Donor/ Recipient Pair . | Restricting Element . | Population Frequency . | Hematopoietic Cells . | Fibroblasts . |
|---|---|---|---|---|---|---|
| A2-1 | PAM-13 | 1 | A2 | 0.70 | + | + |
| A2-2 | ATT-1 | 7 | A2 | 0.47 | + | + |
| A2-3 | ATT-3 | 7 | A2 | 0.28 | + | − |
| A2-4 | ATT-5 | 7 | A2 | 0.17 | + | − |
| A3-1 | DRN-7 | 5 | A3 | 0.74 | + | − |
| A11-1 | SJN-7 | 2 | A11 | (3/7) | + | + |
| A11-2 | SJN-9 | 2 | A11 | (6/7) | + | + |
| B7-1 | ATT-2 | 7 | B7 | 0.31 | + | − |
| B7-2 | ATT-4 | 7 | B7 | 0.92 | + | − |
| B7-3 | ATT-7 | 7 | B7 | 0.85 | + | − |
| B7-4 | ATT-8 | 7 | B7 | 0.77 | + | − |
| B7-5 | ATT-9 | 7 | B7 | 0.60 | + | + |
| B7-6 | DRN-11 | 5 | B7 | 0.56 | + | − |
| B8-1 | MRR-23 | 4 | B8 | 0.50 | + | − |
| B53-1 | DJG-7 | 8 | B53 | (1/4) | + | − |
| B53-2 | DJG-24 | 8 | B53 | (2/4) | + | − |
| Cw7-1 | DRN-14 | 5 | Cw7 | 0.70 | + | − |
| Minor H Antigen . | Representative Clone . | Donor/ Recipient Pair . | Restricting Element . | Population Frequency . | Hematopoietic Cells . | Fibroblasts . |
|---|---|---|---|---|---|---|
| A2-1 | PAM-13 | 1 | A2 | 0.70 | + | + |
| A2-2 | ATT-1 | 7 | A2 | 0.47 | + | + |
| A2-3 | ATT-3 | 7 | A2 | 0.28 | + | − |
| A2-4 | ATT-5 | 7 | A2 | 0.17 | + | − |
| A3-1 | DRN-7 | 5 | A3 | 0.74 | + | − |
| A11-1 | SJN-7 | 2 | A11 | (3/7) | + | + |
| A11-2 | SJN-9 | 2 | A11 | (6/7) | + | + |
| B7-1 | ATT-2 | 7 | B7 | 0.31 | + | − |
| B7-2 | ATT-4 | 7 | B7 | 0.92 | + | − |
| B7-3 | ATT-7 | 7 | B7 | 0.85 | + | − |
| B7-4 | ATT-8 | 7 | B7 | 0.77 | + | − |
| B7-5 | ATT-9 | 7 | B7 | 0.60 | + | + |
| B7-6 | DRN-11 | 5 | B7 | 0.56 | + | − |
| B8-1 | MRR-23 | 4 | B8 | 0.50 | + | − |
| B53-1 | DJG-7 | 8 | B53 | (1/4) | + | − |
| B53-2 | DJG-24 | 8 | B53 | (2/4) | + | − |
| Cw7-1 | DRN-14 | 5 | Cw7 | 0.70 | + | − |
Listed for each minor H antigen are a representative clone defining that minor H antigen, the class I MHC element that restricts recognition of the minor H antigen, the donor/recipient pair from whom this clone was isolated, the estimated phenotype frequency of the minor H antigen in the population bearing the restricting allele, and expression of the minor H antigen in hematopoietic cells and dermal fibroblasts (derived from single donors), as inferred from in vitro cytotoxicity assays. For those clones for which fewer than 10 unrelated EBV-LCL targets bearing the appropriate MHC restricting allele were available, the number of targets recognized/total number of targets tested is listed in parentheses in the place of the population frequency. (+) Greater than 30% lysis at E:T 20:1. (−) Less than 5% lysis at E:T 20:1. All clones were tested against 2 or more types of hematopoietic targets, including EBV-LCL, PHA blasts, and leukemic cells; a (+) appears if greater than 30% specific lysis at E:T 20:1 was demonstrable against any one of these hematopoietic targets.
Identification of class I MHC-restricting elements for four representative CD8+ minor H antigen-specific CTL clones. Each CTL clone was assayed for lytic activity against a panel of EBV-LCL target cells derived from unrelated individuals each of whom shared only one class I MHC allele with the donor/recipient pair from whom the clone was derived. Lytic activity at an E:T ratio of 20:1 against recipient-derived LCL, donor-derived LCL, and LCL derived from unrelated individuals who shared the indicated HLA-A or HLA-B allele with the recipient and the donor is plotted for (A) HLA-A3–restricted clone DRN-7, (B) HLA-B7–restricted clone DRN-11, (C) HLA-B8–restricted clone MRR-2, and (D) HLA-B53–restricted clone DJG-24.
Identification of class I MHC-restricting elements for four representative CD8+ minor H antigen-specific CTL clones. Each CTL clone was assayed for lytic activity against a panel of EBV-LCL target cells derived from unrelated individuals each of whom shared only one class I MHC allele with the donor/recipient pair from whom the clone was derived. Lytic activity at an E:T ratio of 20:1 against recipient-derived LCL, donor-derived LCL, and LCL derived from unrelated individuals who shared the indicated HLA-A or HLA-B allele with the recipient and the donor is plotted for (A) HLA-A3–restricted clone DRN-7, (B) HLA-B7–restricted clone DRN-11, (C) HLA-B8–restricted clone MRR-2, and (D) HLA-B53–restricted clone DJG-24.
The proportion of individuals in the population that express the gene encoding the minor H antigen can be estimated by evaluating the lytic activity of each CTL clone against a large panel of EBV-transformed B-cell targets bearing the relevant class I MHC-restricting allele. For 36 of the 56 clones, an estimate of the phenotype frequency of the minor H antigen in the population was established by evaluating the lysis of EBV-transformed B cells derived from at least 10 unrelated individuals bearing the class I MHC-restricting allele. In some cases, individual CTL clones that used the same class I MHC-restricting element exhibited a different pattern of reactivity against the panel of unrelated EBV-LCL, indicating that distinct minor H antigens were being recognized. For example, four minor H antigen specificities presented by HLA-A2 and six specificities presented by HLA-B7 could be identified by the panel of CTL clones restricted by these alleles (Table 2). The population frequencies of the four HLA-A2–restricted minor H antigens were 0.17, 0.28, 0.47, and 0.70, respectively (Table2).29 The population frequencies of the six HLA-B7–restricted minor H antigens ranged from 0.31 to 0.92 (Table 2). Twenty-three CD8+ CTL clones were generated from donor/recipient pair no. 4 and all recognized a minor H antigen presented by HLA-B8. When these CTL were tested against a panel of 16 EBV-LCL derived from HLA-B8+ donors, they lysed 10 of 10 EBV-LCL from male donors (>60% specific lysis) but 0 of 6 EBV LCL from female donors (<3% specific lysis), suggesting that the minor H antigen recognized by these clones was encoded or regulated by a gene on the Y chromosome.
The analysis of MHC restriction and population phenotype frequency defined at least 17 distinct minor H antigenic specificities, each of which was recognized by one or more of the CD8+ CTL clones generated in this study (Table 2). Multiple CTL clones with identical MHC restriction and patterns of reactivity against the panel of target cells were frequently isolated from a single patient. However, none of the 17 minor H antigens identified in this study was recognized by T cells derived from more than one patient (Table 2). Thus, this analysis indicates that there is a large number of minor H antigen disparities recognized by CD8+ CTL and potentially involved in GVH and GVL reactions after MHC-matched sibling BMT and suggests that isolation of minor H antigen-specific T cells from additional donor/recipient pairs will identify many new specificities.
Recognition of hematopoietic and nonhematopoietic cells by CD3+CD8+ minor H antigen-specific CTL clones.
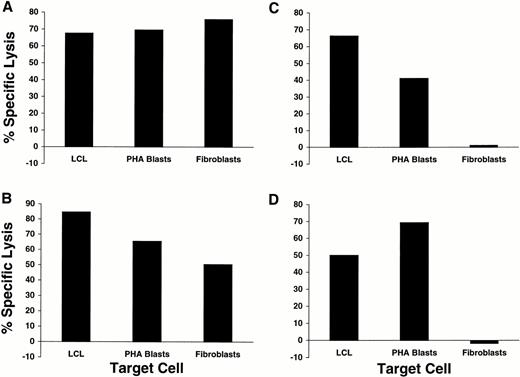
To determine if any of the 17 minor H antigens identified by the CTL isolated in this study were selectively presented by hematopoietic cells, CTL clones were tested for lytic activity against recipient hematopoietic target cells including both EBV-LCL and PHA blasts and against recipient dermal fibroblasts as a representative nonhematopoietic target cell. CD8+ CTL specific for 5 of the 17 minor H antigens lysed the hematopoietic target cells as well as fibroblasts (Table 2). Representative data for two of these clones are shown in Fig 3A and B. However, CD8+ CTL specific for the remaining 12 minor H antigens lysed only the hematopoietic targets but not fibroblasts. Representative data for two of these clones are shown in Fig 3C and D. CTL clones recognizing these 12 minor H antigens with restricted tissue expression were isolated from 4 of the 8 T-cell lines in which T-cell cloning was performed and the class I MHC-restricting elements for these clones included HLA-A2, -A3, -B7, -B8, -B53, and -Cw7 (Table 2).
Recognition of EBV-LCL, PHA blasts, and fibroblasts derived from single donors by four different CD8+ minor H antigen-specific CTL clones. EBV-LCL, PHA blasts, and fibroblasts derived from minor H antigen-positive individuals were used as targets in 51Cr release assays at E:T 20:1 for (A) HLA-A2–restricted clone PAM-13, (B) HLA-A2–restricted clone ATT-1, (C) HLA-A3–restricted clone DRN-7, and (D) HLA-B53–restricted clone DJG-24.
Recognition of EBV-LCL, PHA blasts, and fibroblasts derived from single donors by four different CD8+ minor H antigen-specific CTL clones. EBV-LCL, PHA blasts, and fibroblasts derived from minor H antigen-positive individuals were used as targets in 51Cr release assays at E:T 20:1 for (A) HLA-A2–restricted clone PAM-13, (B) HLA-A2–restricted clone ATT-1, (C) HLA-A3–restricted clone DRN-7, and (D) HLA-B53–restricted clone DJG-24.
Surprisingly, CD8+ CTL clones recognizing the male-specific (H-Y) minor H antigen presented by HLA-B8 lysed hematopoietic target cells but not dermal fibroblasts derived from the same donors (Fig 4). This was not due to a failure of these fibroblasts to present antigens or be lysed by CTL, because a CTL clone specific for an HLA-A2–restricted minor H antigen lysed the same fibroblasts as efficiently as hematopoietic cells (data not shown). Pretreatment of the male fibroblast targets with 500 U/mL interferon-γ (IFN-γ) for 48 or 72 hours did not significantly sensitize them to lysis by any of these HLA-B8–restricted clones (Fig4). These results differ from those obtained with the HLA-A1–, HLA-A2–, and HLA-B7–restricted male-specific (H-Y) CTL clones described by other investigators, which efficiently lysed both hematopoietic and fibroblast target cells derived from male donors.28
The male-specific minor H antigen recognized by HLA-B8–restricted CTL clones is detected in hematopoietic cells but not fibroblasts. Lytic activity of a representative HLA-B8–restricted male-specific CTL clone (MRR-23) against EBV-LCL (□), dermal fibroblasts (□), and IFN-γ–treated fibroblasts (500 U/mL for 48 hours; ▪) derived from four unrelated HLA-B8+ male donors. The effector to target ratio is 20:1.
The male-specific minor H antigen recognized by HLA-B8–restricted CTL clones is detected in hematopoietic cells but not fibroblasts. Lytic activity of a representative HLA-B8–restricted male-specific CTL clone (MRR-23) against EBV-LCL (□), dermal fibroblasts (□), and IFN-γ–treated fibroblasts (500 U/mL for 48 hours; ▪) derived from four unrelated HLA-B8+ male donors. The effector to target ratio is 20:1.
Lysis of leukemic cells by CTL clones specific for tissue-restricted minor H antigens.
The HLA-B53–restricted CD8+ CTL clones isolated from donor/recipient pair no. 8 showed significant lysis of leukemic cells from the recipient (data not shown). To determine if the clones with limited tissue reactivity derived from other recipients also lysed leukemic cells, samples were obtained from 14 HLA-A3+, HLA-B7+, or HLA-B8+ patients with primary refractory or relapsed AML (n = 10), ALL (n = 2), or CLL (n = 2). Flow cytometric analysis of the PB and/or BM mononuclear cells obtained from the 10 individuals with AML showed that greater than 90% of the cells were either CD13+ and/or CD33+ and PBMC from the 4 patients with lymphoid leukemia contained greater than 90% CD19+ and/or CD20+ cells. The leukemic samples were stained for surface expression of class I MHC with the monoclonal antibody W6/32 to determine if complete or partial loss of class I MHC might interfere with the presentation of minor H antigens to CTL. None of the 14 leukemic samples contained a significant population of class I MHClow or class I MHCnegative cells (data not shown).
Four CTL clones which recognized distinct minor H antigens presented by either HLA-A3, -B7, or -B8 were tested for their ability to lyse leukemic cell targets from this panel in vitro. The HLA-A3–restricted clone DRN-7 was assayed against 11 different HLA-A3+ AML, ALL, and CLL samples. Significant lytic activity was observed against 5 of 7 AML samples, 1 of 2 ALL samples, and 1 of 2 CLL samples, and this lytic activity was inhibited in the presence of the anti-pan class I MHC antibody W6/32 (Fig 5A). Clone DRN-7 was also tested for lytic activity against PHA blast populations derived from the same panel of leukemic patients, and lysed PHA blast targets from the 7 patients against whom significant antileukemic activity was also seen, but exhibited negligible lysis of PHA blasts from the patients against whom no antileukemic activity was demonstrable (data not shown).
CD8+ minor H antigen-specific CTL clones demonstrate cytolytic activity against leukemic blasts in vitro that is blocked by antibody to class I MHC. Activity of HLA-A3–restricted clone DRN-7 (A) and HLA-B7–restricted clones ATT-4 (B) and ATT-7 (C) against panels of leukemic cells in the absence (□) or presence (▪) of anti-pan class I MHC antibody W6/32 at 25 μg/mL. The target cell panel in (A) was derived from 11 different HLA-A3+patients: 7 with AML, 2 with ALL, and 2 with CLL. The target cell panel in (B) and (C) was derived from 5 different HLA-B7+patients: 4 with AML and 1 with CLL. E:T was 20:1 in all experiments.
CD8+ minor H antigen-specific CTL clones demonstrate cytolytic activity against leukemic blasts in vitro that is blocked by antibody to class I MHC. Activity of HLA-A3–restricted clone DRN-7 (A) and HLA-B7–restricted clones ATT-4 (B) and ATT-7 (C) against panels of leukemic cells in the absence (□) or presence (▪) of anti-pan class I MHC antibody W6/32 at 25 μg/mL. The target cell panel in (A) was derived from 11 different HLA-A3+patients: 7 with AML, 2 with ALL, and 2 with CLL. The target cell panel in (B) and (C) was derived from 5 different HLA-B7+patients: 4 with AML and 1 with CLL. E:T was 20:1 in all experiments.
The HLA-B7–restricted clones ATT-4 and ATT-7, which are specific for minor H antigens present in 92% and 85% of the population, respectively, were tested for lytic activity against 4 AML samples and 1 CLL sample. Both clones demonstrated significant lytic activity against all of the leukemic targets, and this lytic activity was significantly reduced in the presence of the W6/32 antibody (Fig 5B and C). PHA blast targets derived from the leukemic donors were also lysed (data not shown). Three HLA-B8–restricted, H-Y–specific clones from donor/recipient pair no. 4 were assayed against a panel of 2 male and 3 female HLA-B8+ AML samples. Significant lytic activity was seen against the male but not the female AML targets (data not shown).
DISCUSSION
The CD3+CD8+ class I MHC-restricted minor H antigen-specific CTL clones characterized in this study significantly expand the spectrum of CTL-defined human minor H antigens. Comparison of the results of class I MHC restriction, phenotype frequencies, and distribution of tissue expression for the 17 minor H antigens identified here with those obtained for previously described minor H antigens suggests that the antigens described here represent novel specificities.8,28-30 Similarity exists between two of the HLA-A2–restricted antigens defined by clones ATT-3 and ATT-5 and the previously described HA-5 minor H antigen.28,29 These three minor H antigens are all restricted by HLA-A2 and are detected in hematopoietic cells but not fibroblasts. Clones ATT-3 and ATT-5 recognize distinct specificities as determined by differential recognition of HLA-A2+ target cells from the panel of unrelated donors, but it is conceivable that one of these minor H antigens could be identical to HA-5. The population frequency of 0.07 reported for HA-5 is lower than the frequencies of 0.17 and 0.28 we obtained for the minor H antigen defined by ATT-3 and ATT-5, respectively, although this disparity could be due to the different panel of EBV-LCL used in our analysis. HLA-B7–restricted minor H antigens have also been described in previous studies, but insufficient data were reported on the frequency of these antigens in the population and their tissue expression to determine if any correspond to one of the six distinct HLA-B7–restricted minor H antigens defined by the CTL clones generated and characterized in our study.34 35
The HLA-B8–restricted, male-specific H-Y antigen defined by the CTL clones obtained from donor/recipient pair no. 4 is distinguishable from H-Y antigens described by other investigators.28 CTL clones specific for the HLA-A2– and HLA-B7–restricted H-Y antigens recognize epitopes derived from the human homologue of the murine SMCY gene and lyse both hematopoietic cells and fibroblasts.28,36 37 In contrast, the HLA-B8–restricted H-Y antigen is expressed in hematopoietic cells including EBV-transformed B cells, PHA blasts, and HLA-B8+ AML blasts, but is not expressed sufficiently for CTL recognition in either untreated or IFN-γ–treated fibroblasts. These results suggest that the peptide epitope recognized by these H-Y–specific clones is encoded by a gene distinct from SMCY that is presumably located on the Y chromosome and whose transcription, translation, and/or processing is regulated in a tissue-specific fashion.
In the setting of MHC-matched allogeneic BMT, recipient minor H antigens that are expressed on hematopoietic cells including leukemic cells but are not widely distributed on nonhematopoietic tissues could potentially be targets for adoptive therapy with donor-derived CTL clones to induce GVL activity without causing GVHD. Twelve of the 17 CTL-defined minor H antigens described in this report are detected in recipient hematopoietic cells but not in skin fibroblasts. These 12 tissue-restricted antigens are presented by common class I MHC alleles,31 including HLA-A2, -A3, -B7, -B8, and -Cw7, and several are present in phenotype frequencies that suggest that BMT donor/recipient pairs will often be discordant for one or more of these minor H antigens. In the cohort of patients in this study, CD3+CD8+ CTL clones recognizing tissue-restricted minor H antigens were isolated from 4 of the 8 donor/recipient pairs in whom T-cell cloning was attempted, and it is conceivable that analysis of a larger number of clones would identify such CTL in a higher fraction of patients. Two of the 4 donor/recipient pairs from whom clones with tissue-specific reactivity were isolated (pairs no. 4 and 8) did not develop clinically significant GVHD, in agreement with some38,39 but not all40 previous studies. CD8+ CTL specific for 6 of the tissue-restricted minor H antigens displayed lytic activity against leukemic cells in vitro. CTL defining 4 distinct tissue-resticted minor H antigens were tested against panels of leukemic cells bearing the appropriate MHC-restricting allele and were shown to lyse leukemias of both myeloid and lymphoid phenotypes, demonstrating that, in addition to normal hematopoietic cells such as T cells, B cells, and monocytes, the antigens recognized are expressed in leukemic blasts. Thus, our results significantly extend the number of minor H antigens that could potentially be targeted with CD3+CD8+ CTL clones to selectively induce GVL and demonstrate that a large proportion of BMT patients would be candidates for adoptive T-cell therapy.
A critical issue for the development of this approach to GVL therapy is to demonstrate that expression of the genes encoding candidate target minor H antigens is truly limited to hematopoietic cells. Dermal fibroblasts and keratinocytes have been used as nonhematopoietic target cells because they can be readily obtained from a skin biopsy and cultured in vitro. However, defining the expression of minor H antigens in other tissues using in vitro cytolytic assays is limited by the difficulties inherent in obtaining and culturing samples from other tissue sites. Moreover, data from such in vitro assays could underestimate the expression of minor antigens in tissues in vivo and thus the potential for inducing or aggravating GVHD. Indeed, a recent study identified a significant association of a mismatch of the tissue-restricted HA-1 minor H antigen between donor and recipient with the occurrence of grade II or higher GVHD in adult BMT recipients.41 Thus, the identification of the genes encoding minor H antigens is necessary to permit a comprehensive definition of gene expression in different tissues using molecular techniques. Biochemical methods have been used to isolate and sequence the peptide epitopes bound to MHC molecules and this technology has recently been applied to identify the sequence of human minor H antigen peptides.36,37,42 However, the short peptide sequence obtained with this approach, typically 8 to 11 amino acids in length, does not ensure that the gene encoding the antigen will be identified in available databases. A second approach to identifying genes encoding CTL-defined antigens is based on cDNA expression cloning. In this method, cDNA expression libraries are prepared from antigen-positive cells and divided into small pools; these pools are then cotransfected with a plasmid encoding the class I restricting allele into antigen-negative target cells, and CTL are used to screen the transfectants.43-46 This strategy has been used to identify several genes encoding CTL-defined antigens expressed by melanoma cells and is being adapted in our laboratory to identify genes encoding CTL-defined minor H antigens.
The results of this and other studies have established that minor H antigen-specific CTL clones are cytotoxic for leukemic blasts in vitro, but the extent to which in vitro cytolytic activity will correlate with in vivo antileukemic activity is unknown. New insights into the biology of human AML underscore the potential for the results of in vitro cytotoxicity assays to be misleading. The transplantation of human AML cells into NOD/SCID mice has revealed a hierarchy of cells in the leukemic population with differing potential for establishing leukemic engraftment.47,48 These studies have identified a putative leukemic stem cell that is CD34+, CD38−, present in exceedingly low frequency (<1 in 105 cells) in PB or BM samples from AML patients, and capable of establishing leukemic hematopoiesis in NOD/SCID mice.47 48 This suggests that T cells used in immunotherapy of AML will have to eliminate this rare AML stem cell. The activity of CD8+ minor H antigen-specific CTL clones against the putative AML stem cell cannot easily be addressed with in vitro cytotoxicity assays because of the rarity of this cell, but should be evaluable by analyzing the effect of CTL on leukemic engraftment in the NOD/SCID mouse. Preliminary studies have shown that CTL clones generated in this study prevent engraftment of human AML in the NOD/SCID model (Bonnet and Warren, manuscript in preparation).
The results of this study suggest that there will be a large number of distinct human minor H antigens that could be targets for GVL therapy, and it may not be feasible in all circumstances to pursue gene identification and studies of antileukemic activity in the NOD/SCID mouse model. The ability to genetically modify human T-cell clones with the Herpes simplex virus thymidine kinase gene to confer an inducible toxic phenotype could permit the in vivo elimination of adoptively transferred T cells if they caused severe GVHD.49-51 This strategy should allow clinical evaluation of the antileukemic activity of minor H antigen-specific T-cell clones for patients with relapse of AML or ALL after allogeneic BMT.
ACKNOWLEDGMENT
The authors thank Jennifer Michaels for assistance in preparation of the manuscript and Suzanne Xuereb for technical assistance with many of the experiments described in this report.
Supported by grants from the National Institutes of Health (Grant No. CA18029), the Lady Tata Memorial Trust (E.H.W.), and the Florence A. Carter Fellowship from the American Medical Association-Education and Research Fund (E.H.W.).
Address reprint requests to Edus H. Warren, MD, PhD, Program in Immunology, M-758, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1124 Columbia St, Seattle, WA 98104.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal