Abstract
We investigated the hematopoietic reservoir in 43 severe aplastic anemia (SAA) patients following immunosuppression (IS) (n = 15) or bone marrow transplantation (BMT) (n = 28), at a median interval of 5 years (range, 2-20) from treatment. All patients had normal blood counts, good marrow cellularity, and normal numbers of colony forming unit-granulocyte macrophages (CFU-GM). Burst forming unit-erythroid (BFU-E) and colony forming unit-granulocyte erythroid megakaryocyte macrophages (CFU-GEMM) numbers were reduced when compared with normal controls. However, the most pronounced defect was observed at the level of long-term culture-initiating cells (LTC-IC), which significantly differed from controls (P < .00001) both for IS and BMT patients. Their number did not improve with time and was not affected by transplant or treatment-related variables. When IS patients were compared with BMT we found comparable numbers of CFU-GEMM (P = .8) and LTC-IC (P = .9), but lower numbers of BFU-E and CFU-GM (P = .05 and P = .004, respectively), suggestive of a persistent suppressive mechanism. These data indicate that LTC-IC numbers are severely reduced in BMT and IS patients, contradicting the common belief that the former are fully reconstituted as compared with the latter. In addition, the number of mature cells and committed progenitors does not seem to reflect the real size of the hematopoietic reservoir and few stem cells may be sufficient to guarantee normal hematopoiesis long term.
A STEM CELL DEFECT has been considered for a long time to be the major pathogenetic mechanism resulting in bone marrow failure in patients with severe aplastic anemia (SAA). Early studies using clonogenic cultures demonstrated either a lack or a marked reduction of all types of hematopoietic progenitor cells.1-4 Hematopoietic failure in these patients was also associated with a significant reduction of bone marrow mononuclear cells, phenotypically defined as CD34+ cells and with a lower plating efficiency in comparison with controls.5
These assumptions have been the basis for transplant procedures with syngeneic6 or allogeneic7 normal stem cells considered to be the only curative treatment for this disease. However, hematologic recovery in 50% to 80% of patients following immunosuppression (IS) with anti–lymphocyte-globulin (ALG), cyclosporine A (CyA) with or without growth factors,8-13suggested the possibility that residual stem cells are present in SAA patients and that aplasia may be the result of a toxic or an immune-mediated effect.14,15 Patients recovering after IS therapy exhibit for many years reduced formation of hematopoietic colonies, reduced responsiveness to stem cell factor (SCF) of CD34+ cells, and a decreased survival time in vitro in the presence of FLT3 ligand16 17 despite good marrow cellularity and normal blood counts.
The long-term culture initiating cells (LTC-IC) assay, which is considered the best surrogate method for assessing hematopoietic stem cells allows us to enumerate them with good approximation. The primitive progenitors can be separated, in humans, from the clonogenic compartment with respect to a number of physical and antigenic properties. In the mouse, these progenitor cells can form colonies in vitro with preservation of both their long-term and competitive repopulating ability.18 19
In SAA patients the frequency of these progenitors is markedly reduced when compared with normal controls both using cobblestone area forming cell (CAFC)20 or LTC-IC assays.21
Qualitatively, LTC-IC are also abnormal, showing a profoundly reduced clonogenic capacity that reflects a defect in the supply of mature progenitors from a more primitive cell compartment.21 Both these points may be explained by the concept that stem cell numbers are limited in SAA. In fact, in transplanted mice, the proportion of clones actively contributing to hematopoiesis increases in relation to the size of the donor inoculum and the premature recruitment into mitotic cycle may have a negative effect on the proliferative capacity of stem cell pool.22
This study is an attempt to quantify the hematopoietic reservoir in SAA patients long after IS treatment or allogeneic bone marrow transplantation (BMT), by testing for differences among the two groups of patients in terms of early and committed progenitors.
MATERIALS AND METHODS
Patients
Forty-three SAA patients entered this study: 28 received an unmanipulated HLA-matched sibling transplant, and 15 underwent IS therapy.
Transplanted patients.
There were 18 males and 10 females; median age was 21 years (range, 7 to 36); two patients (UPN 91 and UPN 638) received marrow from a twin. Patients were infused with a median of 4.1 × 108mononuclear cells (MNC)/kg (range, 2.0 to 10.4) and 6.0 × 104 colony forming unit-granulocyte macrophage (CFU-GM)/kg (range, 2.4 to 24: data recovered on 13/28 patients) (Table1). Recipients were prepared for transplant with cyclophospamide (Cy) 200 mg/kg body weight; they did not receive prophylactic growth factors after BMT. Median time from transplant was 5 years (range, 2 to 20).
Bone Marrow Transplant Patients
| UPN . | A/S . | aGvHD . | cGvHD . | CMV . | MNC ×108/kg . | CFU-GM ×104/kg . |
|---|---|---|---|---|---|---|
| 1 | 24/M | 2 | 1 | − | 3.2 | |
| 42 | 15/F | 0 | 0 | − | 10.4 | |
| 52 | 23/M | 2 | 3 | NE | 2.5 | |
| 73 | 7/F | 2 | 1 | − | 4.2 | |
| 91-150 | 22/F | 0 | 0 | − | 2 | |
| 100 | 21/M | 3 | 3 | − | 2.3 | |
| 194 | 9/M | 1 | 0 | − | 2.3 | |
| 324 | 21/F | 1 | 0 | − | 4.3 | |
| 373 | 28/F | 1 | 1 | + | 3.3 | |
| 450 | 26/M | 2 | 3 | − | 2 | |
| 455 | 18/M | 3 | 3 | + | 4.6 | |
| 462 | 14/F | 1 | 1 | − | 3.3 | 3.8 |
| 555 | 36/F | 1 | 1 | − | 4 | |
| 587 | 12/M | 2 | 3 | + | 2.3 | |
| 608 | 34/M | 1 | 1 | − | 3.3 | 5.5 |
| 626 | 20/M | 1 | 1 | − | 3.4 | |
| 638-150 | 19/F | 0 | 0 | + | 5.6 | 6 |
| 639 | 32/M | 2 | 3 | − | 5.6 | 7.6 |
| 657 | 16/M | 0 | 0 | − | 4.1 | 8.5 |
| 682 | 18/F | 0 | 0 | − | 6.1 | |
| 741 | 21/M | 1 | 1 | − | 5.7 | 5.5 |
| 744 | 18/M | 1 | 1 | − | 2.5 | 2.4 |
| 753 | 29/M | 2 | 1 | − | 7.5 | 6.7 |
| 755 | 16/F | 1 | 0 | − | 3.8 | 6.2 |
| 760 | 31/M | 1 | 3 | − | 5.4 | 4.5 |
| 811 | 30/M | 2 | 3 | − | 6 | 24.0 |
| 825 | 21/M | 1 | 1 | + | 7.9 | 3.6 |
| 875 | 23/M | 1 | 1 | − | 6 | 6.6 |
| Median | 21 | 4.1 | 6 | |||
| Range | 7-36 | 2-10.4 | 2.4-24.0 |
| UPN . | A/S . | aGvHD . | cGvHD . | CMV . | MNC ×108/kg . | CFU-GM ×104/kg . |
|---|---|---|---|---|---|---|
| 1 | 24/M | 2 | 1 | − | 3.2 | |
| 42 | 15/F | 0 | 0 | − | 10.4 | |
| 52 | 23/M | 2 | 3 | NE | 2.5 | |
| 73 | 7/F | 2 | 1 | − | 4.2 | |
| 91-150 | 22/F | 0 | 0 | − | 2 | |
| 100 | 21/M | 3 | 3 | − | 2.3 | |
| 194 | 9/M | 1 | 0 | − | 2.3 | |
| 324 | 21/F | 1 | 0 | − | 4.3 | |
| 373 | 28/F | 1 | 1 | + | 3.3 | |
| 450 | 26/M | 2 | 3 | − | 2 | |
| 455 | 18/M | 3 | 3 | + | 4.6 | |
| 462 | 14/F | 1 | 1 | − | 3.3 | 3.8 |
| 555 | 36/F | 1 | 1 | − | 4 | |
| 587 | 12/M | 2 | 3 | + | 2.3 | |
| 608 | 34/M | 1 | 1 | − | 3.3 | 5.5 |
| 626 | 20/M | 1 | 1 | − | 3.4 | |
| 638-150 | 19/F | 0 | 0 | + | 5.6 | 6 |
| 639 | 32/M | 2 | 3 | − | 5.6 | 7.6 |
| 657 | 16/M | 0 | 0 | − | 4.1 | 8.5 |
| 682 | 18/F | 0 | 0 | − | 6.1 | |
| 741 | 21/M | 1 | 1 | − | 5.7 | 5.5 |
| 744 | 18/M | 1 | 1 | − | 2.5 | 2.4 |
| 753 | 29/M | 2 | 1 | − | 7.5 | 6.7 |
| 755 | 16/F | 1 | 0 | − | 3.8 | 6.2 |
| 760 | 31/M | 1 | 3 | − | 5.4 | 4.5 |
| 811 | 30/M | 2 | 3 | − | 6 | 24.0 |
| 825 | 21/M | 1 | 1 | + | 7.9 | 3.6 |
| 875 | 23/M | 1 | 1 | − | 6 | 6.6 |
| Median | 21 | 4.1 | 6 | |||
| Range | 7-36 | 2-10.4 | 2.4-24.0 |
Abbreviations: UPN, unique patient number; A/S, age/sex; aGvHD, acute graft-versus-host disease; cGvHD, chronic graft-versus-host disease; CMV, cytomegalovirus; MNC, mononuclear cells infused; CFU-GM, colony forming unit-granulocyte machrophages infused.
Twins.
IS patients.
There were 11 males and 4 females; median age was 26 years (range, 13 to 53). Each patient received IS therapy as described in Table2; all of them were transfusion independent. Median time from diagnosis was 6 years (range, 2 to 18).
Immunosuppressive Therapy
| PTS . | A/S . | HALG . | RALG . | CyA . | Steroid . | Androgen . | G-CSF . |
|---|---|---|---|---|---|---|---|
| 217-2841 | 23/M | + | + | − | + | − | − |
| 217-2809 | 26/F | + | + | + | − | + | − |
| 217-2802 | 30/F | + | − | − | + | − | − |
| 217-2873 | 40/M | + | − | − | + | − | − |
| 217-2881 | 40/M | − | + | − | + | − | − |
| 217-2967 | 23/M | − | − | + | + | − | − |
| 217-2918 | 53/F | + | − | − | + | − | − |
| 217-2950 | 23/M | − | − | + | − | − | − |
| 217-2960 | 17/M | + | − | + | + | − | + |
| 217-2945 | 45/M | + | − | + | + | − | + |
| 217-2931 | 19/M | + | + | + | − | − | − |
| 796-2001 | 13/M | + | + | + | − | − | + |
| 217-2953 | 18/F | + | + | + | − | − | + |
| 217-2959 | 26/M | + | − | + | + | − | + |
| 217-7802 | 33/M | + | − | + | + | − | + |
| PTS . | A/S . | HALG . | RALG . | CyA . | Steroid . | Androgen . | G-CSF . |
|---|---|---|---|---|---|---|---|
| 217-2841 | 23/M | + | + | − | + | − | − |
| 217-2809 | 26/F | + | + | + | − | + | − |
| 217-2802 | 30/F | + | − | − | + | − | − |
| 217-2873 | 40/M | + | − | − | + | − | − |
| 217-2881 | 40/M | − | + | − | + | − | − |
| 217-2967 | 23/M | − | − | + | + | − | − |
| 217-2918 | 53/F | + | − | − | + | − | − |
| 217-2950 | 23/M | − | − | + | − | − | − |
| 217-2960 | 17/M | + | − | + | + | − | + |
| 217-2945 | 45/M | + | − | + | + | − | + |
| 217-2931 | 19/M | + | + | + | − | − | − |
| 796-2001 | 13/M | + | + | + | − | − | + |
| 217-2953 | 18/F | + | + | + | − | − | + |
| 217-2959 | 26/M | + | − | + | + | − | + |
| 217-7802 | 33/M | + | − | + | + | − | + |
Abbreviations: HALG, horse-antilymphocyte globulin; RALG, rabbit-antilymphocyte globulin; CyA, Cyclosporin A; G-CSF, granulocyte-colony stimulating factor.
Controls
Thirty heparinized marrow samples were obtained from allogeneic donors providing marrow for transplantation, in order to supply both control studies and layers for LTC-IC assay. All donors gave their informed consent. Forty-three long-term survivors after allogeneic BMT (median interval, 6 years; range, 2 to 15) were also tested for in vitro growth. This group of patients consisted of 15 chronic myelogenous leukemia (CML) and 28 acute leukemia (AL) that were prepared for transplant with Cy followed by total body irradiation (TBI).
Cytogenetics
Cytogenetic analysis and standard GTG or QFQ-banding techniques were performed in each case according to standard methods.23
Flow Cytometry Analysis
For the measurement of the expression of GPI-anchored molecules two monoclonal antibodies (MoAb) were used: mouse anti-human PE-conjugated CD14 MoAb (Leu2; Becton Dickinson, Mountain View, CA) with FITC-labeled CD16 Fc-gamma receptor type III (Becton Dickinson).
Clonogenic Assays
Bone marrow mononuclear cells were isolated by centrifugation on 1,077 g/mL Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden) to eliminate the majority of erythrocytes, granulocytes, and platelets. The cells were then washed twice in Iscove's modified Dulbecco's medium (IMDM; MA Product, Walkersville, MD) containing 5% fetal calf serum (FCS; Hyclone, Logan, UT) and resuspended in the same medium. 105MNC were plated in 1.1 mL semisolid culture medium consisting of IMDM supplemented with antibiotics (penicillin/streptomycin, GIBCO, Grand Island, NY), 0.9% methylcellulose (Sigma, St Louis, MO), 30% FCS, 1% crystallized bovine serum albumin (Sigma), 10−4 mol/L mercaptoethanol (2ME, Sigma, final concentration) recombinant human granulocyte macrophage colony-stimulating factor (rhGM-CSF) (10 ng) (Genzyme Corporation, Cambridge, MA), rhIL3 (50 ng) (Sandoz International, Basel CH), rhG-CSF (10 ng) (Hoffman-La Roche, Wyhlen, Germany), and 4U erythropoietin (Genzyme). After 14 days of incubation in a humidified atmosphere at 37°C and 5% CO2, colonies (CFU-GM, BFU-E, and CFU-GEMM) were classified and counted in the same dish using an inverted microscope.
LTC-IC
Aliquots of mononuclear marrow cells (usually 3 × 106MNC/well) were placed in triplicate in 6 well tissue culture dishes (A/S NUNC, Milano) over a murine irradiated cell line (M2-10B4, kindly provided by Dr C. Eaves).24 LTC-IC were maintained for 3 days at 37°C, then switched to 33°C and fed weekly by replacement of half of the growth medium: IMDM + 12.5% FCS + 12.5% Horse Serum (GIBCO) + 10-4 mol/L ME (final concentration) + 10−6 mol/L hydrocortisone-21-hemisuccinate (HC, Sigma, final concentration) + 0.016 mmol/L folic acid (Sigma, final concentration) containing half of the nonadherent cells with fresh growth medium. After 5 weeks, adherent cells were trypsinized and combined with the nonadherent fraction; these cells were then washed and assayed for their CFC content as described above.
Limiting Dilution Assay
The absolute number of LTC-IC was determined using a limiting dilution technique to calculate their proliferative potential.25Briefly, irradiated murine stromal cells were seeded into 96-well flat-bottomed trays; the following day, four dilutions of cells were added to each of 96 wells (total volume 200 μL). A minimum of 12 replicates for each dilution was performed. Cultures were fed at weekly intervals for 5 weeks, then they were overlaid with semisolid culture medium and growth factors as described above. After 18 to 20 days of incubation, wells were examined for the presence of colonies. The frequency of LTC-IC and the mean number of colonies per positive well were calculated by determining the cell dilution that resulted in ≤37% negative wells, equivalent to single hit kinetics (1 LTC-IC/well) according to the Poisson distribution. The clonogenic capacity of a single LTC-IC also was calculated by dividing the numbers of colonies derived from bulk cultures by the frequency of LTC-IC.26 27
Statistical Analysis
Statistical analyses were carried out using a Student's t-test (NCSS package, J.L. Hintze, Kaysville, UT).
RESULTS
At the time of our study all patients were leading a normal life with a Karnowsky score between 80% and 100% and no requirement for blood transfusions.
Clinical Data
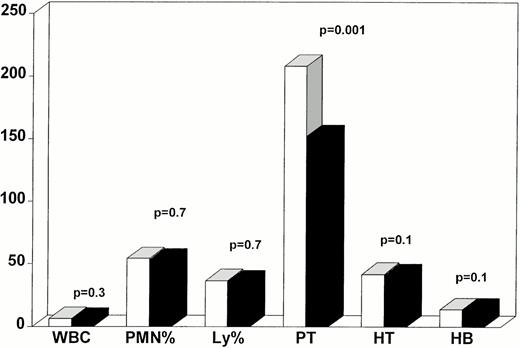
Peripheral blood counts were stable and within normal range in the two groups. Mean WBC numbers were, respectively, for BMT patients 6.9 ± 0.3 × 109/L and 5.6 ± 0.4 × 109/L for IS patients (P = .3). Differential counts were normal and not different in the two groups (BMT, neutrophils 54.2% ± 2.8%, lymphocytes 36.5% ± 2.0%; IS, neutrophils 51.8% ± 2.4%, lymphocytes 36% ± 2.2%). Hematocrit and hemoglobin levels were, respectively, for BMT, 41.5% ± 1.1%–14 ± 0.4 g/dL and for IS, 40.8% ± 1.2%—14 ± 0.5 g/dL (P = .1 andP = .3). Platelet counts were significantly higher in patients who underwent BMT (213 ± 12 v143 ± 12 × 109/L, P = .001) but both values were within normal range (Fig 1).Marrow cellularity ranged between 20% and 100% (median 90%); the three hematologic lineages were well represented and maturation was also normal. Eight of 15 IS patients had evidence of PIG-deficient clones in at least two consecutive determinations. All patients had a normal karyotype at the time of our observations.
Hematopoietic reconstitution in bone marrow transplant (BMT = white bars) and immunosuppression (IS = dark bars) patients. All parameters were within normal range. WBC are expressed × 109/L; PMN are expressed as percentage; Ly are expressed as percentage; platelets (Pt) are expressed × 109/L; hematocrit (HT) is expressed as percentage, hemoglobin (Hb) is expressed g/dL; P values represent differences between BMT and IS patients. (□) BMT; (▪) IS.
Hematopoietic reconstitution in bone marrow transplant (BMT = white bars) and immunosuppression (IS = dark bars) patients. All parameters were within normal range. WBC are expressed × 109/L; PMN are expressed as percentage; Ly are expressed as percentage; platelets (Pt) are expressed × 109/L; hematocrit (HT) is expressed as percentage, hemoglobin (Hb) is expressed g/dL; P values represent differences between BMT and IS patients. (□) BMT; (▪) IS.
Controls
Median laboratory standards were (normal controls, 30): CFU-GM = 58/105 MNC (range, 35 to 198); BFU-E = 12/105MNC (range, 7 to 34); CFU-GEMM = 3/105 MNC (range, 0 to 10); LTC-IC = 34/106 MNC (range, 15 to 238).
BMT Patients
BMT patients grew normal numbers of CFU-GM (P = .5), while significant differences were observed for BFU-E: median 6 (range, 0 to 32) (P = .02) and CFU-GEMM, median 0 (range, 0 to 12) (P = .004) when compared with controls. The reduction of LTC-IC frequency was still more pronounced: median 2 (range, 0 to 16) (P < .00001) (Table 3).
Transplanted Patients
| UPN . | TIME (yr) . | WBC ×109/L . | PT ×109/L . | BM Cellularity % . | CFU-GM/ 105 MNC . | BFU-E/ 105 MNC . | CFU-GEMM/ 105 MNC . | LTC-IC/ 106MNC . |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 4.9 | 189 | 80 | 58 | 15 | 0 | 9 |
| 42 | 16 | 7.8 | 289 | 100 | 38 | 13 | 0 | 3.3 |
| 52 | 15 | 8.9 | 358 | 80 | 130 | 0 | 0 | 6 |
| 73 | 14 | 8.5 | 239 | 60 | 44 | 4 | 0 | 3.3 |
| 91 | 14 | 6.4 | 170 | 100 | 34 | 32 | 0 | 8 |
| 100 | 13 | 5.9 | 198 | 80 | 42 | 15 | 1 | 0.7 |
| 194 | 11 | 6.6 | 243 | 70 | 79 | 11 | 6 | 0 |
| 324 | 9 | 8.7 | 305 | 100 | 95 | 4 | 0 | 0 |
| 373 | 9 | 7.6 | 252 | 90 | 13 | 30 | 2 | 6.7 |
| 450 | 9 | 10.4 | 306 | 100 | 49 | 0 | 0 | 3 |
| 455 | 8 | 4.1 | 176 | 100 | 200 | 0 | 0 | 16 |
| 462 | 7 | 6.8 | 259 | 80 | 59 | 10 | 4 | 0 |
| 555 | 6 | 8.4 | 236 | 80 | 51 | 28 | 12 | 2.1 |
| 587 | 5 | 6.3 | 154 | 100 | 200 | 0 | 0 | 2 |
| 608 | 5 | 6.1 | 134 | 100 | 31 | 8 | 1 | 2 |
| 626 | 5 | 6.3 | 197 | 100 | 55 | 11 | 0 | 0 |
| 638 | 4 | 3.7 | 225 | 80 | 146 | 3 | 0 | 8 |
| 639 | 4 | 6.3 | 102 | 100 | 30 | 9 | 0 | 0 |
| 657 | 4 | 6.5 | 198 | 100 | 34 | 3 | 3 | 1 |
| 682 | 4 | 5.8 | 163 | 80 | 200 | 0 | 0 | 2.1 |
| 741 | 3 | 6 | 246 | 40 | 10 | 3 | 0 | 0 |
| 744 | 3 | 3.3 | 142 | 100 | 145 | 11 | 2 | 10 |
| 753 | 3 | 7.4 | 258 | 100 | 142 | 8 | 0 | 1.2 |
| 755 | 3 | 5 | 188 | 70 | 56 | 10 | 0 | 0 |
| 760 | 3 | 8.3 | 121 | 100 | 17 | 1 | 0 | 1.5 |
| 811 | 3 | 6.7 | 120 | 100 | 58 | 0 | 0 | 3 |
| 825 | 2 | 10.1 | 301 | 90 | 14 | 0 | 0 | 0 |
| 875 | 2 | 10.3 | 204 | 60 | 23 | 0 | 0 | 0.7 |
| Median | 5 | 6.7 | 151 | 95 | 53 | 6 | 0 | 2.1 |
| Range | 2-20 | 3.3-10.4 | 102-358 | 40-100 | 10-200 | 0-32 | 0-12 | 0-16 |
| UPN . | TIME (yr) . | WBC ×109/L . | PT ×109/L . | BM Cellularity % . | CFU-GM/ 105 MNC . | BFU-E/ 105 MNC . | CFU-GEMM/ 105 MNC . | LTC-IC/ 106MNC . |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 4.9 | 189 | 80 | 58 | 15 | 0 | 9 |
| 42 | 16 | 7.8 | 289 | 100 | 38 | 13 | 0 | 3.3 |
| 52 | 15 | 8.9 | 358 | 80 | 130 | 0 | 0 | 6 |
| 73 | 14 | 8.5 | 239 | 60 | 44 | 4 | 0 | 3.3 |
| 91 | 14 | 6.4 | 170 | 100 | 34 | 32 | 0 | 8 |
| 100 | 13 | 5.9 | 198 | 80 | 42 | 15 | 1 | 0.7 |
| 194 | 11 | 6.6 | 243 | 70 | 79 | 11 | 6 | 0 |
| 324 | 9 | 8.7 | 305 | 100 | 95 | 4 | 0 | 0 |
| 373 | 9 | 7.6 | 252 | 90 | 13 | 30 | 2 | 6.7 |
| 450 | 9 | 10.4 | 306 | 100 | 49 | 0 | 0 | 3 |
| 455 | 8 | 4.1 | 176 | 100 | 200 | 0 | 0 | 16 |
| 462 | 7 | 6.8 | 259 | 80 | 59 | 10 | 4 | 0 |
| 555 | 6 | 8.4 | 236 | 80 | 51 | 28 | 12 | 2.1 |
| 587 | 5 | 6.3 | 154 | 100 | 200 | 0 | 0 | 2 |
| 608 | 5 | 6.1 | 134 | 100 | 31 | 8 | 1 | 2 |
| 626 | 5 | 6.3 | 197 | 100 | 55 | 11 | 0 | 0 |
| 638 | 4 | 3.7 | 225 | 80 | 146 | 3 | 0 | 8 |
| 639 | 4 | 6.3 | 102 | 100 | 30 | 9 | 0 | 0 |
| 657 | 4 | 6.5 | 198 | 100 | 34 | 3 | 3 | 1 |
| 682 | 4 | 5.8 | 163 | 80 | 200 | 0 | 0 | 2.1 |
| 741 | 3 | 6 | 246 | 40 | 10 | 3 | 0 | 0 |
| 744 | 3 | 3.3 | 142 | 100 | 145 | 11 | 2 | 10 |
| 753 | 3 | 7.4 | 258 | 100 | 142 | 8 | 0 | 1.2 |
| 755 | 3 | 5 | 188 | 70 | 56 | 10 | 0 | 0 |
| 760 | 3 | 8.3 | 121 | 100 | 17 | 1 | 0 | 1.5 |
| 811 | 3 | 6.7 | 120 | 100 | 58 | 0 | 0 | 3 |
| 825 | 2 | 10.1 | 301 | 90 | 14 | 0 | 0 | 0 |
| 875 | 2 | 10.3 | 204 | 60 | 23 | 0 | 0 | 0.7 |
| Median | 5 | 6.7 | 151 | 95 | 53 | 6 | 0 | 2.1 |
| Range | 2-20 | 3.3-10.4 | 102-358 | 40-100 | 10-200 | 0-32 | 0-12 | 0-16 |
Abbreviations: TIME, time elapsed from bone marrow transplant; WBC, white blood cells count at the time of the study; PT, platelets count at the time of the study.
Graft versus host disease (GvHD) did not influence the number of LTC-IC: patients who had experienced mild or limited acute and chronic GvHD did not have larger numbers of LTC-IC when compared with patients with severe GvHD (P = .5 in both cases). Cytomegalovirus (CMV) infection also did not seem to affect LTC-IC frequency (P = .5).
The cell dose also had no apparent influence; there was no difference in LTC-IC for patients receiving more or less than 4.1 × 108/kg MNC (P = .2) and more or less than 6.0 × 104/kg CFU-GM (P = .4), cut-offs being the median value.
In order to assess whether defective reservoir may be due to underlying disease or to transplant procedure, we also tested 44 long-term survivors transplanted for other malignancies. This group of patients showed the same number of CFU-GM (60 ± 6.2, P = .3) and BFU-E (8.8 ± 0.7, P = .6) than SAA patients. Moreover, similar defective number of CFU-GEMM (1.1 ± 0.3, P = .1) and LTC-IC (2.9 ± 0.6, P = .3) were observed.
IS Patients
CFU-GM numbers were within the normal range (P = .4) but the numbers of BFU-E (median, 0; range, 0 to 39) and CFU-GEMM (median, 0; range, 0 to 3) were significantly lower than in controls (P = .0005 and P = .002). A severe deficiency was observed at the LTC-IC level (median, 1; range, 0 to 23) (P < .00001) Table 4.
IS Patients
| PTS . | TIME (yr) . | WBC ×109/L . | PT ×109/L . | BM Cellularity % . | CFU-GM/ 105 MNC . | BFU-E/ 105 MNC . | CFU-GEMM/ 105 MNC . | LTC-IC/ 105MNC . |
|---|---|---|---|---|---|---|---|---|
| 217-2841 | 18 | 6.5 | 203 | 100 | 52 | 0 | 0 | 23 |
| 217-2809 | 10 | 6.3 | 152 | 100 | 17 | 0 | 0 | 1.9 |
| 217-2802 | 10 | 4.6 | 194 | 70 | 73 | 0 | 0 | 0 |
| 217-2873 | 10 | 4.8 | 242 | 70 | 62 | 0 | 2 | 2.6 |
| 217-2881 | 7 | 4.2 | 130 | 100 | 38 | 3 | 1 | 0 |
| 217-2967 | 6 | 3.6 | 100 | 100 | 100 | 0 | 0 | 0 |
| 217-2918 | 6 | 5 | 136 | 80 | 102 | 5 | 2 | 4.5 |
| 217-2950 | 6 | 4.3 | 116 | 100 | 24 | 0 | 3 | 1 |
| 217-2960 | 5 | 4.2 | 126 | 90 | 23 | 0 | 0 | 0 |
| 217-2945 | 5 | 4.6 | 141 | 70 | 55 | 39 | 2 | 0 |
| 217-2931 | 5 | 7.1 | 131 | 70 | 14 | 0 | 2 | 9 |
| 796-2001 | 3 | 6.0 | 98 | 100 | 39 | 0 | 0 | 2.7 |
| 217-2953 | 2 | 7.3 | 76 | 60 | 13 | 0 | 0 | 0 |
| 217-2959 | 2 | 10.1 | 202 | 20 | 15 | 2 | 0 | 0 |
| 217-7802 | 2 | 6.1 | 103 | 100 | 64 | 4 | 3 | 6.2 |
| Median | 6 | 5 | 131 | 90 | 39 | 0 | 0 | 1 |
| Range | 2-18 | 3.6-10.1 | 76-242 | 20-100 | 13-102 | 0-39 | 0-3 | 0-23 |
| PTS . | TIME (yr) . | WBC ×109/L . | PT ×109/L . | BM Cellularity % . | CFU-GM/ 105 MNC . | BFU-E/ 105 MNC . | CFU-GEMM/ 105 MNC . | LTC-IC/ 105MNC . |
|---|---|---|---|---|---|---|---|---|
| 217-2841 | 18 | 6.5 | 203 | 100 | 52 | 0 | 0 | 23 |
| 217-2809 | 10 | 6.3 | 152 | 100 | 17 | 0 | 0 | 1.9 |
| 217-2802 | 10 | 4.6 | 194 | 70 | 73 | 0 | 0 | 0 |
| 217-2873 | 10 | 4.8 | 242 | 70 | 62 | 0 | 2 | 2.6 |
| 217-2881 | 7 | 4.2 | 130 | 100 | 38 | 3 | 1 | 0 |
| 217-2967 | 6 | 3.6 | 100 | 100 | 100 | 0 | 0 | 0 |
| 217-2918 | 6 | 5 | 136 | 80 | 102 | 5 | 2 | 4.5 |
| 217-2950 | 6 | 4.3 | 116 | 100 | 24 | 0 | 3 | 1 |
| 217-2960 | 5 | 4.2 | 126 | 90 | 23 | 0 | 0 | 0 |
| 217-2945 | 5 | 4.6 | 141 | 70 | 55 | 39 | 2 | 0 |
| 217-2931 | 5 | 7.1 | 131 | 70 | 14 | 0 | 2 | 9 |
| 796-2001 | 3 | 6.0 | 98 | 100 | 39 | 0 | 0 | 2.7 |
| 217-2953 | 2 | 7.3 | 76 | 60 | 13 | 0 | 0 | 0 |
| 217-2959 | 2 | 10.1 | 202 | 20 | 15 | 2 | 0 | 0 |
| 217-7802 | 2 | 6.1 | 103 | 100 | 64 | 4 | 3 | 6.2 |
| Median | 6 | 5 | 131 | 90 | 39 | 0 | 0 | 1 |
| Range | 2-18 | 3.6-10.1 | 76-242 | 20-100 | 13-102 | 0-39 | 0-3 | 0-23 |
IS Versus BMT Patients
When we compared the in vitro growth of IS and BMT patients we observed no difference in CFU-GEMM and LTC-IC numbers (P = .8 andP = .9). However, late progenitors were significantly higher in BMT patients (CFU-GM, P = .004; BFU-E, P = .05).
Limiting Dilution Studies
In our lab each LTC-IC from normal marrow produced a median of 3.0 CFCs (range, 1 to 16) detectable after 5 weeks of culture (no. 12 cases, data not shown). To investigate whether transplanted patients and IS responders had a qualitatively different population, we plated bone marrow cells in limiting dilution as previously described. In transplanted patients (no. 10 cases tested) the median number of CFC derived from each LTC-IC was 3.0 (range, 1.1 to 10), not significantly different from normal controls (P = .2). IS responders (no. 6 cases tested) showed a number of secondary colonies derived from one LTC-IC ranging between 1.0 and 3.3 (median 2.0) that were significantly different from controls (P < .05).
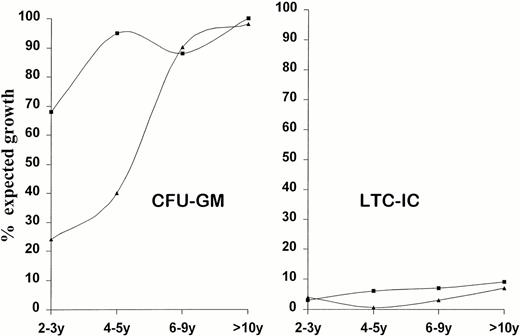
Effect of Time on Hematopoietic Progenitors
The frequencies of CFU-GM and LTC-IC (expressed as percentage of expected growth) were then stratified in different points to study the effect of time elapsed from transplant or from IS therapy on late hematopoietic reconstitution. In IS patients CFU-GM number showed a slow but uniform improvement up to the normal range, which was permanently reached (Fig 2); transplanted patients instead reached and maintained normal values earlier in the first 2 years after transplant (Fig 2). LTC-IC showed the same pattern in the two groups of patients: percentage of expected growth remained far below controls (<10%) and did not improve with time. Two IS patients (no. 217-2809 and no. 217-2945) were studied at different times. Hematologic values were unmodified and within normal range at each time point. The first (217-2809) showed constant low colony formation of both early and late progenitors. The second (217-2945) presented an unexpected normal number of LTC-IC (57.8/106MNC) at the time of an abnormal karyotype (20%: 45X,-Y); BFU-E and CFU-GEMM did not grow. In the following two observations the abnormal clone disappeared (46XY) from the marrow and the LTC-IC number fell to the value expected for aplastic patients. At the same time consistent numbers of BFU-E and CFU-GEMM appeared into the culture.
Effect of time on growth pattern of clonogenic (CFU-GM) and early progenitors (LTC-IC). Patients have been grouped on the basis of treatment (IS or BMT) and time elapsed from. CFU-GM (GM) and LTC-IC (LTC) numbers are expressed as percentage of expected growth; 100% means median laboratory standards (CFU-GM = 58/105 MNC; LTC-IC = 34/106MNC). y = years from treatment. (▴) IS; (▪) BMT.
Effect of time on growth pattern of clonogenic (CFU-GM) and early progenitors (LTC-IC). Patients have been grouped on the basis of treatment (IS or BMT) and time elapsed from. CFU-GM (GM) and LTC-IC (LTC) numbers are expressed as percentage of expected growth; 100% means median laboratory standards (CFU-GM = 58/105 MNC; LTC-IC = 34/106MNC). y = years from treatment. (▴) IS; (▪) BMT.
DISCUSSION
This study demonstrates a severe and long lasting reduction of LTC-IC in the marrow of SAA patients transplanted either from HLA identical siblings or syngeneic twins and in IS responders. In both groups LTC-IC number did not improve with time and were not affected by transplant or treatment-related variables. On the other hand, all SAA patients maintained stable peripheral blood counts, good marrow cellularity and CFU-GM within normal range.
Extremely low LTC-IC numbers, persisting for up to 20 years, may be explained by defective or limited amplification of the stem cell compartment due to the ability of the early hematopoietic progenitors “to count” their divisions and to regulate the production of clonogenic and mature cells when they have reached normal values.28 29 In this way stem cells may protect themselves from exhaustion due to a persistent proliferative stimulus and may allow self-renewal mechanism to take place.
The cell dose and CFU-GM content of the graft did not influence the late hematopoietic reservoir. However, it has been pointed out that a higher degree of stem cell amplification is obtained in the mouse with the smallest number of stem cells transplanted, even though this is insufficient to achieve a comparable level of regeneration of stem cell population by comparison with its size in unperturbed animals.30 On the other hand, recovery of mature blood cell production in vivo may activate negative feedback regulatory mechanism to prematurely limit stem cell self-renewal ability. Other transplant-related variables (CMV infections, GvHD) did not apparently influence late hematopoietic reconstitution.
IS patients had GEMM and LTC-IC frequency not different from transplanted patients but they grew lower number of BFU-E and CFU-GM and had lower platelet counts. These results are in keeping with the fact that proliferative potential of LTC-IC in our SAA patients was below normal. On the other hand, early progenitors may express an intrinsic dysfunction and may give rise only to a limited progeny.31
The different behavior of the two hematopoietic compartments (CFU-GM, LTC-IC) is more evident considering the growth patterns at different times following treatment (BMT or IS). Both groups of patients maintained similar LTC-IC frequency at very low level (10% expected growth) for the entire duration of our observation. The same number of early progenitors, however, gave rise to a progeny of different sizes, in fact in BMT patients clonogenic progenitors reached and maintained normal values within the first 2 years. Instead, IS responders showed a defective CFU-GM growth (20%-40% expected growth) up to 5 years following therapy. Beyond 6 years, CFU-GM numbers were similar in IS and BMT patients, suggesting that the observed growth pattern may be the consequence of a persistent suppressive mechanism limiting the expansion of clonogenic cells, still present a long time after response to therapy. This is in keeping with relapses occurring at discontinuation of CyA in IS patients.32
A persistent suppressive mechanism, associated with a reduced stem cell pool, may also explain late clonal evolution in some SAA patients. In fact, 8 of 15 completely reconstituted patients after immunosuppressive therapy had PIG-negative cells in the blood and one patient (no. 217-2945) occasional karyotypic abnormalities. On the other hand, patients who underwent transplant never developed abnormal clones, suggesting that a reduced stem cell pool does not necessarily imply “stressed hematopoiesis” nor abnormalities of the hematopoietic system. Then, an additional event, such as direct cytotoxic insult to stem cell22 or long-lasting suppression may favor clonal hematologic diseases.
It should be also pointed out that the assay used (LTC-IC) may be unsuitable for the detection and quantification of early progenitors in SAA, thus giving an underestimate of the residual stem cell pool. In fact, one cannot exclude that in SAA patients, stem cells may be kept out of cycle by means of persistent immune-mediated suppression, and this may actually protect them from exhaustion due to excessive proliferation and differentiation. If this is the case, we would detect only early progenitors that have escaped this process without being able to quantify the real size of such population.
Patient no. 217-2945 shows a very interesting pattern of growth; when a cytogenetic abnormality appeared (45X,-Y; 20%) he grew normal number of LTC-IC. This indicates that the LTC-IC compartment of SAA patients may expand as a response to the proliferation of abnormal cell clone; we have observed the same phenomenon in CML in cytogenetic relapse after allogeneic BMT (data not shown). Therefore, the number of early progenitors in SAA may be higher than determined with this assay, or alternatively the ability of LTC-IC to expand may be greater than expected. In keeping with this hypothesis is the fact that mobilized early progenitors greatly exceed in numbers the prediction based on steady state bone marrow analysis.33
Hematologic reconstitution in SAA, whether after BMT or IS, is a process involving a limited number of stem cells that first repopulate marrow spaces and then maintain hematopoiesis long-term. In transplanted patients reconstitution occurs rapidly and the persistent defect at the early progenitor cell level is almost completely masked if only clonogenic cells are evaluated. In IS responders clonogenic growth is defective for many years following therapy despite normal blood counts, possibly due to the continuous effect of a suppressive mechanism. In both cases small numbers of LTC-IC can maintain stable hematopoiesis, suggesting that stem cells are largely in excess under normal conditions and only few of them are active throughout a life time.
Supported by Associazione Italiana per la Ricerca sul Cancro (AIRC), Milano.
Address reprint requests to Marina Podestà, Biol Sci, Divisione Ematologia 2, Ospedale S. Martino, Largo R. Benzi 10, 16132-Genova, Italy.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal