Abstract
Hydroxyurea, interferon, and HLA-identical sibling bone marrow transplantation are common therapies for chronic myelogenous leukemia (CML) in chronic phase. Which is best is controversial. The purpose of this study was to compare survival of patients with CML receiving HLA-identical sibling transplants versus hydroxyurea or interferon. The transplant cohort included 548 recipients of HLA-identical sibling transplants, reported to the International Bone Marrow Transplant Registry. The nontransplant cohort included 196 patients receiving hydroxyurea (n = 121) or interferon (n = 75) on a randomized trial of the German CML Study Group. Survivals were compared using proportional hazards regression with fixed and time-dependent variables to adjust for patient differences and changing risks over time. For the first 18 months after diagnosis, mortality was higher in the transplant than the nontransplant cohort (relative risk [RR], 5.85; P < .0001). From 18 to 56 months, mortality was similar (RR, 0.80; P = .38). After 56 months, mortality was lower in the transplant cohort (RR, 0.16; P < .0001). Seven-year survival probabilities (95% confidence interval) were 58% (50% to 66%) with transplant and 32% (22% to 41%) with hydroxyurea or interferon. There was a significant survival advantage for hydroxyurea or interferon in the first 4 years after diagnosis and for transplants starting 5.5 years after diagnosis. For transplants done within 1 year of diagnosis, the survival advantage for transplantation began earlier. Survival advantage for transplants was greater and occurred earlier in patients with intermediate- and high-risk prognostic features than in those with low-risk features. This study confirms higher early mortality, but a long-term survival advantage for HLA-identical sibling transplants over hydroxyurea or interferon in CML.
OPTIMAL THERAPY for chronic myelogenous leukemia (CML) in chronic phase is controversial. Interferon and/or hydroxyurea are common treatments. Although hydroxyurea controls white blood cell and platelet levels, it rarely produces cytogenetic remissions, prolongs survival only modestly, and does not cure.1-3 Interferon also controls white blood cell and platelet levels; however, in contrast to hydroxyurea, it produces cytogenetic remissions in some patients. In some studies, persons treated with interferon survive longer than those treated with hydroxyurea or busulfan.4-10 It is too early to know if there are cures with interferon, but the frequency will be low. Treatment-related mortality with hydroxyurea and/or interferon is negligible; median survival is 3 to 7 years. Features at diagnosis associated with shorter survival include older age, male sex, high levels of myeloblasts and platelets, and splenomegaly.11-15
HLA-identical sibling bone marrow transplantation is also used to treat CML in chronic phase, especially in younger patients. Five-year leukemia-free survival is 50% to 60%.16-20 This contrasts with hydroxyurea therapy where there is no leukemia-free survival and with interferon therapy where 5-year leukemia-free survival is rare. However, transplants are associated with 20% to 30% treatment-related mortality. Factors associated with lower leukemia-free survival after chronic phase transplants include older age, prior treatment with busulfan, T-cell depletion of donor bone marrow, and intervals from diagnosis to transplant greater than 1 year.16-23
Because most persons with CML can expect 4 or more years survival with hydroxyurea or interferon, the decision to do a transplant early, with its attendant risk of treatment-related mortality, is difficult.24,25 However, delaying transplant increases transplant-related mortality and decreases the likelihood of cure.16 21 We compared survival of 548 people with CML receiving HLA-identical sibling transplants in chronic phase and reported to the International Bone Marrow Transplant Registry (IBMTR) with survival of 196 treated with hydroxyurea or interferon on a randomized trial of the German CML Study Group.
MATERIALS AND METHODS
Transplant cohort.
The study included 548 patients with CML in chronic phase, ≥15 and ≤55 years of age, diagnosed between 1983 and 1991, treated with interferon with or without hydroxyurea (n = 131) or hydroxyurea alone (n = 417) followed by a non–T-cell depleted HLA-identical sibling bone marrow transplant with posttransplant methotrexate and cyclosporine for graft-versus-host disease prophylaxis. Patients were reported to the IBMTR by 116 centers (Table 1). Median interval between diagnosis and transplant was 10.1 (range, 2 to 84) months; 331 (60%) patients were transplanted within 1 year of diagnosis. Median follow-up was 4.3 years.
Comparison of Transplant and Nontransplant Cohorts
| Variable . | Treatment for CML . | P . | |||
|---|---|---|---|---|---|
| No. . | Transplant . | No. . | Hydroxyurea or Interferon . | ||
| No. of patients | 548 | 196 | |||
| Age, median (range) yr | 548 | 35 (15-54) | 196 | 41 (15-55) | <.001 |
| Male sex, No. (%) | 548 | 331 (60) | 196 | 119 (61) | .41 |
| WBC at Dx, median (range) N × 109/L | 548 | 140 (3-850) | 196 | 150 (15-489) | .004 |
| Hemoglobin at Dx, median (range), ×g/dL | 479* | 12 (2-17) | 196 | 12 (4-16) | .06 |
| Platelets at Dx, median (range) N × 109/L | 489* | 419 (2-3,440) | 196 | 412 (94-1,739) | .62 |
| % Blasts at Dx, median (range) | 400* | 1 (0-10) | 196 | 2 (0-10) | <.0001 |
| Spleen size at Dx, median (range) cm | 466* | 3 (0-26) | 196 | 5 (0-30) | .05 |
| Sokal score at Dx, No. (%) | 337* | 196 | .48 | ||
| Low risk | 137 (41) | 72 (37) | |||
| Intermediate risk | 125 (37) | 83 (42) | |||
| High risk | 75 (22) | 41 (21) | |||
| Year of diagnosis <1988, No. (%) | 548 | 294 (41) | 196 | 94 (48) | .05 |
| Variable . | Treatment for CML . | P . | |||
|---|---|---|---|---|---|
| No. . | Transplant . | No. . | Hydroxyurea or Interferon . | ||
| No. of patients | 548 | 196 | |||
| Age, median (range) yr | 548 | 35 (15-54) | 196 | 41 (15-55) | <.001 |
| Male sex, No. (%) | 548 | 331 (60) | 196 | 119 (61) | .41 |
| WBC at Dx, median (range) N × 109/L | 548 | 140 (3-850) | 196 | 150 (15-489) | .004 |
| Hemoglobin at Dx, median (range), ×g/dL | 479* | 12 (2-17) | 196 | 12 (4-16) | .06 |
| Platelets at Dx, median (range) N × 109/L | 489* | 419 (2-3,440) | 196 | 412 (94-1,739) | .62 |
| % Blasts at Dx, median (range) | 400* | 1 (0-10) | 196 | 2 (0-10) | <.0001 |
| Spleen size at Dx, median (range) cm | 466* | 3 (0-26) | 196 | 5 (0-30) | .05 |
| Sokal score at Dx, No. (%) | 337* | 196 | .48 | ||
| Low risk | 137 (41) | 72 (37) | |||
| Intermediate risk | 125 (37) | 83 (42) | |||
| High risk | 75 (22) | 41 (21) | |||
| Year of diagnosis <1988, No. (%) | 548 | 294 (41) | 196 | 94 (48) | .05 |
*Not available for all patients.
Abbreviations: WBC, white blood cell count; Dx, diagnosis.
The IBMTR is a voluntary working group of over 300 transplant centers worldwide that contribute detailed data on their allogeneic and identical twin bone marrow transplants to a Statistical Center at the Medical College of Wisconsin.26 27 Participants are required to report all consecutive transplants. The IBMTR database includes 40% to 45% of allogeneic transplant recipients since 1970. Computerized error checks, physician review of submitted data, and on-site audits of participating centers ensure data quality. Transplant outcomes estimated using IBMTR data are similar to those reported by large nonparticipating centers for comparable patients.
Nontransplant cohort.
The study included 196 patients with CML in chronic phase, ≥15 and ≤55 years of age, diagnosed between 1983 and 1990 and treated with hydroxyurea (n = 121) or interferon (n = 75) on a randomized trial of the German CML Study Group comparing busulfan, hydroxyurea, and interferon for newly diagnosed CML in chronic phase.8 28Details of this trial are published; it enrolled about 10% of the CML cases in West Germany over a 7.5-year period. Patients were treated in 60 centers. In the trial, survival of patients treated with busulfan was inferior to those treated with either hydroxyurea or interferon; there was not a statistically significant difference in survivals of patients receiving hydroxyurea or interferon. Patients receiving busulfan were excluded from the current study. Ninety-five patients receiving hydroxyurea or interferon, but older than 55 years at diagnosis, and 14 with greater than 10% circulating blasts at diagnosis were also excluded from analysis. Median follow-up was 6.5 years. Sixty-five patients in the German study received a transplant at a median interval of 20.0 (range, 3.1 to 77.4) months after diagnosis; they were censored at time of transplant.
Statistical methods.
Characteristics of the transplant and hydroxyurea/interferon groups were compared using the χ2 test for categorical variables and the Wilcoxon two-sample test for continuous variables.
Comparing outcomes in the transplant and nontransplant groups required adjustment for two sources of bias: differences in time to treatment (time to transplant) and differences in baseline characteristics of patients. To address the first source of bias, which results from the fact that patients must survive in chronic phase a sufficient length of time for a transplant to be done, a left-truncated Cox regression model of time to death was used.29 30 At each time point in this model, the risk set in the nontransplant cohort consists of all patients still under study, while the risk set in the transplant cohort includes only those with a waiting time to transplant less than the current time point and who are still under study.
To adjust for differences in baseline characteristics, we used two approaches. First associations between survival and potential prognostic variables were evaluated in each group separately using Cox proportional hazards regression with a backwards stepwise approach. Variables considered were: age (> v ≤35 years), sex, white blood cell count (< 100, 100 to 199, and ≥200 × 109/L), platelets (<150, 150 to 699, and ≥700 × 109/L), blasts (0, 1 to 3, ≥4%), spleen size (0, 1 to 4, 5 to 9, and ≥10 cm below the costal margin), hemoglobin at diagnosis (> v ≤12 g/dL) and year of diagnosis (< and ≥1988). All of these have been reported to affect survival in conventionally treated patients in prior studies. Variables significantly associated with survival in either the transplant or nontransplant cohorts were included as covariates in subsequent survival comparisons. When tests for interaction between significant covariates and type of treatment (transplant v hydroxyurea or interferon) showed significance, covariates were adjusted separately for each treatment. The proportionality assumption of the Cox model was tested by adding a time-dependent covariate for each covariate. The proportionality assumption did not hold for treatment effect, indicating that the relationship between treatment and outcome differed over time. To determine regions of the treatment period where the relative risk (RR) of mortality between the two treatment groups was constant, a series of Cox models with different cut-off points for time-dependent treatment effects were fit.31 The final model chosen was the one giving the largest partial likelihood. In this model, treatment was considered as a time-dependent covariate with different coefficients for 0 to 18, >18 to 56, and >56 months after diagnosis. Adjusted probabilities of survival were then generated from the final Cox model stratified on treatment and weighted averages of covariate values using the sample proportion as the weight function.32 Adjusted probabilities represent predicted outcomes for similar groups of patients receiving each treatment. Estimates and 95% point-wise confidence intervals for differences in survival were obtained by computing estimates of survival for each treatment group separately using the sample proportion as the weight function.
The second strategy to adjust for differences in baseline characteristics was to analyze the impact of Sokal score on outcome in the transplant and nontransplant cohorts and stratify comparisons of treatment effects by Sokal risk group. Sokal score is a widely used predictor of survival in conventionally treated CML.11-13Comparisons in each stratum also used left-truncated Cox regression with time-dependent treatment effects as described above. Survival probabilities for each stratum were calculated using the left-truncated Kaplan-Meier method.
RESULTS
Patient characteristics.
Table 1 compares characteristics of patients receiving transplants with those of patients receiving hydroxyurea or interferon. There were significant differences between the cohorts in distributions of age, white blood cell counts, spleen size, percent blasts in the blood, and year of diagnosis. Table 2 shows results of analyses evaluating associations between patient characteristics and survival. Age, sex, spleen size, and year of diagnosis were significant predictors. Tests of interaction indicated a differential effect of year of diagnosis in the transplant and nontransplant cohorts.
Variables Associated With Survival in the Transplant and/or Nontransplant Cohort
| . | Transplant . | Nontransplant . | ||
|---|---|---|---|---|
| RR of Death . | P . | RR of Death . | P . | |
| Year diagnosis ≥88 | 0.58 | .003 | — | NS |
| Spleen size ≥10 cm | — | NS | 2.11 | <.001 |
| Female sex | 0.65 | .02 | 0.63 | .03 |
| Age >35 years | 1.41 | .04 | — | NS |
| . | Transplant . | Nontransplant . | ||
|---|---|---|---|---|
| RR of Death . | P . | RR of Death . | P . | |
| Year diagnosis ≥88 | 0.58 | .003 | — | NS |
| Spleen size ≥10 cm | — | NS | 2.11 | <.001 |
| Female sex | 0.65 | .02 | 0.63 | .03 |
| Age >35 years | 1.41 | .04 | — | NS |
Other variables considered: platelets at diagnosis (0 to 149, 150 to 699, ≥700 × 109/L, unknown), circulating blasts at diagnosis (0, 1 to 3, ≥4, unknown), WBC at diagnosis (<100, 100 to 199, ≥200 × 109/L).
Abbreviations: RR, relative risk; NS, not significant.
Transplant versus hydroxyurea or interferon.
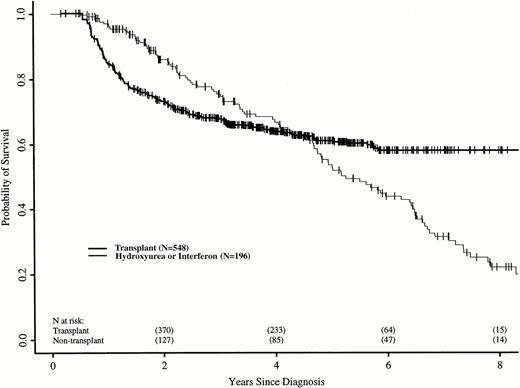
The relative risk of death between the two groups changed with time after diagnosis. Using Cox regression, we identified three discrete time periods after diagnosis with relative risks of death for transplant versus nontransplant treatment that differed: ≤18, >18 to 56, and >56 months. In the first 18 months after diagnosis, patients in the transplant cohort had a higher risk of death than those receiving hydroxyurea or interferon (RR, 5.85; P < .0001). Between 18 and 56 months, the risk of death was similar for patients in the two cohorts (RR, 0.80; P = .38). After 56 months, patients still at risk in the transplant cohort had a lower subsequent risk of death than those still at risk in the nontransplant cohort (RR, 0.16;P < .0001). Figure 1 shows adjusted probabilities of survival after diagnosis, calculated from the Cox models and adjusting for time to transplant, age, sex, spleen size, and year of diagnosis. The 7-year probability of survival (95% confidence interval) was 58% (50% to 65%) with transplant and 32% (22% to 41%) with hydroxyurea or interferon. Figure 2 shows differences in survival probabilities between the two cohorts (survival probability with transplant minus survival probability with hydroxyurea or interferon) over time with 95% point-wise confidence intervals for the differences. Differences greater than zero indicate a survival advantage for transplants at that point in the disease course; those less than zero indicate a survival advantage for hydroxyurea or interferon. The 95% confidence intervals that do not include zero indicate significantly different outcomes. There was a statistically significant survival advantage for hydroxyurea or interferon in the first 2.5 years after diagnosis and a significant advantage for transplants after 5.5 years; survivals were similar between 2.5 and 5.5 years.
Adjusted probabilities (from Cox regression model) of survival after diagnosis of CML in persons receiving HLA-identical sibling bone marrow transplants or nontransplant therapy with hydroxyurea or interferon.
Adjusted probabilities (from Cox regression model) of survival after diagnosis of CML in persons receiving HLA-identical sibling bone marrow transplants or nontransplant therapy with hydroxyurea or interferon.
Differences (with 95% confidence interval) in adjusted probabilities of survival after diagnosis of CML between patients receiving HLA-identical sibling bone marrow transplants versus hydroxyurea or interferon. Differences were calculated as probability of survival with transplant minus probability of survival with nontransplant treatment. A negative difference indicates a survival disadvantage for transplant; a positive difference indicates an advantage for transplants. A 95% confidence interval that does not include zero indicates a statistically significant difference.
Differences (with 95% confidence interval) in adjusted probabilities of survival after diagnosis of CML between patients receiving HLA-identical sibling bone marrow transplants versus hydroxyurea or interferon. Differences were calculated as probability of survival with transplant minus probability of survival with nontransplant treatment. A negative difference indicates a survival disadvantage for transplant; a positive difference indicates an advantage for transplants. A 95% confidence interval that does not include zero indicates a statistically significant difference.
Transplants within 1 year of diagnosis.
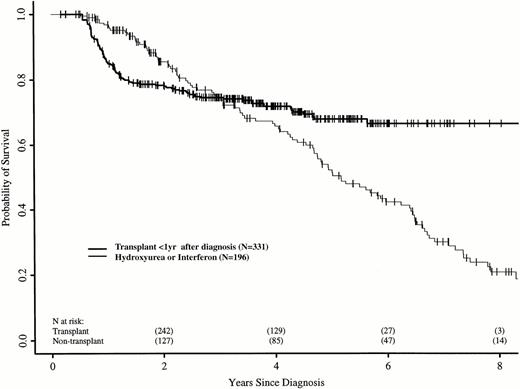
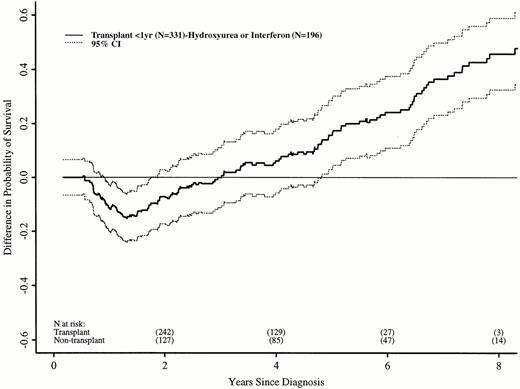
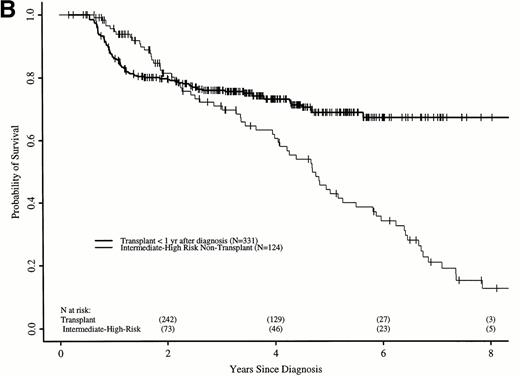
We repeated these analyses, restricting the transplant cohort to 331 patients transplanted ≤ 1 year after diagnosis. These patients have earlier risks of transplant-related death, but better long-term transplant outcomes.16 21 Again, the RR of death in the transplant and nontransplant cohorts differed over time. In the first 18 months after diagnosis, the transplant cohort had a higher risk of death than the nontransplant cohort (RR, 5.01; P < .0001). Between 18 and 56 months after diagnosis, the RR was 0.42 (P = .008), and after 56 months, 0.08 (P = .0005). Figure 3 shows adjusted probabilities of survival after diagnosis, calculated from the Cox models. The 7-year probability of survival was 67% (56% to 75%) with a transplant within 1 year of diagnosis and 30% (21% to 40%) with hydroxyurea or interferon. Figure 4 shows differences in survival probabilities between the two cohorts (transplant in the first year – hydroxyurea or interferon) over time. There was a significant survival advantage for chemotherapy in the first 1.8 years after diagnosis and a significant advantage for transplant after 4.8 years.
Adjusted probabilities (from Cox regression model) of survival after diagnosis of CML in persons receiving HLA-identical sibling bone marrow transplants within 1 year of diagnosis or nontransplant therapy with hydroxyurea or interferon.
Adjusted probabilities (from Cox regression model) of survival after diagnosis of CML in persons receiving HLA-identical sibling bone marrow transplants within 1 year of diagnosis or nontransplant therapy with hydroxyurea or interferon.
Differences (with 95% confidence interval) in adjusted probabilities of survival after diagnosis of CML between patients receiving HLA-identical sibling bone marrow transplants within 1 year of diagnosis versus hydroxyurea or interferon. Differences were calculated as probability of survival with transplant in the first year after diagnosis minus probability of survival with nontransplant treatment. A negative difference indicates a survival disadvantage for transplant; a positive difference indicates an advantage for transplants. A 95% confidence interval that does not include zero indicates a statistically significant difference.
Differences (with 95% confidence interval) in adjusted probabilities of survival after diagnosis of CML between patients receiving HLA-identical sibling bone marrow transplants within 1 year of diagnosis versus hydroxyurea or interferon. Differences were calculated as probability of survival with transplant in the first year after diagnosis minus probability of survival with nontransplant treatment. A negative difference indicates a survival disadvantage for transplant; a positive difference indicates an advantage for transplants. A 95% confidence interval that does not include zero indicates a statistically significant difference.
Association between Sokal score and outcome.
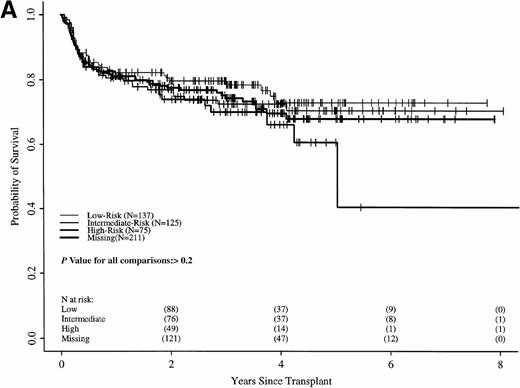
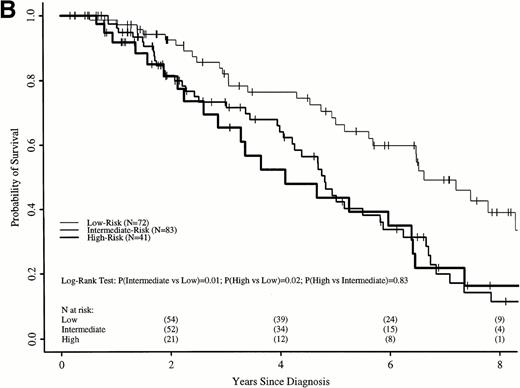
Sokal scores based on sex, spleen size, hematocrit level, platelet count, and percent of blasts in the blood at diagnosis were assigned to each patient in the two cohorts (Table 1). There were insufficient data to assign a Sokal score in 211 transplant recipients; however, survival of these patients was similar to the 337 transplant recipients assigned a Sokal score (Fig 5A). Patients were classified as low-, intermediate-, and high-risk as published.11
Probabilities of survival in persons with CML receiving (A) HLA-identical sibling transplant or (B) nontransplant therapy with hydroxyurea or interferon by Sokal risk group.
Probabilities of survival in persons with CML receiving (A) HLA-identical sibling transplant or (B) nontransplant therapy with hydroxyurea or interferon by Sokal risk group.
Survival in the transplant cohort did not differ among the three risk groups (Fig 5A). Transplant patients were not, therefore, stratified by risk group in subsequent comparisons. In the nontransplant cohort, low-risk patients had significantly longer survivals than intermediate- and high-risk patients, who were similar to each other (Fig 5B).
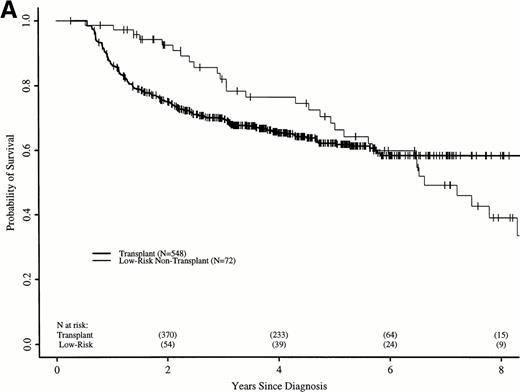
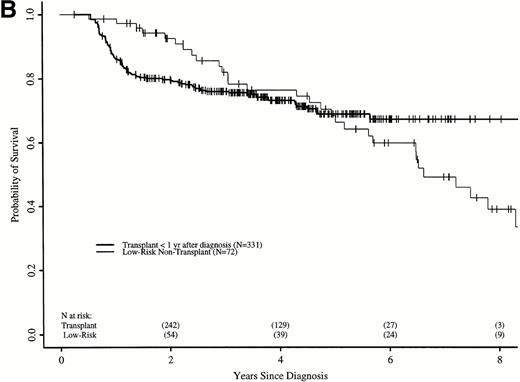
Figure 6A shows probabilities of survival after diagnosis for transplant recipients and low-risk patients receiving hydroxyurea or interferon. The 7-year probability of survival was 58% (52% to 64%) with transplant and 49% (34% to 63%) with hydroxyurea or interferon. Although the curves cross at about 6 years, there was not a statistically significant advantage for transplant until 7.8 years after diagnosis. Figure 6B compares probabilities of survival for patients receiving transplant within 1 year of diagnosis with low-risk patients receiving hydroxyurea or interferon. The 7-year probability of survival was 67% (60% to 73%) with transplant and 49% (34% to 63%) with hydroxyurea or interferon. The curves cross at 5 years, with a statistically significant advantage for transplants after about 6.5 years from diagnosis.
Adjusted probabilities of survival after diagnosis of CML in low-risk persons receiving hydroxyurea or interferon versus persons receiving an HLA-identical sibling bone marrow transplant (A) at any time after diagnosis or (B) within 1 year of diagnosis.
Adjusted probabilities of survival after diagnosis of CML in low-risk persons receiving hydroxyurea or interferon versus persons receiving an HLA-identical sibling bone marrow transplant (A) at any time after diagnosis or (B) within 1 year of diagnosis.
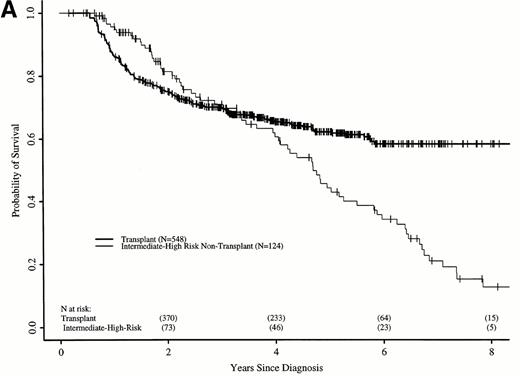
Figure 7A shows probabilities of survival after diagnosis for transplant recipients and intermediate- and high-risk patients receiving hydroxyurea or interferon. The 7-year probability of survival was 58% (52% to 64%) with transplant and 21% (12% to 31%) with hydroxyurea or interferon. The curves cross at 3.5 years, with a statistically significant advantage for transplant after 4.7 years. Figure 7B compares probabilities of survival for patients receiving transplant within 1 year of diagnosis with intermediate- and high-risk patients receiving hydroxyurea or interferon. The 7-year probability of survival was 67% (63% to 73%) with transplant and 21% (12% to 31%) with hydroxyurea or interferon. The curves cross at 2.2 years, with a statistically significant advantage for transplants after about 4 years from diagnosis.
Adjusted probabilities of survival after diagnosis of CML in intermediate- and high-risk persons receiving hydroxyurea or interferon versus persons receiving an HLA-identical sibling bone marrow transplant (A) at any time after diagnosis or (B) within 1 year of diagnosis.
Adjusted probabilities of survival after diagnosis of CML in intermediate- and high-risk persons receiving hydroxyurea or interferon versus persons receiving an HLA-identical sibling bone marrow transplant (A) at any time after diagnosis or (B) within 1 year of diagnosis.
DISCUSSION
The best treatment for CML in chronic phase is controversial. HLA-identical sibling bone marrow transplants can cure a substantial proportion of patients with CML, but have high treatment-related mortality. In contrast, hydroxyurea and interferon have little treatment-related mortality and prolong survival, but cure few, if any, patients. This study confirms the initial survival disadvantage of transplants, but also the later advantage; the latter becomes significant between 4 and 8 years after diagnosis, depending on when in chronic phase transplants are performed and disease-related prognostic factors. Importantly, the early survival disadvantage of transplants is increased and the later survival advantage is decreased by delaying transplants beyond the first year after diagnosis. This results from increased treatment-related mortality and relapse with transplants done later.16 21
The nontransplant cohort in this study included patients receiving hydroxyurea or interferon, treatments with comparable survival in the data set we used.8 This study set differs from other reports in which interferon treatment seemed to have better survival than hydroxyurea in nonrandomized and randomized settings. Differences between the German CML Study and these other studies were recently discussed in detail, including on-study criteria, risk profiles, drug doses, and schedules and frequency of cytogenetic testing.33-36 However, the 62-month median survival of the hydroxyurea and interferon cohort in this study is similar to that reported in other studies of interferon (55 to 72 months) so the comparison between transplant and nontransplant therapy applies to most patients with CML who are ≤55 years old. Additionally, the late advantage of transplants is seen even when compared with nontransplant therapy in low-risk patients in the German trial, who may be more similar to those in other interferon trials. Whether a strategy of early transplant for interferon nonresponders and delayed transplant for interferon-responders would improve survival can only be determined in a prospective trial.
The only type of transplant we studied were those from HLA-identical siblings. Because treatment-related mortality after transplants from other related and unrelated donors is substantially higher,37-39 our conclusions may not apply to these settings. It is likely that the time point after which alternative donor transplants show better survival over hydroxyurea or interferon is later.
This study confirms a long-term survival advantage for transplants and further indicates that the survival advantage is greatest for persons with intermediate- and high-risk prognostic features at diagnosis and when transplants are performed early. It quantifies the trade-off between early mortality and long-term leukemia-free survival with transplants versus hydroxyurea or interferon for newly diagnosed CML and provides data for counseling patients deciding between therapies.
Supported by Public Health Service Grant No. PO1-CA-40053 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; the German Bundesminister für Forschung und Technologie, Förderkennzeichen No. 01ZW044 and 01ZP9001; grants from Alpha Therapeutic Corporation; Amgen, Inc; Anonymous; Astra Pharmaceutical; Baxter Healthcare Corporation; Bayer Corporation; Biogen; Blue Cross and Blue Shield Association; Lynde and Harry Bradley Foundation; Bristol-Myers Squibb Company; Frank G. Brotz Family Foundation; Cancer Center, Medical College of Wisconsin; CellPro, Inc; Centeon; Center for Advanced Studies in Leukemia; Chimeric Therapies, Inc; Charles E. Culpeper Foundation; Eleanor Naylor Dana Charitable Trust; Eppley Foundation for Research; Genentech, Inc; Glaxo Wellcome Company; Hoechst Marion Roussel, Inc; Immunex Corporation; Janssen Pharmaceutica; Kettering Family Foundation; Kirin Brewery Company; Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation; Herbert H. Kohl Charities, Inc; Eli Lilly Company Foundation; Nada and Herbert P. Mahler Charities; Milstein Family Foundation; Milwaukee Foundation/Elsa Schoeneich Research Fund; Samuel Roberts Noble Foundation; Ortho Biotech Corporation; John Oster Family Foundation; Elsa U. Pardee Foundation; Jane and Lloyd Pettit Foundation; Alirio Pfiffer Bone Marrow Transplant Support Association; Pfizer, Inc; Pharmacia and Upjohn; RGK Foundation; Sandoz Pharmaceuticals; Schering-Plough International; Walter Schroeder Foundation; Searle; Stackner Family Foundation; Starr Foundation; Joan and Jack Stein Charities; and Wyeth-Ayerst Laboratories.
Address reprint requests to Mary M. Horowitz, MD, MS, International Bone Marrow Transplant Registry, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal