Abstract
Scott syndrome is an hereditary bleeding disorder characterized by a deficiency in platelet procoagulant activity. Unlike normal blood cells, Scott platelets, as well as erythrocytes and lymphocytes, are strongly impaired in their ability to scramble their membrane phospholipids when challenged with Ca2+. In normal cells this collapse of membrane asymmetry leads to surface exposure of phosphatidylserine. Here we report that Scott erythrocytes show an apparent defect in tyrosine phosphorylation on treatment with Ca2+-ionophore. Diminished tyrosine phosphorylation was also apparent in activated Scott platelets, but much less pronounced than observed in red blood cells. On the other hand, tyrosine phosphorylation profiles observed in Scott red blood cell ghosts after sealing in the presence of adenosine triphosphate (ATP) were indistinguishable from those obtained from normal ghosts. Several observations argue in favor of a mechanism in which tyrosine phosphorylation in red blood cells is facilitated by, rather than required for scrambling of membrane lipids. Staurosporin blocks tyrosine phosphorylation in normal red blood cells, but does not inhibit the lipid scrambling process. White ghosts from normal erythrocytes, resealed in the absence of ATP, exhibit Ca2+-induced lipid scrambling without tyrosine phosphorylation. A selective inhibitor of Ca2+-induced lipid scrambling also showed an apparent inhibition of tyrosine phosphorylation in ionophore-treated normal red blood cells, similar to that observed in Scott erythrocytes. While this inhibitor also suppressed Ca2+-induced lipid scrambling in ghosts that were sealed in the presence of ATP, it did not inhibit tyrosine kinase activity. We conclude that the apparent deficiency in tyrosine phosphorylation in Scott cells is an epiphenomenon, possibly associated with a defect in phospholipid scrambling, but not causal to this defect.
MEMBRANE PHOSPHOLIPID asymmetry is a seemingly ubiquitous phenomenon that is generated by an adenosine triphosphate (ATP)-dependent lipid pump, which specifically shuttles aminophospholipids to the cytoplasmic membrane leaflet. Influx of calcium, however, inhibits this aminophospholipid translocase activity and activates a lipid scramblase that produces a progressive loss of lipid asymmetry. This process exposes phosphatidylserine at the outer surface, which can promote assembly on the membrane of the tenase and prothrombinase complex of the blood coagulation cascade. This assembly dramatically enhances the rate of thrombin formation responsible for the procoagulant activity of activated platelets.1-3
A deficiency in platelet procoagulant activity associated with a bleeding disorder was first described by Weiss et al4,5 and is presently referred to as Scott syndrome. On activation, Scott platelets show a strongly decreased lipid scrambling with little surface exposure of phosphatidylserine.6 Moreover, these platelets express a considerably decreased number of factor Va7 and factor VIIIa8 binding sites and are markedly deficient in their ability to promote both tenase and prothrombinase activity in response to agonists.6 They also exhibit an impaired capacity to shed membrane-derived lipid-symmetric microvesicles, a process often associated with lipid scrambling.9 Recent studies on a newly discovered family suggest that Scott syndrome is an inherited bleeding disorder transmitted as an autosomal recessive trait,10 and this may also be the case in the family of the originally described patient with this disorder.11 The defect is not restricted to platelets, but can also be demonstrated in the patients' erythrocytes and lymphocytes.12,13 The observation that, unlike normal red blood cell ghosts, resealed ghosts from Scott erythrocytes do not show Ca2+-induced scrambling of membrane phospholipids implies that the lesion resides in the plasma membrane or in the tightly associated cytoskeleton.12 Indeed, proteins fractionated from platelet or red blood cell membranes have been reconstituted in artificial lipid vesicles, which exhibited Ca2+-induced lipid scrambling activity.14,15 Recently, it was shown that proteoliposomes reconstituted from red blood cells from Scott syndrome resulted in similar Ca2+-inducible lipid scrambling, as observed for reconstitutes from normal red blood cells.16Moreover, acidification caused Ca2+-independent lipid scrambling in both proteoliposomes and inside-out vesicles derived from normal and Scott erythrocytes, suggesting that the defect in Scott syndrome is caused by an impaired regulatory mechanism rather than the absence of a membrane protein responsible for lipid scrambling.
While hydrolyzable ATP does not seem to be required for lipid scrambling, it has been reported that prolonged ATP depletion of erythrocytes results in markedly diminished Ca2+-induced randomization of lipids.17 18 Because this may suggest a possible involvement of phosphorylated protein(s), it was intriguing to observe a severely impaired tyrosine phosphorylation in Scott erythrocytes after treatment with Ca2+-ionophore. The present study shows that the aberrant tyrosine phosphorylation is a consequence rather than a cause of a defective lipid scrambling process.
MATERIALS AND METHODS
Materials.
Ionomycin and bovine serum albumin (BSA; globulin and fatty acid free) were obtained from Sigma Chemical Co (St Louis, MO). Coagulation factors thrombin, prothrombin, factor Xa, and factor Va were purified from bovine blood as described elsewhere.19Thrombin-specific chromogenic substrate, S2238, was obtained from AB Kabi Diagnostica (Stockholm, Sweden). Sodium orthovanadate was from Janssen Chimica (Geel, Belgium). NBD-PS: 2-(12-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)dodecanoyl-1-oleoyl-sn-glycero-3-phosphoserine was obtained from Avanti Polar Lipids (Alabaster, AL). R5421, ethanimidothioic acid N-[{N-butylthio-N-methylamino]-carbonyloxy}-methyl ester (see insert Fig 3 for formula) was from Dupont-Merck (Wilmington, DE). Monoclonal antiphosphotyrosine antibody (4G10) was obtained from UBI (Lake Placid, NY). Enhanced chemiluminescence (ECL) materials were from Amersham PLC (Aylesbury, UK). All other reagents were of the highest grade commercially available.
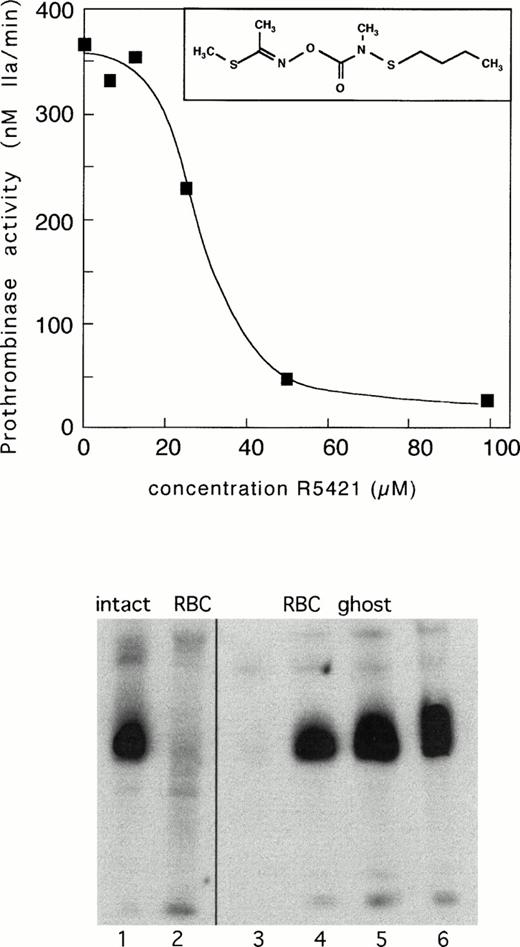
Upper panel: inhibition of Ca2+-ionomycin–induced prothrombinase activity in control erythrocytes by compound R5421. Cells were incubated with increasing concentrations of R5421 for 10 minutes, followed by ionomycin (5 μmol/L) in the presence of Ca2+ (1 mmol/L) for 60 minutes. Subsequently, samples were analyzed for prothrombinase activity as described in Materials and Methods. Insert: structural formula of compound R5421. Lower panel: tyrosine phosphorylation in control erythrocytes incubated for 15 minutes with Ca2+-ionomycin in the absence (lane 1) and presence (lane 2) of R5421 (0.1 mmol/L). Lanes 3 to 6 represent phosphorylation patterns of white ghosts incubated with four different concentrations of ATP (0.05 mmol/L ATP, lane 3; 0.5 mmol/L ATP, lane 4; 1.0 mmol/L ATP, lane 5; and 2.0 mmol/L ATP, lane 6), all in the presence of R5421 (0.1 mmol/L). Contrary to the incubations with intact cells, the experiments with ghosts were performed in the absence of Ca2+ to ensure sufficient levels of phosphorylation (see text).
Upper panel: inhibition of Ca2+-ionomycin–induced prothrombinase activity in control erythrocytes by compound R5421. Cells were incubated with increasing concentrations of R5421 for 10 minutes, followed by ionomycin (5 μmol/L) in the presence of Ca2+ (1 mmol/L) for 60 minutes. Subsequently, samples were analyzed for prothrombinase activity as described in Materials and Methods. Insert: structural formula of compound R5421. Lower panel: tyrosine phosphorylation in control erythrocytes incubated for 15 minutes with Ca2+-ionomycin in the absence (lane 1) and presence (lane 2) of R5421 (0.1 mmol/L). Lanes 3 to 6 represent phosphorylation patterns of white ghosts incubated with four different concentrations of ATP (0.05 mmol/L ATP, lane 3; 0.5 mmol/L ATP, lane 4; 1.0 mmol/L ATP, lane 5; and 2.0 mmol/L ATP, lane 6), all in the presence of R5421 (0.1 mmol/L). Contrary to the incubations with intact cells, the experiments with ghosts were performed in the absence of Ca2+ to ensure sufficient levels of phosphorylation (see text).
Isolation of erythrocytes and preparation of resealed ghosts.
Washed human red blood cells were obtained by differential centrifugation of whole blood collected on citrate (3.8%) from healthy volunteers. Blood from patient MS and appropriate control samples were shipped at 4°C by air express to Maastricht and processed immediately on arrival. After washing in TEMS8 buffer (50 mmol/L TRIS, 120 mmol/L NaCl, 2 mmol/L MgCl2, 0.2 mmol/L EGTA, adjusted at pH 8.0), erythrocytes were finally resuspended at 10% hematocrit and stored on ice. Cells were used within 1 day. Ghosts were prepared by hypotonic shock in ice-cold lysis buffer (5 mmol/L TRIS, 12 mmol/L NaCl, 2 mmol/L MgCl2, 0.2 mmol/L EGTA). After 15 minutes on ice, tonicity was restored by adding one tenth volume 9% NaCl, followed by incubation at 37°C for 15 minutes. In some experiments, ATP (0.05 to 2.0 mmol/L) and/or R5421 (0.1 mmol/L) was added before resealing. Resealed ghosts were collected by centrifugation at 2,250g for 20 minutes and washed three times in TEMS8 buffer. Ghost concentration was adjusted at 1 × 109/mL.
Erythrocytes and resealed ghosts were incubated at a concentration of 1 × 109/mL with ionomycin (final concentration 5 μmol/L) in the presence of 1 mmol/L CaCl2 at 37°C.
Preparation of washed platelets.
Platelets were isolated from patient MS and an appropriate control by differential centrifugation of whole blood, according to Bevers et al.20 Because shipment of the blood samples took approximately 48 hours, the blood was collected in citrate to avoid a prolonged period of acidity. Before centrifugation, acid citrate dextrose (ACD: 0.18 mol/L glucose, 0.08 mol/L trisodium citrate, 0.052 mol/L citric acid) was added to the blood in a ratio 1:5 (vol/vol). Washed platelets were finally resuspended in a HEPES buffer (136 mmol/L NaCl, 2.7 mmol/L KCl, 2 mmol/L MgCl2, 10 mmol/L HEPES, 0.1 mmol/L EGTA, 5 mmol/L glucose, and 0.5 mg/mL human serum albumin, pH 7.5), and diluted to a final concentration of 5 × 108platelets/mL.
Measurement of prothrombinase activity.
The rate of conversion of prothrombin to thrombin by the enzyme complex factor Xa-factor Va has been shown to be a convenient, rapid, and sensitive method to monitor in a semiquantitative way the extent of exposure of phosphatidylserine at the outer cell surface.20Briefly, 1 × 107/mL erythrocytes (or resealed ghosts) were incubated at 37°C with factor Xa and factor Va at a final concentration of 3 and 6 nmol/L, respectively, for 1 minute in the presence of 3 mmol/L CaCl2. The reaction was started by addition of prothrombin (final concentration 4 μmol/L); 1 minute after addition of prothrombin, a sample from the incubation mixture was transferred to a cuvette containing 1 mL buffer composed of 50 mmol/L TRIS, 120 mmol/L NaCl, and 2 mmol/L EDTA (pH 8.0). The amount of thrombin formed was determined by measuring the change in absorbance at 405 nm caused by the conversion of a thrombin-specific substrate, S2238 (0.2 mmol/L).
Measurement of scramblase activity in platelets.
A continuous assay for scrambling of platelet lipids was based on reduction of a fluorescent lipid probe (NBD-PS) by dithionite as previously described by Williamson et al.21Washed platelets resuspended in HEPES buffer in the absence of albumin were preincubated with 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF) before labeling with 1 μmol/L NBD-PS. Addition of PMSF was required to prevent degradation of the probe once it had been internalized by the action of the aminophospholipid translocase. Platelets (2 × 108/mL) were loaded with NBD-PS for 45 minutes at 37°C, followed by an additional incubation period of 45 minutes in the presence or absence of R5421. A total of 50 to 100 μL of NBD-PS–labeled platelets were diluted in 2 mL of HEPES buffer and placed in the fluorimeter at 37°C under continuous stirring and fluorescence was monitored at 534 nm (λex 472 nm). At 60 seconds, dithionite was added to a final concentration of 5 mmol/L to quench NBD-PS present in the membrane outer leaflet. At t=90 seconds, ionomycin was added (1 μmol/L final concentration), and the scrambling process was started by the addition of 1 mmol/L CaCl2 at t= 120 seconds. Fluorescence intensity was monitored for 7.5 minutes, after which Triton X100 was added to a final concentration of 1% to make all of the NBD-PS available to dithionite. To determine IC50 and half-time of R5421 inhibition, the rate of scrambling activity was analyzed as the initial slope of the fluorescence decay after addition of Ca2+.
Tyrosine phosphorylation.
Protein-tyrosine phosphorylation was detected after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Briefly, after the ionomycin treatment of intact erythrocytes, 0.2 mmol/L PMSF was added and cells were lysed by the addition of 20 vol ice-cold lysis buffer. Ghosts were collected and washed once by centrifugation (30,000g for 15 minutes). After resuspension, one fifth volume of electrophoresis sample buffer (312.5 mmol/L TRIS, 50% (vol/vol) glycerol, 15% (wt/vol) SDS, 0.17 mg/mL Bromphenol blue, 50 mmol/L dithiothreitol, pH 6.8) was added. Samples for electrophoresis from incubations with resealed ghosts (1 × 109/mL) or platelets (5 × 108/mL) were prepared by addition of one fifth volume of electrophoresis buffer to the incubation mixture. Proteins were separated by SDS-PAGE on 7.5% gels and transferred to nitrocellulose. After blocking with 3% BSA for 1 hour, blots were incubated with the antiphosphotyrosine antibody, 4G10 (0.04 μg/mL) and after extensive washing, incubated with a biotinylated goat antimouse antibody. Immunoreactive proteins were detected by streptavidine horseradish peroxidase, visualized by ECL (Amersham) according to the manufacturer's procedure.
RESULTS AND DISCUSSION
Incubation of human erythrocytes with Ca2+-ionophore results in a rapid phosphorylation of tyrosine residues in certain membrane proteins, of which band 3 appears to be the most prominent.22 23 We have compared the phosphorylation patterns of erythrocytes from healthy individuals with those from a patient with Scott syndrome by Western blots using a monoclonal antibody specific for phosphotyrosine residues. Figure 1, upper panel, shows that tyrosine phosphorylation is severely impaired in the patients' red blood cells after treatment with Ca2+-ionophore (lanes 1 to 4). This raises the question whether this impaired phosphorylation is related to the lack of Ca2+-induced lipid scrambling in the patients' blood cells, as shown by the lack of phosphatidylserine exposure monitored by prothrombinase (Fig 1, lower panel).
Tyrosine phosphorylation of membrane proteins and development of prothrombinase activity in normal erythrocytes and erythrocytes from Scott syndrome incubated with Ca2+-ionomycin. Washed cells were incubated with ionomycin in the presence of Ca2+ as described in Materials and Methods, and samples were taken at 15 minutes for analysis of tyrosine phosphorylation and at various time intervals for measuring prothrombinase activity as a measure for surface exposed phosphatidylserine. Phosphorylation patterns in red blood cells did not appreciably change on varying the incubation time from 5 to 30 minutes. Upper panel: normal erythrocytes in the absence (lane 1) and presence of Ca2+-ionomycin (lane 2) and Scott erythrocytes in the absence (lane 3) and presence of Ca2+-ionomycin (lane 4). Lower panel: time course of appearance of prothrombinase activity in normal (▪) and Scott erythrocytes (•).
Tyrosine phosphorylation of membrane proteins and development of prothrombinase activity in normal erythrocytes and erythrocytes from Scott syndrome incubated with Ca2+-ionomycin. Washed cells were incubated with ionomycin in the presence of Ca2+ as described in Materials and Methods, and samples were taken at 15 minutes for analysis of tyrosine phosphorylation and at various time intervals for measuring prothrombinase activity as a measure for surface exposed phosphatidylserine. Phosphorylation patterns in red blood cells did not appreciably change on varying the incubation time from 5 to 30 minutes. Upper panel: normal erythrocytes in the absence (lane 1) and presence of Ca2+-ionomycin (lane 2) and Scott erythrocytes in the absence (lane 3) and presence of Ca2+-ionomycin (lane 4). Lower panel: time course of appearance of prothrombinase activity in normal (▪) and Scott erythrocytes (•).
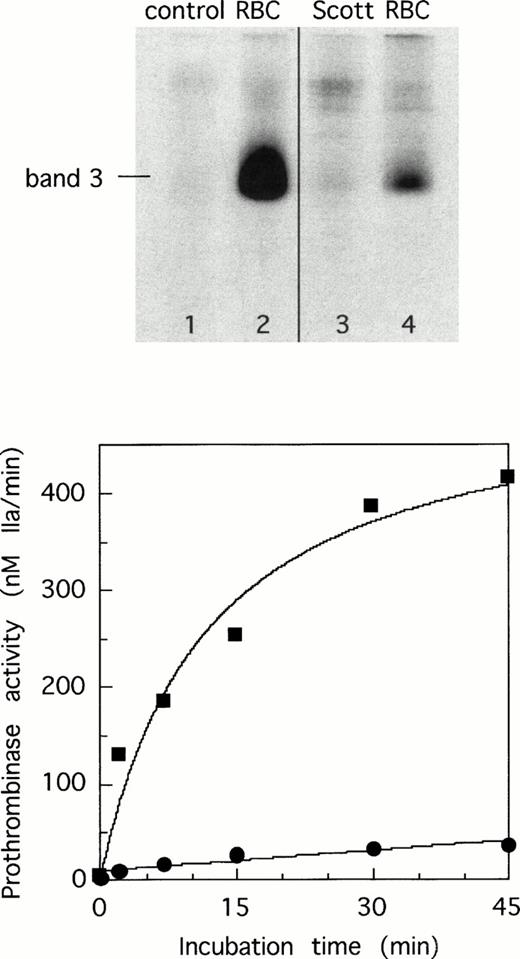
To investigate if the impaired tyrosine phosphorylation observed in Scott erythrocytes is caused by a deficiency in one or more of the tyrosine kinases, we tested tyrosine phosphorylation in hemoglobin-free (‘white’) erythrocyte ghosts. The major tyrosine kinase activity in erythrocytes appears membrane-associated, and direct phosphorylation can be observed in nonsealed ghost preparations after addition of Mg-ATP.24 For this purpose, we used Ca2+-free ghosts as a substrate because tyrosine phosphorylation in Ca2+-loaded ghosts is too low to detect a possible deficiency of tyrosine kinase activity in Scott syndrome. As shown in Fig 2, similar phosphorylation patterns were found for both Scott and control ghosts when incubated with increasing amounts of ATP. Because red blood cell ghosts still contain protein tyrosine phosphatase activity associated with band 3,24 the level of protein phosphotyrosine is determined by the activity of protein tyrosine kinases and the opposing activity of protein tyrosine phosphatases. As expected, addition of Mn2+ and VO43-, known inhibitors of tyrosine phosphatase,23,24 causes higher levels of phosphorylation, but again, no difference was found between control and Scott ghosts. While these data suggest that neither enzyme is defective in the patients' red blood cell ghosts, it can at present not be excluded that diminished phosphorylation in Scott erythrocytes reflects a difference in either a cytosolic kinase or phosphatase (or regulatory component) that is washed away from intact erythrocytes on preparing ghosts. However, the data from both intact cells and ghosts strongly suggest that tyrosine phosphorylation is not required for lipid scrambling. This is supported by two additional observations. First, incubation of normal erythrocytes with staurosporin at a concentration of 5 μmol/L, which completely blocks tyrosine kinase activity, does not prevent the generation of prothrombinase activity induced by Ca2+-ionophore. Second, white ghosts from normal erythrocytes resealed in the absence of ATP retain the ability to scramble their phospholipids on Ca2+-influx,12while no tyrosine phosphorylation can occur under these conditions.
Western blots showing tyrosine phosphorylation in control (lanes 1 to 4) and Scott erythrocyte ghosts (lanes 5 to 8) after a 15-minute incubation period with increasing concentrations of ATP: 0.05 mmol/L (lanes 1 and 5); 0.5 mmol/L (lanes 2 and 6); 1.0 mmol/L (lanes 3 and 7) and 2.0 mmol/L (lanes 4 and 8).
Western blots showing tyrosine phosphorylation in control (lanes 1 to 4) and Scott erythrocyte ghosts (lanes 5 to 8) after a 15-minute incubation period with increasing concentrations of ATP: 0.05 mmol/L (lanes 1 and 5); 0.5 mmol/L (lanes 2 and 6); 1.0 mmol/L (lanes 3 and 7) and 2.0 mmol/L (lanes 4 and 8).
It should be realized, however, that the identity of the (membrane) protein(s) involved in the collapse of membrane lipid asymmetry is still obscure, and these proteins may escape detection by the phosphotyrosine Western blots. Although our observations rule out that lipid scrambling requires stimulus-response coupled tyrosine phosphorylation, it cannot be excluded that the putative protein(s) involved in scramblase activity are already phosphorylated before cell activation. Such involvement of constitutively phosphorylated proteins may explain why red blood cells gradually lose their ability to undergo Ca2+-induced lipid scrambling on prolonged ATP deprivation.17 18
While impaired tyrosine phosphorylation does not underlie defective Ca2+-induced lipid scrambling in erythrocytes from Scott syndrome, it cannot be ruled out that the impaired tyrosine phosphorylation results from defective scrambling. To address this question, experiments were performed using R5421, which was found to inhibit Ca2+-induced lipid scrambling in erythrocytes and platelets. R5421 was found after screening a variety of organic compounds using both the prothrombinase assay and the assay for scramblase activity described in Materials and Methods. Its pharmacology and mechanism of action is as yet unknown. Figure 3 (upper panel) shows the effect of increasing concentrations of R5421 on the Ca2+-ionophore–induced prothrombinase activity in intact normal erythrocytes. An IC50 of 35 μmol/L was found, while complete inhibition was observed at a concentration of 100 μmol/L. Similar results were obtained when intact erythrocytes were replaced by resealed ghosts. R5421 at concentrations up to 200 μmol/L did not inhibit the prothrombinase assay in the presence of artificial lipid vesicles. As shown in Fig 3 (lower panel), R5421 causes a dramatic decrease in tyrosine phosphorylation on Ca2+-influx into normal erythrocytes (lanes 1 and 2). To investigate whether R5421 is a direct inhibitor of the major tyrosine kinase in erythrocytes (or activator of tyrosine phosphatase), we measured the effect of R5421 on tyrosine phosphorylation in ‘white’ ghosts incubated with ATP. As shown in Fig 3 (lower panel, lanes 3 to 6), no appreciable difference relative to ghosts without R5421 (Fig 2) was found. Again, these findings do not rule out a possible effect of R5421 on cytosolic kinase or phosphatase activities.
The present data may indicate that Ca2+-induced scrambling of lipids facilitates protein tyrosine phosphorylation in intact erythrocytes. Because lipid scrambling is associated with membrane unpacking,25 it can be speculated that this increases susceptibility of tyrosine residues for kinases. However, it can at present not be excluded that erythrocytes from Scott syndrome (or normal cells treated with R5421) are affected at an early step that leads to both the collapse of lipid asymmetry and tyrosine phosphorylation.
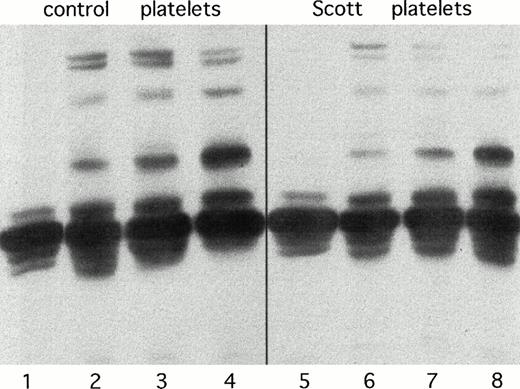
Originally, impaired lipid scrambling in Scott syndrome was discovered in the patients' platelets.4 These platelets do not develop appreciable procoagulant activity on activation with Ca2+-ionophore and have a strongly diminished procoagulant activity in response to the combined action of collagen and thrombin.6 As shown in Fig 4, Scott platelets in response to various agonists consistently show a lower degree of phosphorylation in comparison to healthy controls. This effect is much less pronounced than observed for the patients' erythrocytes, but this may be due to the presence of a wide variety of different tyrosine kinases in platelets,26 27 which may obscure a decreased activity of a single kinase. Also, platelets show a significant extent of constitutive tyrosine phosphorylation.
Tyrosine phosphorylation in normal (lanes 1 to 4) and Scott syndrome platelets (lanes 5 to 8). Lanes 1 and 5 represent unstimulated platelets; lanes 2 and 6, platelets stimulated with 4 nmol/L thrombin; lanes 3 and 7, platelets treated with 70 nmol/L thapsigargin and subsequently activated with 4 nmol/L thrombin; lanes 4 and 8, platelets activated with 10 μg/mL collagen.
Tyrosine phosphorylation in normal (lanes 1 to 4) and Scott syndrome platelets (lanes 5 to 8). Lanes 1 and 5 represent unstimulated platelets; lanes 2 and 6, platelets stimulated with 4 nmol/L thrombin; lanes 3 and 7, platelets treated with 70 nmol/L thapsigargin and subsequently activated with 4 nmol/L thrombin; lanes 4 and 8, platelets activated with 10 μg/mL collagen.
Consistent with the observations in intact erythrocytes, R5421 also inhibits prothrombinase activity in platelets stimulated with several agonists including thrombin and Ca2+-ionophore (IC50, 23 μmol/L). To further assess the effect of R5421 on scramblase activity in platelets, we used a continuous assay recently described by Williamson et al,21 which allows monitoring real time movement of a fluorescent lipid analog, NBD-PS, from the inner to the outer leaflet of the plasma membrane. As shown in Fig 5, preincubation of NBD-PS–loaded platelets with 50 μmol/L R5421 inhibited Ca2+-ionophore–induced transbilayer movement of PS by more than 90% (curves a and b). For comparison, no scrambling of NBD-PS was found when Scott platelets were stimulated with Ca2+-ionophore (Fig 5, curve c). Subsequent experiments showed that the extent of inhibition by R5421 was time-dependent, with 50% inhibition obtained at 20 μmol/L for 60 minutes (Fig 5, insert). The inhibitory effect of R5421 could not be reversed by washing the platelets, suggesting that R5421 is an irreversible inhibitor of the scramblase.
Effect of R5421 on Ca2+-dependent scrambling of NBD-PS in human platelets stimulated with Ca2+-ionomycin. Platelets were preincubated with 50 μmol/L R5421 for 60 minutes. At arrow 1, dithionite (5 mmol/L) was added; at arrow 2, ionomycin (1 μmol/L); at arrow 3, CaCl2 (1 mmol/L); and at arrow 4, Triton X100 (1%). Curve a: control platelets in the absence of R5421, curve b in the presence of R5421. Curve c represents Scott platelets stimulated with Ca2+-ionophore. Insert: effect of preincubation time with R5421 (20 μmol/L) on rate of scrambling.
Effect of R5421 on Ca2+-dependent scrambling of NBD-PS in human platelets stimulated with Ca2+-ionomycin. Platelets were preincubated with 50 μmol/L R5421 for 60 minutes. At arrow 1, dithionite (5 mmol/L) was added; at arrow 2, ionomycin (1 μmol/L); at arrow 3, CaCl2 (1 mmol/L); and at arrow 4, Triton X100 (1%). Curve a: control platelets in the absence of R5421, curve b in the presence of R5421. Curve c represents Scott platelets stimulated with Ca2+-ionophore. Insert: effect of preincubation time with R5421 (20 μmol/L) on rate of scrambling.
Unlike the slightly diminished phosphorylation patterns observed with Scott platelets, incubation of normal platelets with R5421 (100 μmol/L) does not cause appreciable changes in tyrosine phosphorylation patterns obtained on activation with a variety of agonists. However, inhibition of scramblase activity by these high levels of R5421 is not complete (compare curves b and c in Fig 5). If this would be accompanied by a lower extent of inhibition of (a single?) kinase, the effect of this compound may no longer be visible on Western blots.
Of note, as in erythrocytes, aminophospholipid translocase activity in platelets was not affected by R5421 up to a concentration of 100 μmol/L.
In summary, we found that the impaired Ca2+-induced lipid scrambling in Scott syndrome is accompanied by a diminished tyrosine phosphorylation, an effect that was most pronounced in the patients' erythrocytes. We conclude that the apparent deficiency in tyrosine phosphorylation in Scott syndrome cells is an epiphenomenon, possibly associated with defective lipid scrambling, but is not causal to this defect. The data do not exclude the possibility that deficient lipid scrambling in Scott syndrome directly affects tyrosine phosphorylation or that a step required for collapse of lipid asymmetry and tyrosine phosphorylation is aberrant.
Supported in part by Grant No. NHS 93.166 from The Netherlands Heart Foundation (The Hague) (to W.M.J.V.).
The authors wish to dedicate this report to the memory of Mary Ann Scott.
Address reprint requests to Edouard M. Bevers, PhD, Department of Biochemistry, Cardiovascular Research Institute Maastricht, Maastricht University, PO Box 616, 6200 MD Maastricht, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal