Abstract
A photochemical treatment (PCT) process using a novel psoralen and long wavelength ultraviolet light (UVA, 320-400 nm) has been developed to inactivate bacteria and viruses in platelet concentrates. This study evaluated the efficacy of PCT for inactivation of leukocytes that contaminate platelet preparations. Three psoralens, 8-methoxypsoralen (8-MOP), 4′-aminomethyl 4,5′,8-trimethylpsoralen (AMT), and the novel psoralen S-59, were compared using the following four independent but complementary biological and molecular assays. (1) T-cell viability: Treatment with 150 μmol/L S-59 and 1.0 to 3.0 Joules/cm2 UVA inactivated >5.4 ± 0.3 log10 of T cells in full-sized single-donor plateletpheresis units. Using 1.0 Joule/cm2 UVA, the lowest dose of S-59, AMT and 8-MOP required to reduce the number of T cells to the limit of detection was 0.05 μmol/L, 1.0 μmol/L, and 10.0 μmol/L, respectively. (2) Cytokine synthesis: Treatment with 1.9 Joules/cm2 UVA and 150 μmol/L S-59 or AMT completely inhibited synthesis of the cytokine IL-8 by contaminating leukocytes during 5 days of platelet storage. After treatment with 75 μmol/L 8-MOP and 1.9 Joules/cm2 UVA, only low levels of IL-8 were detected. (3) Psoralen-DNA adduct formation: The combination of 1.9 Joules/cm2 UVA and 150 μmol/L S-59, AMT, or 8-MOP induced 12.0 ± 3.0, 6.0 ± 0.9, and 0.7 psoralen adducts per 1,000 bp DNA, respectively. (4) Replication competence: Polymerase chain reaction (PCR) amplification of small genomic DNA sequences (242-439 bp) after PCT was inhibited. The degree of PCR amplification inhibition correlated with the level of adduct formation (S-59 > AMT > 8-MOP). In contrast, 2,500 cGy gamma radiation, a dose that inactivates >5 log10 of T cells in blood products, had minimal effect on cytokine synthesis and did not induce sufficient DNA strand breaks to inhibit PCR amplification of the same small DNA sequences. These results demonstrate that leukocytes are sensitive to PCT with psoralens and among the psoralens tested S-59 is the most effective. Therefore, PCT has the potential to reduce the incidence of leukocyte-mediated adverse immune reactions associated with platelet transfusion.
TRANSFUSION OF CELLULAR blood products is associated with a number of adverse immune reactions. Transfusion-associated graft-versus-host disease (TA-GVHD) has been well documented for severely immunocompromised patients1-3as well as immunocompetent patients who have received blood from donors homozygous for shared HLA haplotypes.4-7 T cells contaminating cellular blood components have been implicated as the initiating agents for TA-GVHD.8,9 There is no effective therapy for TA-GVHD which is 80% to 90% fatal.10 At present, the primary prophylactic measure is irradiation of cellular blood products using a gamma source.11,12 Based on more than 30 years of clinical practice, gamma radiation has been shown to be effective in reducing the incidence of TA-GVHD. The effective clinical dose of gamma radiation has been determined to be 2,500 cGy based on a clonogenic expansion assay using limiting dilution analysis (LDA).8 13 This dose is required for the inactivation of >5 log10 of T cells in cellular blood products.
While gamma irradiation is efficacious in reducing the incidence of TA-GVHD, transfusion-associated viral and bacterial diseases remain a persistent problem for blood products.14 To reduce the risks of viral and bacterial diseases associated with platelet transfusion, a photochemical treatment (PCT) process using psoralens and long wavelength ultraviolet radiation (UVA 320-400 nm) has been developed.15-17 Psoralens are planar, aromatic molecules that can bind reversibly to nucleic acids by intercalation.18 On illumination with UVA, intercalated psoralens form covalent monoadducts and interstrand crosslinks with RNA and DNA. In the absence of repair, the psoralen-modified genomes of viruses and bacteria are inactivated because replication cannot occur. Because platelets do not have nuclei, they are unaffected by treatment with psoralens and UVA. Novel psoralens have been synthesized to maximize viral inactivation efficiency.19-22 PCT with a novel psoralen, S-59, has been shown to be effective in inactivating a broad spectrum of viruses and bacteria without adversely affecting in vitro or in vivo platelet function.15 23
This report describes the additional benefits derived from a PCT process that is antiviral and antibacterial. Because psoralens are nucleic acid specific reagents, contaminating nucleated leukocytes in platelet concentrates are susceptible targets for inactivation. In this study, the inactivation of leukocytes by PCT with psoralens is evaluated using four independent but complimentary biological and molecular assays. Evidence is presented to demonstrate that (1) T cells in platelet concentrates are extremely susceptible to PCT inactivation; (2) cytokine synthesis by PCT-inactivated leukocytes is inhibited during platelet storage because PCT is performed before storage; (3) leukocyte genomic DNA is heavily modified by psoralens after PCT; and (4) psoralen-DNA adducts block DNA polymerase activity and inhibit the polymerase chain reaction (PCR). Three psoralens, 8-methoxypsoralen (8-MOP), 4′-aminomethyl 4,5′,8-trimethylpsoralen (AMT), and S-59,24 were evaluated for their relative efficiency in leukocyte inactivation. In addition, the effect of 2,500 cGy gamma radiation was evaluated in parallel for its effect on cytokine synthesis by leukocytes during platelet storage and on DNA amplification by PCR. Results from this study suggest that PCT with S-59 has the potential to serve as a superior alternative to gamma radiation for prevention of TA-GVHD.
MATERIALS AND METHODS
Preparation of Platelet Concentrates in 35% Plasma
Random donor platelet concentrates.
Five freshly drawn ABO- matched random donor platelet concentrates (Alameda Contra Costa Medical Association, Oakland, CA) were pooled and transferred to sterile 50 mL polypropylene centrifuge tubes in 30 mL aliquots. After centrifugation (3,000g for 6 minutes at 22°C), the supernatant plasma concentration was adjusted to 35% by removing 65% of the total volume and replacing it with a platelet additive solution (PAS III) and then resuspending the pelleted material. PAS III is a modified platelet synthetic medium (115 mmol/L Na chloride, 30 mmol/L Na acetate, 10 mmol/L Na citrate, and 26 mmol/L Na phosphate) similar to that used by others.25 The pH of PAS III is 7.2 and the osmolarity is 300 mOsm/L. The platelet counts of concentrates ranged between 1.5 to 2.5 × 106/μL.
Single donor plateletpheresis units.
Single donor plateletpheresis units were collected using a CS3000 Blood Cell Separator (Baxter-Fenwal, Round Lake, IL) equipped with a PLT30 collection chamber with a modified procedure (Sacramento Blood Center, Sacramento, CA). The platelets (2.5 to 5.0 × 1011) were collected in approximately 105 mL of plasma. After resuspension, 195 mL of PAS III was added. The final platelet concentrate in approximately 300 mL was transferred into a 1-L PL2410 plastic container (Baxter-Fenwal) and placed on a reciprocal platelet shaker (Helmer Labs, Nobelsville, IN) for storage with temperature control (20°C to 24°C) until use. Concurrent plasma (100 to 150 mL) was also collected and centrifuged (3,000g for 10 minutes at 22°C) to make platelet poor plasma (PPP). The platelet counts of SDP concentrates were similar to random donor platelet concentrates.
Characterization of Platelet Concentrates
A 0.5 mL aliquot was withdrawn from each platelet concentrate using aseptic techniques to measure plasma pH (CIBA-Corning Blood Gas System, CIBA-Corning Diagnostics Corp, Alameda, CA). The platelet and white blood cell counts were determined using an electrical impedance cell counter (Hematology Analyzer, SysmexTM F-800, TOA Medical Electronics Co, Los Alamitos, CA).
Preparation of Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs were isolated from freshly drawn whole blood or buffy coat (Alameda Contra Costa Medical Association) by Ficoll (Sigma, St Louis, MO) density gradient centrifugations according to the manufacturer's instructions. The buffy coat was diluted 4× with phosphate buffered saline (PBS), pH 7.2 (Mediatech, Herndon, VA) before use. The final PBMC pellet was resuspended in the appropriate volume of a mixture made of 35% autologous PPP and 65% PASIII.
Preparation of Psoralen Stock Solutions
Stock aqueous solutions of AMT (HRI Associates, Concord, CA), 8-MOP (Sigma), and S-59 (Cerus Corp, Concord, CA) were optically measured with a Shimadzu UV160U spectrophotometer (Shimadzu Scientific Instruments, Pleasanton, CA). The concentration was calculated using the absorbance at 250 nm and the extinction coefficient of 26,900 M−1cm−1 for S-59, 25,000 M−1cm−1 for AMT, and 22,900 M−1cm−1 for 8-MOP. S-59 and AMT are water soluble. In aqueous medium, 8-MOP saturates at approximately 30 μg/mL. Concentrated stock solutions of 8-MOP were made in dimethyl sulfoxide (DMSO) (Research Industries Corp, Salt Lake City, UT). The structure and synthesis of the novel psoralen S-59 are as described previously.24 S-59 is an amino alkylated psoralen formulated as a hydrochloride salt; it is an odorless, white microcrystalline powder, with solubility greater than 50 mg/mL in 0.9% NaCl. Solid S-59 is stable at high temperature. Aqueous solutions of S-59 show good long-term stability and can be terminally sterilized by autoclaving.
UVA Illumination
After adding psoralens at the indicated concentrations in platelet concentrates, they were illuminated with UVA on a modified Baxter Ultraviolet Irradiation System (Model #4R4440; Baxter-Fenwal) while being mixed. This illumination device is air-cooled, mounted on a reciprocal platelet shaker (Helmer Labs), and capable of maintaining the temperature rise of the platelet concentrate to less than 1°C per Joule/cm2 during the course of the illumination. There are two opposing banks of seven F15T12-BL fluorescent lamps (Spectronics, Westbury, NY) mounted approximately 8 inches from each other. Platelet samples were placed in the center on a piece of glass between the two banks of lights. The output of this device was approximately 15 to 20 mW/cm2 permitting delivery of 3 Joules/cm2 in approximately 3 to 4 minutes.
UVA Dosimetry
Chemical actinometry was used to normalize UVA doses for platelet concentrates with volumes less than 300 mL. The configuration that has been validated for viral and bacterial inactivation while preserving in vitro and in vivo platelet function is 3 Joules/cm2 UVA with 150 μmol/L S-59 for a 300 mL platelet concentrate.15To deliver an equivalent dose of UVA for a 20 mL platelet concentrate, only 1.9 Joules/cm2 was required. LDA experiments with 30 mL platelet concentrates received 1.0 Joule/cm2 of UVA, which is equivalent to a dose of 1.4 Joules/cm2 for a 300 mL platelet concentrate.
Limiting Dilution Analysis (LDA)
Preparation of allostimulator cells.
Whole blood was drawn into two 10 mL ACD collection tubes (Vacutainer, Rutheford, NJ) from each of 10 volunteers. The PBMCs were isolated by Ficoll density gradient centrifugation as described above. After two washings (250g, 20 min, 22°C) in PBS, the final PBMC pellets were pooled in RPMI 1640 (Mediatech) supplemented with 2.0 mmol/L L-glutamine (Sigma), 50 μg/mL penicillin, 50 U/mL streptomycin (GIBCO Life Technologies, Baltimore, MD), and 20% fetal bovine serum (FBS) (Hyclone, Logan, UT) (RPMI/20% FBS) and counted with the Hematology Analyzer. DMSO was added to a final volume of 10%. Aliquots of 2.0 × 106 cells/mL were placed into 1.5 mL sterile cryotubes (Sarstedt, Newton, NC) and frozen at −80°C.
Plating of allostimulator cells.
On the day of each assay, the required number of allostimulator cells was thawed and washed with 6 to 7 volumes of RPMI/20% FBS. The cells were pooled, resuspended in 10 to 15 mL of RPMI/20% FBS, and irradiated with 5,000 cGy of gamma using a Nordion Gamma Cell-1000 irradiator (Alameda Contra Costa Medical Association). After gamma-irradiation, the allostimulator cells were centrifuged at 250g for 10 minutes at 22°C and resuspended in 2× T-cell medium (80% RPMI/20% FBS, 20% TCGF [Cellular Products Inc, Buffalo, NY], 100 U/mL rIL-2 [Cellular Products Inc], and 16 μg/mL PHA-M [Sigma]). To each well of the 96-well flat bottom tissue culture plates (VWR Scientific, Foster City, CA) 1.0 × 105 cells in 100 μL of 2× T-cell medium were plated.
Isolation of leukocytes from platelet concentrates.
The leukocytes from a platelet concentrate sample were pelleted (350g for 5 minutes at 22°C) and resuspended in 20 mL of PBS. This cell suspension was underlayed with 20 mL of 1-Step Platelets (Accurate Chemical and Scientific, Westbury, NY) and centrifuged at 350g for 15 minutes at 22°C. The resulting cell pellet was washed with 40 mL PBS (250g for 10 minutes at 22°C), resuspended in 20 mL PBS, and underlayed with 10 mL of Ficoll (Sigma). After centrifuging at 400g for 30 minutes at 22°C, the buffy coat (8 to 10 mL) was removed and diluted 3 to 4× with PBS. The cells were recovered by centrifugation (250g for 20 minutes at 22°C) and washed twice with 40 mL of PBS (250g for 10 minutes at 22°C). The final cell pellet was resuspended in 2 to 10 mL of RPMI/20% FBS for plating.
Plating of control samples.
Leukocytes from control platelet concentrates that contained no psoralen and were not UVA illuminated were diluted in RPMI/20% FBS to achieve the following concentrations: 3,000, 1,000, 333, 110, and 37 cells/mL. Leukocytes from platelet concentrates treated with UVA alone (1.0 Joule/cm2 or 4.0 Joules/cm2) and from platelet concentrates treated with S-59 alone (1.0 μmol/L or 0.05 μmol/L) were diluted in RPMI/20% FBS to achieve the following concentrations: 10,000, 1,000, 100 cells/mL. Aliquots of 100 μL of each dilution were plated in ten replicates into wells containing 1.0 × 105 allostimulator cells plated previously as described above. To the allostimulator cells control wells, 100 μL of RPMI/20% FBS was added.
Plating of treated samples.
Leukocytes from platelet concentrates (30 mL) treated with low doses of psoralen and 1 Joule/cm2 UVA were serially diluted (1:10) in RPMI/20% FBS to achieve the following range of concentrations: 106 to 101 cells/mL. For each dilution 100 μL was plated in each of 10 wells containing 1.0 × 105allostimulator cells. Two experiments were performed with two independent sources of platelet concentrate. Leukocytes from platelet concentrates (300 mL) treated with 150 μmol/L S-59 and 3 Joules/cm2 UVA were adjusted to 106 cells/mL with RPMI/20% PBS and 100 μL aliquots were plated into each of 110 wells containing 1.0 × 105 allostimulator cells. Four replicate experiments were performed using four independent 300 mL units of single donor platelet concentrate.
Incubation and feeding.
Cells were incubated at 37°C for 3 weeks in a humidified 5% CO2 incubator (Forma Scientific, Marietta, OH). After 3 days, 1 week, and 2 weeks, cells in each well were fed with 25 μL of media consisting of 50% FBS, 50% TCGF, 500 U/mL rIL-2, and 80 μg/mL PHA-M.
Data analysis.
LDA plates were scored visually after 3 weeks using an inverted microscope (Model # CK2, Olympus, Japan). Wells with at least one T-cell clone were scored positive. Wells without a T-cell clone were scored negative. The T-cell frequencies were calculated by minimum chi-square analysis based on a Poisson distribution.13 26The log10 T-cell reduction was calculated as log10 (fcontrol/ftreated), where fcontrol is the T-cell frequency of the control platelet concentrate and ftreated is the T-cell frequency of the photochemically treated platelet concentrate. Mean and standard deviations were calculated using standard methods.
Measurement of Cytokine Levels
These experiments were performed with random donor platelet concentrates with higher levels of contaminating leukocytes. The leukocyte level was quantified using a procedure as previously described.27 If necessary the platelet concentrates were supplemented with leukocytes isolated from a buffy coat unit so that the final pooled leukocyte count was 4.33 × 106/mL for all of the samples.
Twenty milliliter aliquots of the pooled random donor platelet concentrates in 35% plasma were transferred into mini PL2410 plastic containers. One aliquot was not treated and served as the control. A second aliquot was treated with 2,500 cGy gamma radiation. The other aliquots were treated with 150 μmol/L S-59, 150 μmol/L AMT, or 75 μmol/L 8-MOP. The samples containing psoralen were illuminated with 1.9 Joules/cm2 UVA. After treatment, they were stored in parallel with the control platelet concentrate on a reciprocal platelet shaker with temperature control (20°C to 24°C). Samples (1.0 mL) from each mini- platelet unit were taken on day 0 and day 5 and centrifuged at 10,000 rpm for 5 minutes at room temperature (IEC Micromax centrifuge, Needham Heights, MA). The supernatant was stored at −70°C for later analysis of the level of cytokine IL-8 by ELISA (R&D Systems, Minneapolis, MN). After thawing, the plasma samples were centrifuged at 5,000 rpm for 5 minutes at room temperature and the clarified supernatant samples were used for analysis. ELISA assays were performed according to the protocol supplied by the manufacturer and the absorbance measured at 450 nm (Bio-Tek EL 312 Microplate reader, Bio-Tek Instruments Inc, Winooski, VT). Results were evaluated by comparing absorbance measurements for each sample to a standard curve generated by cytokine standards that were supplied with each kit.
Measurement of Psoralen-DNA Adduct Formation
The platelet concentrates were spiked with 3H-labeled psoralens (HRI Associates) to achieve a final specific radioactivity for each psoralen of 5 mCi/mmol. Platelet concentrates were treated with 10 μmol/L, 75 μmol/L, 100 μmol/L and 150 μmol/L S-59; 150 μmol/L AMT; or 75 μmol/L and 150 μmol/L 8-MOP. After photochemical treatment, 1.0 mL samples were centrifuged as described above. The pelleted material was used for measurement of psoralen-DNA adduct formation.
The cell pellets were resuspended in 3 mL of 10 mmol/L Tris-HCl (pH 8.0) containing 1 mmol/L EDTA and 0.1 mg/mL Proteinase K (Sigma), and incubated at room temperature overnight. DNA was isolated from the digest by equal volume extractions (2×) with phenol-chloroform (Sigma), followed by equal volume extractions with chloroform and then ether, and three ethanol precipitations. The ethanol precipitate was redissolved in 10 mmol/L Tris pH 8.0, 100 mmol/L NaCl, 1 mmol/L EDTA between precipitation steps and in water after the final precipitation. The DNA content of each sample was determined by absorbance measurements at 260 nm (1 O.D. unit = 50 μg/mL). The number of psoralen adducts was calculated from residual radioactivity levels in the DNA samples determined by liquid scintillation (Wallac Scientific, Gaithersberg, MD) counting. Data is from two independent experiments.
PCR Amplification Inhibition Assay
Samples containing 1.0 μg DNA obtained from above were amplified for a 242 bp sequence in the HLA-DQα locus using the primer pair GH26/GH2728 and for a 439 bp sequence in the β-globin gene using the primer pair PC03/RS42.29 PCR reactions were set up in 50 μL of 1× Taq Buffer (Perkin Elmer, San Francisco, CA) containing 200 μmol/L each of dATP, dCTP, dGTP, and dTTP (Perkin Elmer), 0.5 μmol/L each of the respective primer set and 2.5 U Taq polymerase (Perkin Elmer). The control DNA sample was serially diluted (1:10) and then amplified. The treated DNA samples were amplified undiluted. Amplification was carried out to 35 cycles on a Perkin Elmer Cetus DNA thermal cycler with the denaturing temperature at 95°C (30 seconds), the annealing temperature at 55°C (30 seconds), and the extension temperature at 72°C (1 minute). The amplification products were analyzed by electrophoresis on a 2.5% NuSieve agarose gel (FMC bioproducts, Rockland, ME). The extent of PCR signal reduction was estimated by comparing the degree of ethidium bromide staining of the amplification products of treated samples with the serially diluted untreated samples.
RESULTS
Photochemical Inactivation of T Cells in Platelet Concentrates
PBMCs isolated from freshly prepared whole blood by Ficoll density gradient centrifugation were spiked into each of four full-sized (300 mL) single donor plateletpheresis units to achieve final leukocyte concentrations of 4.8 × 105 to 3.4 × 106/mL. After photochemical treatment with 150 μmol/L S-59 and 1.0 or 3.0 Joules/cm2 UVA, no viable T cells were detected using the LDA assay. The mean ± standard deviation of T-cell inactivation under these conditions was greater than 5.4 ± 0.3 log10 in four replicates (Table1). These results indicate that photochemical treatment conditions (150 μmol/L S-59 and 3.0 Joules/cm2 UVA) that are virucidal and bactericidal are capable of inactivating high levels of T cells in platelet concentrates.15
Inactivation of T Cells in Platelet Concentrates by Photochemical Treatment With 150 μmol/L S-59 and UVA Light (N = 4)
| Replicate . | Number of Leukocytes per mL* . | T-Cell Frequency . | Log10-Reduction of Viable T Cells-153 . | |
|---|---|---|---|---|
| fcontrol-151 . | ftreated-152 . | |||
| 1 | 1.5 × 106 | 1/22 | <1/1.1 × 107 | >5.70 |
| 2 | 1.3 × 106 | 1/41 | <1/1.1 × 107 | >5.43 |
| 3 | 4.8 × 105 | 1/43 | <1/1.1 × 107 | >5.41 |
| 4 | 3.4 × 106 | 1/110 | <1/1.1 × 107 | >5.00 |
| Mean ± SD | 1.7 ± 1.2 × 106 | 1/39 ± 1/67 | <1/1.1 × 107 | >5.39 ± 0.29 |
| Replicate . | Number of Leukocytes per mL* . | T-Cell Frequency . | Log10-Reduction of Viable T Cells-153 . | |
|---|---|---|---|---|
| fcontrol-151 . | ftreated-152 . | |||
| 1 | 1.5 × 106 | 1/22 | <1/1.1 × 107 | >5.70 |
| 2 | 1.3 × 106 | 1/41 | <1/1.1 × 107 | >5.43 |
| 3 | 4.8 × 105 | 1/43 | <1/1.1 × 107 | >5.41 |
| 4 | 3.4 × 106 | 1/110 | <1/1.1 × 107 | >5.00 |
| Mean ± SD | 1.7 ± 1.2 × 106 | 1/39 ± 1/67 | <1/1.1 × 107 | >5.39 ± 0.29 |
*PBMCs isolated from whole blood were spiked into each of four single donor plateletpheresis units in 300 mL of 35% autologous plasma and 65% PASIII to obtain final leukocyte counts that ranged between 4.8 × 105 and 3.4 × 106 per mL. An aliquot was withdrawn from each unit for measurement of the control viable T-cell frequency by the LDA assay as described in Materials and Methods. S-59 was then added to a final concentration of 150 μmol/L and the platelet units illuminated with 1 or 3 Joules/cm2UVA. Aliquots were withdrawn after each dose of UVA for measurement of the treated viable T-cell frequency by LDA. N = 4.
fcontrol = control viable T-cell frequency.
ftreated = treated viable T-cell frequency.
The log10-reduction is calculated as log10(fcontrol/ftreated).
The minimum dose of 8-MOP, AMT, or S-59 required to inactivate T cells was determined. The sensitivity of T cells to photochemical treatment was evaluated in two dose response experiments using 1 Joule/cm2 UVA and 0.0005 to 1.0 μmol/L S-59, 0.001 to 1.0 μmol/L AMT, and 0.0002 to 100 μmol/L 8-MOP. The platelet concentrates used for these studies had an average leukocyte count of 3.9 ± 2.0 × 106/mL. The viable T-cell frequencies of the untreated (no psoralen, no UVA) controls in four replicates were 1/19, 1/27, 1/34, and 1/88. In the first experiment, the UVA only (1.0 Joule/cm2) control and S-59 only (1.0 μmol/L) control reduced the number of viable T cells by 0.8 log10 and 0.7 log10, respectively. In the second experiment, the UVA only (1.0 Joule/cm2 and 4.0 Joules/cm2) control and S-59 only (0.05 μmol/L) control did not cause a reduction in the number of viable T cells. These results indicate that T cells are essentially stable to UVA or psoralen treatment alone.
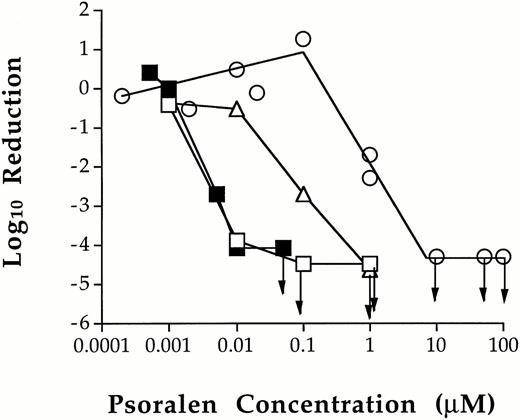
However, T cells were sensitive to treatment with a combination of psoralen and 1.0 Joule/cm2 UVA in platelet concentrates (Fig 1). In one experiment, 0.10 μmol/L S-59 reduced the number of viable T cells to the limit of detection by LDA. There were no viable T-cell clones present in any of the 10 wells plated (>4.5 log10 of reduction). In another experiment, the lowest dose required to inactivate T cells to the limit of detection by LDA (>4.1 log10 of reduction) was 0.05 μmol/L S-59. This concentration of S-59 is 3,000-fold lower than the 150 μmol/L used to inactivate viral and bacterial contaminants in platelet concentrate.15 AMT was slightly less efficient. At 0.1 μmol/L, AMT reduced the T-cell frequency by 2.7 log10. The T-cell frequency was reduced to the limit of detection at 1.0 μmol/L AMT (>4.7 log10 of reduction). Thus, AMT is approximately 20-fold less effective than S-59 for the inactivation of T cells in platelet concentrates. When 8-MOP was used, no T-cell inactivation was obtained up to 0.1 μmol/L. At 1.0 μmol/L, 8-MOP reduced the number of viable T cells by approximately 2.0 log10, and accomplished inactivation to the limit of detection at 10.0 μmol/L (>4.4 log10 of reduction). These results demonstrate the following relative psoralen efficiency for T-cell inactivation: S-59 > AMT > 8-MOP.
Inhibition of T-cell proliferation by photochemical treatment of platelet concentrates with various psoralens. The dose related effect of photochemical treatment with S-59 (□ and ▪), AMT (▵), and 8-MOP (○) on T cells in platelet concentrates was characterized. Leukocytes from photochemically treated and untreated pooled random donor platelet concentrates were plated in the LDA assay. The minimum psoralen concentrations required to inactivate T cells to the limit of detection by LDA (→) after 1.0 Joule/cm2 UVA were 0.05 μmol/L S-59, 1.0 μmol/L AMT, and 10.0 μmol/L 8-MOP. The results in Fig 1 are from two independent experiments. Arrows indicate the psoralen concentrations at which no viable T cells were found.
Inhibition of T-cell proliferation by photochemical treatment of platelet concentrates with various psoralens. The dose related effect of photochemical treatment with S-59 (□ and ▪), AMT (▵), and 8-MOP (○) on T cells in platelet concentrates was characterized. Leukocytes from photochemically treated and untreated pooled random donor platelet concentrates were plated in the LDA assay. The minimum psoralen concentrations required to inactivate T cells to the limit of detection by LDA (→) after 1.0 Joule/cm2 UVA were 0.05 μmol/L S-59, 1.0 μmol/L AMT, and 10.0 μmol/L 8-MOP. The results in Fig 1 are from two independent experiments. Arrows indicate the psoralen concentrations at which no viable T cells were found.
Inhibition of Cytokine Synthesis by Photochemical Treatment
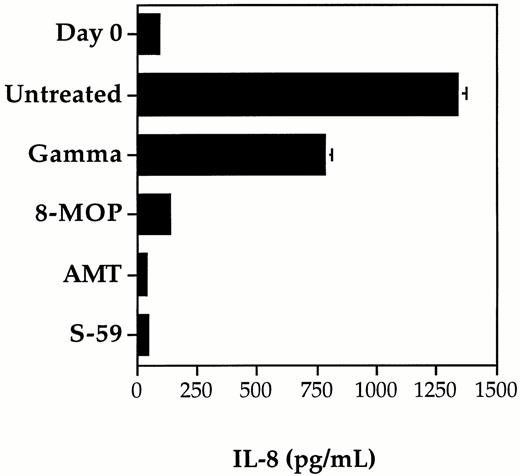
The inhibition of cytokine IL-8 synthesis by contaminating leukocytes in control and photochemically treated random-donor platelet concentrates was compared using S-59, AMT, and 8-MOP (Fig2). Although IL-1β, IL-6, and TNF-α have also been detected in platelet concentrate after storage, we chose to measure IL-8 because it is produced in larger quantities during platelet concentrate storage. Concentrations of IL-8 were found to increase to high levels in the control platelet concentrate with leukocyte counts greater than 1.0 × 106/mL. The level of IL-8 increased from day 0 to day 5 suggesting that active synthesis of IL-8 by viable leukocytes was occurring during storage. Similar results, although with partially reduced levels of IL-8 synthesis, were obtained for the platelet concentrate treated with the clinical dose of gamma radiation (2,500 cGy). In contrast, photochemical treatment with 150 μmol/L AMT or 150 μmol/L S-59 and 1.9 Joules/cm2UVA resulted in complete inhibition of IL-8 synthesis. During the five days of platelet storage, the IL-8 level did not rise above the day 0 baseline level. After photochemical treatment with 75 μmol/L 8-MOP and 1.9 Joules/cm2 UVA, IL-8 levels rose slightly by day 5. These results are consistent with inhibition of leukocyte protein synthesis and correlate with the level of psoralen-DNA adduct formation after photochemical treatment of platelet concentrates.
Inhibition of cytokine synthesis by photochemical treatment and gamma irradiation. The synthesis of cytokine IL-8 was measured after 5 days of storage in pooled random donor platelet concentrates treated separately with S-59, AMT, or 8-MOP plus 1.9 Joules/cm2 UVA, and 2,500 cGy gamma radiation. The level of IL-8 measured on day 0 before treatment served as a baseline measurement. IL-8 levels increased significantly after 5 days of storage in untreated platelet samples. Photochemical treatment with 150 μmol/L S-59 or AMT completely inhibited IL-8 production during storage. Low levels of IL-8 were present after treatment with 75-μmol/L 8-MOP. A partial reduction in IL-8 production was obtained after 2,500 cGy gamma radiation. Each data point is the average of duplicate ELISA measurements. Error bars indicate the standard deviation which is only significant for the untreated and gamma groups after 5 days of storage (error bars for the other groups are not large enough to resolve on the scale in this figure). The data shown is from a representative of five experiments.
Inhibition of cytokine synthesis by photochemical treatment and gamma irradiation. The synthesis of cytokine IL-8 was measured after 5 days of storage in pooled random donor platelet concentrates treated separately with S-59, AMT, or 8-MOP plus 1.9 Joules/cm2 UVA, and 2,500 cGy gamma radiation. The level of IL-8 measured on day 0 before treatment served as a baseline measurement. IL-8 levels increased significantly after 5 days of storage in untreated platelet samples. Photochemical treatment with 150 μmol/L S-59 or AMT completely inhibited IL-8 production during storage. Low levels of IL-8 were present after treatment with 75-μmol/L 8-MOP. A partial reduction in IL-8 production was obtained after 2,500 cGy gamma radiation. Each data point is the average of duplicate ELISA measurements. Error bars indicate the standard deviation which is only significant for the untreated and gamma groups after 5 days of storage (error bars for the other groups are not large enough to resolve on the scale in this figure). The data shown is from a representative of five experiments.
Psoralen Modification of Leukocyte Genomic DNA After Photochemical Treatment
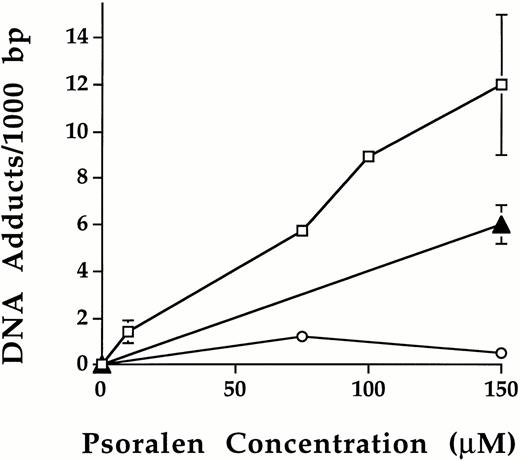
Following photochemical treatment, the level of psoralen-DNA adduct formation was measured using leukocytes isolated from platelet concentrates (Fig 3). No psoralen-DNA adducts were detected in leukocytes of platelet samples either untreated or treated with psoralen or UVA alone. After treatment with psoralen plus UVA, psoralen-DNA adducts were formed and the level of psoralen-DNA adduct formation increased as a function of psoralen concentration. Using 1.9 Joules/cm2 UVA, 10 μmol/L, 75 μmol/L, 100 μmol/L, and 150 μmol/L S-59 induced 1.4 ± 0.5, 5.7, 8.9 and 12 ± 3.0 adducts per 1,000 bp, respectively. At 150 μmol/L plus 1.9 Joules/cm2, the number of psoralen-DNA adducts were 6.0 ± 0.9/1,000 bp and 0.7/1,000 bp for AMT and 8-MOP, respectively. These results indicate the following order of DNA binding efficiency: S-59 > AMT > 8-MOP.
Psoralen-DNA adduct formation following photochemical treatment with various psoralens in platelet concentrates. Psoralen adducts on leukocyte genomic DNA were measured after photochemical treatment in pooled random donor platelet concentrates by using3H radiolabeled S-59 (□), AMT (▴), and 8-MOP (○) plus 1.9 Joules/cm2 UVA. Photochemical treatment with 150 μmol/L S-59, AMT, and 8-MOP induced 12.0 ± 3.0, 6.0 ± 0.9, and 0.7 adducts/1,000 bp of DNA, respectively. Points with error bars (standard deviation) represent averages of duplicate samples obtained from two independent experiments.
Psoralen-DNA adduct formation following photochemical treatment with various psoralens in platelet concentrates. Psoralen adducts on leukocyte genomic DNA were measured after photochemical treatment in pooled random donor platelet concentrates by using3H radiolabeled S-59 (□), AMT (▴), and 8-MOP (○) plus 1.9 Joules/cm2 UVA. Photochemical treatment with 150 μmol/L S-59, AMT, and 8-MOP induced 12.0 ± 3.0, 6.0 ± 0.9, and 0.7 adducts/1,000 bp of DNA, respectively. Points with error bars (standard deviation) represent averages of duplicate samples obtained from two independent experiments.
Psoralen-DNA Adducts Inhibit PCR DNA Amplification
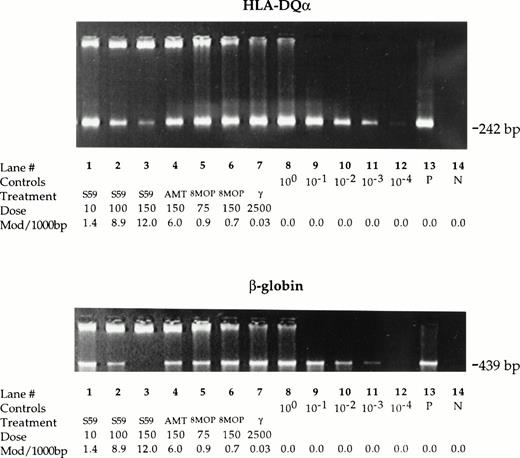
Leukocyte DNA samples isolated from control, photochemically treated, and gamma-irradiated platelet samples were amplified under standard PCR conditions using a primer set for a 242 bp sequence in the HLA-DQα locus and for a 439 bp sequence in the β-globin gene (Fig4). Similar results were obtained for both sequences. While the PCR amplification proceeded normally to plateau values with DNA of control untreated platelet samples, the amplification of DNA of photochemically treated samples was reduced. The degree of reduction in PCR signal varied depending on the psoralen and treatment conditions. After 35 cycles of amplification, treatment with 150 μmol/L S-59 plus 1.9 Joules/cm2 UVA resulted in >103-fold reduction (lane 3 v lane 11) while treatment with 150 μmol/L AMT plus 1.9 Joules/cm2 UVA showed 101- to 102-fold reduction (lane 4v lanes 9, 10) in PCR signal. Treatment with 100 μmol/L S-59 plus 1.9 Joules/cm2 UVA resulted in a PCR signal equivalent to approximately a 102-fold inhibition (lane 2 vlane 10). Treatment with 75 μmol/L and 150 μmol/L 8-MOP resulted in low but detectable PCR reduction (100- to 101-fold reduction, lanes 5, 6 v lanes 8, 9) only with the 439 bp amplicon. However, after 30 cycles of amplification the PCR signal reduction was more readily detectable for both amplicons (101-fold inhibition, data not shown). In contrast, leukocyte DNA of the platelet sample treated with 2,500 cGy gamma radiation amplified to plateau values similar to DNA of control untreated platelet concentrates even with 30 cycles of amplification (lane 7 v lane 8). These PCR DNA amplification inhibition results correlated qualitatively with the number of psoralen-DNA adducts.
Psoralen-DNA adducts inhibit PCR DNA amplification. Genomic DNA was isolated from leukocytes in pooled random donor platelet concentrates. Platelet concentrate samples were either untreated or treated with 10, 100, and 150 μmol/L S-59, 150 μmol/L AMT, 75 or 150 μmol/L 8-MOP plus 1.9 Joules/cm2 UVA, or 2,500 cGy gamma radiation. Inhibition of PCR DNA amplification for the 242 bp amplicon in the HLA-DQα locus and the 439 bp amplicon in the β-globin gene was measured by comparing the band intensity of treated samples with serially diluted (1:10) untreated samples. After 35 cycles, 150 μmol/L S-59 resulted in >103-fold signal reduction for both the HLA-DQα and β-globin amplicons. AMT at a dose of 150 μmol/L reduced the PCR signal by a factor of 101 to 102. With 8-MOP at 75 μmol/L or 150 μmol/L detectable reduction in PCR DNA amplification (∼101-fold reduction) was observed. The reduction was more readily detectable at 30 cycles of amplification (data not shown) and for the 429 bp amplicon in the β-globin gene. Treatment with 2,500 cGy did not result in inhibition of PCR DNA amplification. One microgram human placental DNA was amplified in the positive control sample (P). A reagent-only control sample contained no DNA (N).
Psoralen-DNA adducts inhibit PCR DNA amplification. Genomic DNA was isolated from leukocytes in pooled random donor platelet concentrates. Platelet concentrate samples were either untreated or treated with 10, 100, and 150 μmol/L S-59, 150 μmol/L AMT, 75 or 150 μmol/L 8-MOP plus 1.9 Joules/cm2 UVA, or 2,500 cGy gamma radiation. Inhibition of PCR DNA amplification for the 242 bp amplicon in the HLA-DQα locus and the 439 bp amplicon in the β-globin gene was measured by comparing the band intensity of treated samples with serially diluted (1:10) untreated samples. After 35 cycles, 150 μmol/L S-59 resulted in >103-fold signal reduction for both the HLA-DQα and β-globin amplicons. AMT at a dose of 150 μmol/L reduced the PCR signal by a factor of 101 to 102. With 8-MOP at 75 μmol/L or 150 μmol/L detectable reduction in PCR DNA amplification (∼101-fold reduction) was observed. The reduction was more readily detectable at 30 cycles of amplification (data not shown) and for the 429 bp amplicon in the β-globin gene. Treatment with 2,500 cGy did not result in inhibition of PCR DNA amplification. One microgram human placental DNA was amplified in the positive control sample (P). A reagent-only control sample contained no DNA (N).
DISCUSSION
To reduce the risk of viral and bacterial disease transmission through platelet transfusion, a photochemical treatment process using a novel psoralen, S-59, and UVA was developed.15,19-23 Treatment conditions have been optimized for platelet concentrates to inactivate high levels of a broad spectrum of viruses and bacteria while preserving platelet function.15 This study demonstrates that contaminating leukocytes in platelet concentrates are inactivated after treatment with psoralens and UVA. More importantly, the virucidal and bactericidal conditions provide a large margin of safety for leukocyte inactivation.
Contaminating leukocytes cause a number of adverse immune reactions associated with platelet transfusions. Viable T cells are implicated as the primary cause of TA-GVHD.1 Gamma irradiation has been used as the primary prophylactic treatment for prevention of TA-GVHD.10,11 A clonal T-cell expansion assay, LDA, has been used to correlate the extent of T-cell inactivation with the dose of gamma radiation.13 A dose of 2,500 cGy was shown to inactivate >5 log10 of T cells in packed red blood cell units. The same LDA method was used in the present study to measure the level of T-cell inactivation in platelet concentrates by treatment with S-59 and UVA. Under the virucidal and bactericidal treatment conditions (150 μmol/L S-59 and 3 Joules/cm2 UVA), greater than 5.4 ± 0.3 log10 of T cells were inactivated in full-sized single-donor plateletpheresis units. This finding suggests that photochemical treatment with S-59 and UVA, similar to gamma irradiation treatment, inactivates high levels of T cells in platelet concentrates and thus has the potential to prevent TA-GVHD.
In addition, results from this study indicate that T cells are extremely sensitive to photochemical treatment. At an S-59 dose (0.05 μmol/L) that is 3,000-fold lower than that used for inactivation of viruses and bacteria, T cells are inactivated to an undetectable level by LDA after 1 Joule/cm2 of UVA illumination. Therefore, the treatment dose for viral and bacterial inactivation of platelet concentrates provides a large safety margin for T-cell inactivation. In contrast, the safety margin for the 2,500 cGy gamma radiation is limited. TA-GVHD has been reported in patients who received blood products that were irradiated with 2,000 cGy.30
The accumulation of inflammatory cytokines synthesized by leukocytes during storage of platelet concentrates has been implicated as the cause of febrile nonhemolytic transfusion reactions (FNHTR).31,32 FNHTR are the most frequently reported adverse immune reactions associated with platelet transfusion.32 Leukodepletion filters used to reduce the number of leukocytes transfused are not effective in preventing FNHTR if they are used after storage.33,34 Photochemical treatment with 150 μmol/L S-59 and 1.9 Joules/cm2 UVA completely inhibited synthesis of the cytokine IL-8 by leukocytes during platelet storage. Similar results with IL-8 IL-1β, TNF-α, and IL-6 have been reported previously.35 Suppression of cytokine production by peripheral blood mononuclear cells treatedin vivo or in vitro by 8-MOP and UVA was also reported by other investigators.36 These results suggest that photochemical treatment may provide the potential to reduce the incidence of FNHTR associated with platelet transfusion, a benefit that is lacking by treatment with gamma radiation. Irradiating platelet concentrates with 2,500 cGy of gamma only partially reduced the level of cytokine synthesis during storage. Although PCT has no effect on preformed cytokines or complement (unpublished data) PCT with S-59 and UVA should be performed before storage before pro-inflammatory cytokines accumulate in the stored platelets. Bacterial contamination, which can induce cytokine secretion in stored platelets, has been implicated as the cause of adverse transfusion reactions.37PCT performed before storage will also prevent bacterial proliferation and thus reduce the incidence of these platelet transfusion associated adverse immune reactions.17
Leukocyte inactivation by photochemical treatment was also confirmed at the molecular level. The extent of S-59 photomodification of leukocyte genomic DNA correlated with the concentration of S-59. After treatment with 150 μmol/L S-59 and 1.9 Joules/cm2 UVA, DNA was modified by S-59 at the level of 12 ± 3 covalent adducts per 1,000 bp. This level of modification overwhelms cellular repair mechanisms and is able to inhibit gene expression.18 This finding is consistent with the observation that synthesis of IL-8 by leukocytes during platelet storage was completely eliminated after photochemical treatment with S-59. In comparison, gamma radiation (2,500 cGy) induces DNA strand breaks at a level of one per 37,000 bp.38 Therefore, the short DNA coding sequence for IL-8, a peptide of approximately 80 amino acids, is not likely to be modified extensively. Therefore, only partial reduction in IL-8 synthesis after gamma treatment was obtained.
The effect of psoralen-DNA adducts on nucleic acid replication was further demonstrated by a PCR inhibition assay. Because psoralen-DNA adducts block DNA polymerase and inhibit replication, PCR amplification of psoralen-modified DNA is affected.39 Using two primer pairs, one pair encoding a 242 bp sequence in the HLA-DQα locus and the other pair encoding a 439 bp sequence in the β-globin gene, the extent of PCR signal reduction was found to correlate with the number of psoralen-DNA adducts. Therefore, by selecting PCR primers encoding a sufficiently long sequence, the reduction in PCR amplification signal can be used as an indirect measure of the psoralen-DNA modification density. For DNA of gamma-treated samples, PCR was not affected by using the same primer pairs. This finding is consistent with the relatively sparse strand breaks on the DNA caused by gamma irradiation. The results obtained from psoralen-DNA adduct formation and from PCR inhibition assay showed that the DNA of leukocytes treated with S-59 and UVA is more extensively modified than DNA of leukocytes treated with gamma radiation.
This study also compared the efficacy of S-59 with that of two commonly used psoralens, 8-MOP and AMT. Results obtained from all of the parameters evaluated are in agreement with respect to the level of relative biological and molecular inactivation achieved. S-59 was the most efficient psoralen of the three. Using the same dose of UVA, the concentration of S-59 required to completely inactivate T cells in platelet concentrates is approximately 20-fold lower than that required for AMT and approximately 200-fold lower than that required for 8-MOP. Under equivalent treatment conditions, DNA modification density was lowest with 8-MOP demonstrating that 8-MOP binds to DNA less efficiently than either AMT or S-59. Therefore 8-MOP is less efficient than S-59 or AMT with respect to its ability to completely inhibit cytokine synthesis.
HLA-alloimmunization that can lead to refractoriness to platelet transfusion is another adverse immune reaction mediated by leukocytes.40 In vitro and in vivo animal studies performed with 8-MOP + UVA suggest that psoralen + UVA treatment of platelet concentrate has the potential to reduce HLA alloimmunization in platelet transfusion recipients.41 Studies with S-59 and UVA are underway to investigate the effect of S-59 PCT on the incidence of HLA-alloimmunization.
Results presented here clearly showed that PCT with S-59 and UVA inactivates T cells with greater potency than gamma radiation, the current method of TA-GVHD prophylaxis in platelet concentrate transfusion recipients. Although PCT provides an alternative method for the prevention of TA-GVHD, some physicians may choose to gamma irradiate PCT-treated platelets. In vitro platelet function studies have shown no adverse synergistic effects due to the combined treatment of PCT and gamma radiation over 5 days of platelet storage (unpublished data).
In summary, the results obtained from biological and molecular assays demonstrate that photochemical treatment with S-59 has the potential to replace gamma radiation for preventing TA-GVHD associated with platelet transfusion. PCT provides a much larger margin of safety for the inactivation of T cells than gamma radiation, while simultaneously inactivating high levels of a wide variety of infectious agents contaminating platelet concentrate. In addition, photochemical treatment with S-59 may also provide a method that could reduce the incidence of platelet transfusion related FNHTR. Further in vivo transfusion studies using immunocompetent and immunocompromised mice42-44 are underway to confirm the utility of photochemical treatment in preventing TA-GVHD and other adverse immune reactions associated with platelet transfusions.
ACKNOWLEDGMENT
The authors acknowledge the excellent technical assistance of Terri Anderson in performing the LDA assay. Lainie Corten provided critical contributions during the development phase of the LDA assay. The authors also thank Dr John Hearst for his constant encouragement and valuable suggestions throughout this study. The cooperation of Dr Paul Holland, Vangie Schoening, and Barbara Evans of the Sacramento Blood Center, Sacramento, CA and Dr Sherri Evans of the Alameda Contra Costa Medical Association, Oakland, CA in supplying platelet concentrates for this study was greatly appreciated. The Alameda Contra Costa Medical Association also provided services for gamma irradiation of cellular samples with the Nordion Gamma Cell-1000. David Drothler of the Children's National Medical Center, Washington DC provided critical information regarding the LDA assay and the IBM PC software program for calculation of T-cell frequency from the LDA results.
Supported in part by National Institutes of Health Grant No. HL 43320.
Address reprint requests to Lily Lin, PhD, Cerus Corp, 2525 Stanwell Dr, Suite 300, Concord, CA 94520.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal