Abstract
We generated monoclonal antibodies against the human Flt3 receptor and used them to study the characteristics of normal human bone marrow cells resolved based on Flt3 expression. Human CD34+ or CD34+lin− marrow cells were sorted into two populations: cells expressing high levels of Flt3 receptor (Flt3high) and cells with little or no expression of Flt3 receptor (Flt3low). Flt3 receptor was detected on a subset of CD34+CD38− marrow cells, as well as on CD34+CD19+ B lymphoid progenitors and CD34+CD14+CD64+ monocytic precursors. Flt3 receptor was also present on more mature CD34−CD14+ monocytes. In colony-forming assays, Flt3high cells gave rise mainly to colony-forming unit–granulocyte-macrophage (CFU-GM) colonies, whereas Flt3low cells produced mostly burst-forming unit-erythroid colonies. There was no difference in the number of multilineage CFU-Mix colonies between the two cell fractions. Cell cycle analysis showed that a large number of the Flt3low cells were in the G0 phase of the cell cycle, whereas Flt3highcells were predominantly in G1. Cell numbers in the suspension cultures initiated with Flt3high cells were maintained in the presence of Flt3 ligand (FL) alone, and increased in response to FL plus kit ligand (KL). In contrast, cell numbers in the suspension cultures started with Flt3low cells did not increase in the presence of FL, or FL plus KL. Upregulation of Flt3 receptor on Flt3low cells was not detected during suspension culture. CD14+ monocytes were the major cell type generated from CD34+lin−Flt3high cells in liquid suspension culture, whereas cells generated from CD34+lin−Flt3low cells were mainly CD71+GlycA+ erythroid cells. These results show clear functional differences between CD34+Flt3high and CD34+Flt3low cells and may have implications concerning the in vitro expansion of human hematopoietic progenitor cells.
PLURIPOTENT HEMATOPOIETIC stem cells are characterized by the ability to self-renew as well as differentiate into various lineage-committed progenitor cells, which in turn produce large numbers of mature blood cells.1 The majority of hematopoietic stem and progenitor cells in the bone marrow are in cell cycle dormancy.1-3 These cells are thought to enter active cell cycle in response to certain cytokines, and subsequent stages of differentiation are associated with upregulation of growth factor receptors on the cell surface.3 4 To date, several hematopoietic growth factor receptors involved in the proliferation and differentiation of early progenitor cells have been identified. Knowledge of the cellular expression of these receptors may make it possible to delineate the earliest signalling events in hematopoiesis and further characterize the pluripotent stem cell.
One such receptor that plays a role in regulating early hematopoiesis is the tyrosine kinase Flt3 (Flk2/STK-1), cloned by three groups including our own.5-8 Flt3 receptor has also been designated CD135.9 It is structurally related to c-kit (kit, CD117), the receptor for kit ligand (KL). By themselves, Flt3 ligand (FL) or KL show only weak proliferative effects.10-12 However, they synergize with each other and various other factors (eg, interleukin-3 [IL-3], IL-6, and granulocyte-macrophage colony-stimulating factor [GM-CSF]) to stimulate proliferation and colony formation of early progenitor cells.10-17 The Flt3 and c-kit receptors share structural features and have similar effects, but clear differences exist between the two receptors and their respective ligands. Both FL and KL induce the proliferation of myeloid progenitors.14-19 However, KL but not FL stimulates mast cells20 and erythroid precursors,21,22 and FL but not KL promotes the expansion of early B lymphoid progenitors23-25 and dendritic cells.26 It has been found that primitive murine hematopoietic progenitors that are quiescent express low levels of kit, whereas actively cycling progenitor cells are kithigh.27,28 Expression of kit is downregulated as progenitor cells mature, resulting in stage-related expression of murine kit receptor.27 Similarly, the majority of murine stem cells residing in the G0 stage of the cell cycle show little or no expression of Flt3 receptor,29 whereas actively cycling murine stem cells have increased Flt3 mRNA and protein expression.29 30
Recently, it has been reported that human CD34+stem/progenitor cells possessing long-term engrafting ability express detectable but low levels of kit receptor.18 Whether Flt3 receptor is expressed on human hematopoietic stem cells is still a matter of controversy. Early studies showed that antisense oligonucleotides directed against the gene for human Flt3 receptor suppressed colony formation by CD34+ progenitor cells in semisolid culture and strongly inhibited the generation of granulo-monocytic colonies from long-term bone marrow cultures.6 More recently, it has been shown that FL alone increases the number of primitive long-term culture-initiating cells in cultures initiated from CD34+CD38−cells,19 fetal liver cells,31 or peripheral blood stem/progenitor cells.32 Taken together, these results indicate that signalling through Flt3 receptor is important in very early human hematopoiesis, possibly involving the earliest stem cells.
In the present study, we used monoclonal antibodies (MoAbs) against the human Flt3 receptor to examine its expression on human CD34+ bone marrow cells. CD34+ progenitor cells were fractionated into two subpopulations: cells expressing high levels of Flt3 receptor (Flt3high) and cells with little or no expression of Flt3 receptor (Flt3low). The results show that CD34+Flt3low cells clearly differ from CD34+Flt3high cells with respect to differentiation potential, cell cycle status, and responsiveness to FL.
MATERIALS AND METHODS
Cells.
Human bone marrow cells were isolated by density-gradient centrifugation (Ficoll-Hypaque; Pharmacia, Piscataway, NJ). Human marrow was obtained from the posterior iliac crests of consenting healthy adults under an Institutional Review Board approved protocol. CD34+ cells were isolated using immunomagnetic microspheres as previously described.33 The purity of the isolated CD34+ cell preparations ranged from 88% to 95%. CD34+ cells were further depleted of more mature cells expressing CD3, CD14, CD15, CD19, and CD71 lineage antigens by using Dynabeads (Dynal, Lake Success, NY) following the manufacturer's instructions. The human hematopoietic cell lines ML-1 and TF-1 and the murine cell line 32D were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) as were TF-1 cells and 32D cells transfected with the gene for human Flt3 receptor (TF-1/S.9; 32D-Flk2). Media for growth of TF-1 and TF-1/S.9 cells also contained 10 ng/mL recombinant human IL-3; media for growth of 32D and 32D-Flk2 cells contained 10 ng/mL recombinant murine IL-3. Parental human 293 cells and 293 cells transfected with human Flt3 receptor (293-Flk2) were maintained in Dulbecco's modified Eagle's medium containing 10% FBS. G418 (0.5 mg/mL) was added to selective media of transfected cell lines.
MoAbs against Flt3 receptor.
For production of MoAbs 2G3.9.14 (IgG2b) and 4A6.6.3 (IgG2a), BALB/c mice were immunized in each rear footpad with either 106 transfected 293-Flk2 cells, or 106 transfected 32D-Flk2 cells on days 0, 7, 14, 27, and 76 or days 0, 3, 7, 10, and 101, respectively. To produce MoAb 1E7.3.2 (IgG1), BALB/c mice were immunized in each rear footpad with 2.5 μg of soluble human Flt3/Flk2-IgG suspended in MPL/TDM adjuvant on days 0, 3, 7, 14, 17, 21, and 122. Four days after the last immunization, lymph nodes were harvested and fused with P3/X63-Ag8U1 myeloma cells34 with 35% polyethyleneglycol as described.35 Hybridoma cell lines secreting antibody specific for human Flt3/Flk2 receptor were assayed either by enzyme-linked immunosorbent assay using CD4-IgG versus Flt3/Flk2-IgG or by flow cytometry using transfected 293-Flk2 or 32D-Flk2 cells versus control cells. Selected hybridomas were cloned twice by limiting dilution then further characterized for their binding capabilities. Ascites was produced in BALB/c mice, and MoAbs were purified on a protein G affinity column. Protein concentration was determined by absorbance at 280 nm using an extinction coefficient of 1.4.
Other antibodies.
Phycoerythrin (PE)-conjugated purified mouse antihuman CD34, CD3 and secondary rat antimouse IgG1, and IgG2a plus IgG2b, as well as Peridinin Chlorophyll Protein (PerCP)-conjugated mouse antihuman CD34 and matching IgG1 isotype control, were purchased from Becton Dickinson (San Jose, CA). Fluorescein isothiocyanate (FITC)-conjugated purified secondary goat antimouse Ig (polyclonal) and CD71 were also obtained from Becton Dickinson. PE-conjugated mouse antihuman CD19, CD45, and CD117 were bought from Pharmingen (San Diego, CA), as were CyChrome-conjugated CD38, CD19, CD64, and IgG isotype controls. PE-conjugated purified mouse antihuman CD14 and CD15 were purchased from Dako (Carpinteria, CA). PE-conjugated and FITC-conjugated isotype control antibodies were obtained from Sigma (St Louis, MO). A pool of polyclonal murine antibodies (containing IgG1, IgG2a, IgG2b, and IgG3 subclasses) used as a negative control was also purchased from Sigma.
Immunophenotyping.
Two- or three-color flow cytometry was performed on a FACSort flow cytometer (Becton Dickinson) using the CellQuest (Becton Dickinson) software for data acquisition and analysis. Instrument alignment and compensation were accomplished using Calibrite beads (Becton Dickinson) following the manufacturer's instructions. Directly conjugated MoAbs were used to stain hematopoietic cells for all antigens except Flt3 receptor on which an indirect staining procedure was used as described previously.36 Briefly, cells were resuspended in immunofluorescence assay (IFA) medium (0.01 mol/L HEPES, 0.15 mol/L NaCl, 0.1% NaN3, 4% bovine serum, pH 7.4) containing 2% human AB+ serum. Indirect staining was performed by adding unconjugated anti-Flt3 receptor antibodies to CD34+ cells for 30 minutes at 4°C. Cells were then washed twice in IFA medium and resuspended in 100 μL of either a PE-conjugated rat or FITC-conjugated goat antimouse IgG secondary antibody diluted 1:50 in IFA medium containing 2% human AB+ serum and either 2% rat or goat serum, respectively. After a 30-minute incubation at 4°C, cells were washed twice, and 15 μg of mouse IgG1(Sigma) was added to each tube to block unsaturated valencies of antimouse IgG.37 After 15 minutes, directly conjugated antibodies were added to the cells for 30 minutes at 4°C, followed by 2 washes with 2 mL of ice-cold IFA medium and fixation with 1% formaldehyde. Determinations of background staining using matching isotype control MoAbs at the appropriate concentrations were performed in parallel in every experiment. For control of indirect staining, a pool of polyclonal mouse antibodies was used as the primary antibody. The same secondary antibody (PE or FITC) used for Flt3 staining was used.
Immunostained cells were analyzed by flow cytometry. For cell sorting, 104 events were acquired from the sample tube and isotype control tube to set gates. Fluorescence-activated cell sorting (FACS) gates were defined to include the 30% brightest Flt3-expressing cells (defined as Flt3high) versus the 30% lowest Flt3-expressing cells (defined as Flt3low) within the CD34+ cell population (see Fig 5).
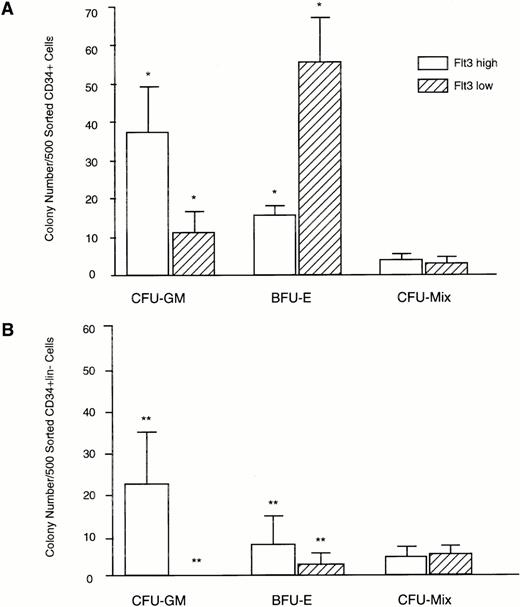
Clonogenic capacity of CD34+Flt3high versus CD34+Flt3low marrow cell subpopulations. Cells were FACS sorted as shown in Fig 4 and plated at a density of 500 cells per culture dish in methylcellulose. Colonies were scored after 14 days of culture. (A) Colony formation from CD34+Flt3high versus Flt3lowcells. Data represent mean ± SD of triplicate cultures from three individual experiments. (B) Colony formation of CD34+lin−Flt3high versus CD34+lin−Flt3low cells. Data represent mean ± SD of triplicate cultures from one experiment. *P < .001; ** P < .004.
Clonogenic capacity of CD34+Flt3high versus CD34+Flt3low marrow cell subpopulations. Cells were FACS sorted as shown in Fig 4 and plated at a density of 500 cells per culture dish in methylcellulose. Colonies were scored after 14 days of culture. (A) Colony formation from CD34+Flt3high versus Flt3lowcells. Data represent mean ± SD of triplicate cultures from three individual experiments. (B) Colony formation of CD34+lin−Flt3high versus CD34+lin−Flt3low cells. Data represent mean ± SD of triplicate cultures from one experiment. *P < .001; ** P < .004.
Clonal colony assays.
Freshly isolated, FACS sorted subsets of CD34+ cells cultured in vitro for 3 to 9 days were plated in 1 mL methylcellulose containing 30% FBS, 1% bovine serum albumin (BSA), 10−4 mol/L 2-mercaptoethanol, 2 mmol/L L-glutamine and human cytokines (Stem Cell Technologies Inc, Vancouver, Canada) in 35 mm2 tissue culture dishes in triplicate and incubated at 37°C, 5% CO2. Human cytokines were present at the following concentrations: KL 50 ng/mL, GM-CSF 20 ng/mL, IL-3 20 ng/mL, IL-6 20 ng/mL, granulocyte colony-stimulating factor (G-CSF) 20 ng/mL, and erythropoietin (Epo) 3 U/mL. After 14 days of culture, plates were scored for the presence of erythroid (BFU-E), granulo-monocytic (CFU-GM), and multilineage (CFU-Mix) colonies by standard morphological criteria using an inverted microscope. To determine the replating efficiency of populations derived from each FACS sorted cell fraction, day-14 colonies were replated into secondary and tertiary clonal cultures. For this, methylcellulose plates were flushed several times with RPMI medium until all cells were removed. Cells were then pooled, washed twice in RPMI to remove residual methylcellulose, counted, and replated in triplicate as described previously. Two types of colonies were scored in these 2o and 3o cultures: “CFU-C,” which were defined as colonies containing ≥50 cells/colony, and “blast clusters,” consisting of 10 to 50 cells/colony.31
Liquid suspension cultures.
For suspension cultures, CD34+ cells sorted on the basis of Flt3 receptor expression were cultured in 24-well tissue culture plates containing RPMI supplemented with 10% FBS and various growth factor combinations for 3 to 9 days. Human growth factors were used at the following concentrations: FL 100 ng/mL (generously provided by Dr Stewart Lyman, Immunex Corp, Seattle, WA), KL 50 ng/mL, IL-3 20 ng/mL, IL-6 20 ng/mL, GM-CSF 20 ng/mL, and Epo 3 U/mL (Amgen, Thousand Oaks, CA). At various timepoints, cells were removed from the wells by vigorous pipetting. After washing, cells were counted by trypan blue dye exclusion, stained, and analyzed for expression of leukocyte differentiation antigens by flow cytometry as described previously. Cells were also plated in methylcellulose (see above). In some experiments, cytocentrifuge slides were prepared and cells were stained with Wright-Giemsa for morphological analysis.
Cell cycle analysis.
CD34+ cell samples were stained and analyzed for simultaneous expression of the proliferation-associated nuclear antigen Ki67, DNA content, and a cell surface antigen (ie, Flt3 receptor) using a modification of the flow cytometric assay described by Jordan et al.38 Briefly, cells were washed after isolation and resuspended in IFA medium. All subsequent steps were carried out at 4°C. Cells were first stained indirectly with MoAbs against Flt3 receptor (or isotype control) as described previously using a PE-conjugated secondary antibody. After the final wash, cells were fixed in 1% formaldehyde (ultra-pure, EM grade; Polysciences, Warrington, PA) in phosphate-buffered saline (PBS) and incubated on ice for 30 minutes. An equal volume of 0.2% Triton X-100 in PBS was added, and samples were left at 4°C overnight for permeabilization. The following day, the fixed and permeabilized samples were washed and resuspended in IFA buffer and stained with anti-Ki67–FITC (Dako Corp, Carpinteria, CA). Finally, cells were washed and resuspended in PBS containing 1% FBS and 0.5 μg/mL 7-actinomycin D (7-AAD) then incubated at 4°C overnight before analysis. Peripheral blood lymphocytes (resting cells) as well as lymphocytes stimulated into cycle with phytohemagglutinin were used as negative and positive controls for Ki67 staining, respectively.39 An isotype-matched, irrelevant control mouse hybridoma supernatant (MOPC 21; Sigma) was used as an additional control.
Replicate samples were stained for Flt3 receptor versus propidium iodide (PI). For this, cell samples were first stained for Flt3 receptor using the same MoAb cocktail and an FITC-conjugated secondary antibody as described previously, washed twice and resuspended in 400-μL ice-cold PBS. Cells were fixed by the addition of 1.2 mL ice-cold 95% ethanol and kept on ice for 20 minutes. Cells were then spun down and resuspended in 1 mL cold PBS containing 0.05 mg/mL PI. DNAse-free RNAse was added to a concentration of 50 μg/mL, and cells were incubated for 30 minutes at room temperature.
Samples were stored at 4°C until analysis by flow cytometry. DNA histograms were analyzed using ModFit LT software (Verity Software House Inc, Sunnyvale, CA) to determine the distribution of cells in G0/G1, S, and G2/M phases of the cell cycle.
Statistical analysis.
Statistical analysis was performed using the Stata software program (Stata Press, College Station, TX). Results are expressed as mean ± standard deviation (SD) or standard error (SE). Results were considered significant if the P value was ≤.05. We used the two-sample Wilcoxon rank-sum test (Mann-Whitney two-sample statistic) to compare clonogenic output of the Flt3high versus Flt3low cell populations.
RESULTS
Specificity of MoAbs against Flt3 receptor.
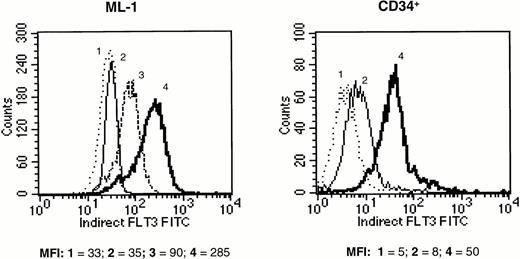
Screened candidate murine monoclonal antihuman Flt3 receptor antibodies, produced and initially identified as described previously, were retested for their ability to specifically bind to Flt3 receptor on the surface of hematopoietic cell lines previously characterized for Flt3 mRNA expression. The human erythroleukemia TF-1 cell line had been previously transfected with the full length human Flt3 receptor. Transfected TF-1 cells (TF-1/S.9) expressed Flt3 receptor by Western blot (data not shown). Because parental TF-1 cells normally express kit, but not Flt3 receptor, staining for kit was used as a positive control for both the transfected and parental TF-1 cells. All 11 monoclonal antihuman Flt3 MoAb clones tested selectively stained the Flt3-transfected TF-1/S.9 cells but not parental TF-1 cells, as determined by flow cytometry. In contrast, antihuman kit MoAbs stained both parental and TF-1/S.9 cells (data not shown). The same anti-Flt3 MoAbs also stained (previously) transfected Flt3-transfected 32D cells, but not parental 32D (murine) cells. This confirmed that the anti-Flt3 MoAbs were specific for Flt3 receptor. Staining intensity was relatively weak, likely because of low expression of Flt3 receptor on the transfected cells. Therefore, the anti-Flt3 MoAb preparations were titered using the human myeloid leukemia ML-1 cell line, which has been shown to express Flt3 receptor at high levels.6 40 Staining with optimal concentrations of single MoAbs was bright on ML-1 cells but weak on human CD34+ cells. Therefore, antibodies were tested in combinations, in an attempt to maximize Flt3 receptor labeling intensity. Three of the antibodies tested (clones 4A6.6.3 [IgG2a], 1E7.3.2 [IgG1], and 2G3.9.14 [IgG2b]) produced additive staining when used in combination, and the cocktail of these three MoAbs produced the highest intensity of staining of any combination, both on ML-1 and CD34+ cells (Fig 1). Therefore, this MoAb cocktail was used to label Flt3 receptor in all subsequent experiments.
Fluorescence intensity of human ML-1 cells (left panel) and purified human CD34+ marrow cells (right panel) labeled with MoAb against Flt3 receptor and stained with FITC-conjugated secondary antibody. (1) Unstained cells (dotted line), (2) cells stained with mouse isotype control antibody (thin solid line), (3) cells stained with a single MoAb against Flt3 receptor (MoAb 4A6.6.3; dashed line, shown only for ML-1 cells), (4) cells stained with a combination of three anti-Flt3 receptor MoAbs (MoAbs 4A6.6.3, 1E7.3.2, and 2G3.9.14; thick solid line). The mean fluorescence intensity (MFI) of each histogram is provided below the panels. The results shown are representative of two experiments.
Fluorescence intensity of human ML-1 cells (left panel) and purified human CD34+ marrow cells (right panel) labeled with MoAb against Flt3 receptor and stained with FITC-conjugated secondary antibody. (1) Unstained cells (dotted line), (2) cells stained with mouse isotype control antibody (thin solid line), (3) cells stained with a single MoAb against Flt3 receptor (MoAb 4A6.6.3; dashed line, shown only for ML-1 cells), (4) cells stained with a combination of three anti-Flt3 receptor MoAbs (MoAbs 4A6.6.3, 1E7.3.2, and 2G3.9.14; thick solid line). The mean fluorescence intensity (MFI) of each histogram is provided below the panels. The results shown are representative of two experiments.
Flt3 receptor expression on normal human marrow mononuclear cells.
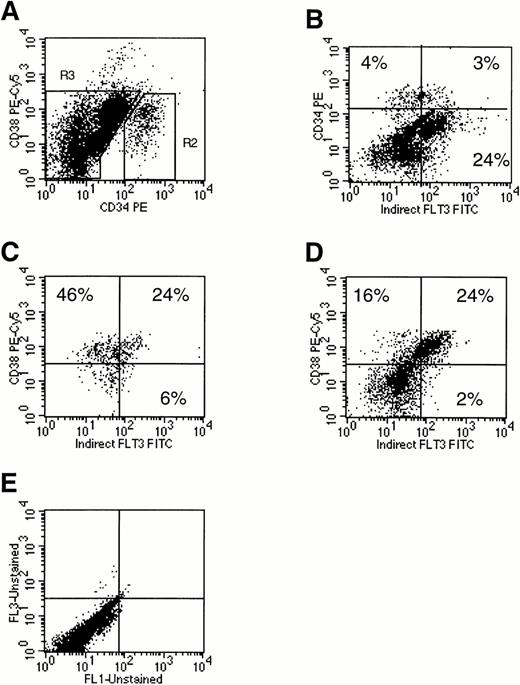
Flt3 receptor was present at very low levels on 32% of human marrow CD34+CD38− cells and at relatively high levels on 52% of CD34+CD38+ cells (Fig 2C: comparison of the left versus right lower quadrants). Among the CD34+ cells, the highest levels of Flt3 receptor were found on cells coexpressing high amounts of CD38. Flt3 receptor was also expressed at moderate to high levels on 75% of the mononuclear cell subsets expressing little or no CD34 but high levels of CD38 (Fig 2D: comparison of the left versus right upper quadrants). Dual light scatter analysis suggested that these CD34−CD38++Flt3high cells were monocytes. Confirming this, marrow mononuclear cells strongly positive for the CD14 monocyte marker coexpressed Flt3 (Fig 3A). Flt3 receptor was not present either on mature CD15+ granulocytes, or on mature CD34−CD19+ B lymphocytes (data not shown). Finally, Flt3 receptor was detected on a small percentage of mature CD34−CD14− cells, which were not further characterized (Figs 2D and 3A).
Analysis of Flt3 receptor expression on human marrow mononuclear cells using three-color flow cytometry. Cells were stained for CD34, CD38, and Flt3 receptor. A scatter gate was first drawn to exclude dead cells (R1, not shown). (A) Expression of CD34 and CD38 on all live marrow mononuclear cells. Two cell populations were defined by gates: (R2) CD34+ and (R3) CD34−. (B) Expression of CD34 and Flt3 receptor on total marrow mononuclear cells. Using the gates set in (A), expression of Flt3 receptor versus CD38 was examined on gated CD34+ cells (C) or CD34−cells (D). Percentages of cells are provided in each plot by quadrant. Quadrants were drawn based on isotype control profiles (E). The results shown are representative of three experiments.
Analysis of Flt3 receptor expression on human marrow mononuclear cells using three-color flow cytometry. Cells were stained for CD34, CD38, and Flt3 receptor. A scatter gate was first drawn to exclude dead cells (R1, not shown). (A) Expression of CD34 and CD38 on all live marrow mononuclear cells. Two cell populations were defined by gates: (R2) CD34+ and (R3) CD34−. (B) Expression of CD34 and Flt3 receptor on total marrow mononuclear cells. Using the gates set in (A), expression of Flt3 receptor versus CD38 was examined on gated CD34+ cells (C) or CD34−cells (D). Percentages of cells are provided in each plot by quadrant. Quadrants were drawn based on isotype control profiles (E). The results shown are representative of three experiments.
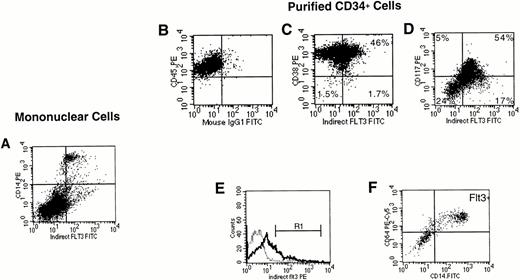
FACS analysis of Flt3 labeling of unfractionated marrow mononuclear cells and purified CD34+ cells. (A) Expression of Flt3 receptor versus CD14 on unfractionated marrow mononuclear cells. Cells were first gated to exclude dead cells (not shown). Quadrants were drawn as shown in (B). In the results of immunostaining of purified CD34+ cells, expression of Flt3 receptor was correlated with (C) CD38, or (D) CD117 (kit). For (C) and (D), a CD34+ cell gate had been set first (not shown). (E) A blast gate was set (not shown), then a gate was set on the brightest Flt3+ cells (R1). Expression of CD14 and CD64 on CD34+ cells gated on R1 (F). The results shown are representative of two (A) or three (B to F) experiments.
FACS analysis of Flt3 labeling of unfractionated marrow mononuclear cells and purified CD34+ cells. (A) Expression of Flt3 receptor versus CD14 on unfractionated marrow mononuclear cells. Cells were first gated to exclude dead cells (not shown). Quadrants were drawn as shown in (B). In the results of immunostaining of purified CD34+ cells, expression of Flt3 receptor was correlated with (C) CD38, or (D) CD117 (kit). For (C) and (D), a CD34+ cell gate had been set first (not shown). (E) A blast gate was set (not shown), then a gate was set on the brightest Flt3+ cells (R1). Expression of CD14 and CD64 on CD34+ cells gated on R1 (F). The results shown are representative of two (A) or three (B to F) experiments.
Flt3 receptor expression on purified CD34+ and CD34+lin− marrow cells.
As was observed above in analyses of unfractionated mononuclear cells, analysis of purified stem/progenitor cells showed that Flt3 receptor was clearly present, albeit at low levels, on a subset of CD34+CD38+ cells (Fig 3C). Fifty-three percent of cells in the CD34+CD38− cell fraction expressed Flt3 receptor (ie, in Fig 3C, comparison of the lower quadrants showed that a total of 1.7% of the CD34+ cells had the phenotype CD34+CD38−Flt3+, whereas 1.5% were CD34+CD38−Flt3−). Of the CD34+CD38+ cells, 46% expressed Flt3 receptor. Those cells exhibiting the highest level of Flt3 expression also stained brightest for CD38. CD34+ cells that were low or negative for kit were also low or negative for Flt3 receptor, whereas the brightest kit+ cells were Flt3++. However, those CD34+ cells staining most intensely for Flt3 receptor had an intermediate level of kit fluorescence (Fig 3D). This population exhibited the light scattering properties of monocytic cells. Coexpression of CD14 and CD64 confirmed that these CD34+Flt3+ cells were monocytic precursors (Fig3E and F). CD34+Flt3+kit+ cells expressed low levels of CD71, whereas very high levels of CD71 were observed on a fraction of CD34+Flt3−kit+ cells, suggesting that they were committed erythroid precursors (data not shown). Flt3 receptor was also detected on a subset of CD34+CD19+ B lymphoid precursors (data not shown).
We further studied the expression of Flt3 receptor on CD34+bone marrow cells further depleted of the relatively mature cells expressing lineage antigens (ie, CD3, CD14, CD15, CD19, and CD71), hereafter referred to as CD34+lin− cells. The lineage-depleted cells were highly purified (97% CD34+lin−), and enriched in CD34+CD38− cells (5.2% in the CD34+lin− versus 2.9% in the CD34+ cell population, data not shown). There were no cells expressing CD14, CD15, CD19, or CD3 detectable in the CD34+lin− cell fraction (data not shown). CD71 was expressed in low amounts on some CD34+lin− cells. Lineage depletion did not change the percentage of cells with the phenotype CD34+CD38−Flt3+significantly, but the percentage of cells that were CD34+CD38−Flt3−increased from 1.5% to 3.5% (ie, 64% of the cells in the CD34+CD38−lin− cell population were Flt3− compared with 47% of CD34+CD38− cells, data not shown). In the CD34+CD38+ cell population, the expression of Flt3 receptor did not change significantly.
FACS sorting of Flt3high versus Flt3lowCD34 and CD34+lin− bone marrow cells.
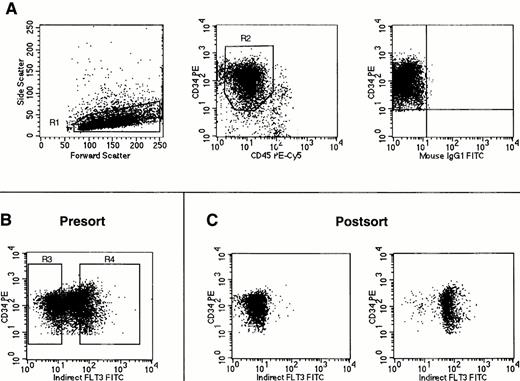
CD34+ or CD34+lin− bone marrow cells were sorted by flow cytometry into two populations based on expression of Flt3 receptor. After gating on CD34+ cells with low side light scatter and low to moderate forward light scatter properties (“blast gate”), sorting gates were set to obtain the 30% brightest Flt3+ cells (operationally defined as Flt3high) and the 30% of cells negative or dimmest for Flt3 receptor (Flt3low) within the total CD34+population (Fig 4). The cell population expressing intermediate amounts of Flt3 was not obtained. For each population obtained by FACS sorting, a small number of cells was reanalyzed by flow cytometry (Fig 4C). Dual light scatter analysis of the reanalyzed cell populations showed that the Flt3lowcells were consistently smaller than the Flt3high cells. This difference in cell size was confirmed by Wright-Giemsa staining of cytospins, which in addition showed that CD34+Flt3high cells also had a broader rim of cytoplasm than CD34+Flt3low cells (data not shown).
FACS sorting of Flt3low and Flt3high CD34+ cells. Cells were stained using antibodies against CD34, CD45, and Flt3 receptor. (A) Gates were first set on light scattering (R1) and fluorescence (R2) to acquire only CD34+ cells with low side light scatter and low to moderate forward light scatter properties (“blast gate”) with the isotype control staining for these cells shown in the rightmost panel. (B) With gates R1 and R2 activated, sorting gates were set for acquistion of CD34+ cells negative or dimmest for Flt3 receptor (Flt3low = R3) and CD34+ cells brightest for Flt3 receptor (Flt3high = R4). (C) Reanalysis of CD34+ cells obtained after FACS sorting for Flt3 receptor expression using the gates shown in (B). No gates were activated for this reanalysis. Cells obtained by gating on R3 (Flt3low) are shown in the left plot, and cells obtained by gating on R4 (Flt3high) are shown on the right. The cell staining profiles in each FACS sorting experiment performed were similar to those shown here. Gates R3 and R4 were consistently drawn to each contain 30% of cells within the total population defined by gates R1 and R2.
FACS sorting of Flt3low and Flt3high CD34+ cells. Cells were stained using antibodies against CD34, CD45, and Flt3 receptor. (A) Gates were first set on light scattering (R1) and fluorescence (R2) to acquire only CD34+ cells with low side light scatter and low to moderate forward light scatter properties (“blast gate”) with the isotype control staining for these cells shown in the rightmost panel. (B) With gates R1 and R2 activated, sorting gates were set for acquistion of CD34+ cells negative or dimmest for Flt3 receptor (Flt3low = R3) and CD34+ cells brightest for Flt3 receptor (Flt3high = R4). (C) Reanalysis of CD34+ cells obtained after FACS sorting for Flt3 receptor expression using the gates shown in (B). No gates were activated for this reanalysis. Cells obtained by gating on R3 (Flt3low) are shown in the left plot, and cells obtained by gating on R4 (Flt3high) are shown on the right. The cell staining profiles in each FACS sorting experiment performed were similar to those shown here. Gates R3 and R4 were consistently drawn to each contain 30% of cells within the total population defined by gates R1 and R2.
Clonogenic capacity of FACS sorted Flt3high versus Flt3low populations.
FACS sorted CD34+Flt3high versus CD34+Flt3low cells were plated in hematopoietic colony-forming assays. CD34+Flt3high cells gave rise primarily to granulo-monocytic colonies (38 ± 13 CFU-GM, 16 ± 4 BFU-E per 500 sorted cells, P < .001), whereas CD34+Flt3low cells formed mostly erythroid colonies (12 ± 7 CFU-GM, 56 ± 12 BFU-E per 500 sorted cells,P < .001; Fig 5). There was no significant difference in the numbers of more primitive multilineage colonies from CD34+Flt3high versus CD34+Flt3low cells (4 ± 1 and 3 ± 2 CFU-Mix per 500 sorted cells, respectively, P > .1).
In a separate experiment (Fig 5B), the FACS sorted CD34+lin−Flt3high cell population produced more granulo-monocytic colonies than erythroid colonies (23 ± 13 CFU-GM, 8 ± 6 BFU-E per 500 sorted cells,P < .004). In the CD34+lin−Flt3low population, few colonies were seen (0 CFU-GM, 2 ± 2 BFU-E per 500 sorted cells,P < .004). There was no significant difference in the number of CFU-Mix in the CD34+lin+Flt3highversus Flt3low populations (4 ± 4 CFU-Mix in the Flt3low fraction, 4 ± 2 CFU-Mix in Flt3highcell fraction per 500 cells, P > .1).
In three experiments performed starting with CD34+lin− cells, colonies formed from CD34+lin−Flt3low versus CD34+lin−Flt3high cells after 14 days in methylcellulose were replated into secondary and tertiary cultures. The Flt3high and Flt3low cell populations formed approximately the same number of blast clusters in 2o culture (6 ± 7 blast clusters from Flt3low, 11 ± 6 from Flt3high cells,P = .07). In 3o culture, the number of blast clusters generated was very low (1 ± 1 blast clusters from Flt3low, 3 ± 1 from Flt3high cells,P = .07). However, all the plates contained many single blast cells, which were too numerous to be enumerated. There was no significant difference in the number of CFU-C formed in 2oculture (17 ± 10 CFU-C from Flt3low, 23 ± 10 from Flt3high cells, P = .1) or 3o culture (2 ± 2 colonies from Flt3low, 3 ± 1 colonies from Flt3high, P = .7).
Cell cycle analysis of FACS sorted cell populations.
We used surface, intracellular, and DNA (SID) analysis to examine the cell cycle status of purified human CD34+ and CD34+lin− bone marrow cells with respect to Flt3 receptor expression. This assay, as described by Jordan et al,38 allows for discrimination between G0 and G1 stages of the cell cycle. Samples were stained for Ki67 antigen, which is not expressed in quiescent cells but is upregulated when cells enter cell cycle. Simultaneous staining with 7-amino actinomycin D (7-AAD; Molecular Probes, Eugene, OR) was used to measure cellular DNA content. Using these two parameters in combination with cell surface staining for Flt3 receptor, all phases of the cell cycle were delineated for each cell population of interest.
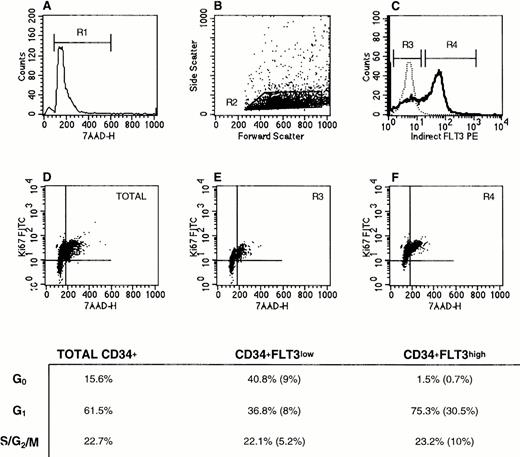
As shown for a representative experiment in Fig 6, SID analysis showed that CD34+Flt3low cells had a greater percentage of cells in G0 (41%) compared with Flt3high cells (1% in G0). The percent cells in G1 was larger in the Flt3high population than in the Flt3lowcell fraction (75% versus 37%, respectively). There were no appreciable differences in the percentage of cells in S/G2/M phases in these three subpopulations (total CD34+ versus Flt3high versus Flt3low). However, the proportion of the CD34+cells that were Flt3high and in G2/S/M phases was twice as high as those that were Flt3low and in G2/S/M (10% versus 5%).
Cell cycle status of CD34+Flt3high versus CD34+Flt3low cells. (A) Based on 7-AAD labeling, a gate was set to exclude dead cells (R1). (B) Next, a “blast gate” was set (R2). (C) With both R1 and R2 activated, gates were drawn to discriminate Flt3low (R3) from Flt3high (R4) cells. Histograms of Flt3 receptor (bold line) and IgG isotype control (dashed line) staining are shown. The plots in the lower panels show the Ki67 versus 7-AAD staining profiles for (D) total CD34+ cells, (E) CD34+Flt3low, and (F) CD34+Flt3high subpopulations. Cells in the lower left quadrant of each plot are defined as being in G0, cells in the upper left quadrant as G1, and cells in the upper right quadrant as G2, S, or M phases of the cell cycle. For the three surface marker–defined subpopulations, the percentage of cells in each phase of the cell cycle is provided in the table. The numbers in parentheses represent the percentages of cells with the given immunophenotype and cell cycle stage within the total CD34+ cell population. This experiment was performed three times with CD34+ cells and twice with CD34+lin− cells. Staining profiles were similar each time.
Cell cycle status of CD34+Flt3high versus CD34+Flt3low cells. (A) Based on 7-AAD labeling, a gate was set to exclude dead cells (R1). (B) Next, a “blast gate” was set (R2). (C) With both R1 and R2 activated, gates were drawn to discriminate Flt3low (R3) from Flt3high (R4) cells. Histograms of Flt3 receptor (bold line) and IgG isotype control (dashed line) staining are shown. The plots in the lower panels show the Ki67 versus 7-AAD staining profiles for (D) total CD34+ cells, (E) CD34+Flt3low, and (F) CD34+Flt3high subpopulations. Cells in the lower left quadrant of each plot are defined as being in G0, cells in the upper left quadrant as G1, and cells in the upper right quadrant as G2, S, or M phases of the cell cycle. For the three surface marker–defined subpopulations, the percentage of cells in each phase of the cell cycle is provided in the table. The numbers in parentheses represent the percentages of cells with the given immunophenotype and cell cycle stage within the total CD34+ cell population. This experiment was performed three times with CD34+ cells and twice with CD34+lin− cells. Staining profiles were similar each time.
Replicate samples were stained for Flt3 versus PI. Analysis of PI staining was confirmatory (data not shown).
In vitro expansion of FACS sorted cells in response to FL.
Based on the cell cycle analyses, it was of interest to determine whether Flt3high versus Flt3low cells proliferate in response to FL, and whether Flt3lowcells upregulate expression of Flt3 receptor. FACS sorted CD34+lin−Flt3high versus CD34+lin−Flt3low cells were incubated in RPMI medium containing 10% FBS and various growth factor combinations. Figure 7 shows the cell counts in these cultures over 9 days in three independent experiments.
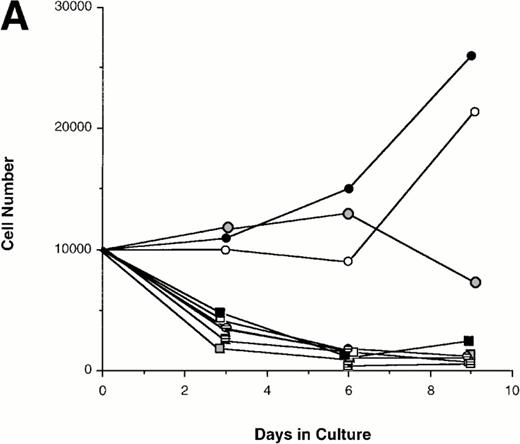
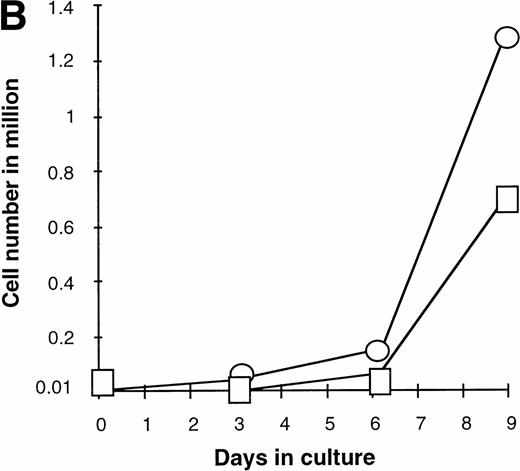
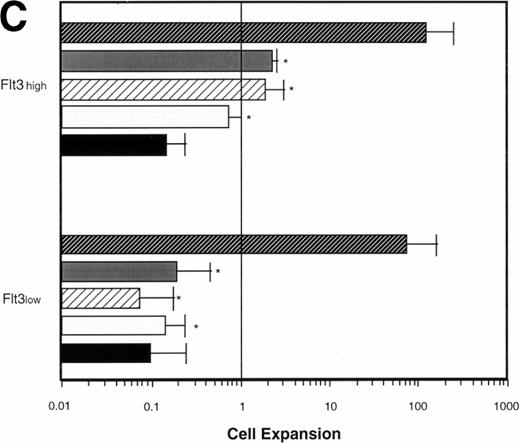
Effect of various cytokines on the survival and proliferation of FACS sorted CD34+lin−Flt3high versus CD34+lin−Flt3low cells in liquid suspension culture. Ten thousand Flt3high or Flt3low cells were cultured in RPMI containing 10% FBS and the designated cytokines, as described in Materials and Methods. Cells were counted by trypan blue exclusion. (A) and (B) time course of proliferation. Numbers shown are mean values of three independent experiments. The combination of FL plus five growth factors is shown separately in (B), because it would be off-scale in (A). (C) Fold expansion of sorted cells after 9 days in suspension culture in the same three experiments. The bars show cell expansion as the mean number of live cells per input cell. (A) (▤) Flt3 low; (▩) Flt3 low + FL; (□) Flt3 low + FL + KL; (▪) Flt3 low FL + KL + IL-3; (⦵) Flt3 high; (◍) Flt3 high FL; (○) Flt3 high FL + KL; (•) Flt3 high FL + KL + IL-3. (B) (□) Flt3 low; (○) Flt3 high. (C) (▨) FL + KL, IL-3 + GM-CSF + Epo; (▩) FL + KL + IL-3; (▨) FL + KL; (▧) FL; (▪) medium only. Error bars indicate the standard errors.
Effect of various cytokines on the survival and proliferation of FACS sorted CD34+lin−Flt3high versus CD34+lin−Flt3low cells in liquid suspension culture. Ten thousand Flt3high or Flt3low cells were cultured in RPMI containing 10% FBS and the designated cytokines, as described in Materials and Methods. Cells were counted by trypan blue exclusion. (A) and (B) time course of proliferation. Numbers shown are mean values of three independent experiments. The combination of FL plus five growth factors is shown separately in (B), because it would be off-scale in (A). (C) Fold expansion of sorted cells after 9 days in suspension culture in the same three experiments. The bars show cell expansion as the mean number of live cells per input cell. (A) (▤) Flt3 low; (▩) Flt3 low + FL; (□) Flt3 low + FL + KL; (▪) Flt3 low FL + KL + IL-3; (⦵) Flt3 high; (◍) Flt3 high FL; (○) Flt3 high FL + KL; (•) Flt3 high FL + KL + IL-3. (B) (□) Flt3 low; (○) Flt3 high. (C) (▨) FL + KL, IL-3 + GM-CSF + Epo; (▩) FL + KL + IL-3; (▨) FL + KL; (▧) FL; (▪) medium only. Error bars indicate the standard errors.
Cultures of CD34+lin−Flt3highcells had approximately constant cell numbers for 6 days in the presence of FL alone, but cell numbers declined by day 9. In the presence of FL and KL, cell numbers were maintained for 6 days then increased twofold from day 6 to day 9 of culture. The addition of IL-3 enhanced the effects of FL and KL, with moderate cell expansion at 6 days of culture and a 2.5-fold increase in cell number at day 9. In contrast, the numbers of cells in the cultures of CD34+lin−Flt3low cells fell rapidly in the presence of FL alone, FL plus KL, or FL plus KL plus IL-3 (Fig 7A and C).
Extensive proliferation was observed when either CD34+lin−Flt3high and Flt3low cells were cultured in the combination of FL plus five other growth factors (GFs; KL, IL-3, IL-6, GM-CSF, and Epo). Although the cultures of CD34+lin−Flt3low cells had a slightly longer initial lag phase, cultures of both subpopulations had expanded 100-fold after 9 days of culture (Fig 7B and C).
Cell surface antigen expression of cells generated in suspension culture.
We also examined the cell surface antigen expression of cells produced in the above suspension cultures (Table 1). CD34+lin−Flt3high cells cultured in FL plus five GFs developed predominantly into CD14+ monocytes, although a few erythroid cells were also detected. The expression of Flt3 receptor gradually decreased over time in cultures of Flt3high cells, but a population of cells expressing Flt3 receptor at moderate levels was still present after 9 days in culture. A similar pattern of downregulation was observed for kit.
Predominant Immunophenotypes of Flt3highVersus Flt3low CD34+lin−Populations in Suspension Culture
| Cytokines . | Day 3 . | Day 6 . | Day 9 . | |||
|---|---|---|---|---|---|---|
| Flt3high . | Flt3low . | Flt3high . | Flt3low . | Flt3high . | Flt3low . | |
| FL | ND-150 | ND | ND | ND | Flt3++c-kit++ | ND |
| Flt3+c-kit+ | ||||||
| FL + KL | ND | ND | ND | ND | Flt3++c-kit++ | ND |
| Flt3+c-kit+ | ||||||
| FL + 5 GF-151 | Flt3++c-kit++ | Flt3−c-kit++ | Flt3+c-kit+ | Flt3−c-kit+ | Flt3+c-kit+ | Flt3−c-kit+ |
| -152 | -152 | Flt3+CD14+ | Flt3−CD14− | Flt3−c-kit− | Flt3−c-kit− | |
| Flt3+CD71+ | Flt3−CD71++ | Flt3+CD14+ | Flt3−CD14− | |||
| CD71+GlyA− | CD71++GlyA+ | |||||
| Cytokines . | Day 3 . | Day 6 . | Day 9 . | |||
|---|---|---|---|---|---|---|
| Flt3high . | Flt3low . | Flt3high . | Flt3low . | Flt3high . | Flt3low . | |
| FL | ND-150 | ND | ND | ND | Flt3++c-kit++ | ND |
| Flt3+c-kit+ | ||||||
| FL + KL | ND | ND | ND | ND | Flt3++c-kit++ | ND |
| Flt3+c-kit+ | ||||||
| FL + 5 GF-151 | Flt3++c-kit++ | Flt3−c-kit++ | Flt3+c-kit+ | Flt3−c-kit+ | Flt3+c-kit+ | Flt3−c-kit+ |
| -152 | -152 | Flt3+CD14+ | Flt3−CD14− | Flt3−c-kit− | Flt3−c-kit− | |
| Flt3+CD71+ | Flt3−CD71++ | Flt3+CD14+ | Flt3−CD14− | |||
| CD71+GlyA− | CD71++GlyA+ | |||||
Ten thousand CD34+lin− bone marrow cells sorted into Flt3high and Flt3low subpopulations were cultured in the presence of the designated cytokines. On day 3, 6, and 9 of culture, cells were analyzed for the expression of leukocyte differentiation antigens by flow cytometry.
Abbreviations: ND, not determined; GF, growth factors.
Values are not determinable because of low cell number (see Fig 8).
Growth factors: KL, IL-6, IL-3, GM-CSF, and Epo.
The presence of other leukocyte differentiation antigens could not be examined because of low cell number.
When CD34+lin−Flt3high cells were cultured in FL alone, or in FL plus KL, most cells still expressed Flt3 and kit receptors after 9 days. In fact, a subset of these cells remained strongly positive for Flt3 and kit receptor. CD34+cells were not detected after 9 days with any of the GF combinations used.
The numbers of cells in the cultures initiated with CD34+lin−Flt3low cells decreased or did not increase to a significant extent when cultured with FL alone or FL plus KL, making it impossible to assess the phenotype of these small numbers of cells. CD34+lin−Flt3low cells cultured in FL plus five GFs produced mainly erythroid cells, which were kit+, CD71++, and Glycophorin A++. CD14 was not detected on any cells generated from CD34+lin−Flt3low cells. Interestingly, upregulation of Flt3 receptor was not observed on cells in cultures started with Flt3low cells by using any combination of cytokines at any of the timepoints examined. The expression of Flt3 receptor on cultured CD34+lin−Flt3low cells remained low to negative, whereas the expression of kit receptor decreased from high levels at day 3 to moderate or negative levels at day 9.
Clonogenic capacity of CD34+lin−Flt3highversus Flt3low marrow cells after suspension culture.
Cells generated in suspension cultures in the experiments above were assayed in colony-forming assays (Table 2). The total numbers of blast cluster-forming cells (see Methods for definition31) increased from day 6 to day 9 of suspension culture in the combination of FL plus five GFs for both the initially cultured Flt3high (sixfold) and Flt3low(twofold) cells. The total numbers of CFU-C (see Methods for definition31) formed from the cultured cells derived from both the CD34+lin−Flt3high and Flt3low cell populations increased dramatically (27-fold for Flt3high and 37-fold for Flt3low cells) from day 0 to day 6. At day 9, the total numbers of CFU-C were still 13-fold greater than initial levels for the Flt3high and threefold for the Flt3low cultures.
Clonogenic Capacity of CD34+lin−Flt3high Versus Flt3low Marrow Cells After Suspension Culture
| Cytokines . | Day 6 . | Day 9 . | ||||||
|---|---|---|---|---|---|---|---|---|
| Flt3high . | Flt3low . | Flt3high . | Flt3low . | |||||
| Clusters . | CFU-C . | Clusters . | CFU-C . | Clusters . | CFU-C . | Clusters . | CFU-C . | |
| FL | ND | ND | ND | ND | 1500 | 1500 | 11 | 8 |
| FL + KL | ND | ND | ND | ND | 21,000 | 840 | 49 | 84 |
| FL + 5 GF* | 868 | 31,640 | 730 | 19,856 | 5,200 | 15,600 | 1,410 | 1,833 |
| Cytokines . | Day 6 . | Day 9 . | ||||||
|---|---|---|---|---|---|---|---|---|
| Flt3high . | Flt3low . | Flt3high . | Flt3low . | |||||
| Clusters . | CFU-C . | Clusters . | CFU-C . | Clusters . | CFU-C . | Clusters . | CFU-C . | |
| FL | ND | ND | ND | ND | 1500 | 1500 | 11 | 8 |
| FL + KL | ND | ND | ND | ND | 21,000 | 840 | 49 | 84 |
| FL + 5 GF* | 868 | 31,640 | 730 | 19,856 | 5,200 | 15,600 | 1,410 | 1,833 |
CD34+lin− marrow cells sorted into Flt3high and Flt3low subpopulations were cultured at a density of 104 cells/well in 24-well dishes in the presence of the designated cytokines at concentrations described in Materials and Methods. On days 6 and 9 of culture, 500 cells from each well were plated in methylcellulose in triplicates. The values shown are the total numbers of clusters and CFU-C (as defined in Material and Methods31) per suspension culture. On day 0 (before the start of the suspension cultures), there were 1,172 CFU-C in the Flt3high and 536 CFU-C in the Flt3lowsubpopulations; blast colonies were not determined.
Abbreviation: ND, Not determined.
GF, growth factors: KL, IL-6, IL-3, GM-CSF, Epo.
In contrast, when Flt3high cells were cultured in FL alone for 9 days, the total numbers of CFU-C did not change significantly from day 0 to day 9. The number of CFU-C produced by initially Flt3high cells cultured in FL plus KL was lower than with FL alone, but the formation of blast clusters from initially Flt3high cells was increased 14-fold by the additional presence of KL in the suspension culture.
The production of CFU-C and clusters from initially Flt3lowcells cultured in FL alone or FL plus KL declined significantly from day 0 to day 9. This paralleled the decline in the total cell numbers in these cultures. Both the numbers of CFU-C and clusters from initially Flt3low cells were increased in the presence of FL plus KL in contrast to the cultures containing FL alone (10-fold and 4-fold, respectively).
DISCUSSION
In this study, we assessed the phenotype and biology of normal human bone marrow CD34+ cells expressing high versus low levels of Flt3 receptor. Previously published results have suggested that Flt3 receptor is present on all human CD34+ cells.16However, biotinylated FL, not antibody, was used to label cells in that study, and staining intensity was low. The production of MoAbs against the human Flt3 receptor enabled us to label Flt3+ versus Flt3− cells and to FACS sort CD34+progenitor cells into two subpopulations: cells expressing high levels of Flt3 receptor (Flt3high) and cells with little or no expression of Flt3 receptor (Flt3low). Cells expressing intermediate levels of Flt3 were not studied, because our goal was to compare cell subsets that differed distinctly in Flt3 expression levels rather than to comprehensively study the entire spectrum of marrow cell types.
Hematopoietic colony-forming cell assays showed a clear difference between Flt3high and Flt3low populations. CD34+Flt3high cells contained mainly granulo-monocytic colonies, whereas CD34+Flt3low cells contained erythroid colonies almost exclusively. This observation is in line with previous studies showing that FL has no effect on human erythropoiesis.11,12,16 Flt3 was detected on monocytic precursors (CD34+CD64+CD14+) and B lymphoid precursors (CD34+CD19+), as well as on more mature monocytic cells (CD34−CD38+CD14+). This agrees with reports that FL induces proliferation and differentiation of monocytic precursors.32 Furthermore, cells generated from CD34+lin−Flt3high cells in liquid culture were predominantly CD14+ monocytes, whereas cells generated from CD34+lin−Flt3low cells were erythroid (CD71+, Glycophorin A+). The detection of Flt3 receptor on mature CD34−CD14+ cells is in apparent contrast to earlier results from our laboratory, in which Flt3 mRNA was not detected in bone marrow cells that had been twice (doubly) depleted of CD34+ cells.6 It is possible that, as progenitor cells mature, Flt3 protein is still expressed on the cell surface, although Flt3 mRNA has already declined to undetectable levels. Alternatively, the discrepancy may be caused by differences in sensitivity of the methods used, or monocytic cells may have been nonspecifically depleted in the prior study.
It has been postulated that Flt3 receptor is a marker for hematopoietic stem cells.5-8 Although we could detect Flt3 receptor on a subset of CD34+CD38− cells, a population enriched for primitive stem/progenitor cells, the staining intensity was relatively weak. Levels of Flt3 receptor increased concomitantly with increasing levels of CD38. In addition, depletion of the more mature component of the CD34+ cell population (ie, those CD34+ cells expressing “lineage antigens”) resulted in an enrichment of CD34+CD38−Flt3− cells. Analysis of FACS sorted cell populations also showed that Flt3low cells were consistently smaller than Flt3high cells, with only a narrow halo of cytoplasm. Taken together, these results suggest that the earliest progenitor cells may lack or have only low levels of Flt3 receptor. To further investigate this hypothesis, we performed cell cycle analyses.
Transplantable murine stem cells expressing Flt3 receptor have previously been shown to be actively cycling, whereas murine stem cells negative for Flt3 receptor were predominantly in G0.29 The same study also showed that the repopulating capacity of murine Flt3+ stem cells was significantly less than that of murine Flt3−cells.29 Based on these observations, it was postulated that expression of Flt3 receptor was stage related on murine hematopoietic stem cells with a pattern similar to that of kit.27 Our analysis of the cell cycle status of human CD34+ (and CD34+lin−) marrow cells showed that CD34+Flt3low progenitor cells are primarily in cell cycle dormancy, whereas the majority of CD34+Flt3high cells are actively cycling. We do not think it is likely that our procedure of labeling cells with anti-Flt3 MoAbs and FACS sorting activated the cells to begin cell cycling, because Flt3high cells did not proliferate detectably in the absence of FL (see below and Fig 7). However, we have not determined whether one or more of the antibodies used in our study can activate Flt3 receptor (ie, act as an agonist imitating the effect of FL) by other tests such as ability to induce proliferation of transfected cell lines.
FACS sorted CD34+Flt3high cells gave rise primarily to granulo-monocytic colonies, whereas CD34+Flt3low cells formed mostly erythroid colonies. Removal of the relatively mature cells expressing lineage antigens from the CD34+ cell population before FACS separation of Flt3high versus Flt3low cells resulted in depletion of a large proportion of the colony-forming cells, somewhat obscuring this difference (Fig 4). It is not entirely clear from our results whether CD34+ cells expressing high levels of Flt3 receptor are already definitely committed to differentiate towards the granulo-monocytic lineage rather than the erythroid lineage, or if the presence of Flt3 receptor is simply permissive for differentiation.
When CD34+lin−Flt3high cells were incubated in short-term liquid suspension cultures, the cell numbers in these cultures remained stable over several days in FL alone and increased moderately in response to FL plus KL. This is in accordance with the recent observation that FL induces proliferation of human CD34+CD38− progenitor cells,41 and can accelerate cell cycling of hematopoietic progenitors.42 Taken together, these results suggest that Flt3 receptor is expressed on those progenitor cells poised to enter cell cycle and proliferate.43 The numbers of cells in the cultures initiated with CD34+lin−Flt3low cells did not increase in response to FL alone or FL plus KL. Interestingly, we did not detect upregulation of Flt3 receptor on Flt3lowcells. This would seem to argue against a stage-related expression of Flt3 receptor on human hematopoietic progenitor cells. It is possible that the conditions needed to induce expression of Flt3 receptor on Flt3low cells (eg, additional perhaps unknown cytokines, adhesion factors, or bone marrow microenvironmental factors) were not present in our in vitro system. In a recently published work, primitive murine lin− progenitor cells also failed to proliferate in response to FL as the sole growth factor.44
The clonogenic output of the FACS sorted CD34+Flt3high cells after liquid suspension culture was higher than that of the CD34+Flt3low cells, no matter what was the combination of cytokines used in the cultures. The lower clonogenic output of the Flt3low cells cultured in the presence of FL alone or FL plus additional GFs may be a reflection of the unresponsiveness of Flt3low cells to FL. In addition, the phenotypic analysis of cells generated in suspension culture showed that a fraction of Flt3high cells remained strongly positive for Flt3, as well as for kit, when cultured in FL plus KL, suggesting that at least some of these cells did not differentiate further. This is in line with previous work showing severely impaired hematopoiesis in mice deficient for both Flt3 and kit receptor43 and may have implications for ex vivo expansion of human progenitor cells for clinical use. In our study, both CD34+Flt3high and CD34+Flt3low cell populations contained primitive progenitor cells as assayed by replating experiments. However, the number of colonies obtained was low for each sorted subpopulation. It is possible that a majority of the more primitive progenitor cells were in the CD34+ cell fraction expressing intermediate levels of Flt3 receptor, and that these cells were, thus, in neither of the two FACS sorted populations we obtained. Taken together, the data presented here indicate that FL as well as KL can preserve the clonogenic capacity of human hematopoietic progenitor cells in liquid suspension culture.
Those hematopoietic progenitor cells that do not respond to known cytokines are believed to include the earliest stem cells.45 The results presented in this study imply that primitive, quiescent human hematopoietic progenitor cells may lack expression of Flt3 as well as kit. Whether this is also true of pluripotent hematopoietic stem cells remains to be established. Further experiments such as transplantation of FACS sorted human hematopoietic cell populations into immunodeficient mice46 or fetal sheep47 are needed to investigate this issue.
ACKNOWLEDGMENT
We thank Dr Stewart Lyman for generously providing human Flt3 ligand and Dr Wing Leung for assistance with the statistical analysis.
Supported by a research grant from Gryphon Pharmaceuticals Inc. K.S.G. was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG).
Gryphon Pharmaceuticals, Inc provided partial funding for the study described in this paper. Under an agreement between Gryphon Pharmaceuticals, Inc and the Johns Hopkins University, the University and Drs Civin and Small will receive a share of sales royalty. The University and Drs Civin and Small also own Gryphon Pharmaceuticals stock, the sale of which is subject to certain restrictions under University policy. Drs Civin and Small also serve on the Board of Directors and on the Scientific Advisory Board of Gryphon. The terms of this arrangement are being managed by the University in accordance with its conflict of interest policies.
Address reprint requests to Curt I. Civin, MD, Oncology 3-109, Johns Hopkins Hospital, 600 N Wolfe St, Baltimore, MD 21287-5001.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal