Abstract
Recent work has enabled us to quantitate the four variables (2,3-DPG concentration, pHi, non-S hemoglobin composition, and O2 saturation) that modulate the equilibrium solubility (csat) of Hb S inside sickle erythrocytes (SS RBCs). Using measured values of mean corpuscular hemoglobin concentration (MCHC), 2,3-DPG concentration, and %Hb (F+A2), along with estimates of pHiand the Δcsat due to partial oxygenation of SS RBCs in the microcirculation, we calculated the mean polymer fraction (fp) in erythrocytes from 46 SS homozygotes. Values of fp derived from the conservation of mass equation ranged from 0.30 to 0.59. MCHC and %Hb F were major determinants of the magnitude of fp; 2,3-DPG concentration and pHialso contributed, but to a lesser extent. A clinical severity score (CSS) was assigned to each patient based on mean hospitalization rate. There was a weak, but statistically significant, negative correlation between fp and steady state hematocrit (P = .017), but none between fp and whole blood hemoglobin concentration (P = .218). Although there was no correlation between fp and mean number of hospitalization days per year, patients with the greatest number of admissions and hospitalization days were found only among those who had an fp > 0.45. All five patients who died during the follow-up period (median, 7 years; range, 3 to 10 years) had fp values ≥0.48. However, patients with few admissions, low hospitalization days, and long survivals occurred at all fp levels. These results suggest that the clinical course of homozygous SS disease cannot be predicted by mean fpcalculations, which assume a homogeneous distribution of the five variables that modulate intraerythrocytic polymerization. A heterogeneous distribution is more likely; so the amount of polymerized Hb S could vary considerably among cell populations. Factors such as membrane abnormalities and endothelial cell interactions may also contribute to clinical severity.
THE PRIMARY CAUSE of the clinical symptomatology of sickle cell anemia is the intracellular polymerization of sickle hemoglobin (Hb S) that occurs when sickle erythrocytes (SS RBCs) are partially deoxygenated under the hypoxic conditions of the microcirculation. This, in turn, makes SS RBCs less deformable and ultimately results in the debilitating microvascular occlusions and hemolytic anemia characteristic of the disease. Recent work1-3 has enabled us to quantitate the four variables (2,3-DPG concentration, pHi, non-S hemoglobin composition, and O2 saturation) that modulate the equilibrium solubility (csat) of Hb S inside sickle erythrocytes (SS RBCs).
The requirements for therapeutic inhibition of Hb S gelation have been set forth by Sunshine et al,4 who showed that there is a relationship between the kinetics of polymerization (td) and the solubility (csat) under various cellular conditions. They also showed the extent by which td must be increased to provide an amelioration of homozygous sickle cell disease to the less severe clinical conditions of S/β+-thal, S/HPFH, and A/S trait. Although there was a strong correlation with non-Hb S hemoglobin composition, there was only a weak one at constant hemoglobin concentration. A detailed analysis of the nucleation-controlled polymerization that underlies sickling has been elucidated by these same investigators.5 6
Attempts by a variety of investigators7-16 to correlate hematologic parameters with the clinical severity of various sickling disorders have, for the most part, been only partially successful. Some earlier studies used only painful crises to score illness severity.17-19 Others20,21 used a composite vasoocclusive severity score to assign weighted values to the presence of various vasoocclusive events in a cohort of patients. This assessment of clinical severity showed no correlation with any laboratory parameters; however, the clinical severity score (CSS) and erythrocyte adherence to endothelial cells were strongly correlated.22
Eaton et al23 showed in a series of reports that the polymer content of the sickle erythrocyte depends on hemoglobin concentration and composition,24 as well as O2saturation.25 The quantitative relationships among these three cellular variables and the equilibrium aggregation of deoxy-Hb S may be found in Eaton and Hofrichter.5,6 Subsequently, Noguchi et al,26-31 using the conservation of mass equation of Ross et al32 and the activity coefficients of Ross and Minton,33 were able to quantitate intraerythrocytic polymer fraction (fp) by a theoretical analysis of Hb S solubility that accounted for these three cellular variables. A series of reports that used this framework to quantitate intracellular polymer content in various sickling syndromes,31,34 as well as in erythrocytes from individual patients,35 has shown an inverse correlation between fp and whole blood hemoglobin concentration, a hematologic index of hemolytic severity. Furthermore, in the latter study,35 a visual analogue scale (VAS) was used on a cohort of 30 patients to indicate perceived disease severity. The VAS score showed significant positive correlations with the calculated values of fp at both the venous pO2(40 mm Hg) and P50.
In addition to the three major determinants of the polymerization tendency of sickle erythrocytes cited above, we have shown that two other cellular variables (2,3-diphosphoglycerate [2,3-DPG] concentration and intracellular pH [pHi]) exert separate, but interdependent, effects on the equilibrium solubility (csat) of unliganded Hb S.1 In a separate study, we were able to quantitate the sparing effect of non-S hemoglobins on the solubility of partially liganded Hb S in the region of pathophysiologic interest (25% to 70% saturation2). It was found that hemoglobins F and A2 are equipotent in their effects on csat, as are hemoglobins A and C, but to a lesser extent. Thus, all five cellular variables (2,3-DPG, pHi, non-S hemoglobins, O2 saturation, and mean corpuscular hemoglobin concentration [MCHC]) that determine the polymerization tendency of sickle erythrocytes can now be quantitated by use of the requisite experimental values.3
Our earlier study3 showed that depletion of intracellular 2,3-DPG produced a consistent reduction in the sickling tendency of erythrocytes from four sickle cell anemia patients with widely differing hematologic features. Furthermore, estimates were made of the decrease in fp evoked by loss of 2,3-DPG by using appropriate values of the four variables that interact to affect polymerization in a 2,3-DPG–dependent manner (2,3-DPG concentration, pHi, O2 saturation, and MCHC).
In the present study, we used similar laboratory data, plus those for non-S hemoglobin composition, for a cohort of 46 homozygous sickle cell anemia patients, to quantitate all five cellular variables that modulate the intracellular polymer content of SS RBCs. We then attempted to correlate the values of fp so obtained with hemolytic and vasoocclusive severity for each patient.
MATERIALS AND METHODS
General methods.
Venous blood from 46 SS patients in the steady state was collected in standard heparin Vacutainer tubes and sampled within 2 hours. Informed consent was obtained before blood collection. All patients were adults, age 18 years or greater (mean age, 30.4 ± 8.9 years; range, 18 to 51 years). The male to female ratio was 0.64. No patient had undergone a blood transfusion for at least at least 3 months before blood collection. Hemoglobin concentrations were measured with Drabkin's reagent36 and used, along with the spun hematocrit, to calculate MCHC. Red blood cell adenosine triphosphate (ATP) and 2,3-DPG concentrations were measured using Sigma kits (Sigma, St Louis, MO). Hemoglobins F and A2 were quantitated in hemolysates by alkali denaturation37 and elution from diethylaminoethyl (DEAE)-cellulose columns,38 respectively. Intracellular pH (pHi) was estimated by use of the factor relating pHi to 2,3-DPG concentration.3
Mean polymerization tendency of sickle erythrocytes from individual patients.
A quantitative approach to sickle cell disease severity must take into account the extent of polymer formation at equilibrium in the circulating erythrocytes of individual SS patients. Of the five cellular variables that determine intracellular polymer content,3 three (2,3-DPG concentration, % non-S hemoglobins [F and A2], and MCHC) were measured directly. Values of pHi were estimated from the 2,3-DPG concentration and the loss of Bohr protons due to partial ligation and depolymerization at the O2 tension of the microcirculation (pO2 = 20 mm Hg1). The O2saturation corresponding to this partial pressure (≈25%) was assumed to be the same for each patient's erythrocytes. Knowledge of four of these five variables (2,3-DPG concentration, pHi, non-S Hb composition, and O2 saturation) permits one to determine csat, the equilibrium solubility of intraerythrocytic Hb S at 25% O2 saturation, without measuring it directly.3
One can derive increments of csat for each of these four parameters by use of various empirical relationships we deduced (ie, the interdependence of 2,3-DPG concentration and pHi1,3 and the sparing effect of non-S hemoglobins2,24,39) and the effect of ligation on solubility deduced by others.23 Thus: csat = csato + Δcsat2,3-DPG + ΔcsatHb(F+A2) + Δcsat25%O2 satn. The relevant csat is then obtained as the sum of the increments due to cellular modulators of solubility plus csato, the intraerythrocytic solubility for unliganded, 2,3-DPG-saturated Hb S at pH 7.41, which has a value of 18.0 g/dL (this baseline solubility was deduced in Poillon and Kim1).
For our patient with the lowest fp (0.30), each of these increments can be estimated: keeping in mind that the Bohr effect for SS red blood cells is about twice that for AA red blood cells (ie, −0.99 and −0.42, respectively40) and that this translates into a loss of approximately four and two Bohr protons on complete ligation, one can estimate the pH decrement for 25% oxygenation of SS red blood cells as follows: the Bohr protons released at this saturation = 25/89 × 4H+/tetramer = 1.12H+/tetramer, where 89% is the O2saturation at which fp = 0. Then ΔpH/ΔH+ = −0.28 × 1.12/4 = −0.078 pH unit, where −0.28 is the pH change for release of all four Bohr protons at 89% O2 saturation1 and Δcsat/ΔpH = 10.8 × −0.078 = −0.85 g/dL (where 10.8 g/dL is the increment in csat per pH unit1).
Effects of non-S hemoglobins (F and A2) and the degree of ligation on csat are as follows: Δcsat/ΔHb(F+A2) = 0.334 × 18.5 = 6.18 g/dL (where 0.334 is the csat increment for 1% Hb[F+A2]2 and 18.5 is the % Hb[F+A2] for this patient; Δcsat/Δ25% O2 saturation = 2.46 g/dL [derived from the empirical relationship between csat and O2 saturation deduced in Sunshine et al25]). Thus, the overall solubility at 25% O2 saturation for Hb S in SS erythrocytes from this particular patient is: 18.0 − 0.85 + 6.18 + 2.46 = 25.8 g/dL.
The conservation of mass equation fp = cp(ct − csat)/ct(cp − csat) shows the relationship among three intracellular concentrations in determining the polymer content of sickle erythrocytes at any degree of oxygenation: cp, the polymer concentration, 69.3 g/dL (taken from Sunshine et al24); ct, the intracellular hemoglobin concentration or MCHC, which has a value of 31.8 g/dL for this particular patient; and csat, the equilibrium solubility, which has a value of 25.8 g/dL here. Substitution of these values into the conservation of mass equation gives fp = 0.30.
This equation has general use for estimating the mean fpfor unfractionated SS erythrocytes. That is, the two variables that show the strongest correlation with fp (see Results) are non-S hemoglobin composition and MCHC. Thus, one must have accurate values for these parameters to obtain a reliable estimate of fp. For the other two parameters, O2 saturation is held constant at 25% (corresponding to a Δcsat of 2.46 g/dL) and the decrement in pH evoked by 2,3-DPG and H+loss at 25% O2 saturation (−0.078 pH unit) is considered constant and corresponds to a decrement in csatof −0.85 g/dL.
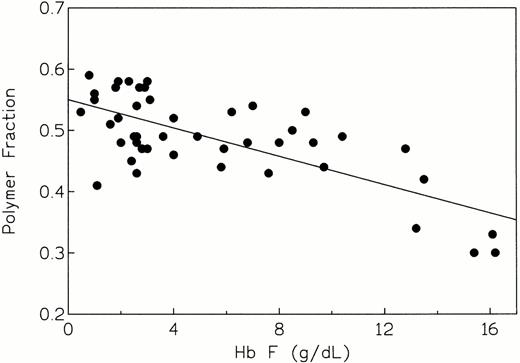
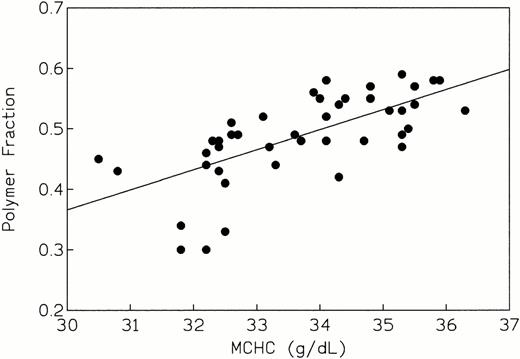
Our findings that polymer fraction correlates well with both Hb F (Fig1) and MCHC (Fig 2) have been shown by us2 and by others.24 39 Although these are strong associations, the simultaneous variation in other parameters that influence fp may introduce a certain amount of noise to the overall expression of polymer fraction.
Polymer fraction fp versus Hb F concentration for a cohort of homozygous SS patients (n = 46). Calculation of fp is described in the text. Linear regression analysis of these data gave values of r = −0.710 and P < 10−4, indicating a highly significant association between these variables.
Polymer fraction fp versus Hb F concentration for a cohort of homozygous SS patients (n = 46). Calculation of fp is described in the text. Linear regression analysis of these data gave values of r = −0.710 and P < 10−4, indicating a highly significant association between these variables.
fp versus intracellular hemoglobin concentration (r = 0.677, P < 10−4, indicating a highly significant association between these variables).
fp versus intracellular hemoglobin concentration (r = 0.677, P < 10−4, indicating a highly significant association between these variables).
Assessment of clinical severity.
Because painful crisis is the most common acute event experienced by SS patients, the number of pain episodes requiring hospitalization and administration of narcotic analgesics was used to assess clinical severity for the cohort of 46 patients who were followed for up to 10 years (1986 to 1995). The median follow-up time was 7 years, with a range of 3 to 10 years. The mean number of admissions and mean hospitalization days were determined retrospectively. For the purposes of this study, we assumed each hospital admission to be due to painful crisis because 87% of all hospital discharges of sickle cell patients listed sickle cell crisis as a discharge diagnosis during the follow-up period. A modification of the vasoocclusive severity score of Hebbel et al20 was used to assign an index of clinical severity for each patient. Our CSS was assigned solely on the basis of hospitalizations and did not include contributions from organ dysfunction due to specific vasoocclusive events. To compute the average number of hospitalizations per year, we divided the number of hospital admissions during the follow-up period (regardless of length of hospital stay) by the number of years of follow-up. Then, CSS (Table1, column 10) was assigned as follows: 15 patients with no hospitalizations or less than one per year, 0 points; those with means of 1 to 5 per year (25 patients), 1 point; 6 to 10 per year (5 patients), 2 points; more than 10 per year (1 patient), 3 points. A second clinical severity measurement was also used: the mean number of hospital days per year (Table 1, column 9), obtained by dividing total number of days each subject was an inpatient during the follow-up period by the number of years of follow-up.
Mean Values of Red Blood Cell Variables That Affect Polymerization and Clinical Severity for a Cohort of Adult Sickle Cell Anemia Patients
| . | Hct (%) . | Hb (g/dL) . | MCHC (g/dL) . | ATP (mmol/L) . | 2,3-DPG (mmol/L) . | Hb F-150 (%) . | Hb A2 (%) . | fp-151 . | Hosp. (d/yr) . | CSS-152 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 26.1 ± 4.3 | 8.7 ± 1.3 | 33.7 ± 1.5 | 1.4 ± 0.2 | 6.1 ± 0.8 | 5.5 ± 4.4 | 3.0 ± 0.6 | 0.49 ± 0.07 | 21.8 ± 27.8 | 0.83 ± 0.71 |
| No. | 46 | 46 | 46 | 43 | 46 | 46 | 44 | 46 | 46 | 46 |
| Range | 18.0-39.6 | 6.4-12.7 | 30.5-36.3 | 1.1-1.9 | 4.3-9.5 | 0.8-16.2 | 1.5-3.9 | 0.30-0.59 | 0-140 | 0-3 |
| . | Hct (%) . | Hb (g/dL) . | MCHC (g/dL) . | ATP (mmol/L) . | 2,3-DPG (mmol/L) . | Hb F-150 (%) . | Hb A2 (%) . | fp-151 . | Hosp. (d/yr) . | CSS-152 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 26.1 ± 4.3 | 8.7 ± 1.3 | 33.7 ± 1.5 | 1.4 ± 0.2 | 6.1 ± 0.8 | 5.5 ± 4.4 | 3.0 ± 0.6 | 0.49 ± 0.07 | 21.8 ± 27.8 | 0.83 ± 0.71 |
| No. | 46 | 46 | 46 | 43 | 46 | 46 | 44 | 46 | 46 | 46 |
| Range | 18.0-39.6 | 6.4-12.7 | 30.5-36.3 | 1.1-1.9 | 4.3-9.5 | 0.8-16.2 | 1.5-3.9 | 0.30-0.59 | 0-140 | 0-3 |
For the 12 patients evaluated more than once (one, 4 times; two, 3 times; nine, twice), mean values were used here.
Seven of the 46 blood samples used for this study had Hb F levels >10%. The possibility that these patients may be double heterozygotes (S/HPFH or S/δβ-thal) rather than SS homozygotes has not been ruled out.
Polymer fraction at the nominal O2 tension of the microvasculature (pO2 = 20 mm Hg, corresponding to 25% O2 saturation); calculated from the conservation of mass relationship: fp = cp(ct − csat)/ct(cp − csat) where cp, ct, and csat are the polymer concentration (69.3 g/dL), the total hemoglobin concentration (MCHC), and the equilibrium solubility at 25% O2 saturation, respectively.
Clinical severity score based on average frequency of hospitalizations for pain crises per year: 0 points for none or less than one; 1 point for 1-5 crises per year; 2 points for 6-10; 3 points for more than 10.
Statistical methods.
Analyses of variance were performed and used unpaired Student'st-test (two tailed). To show linear relationships between fp and other variables, both ordinary least squares linear regression and nonparametric tests (Spearman rank correlation) were performed. These tests then generated correlation coefficients andP values (two tailed) for the slope. Except where noted, all correlation coefficients and P values are reported for ordinary linear regression.
RESULTS
Mean values for laboratory and clinical parameters.
Because of its length, the table showing composite laboratory and clinical data for the entire cohort of 46 SS patients is not shown here in full detail. Instead, we have compiled in Table1 the mean values for three of the five cellular variables (MCHC, 2,3-DPG concentration, and % Hb[F+A2]) that interact to determine polymer content, as well as those for whole blood hemoglobin and ATP concentrations and for hospitalization days and clinical severity score. The mean values of hematocrit, intracellular hemoglobin concentration (MCHC), %Hb F, and %Hb A2 shown in Table 1(26.1 ± 4.3, 33.7 ± 1.5 g/dL, 5.5% ± 4.4%, and 3.0% ± 0.6%, respectively) are in the range found in other studies of this nature.7-15 Mean values for ATP and 2,3-DPG concentrations are 1.4 ± 0.2 and 6.1 ± 0.8 mmol/L, respectively. These values are elevated by ≈25% relative to those for normal adult blood, as was shown in our earlier study1and by Steinberg et al.13 The mean value of hospitalization days per year was 21.8 ± 27.8 and the mean value of CSS was 0.83 ± 0.71.
Dependence of polymer fraction on Hb F concentration and MCHC.
Linear regression plots of fp as a function of %Hb F and MCHC are shown in Figs 1 and2, respectively. The corresponding correlation coefficients were −0.710 and 0.677, and the slopes were significantly different from zero (P < 10-4) in each case. Thus, a highly significant correlation exists between intracellular polymer content and non-S hemoglobin composition, as well as intracellular hemoglobin concentration. The strong dependence of polymer fraction on Hb F concentration and MCHC is well known and has been documented by us2 and by others.24 39
By contrast, for a plot of fp versus 2,3-DPG concentration (data not shown), there was no association between these two variables (correlation coefficient = 0.199; P = .185).
Hemolytic and clinical severity: Is there a correlation with polymerization tendency?
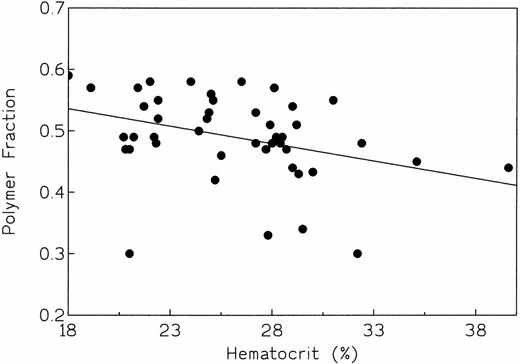
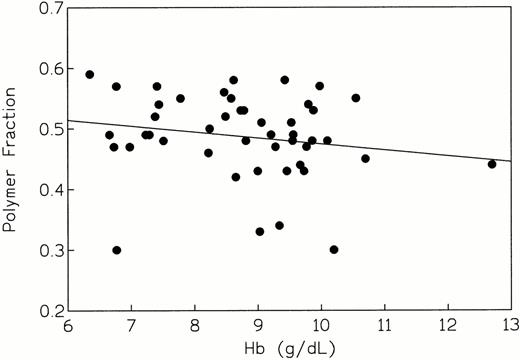
An attempt was made to correlate polymer fraction fp with hematocrit and whole blood hemoglobin concentration, the laboratory parameters that best reflect hemolytic severity in sickle cell anemia. The linear regression plot of fp versus hematocrit (Fig 3), gave a correlation coefficient of −0.350 and a P value of .0173 for the slope, indicating a negative relationship between fp and hematocrit; for the Spearman rank correlation test, the values were r = −0.403 and P = .0054. The linear regression plot of fp versus whole blood hemoglobin concentration (Fig4) also showed a negative correlation coefficient of −0.198. However, the P value was .187, indicating no statistically significant association between fp and hemoglobin concentration in this group of patients; nonparametric tests gave essentially the same results (r = −0.225; P = .133) indicating no statistically significant association between fp and hemoglobin concentration in this group of patients.
fp versus hematocrit (r = −0.350,P = .0173, indicating a significant association between these variables).
fp versus hematocrit (r = −0.350,P = .0173, indicating a significant association between these variables).
fp versus whole blood hemoglobin concentration (r = −0.198, P = .187, indicating no association between these variables).
fp versus whole blood hemoglobin concentration (r = −0.198, P = .187, indicating no association between these variables).
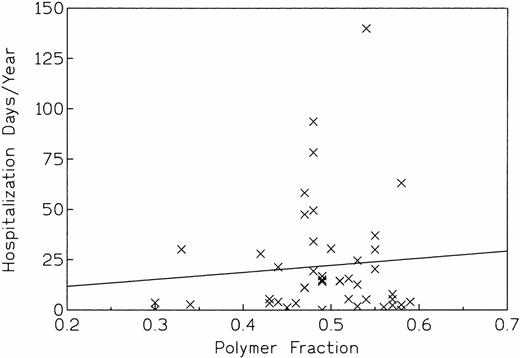
An attempt was made to correlate fp with hospitalization data as an index of clinical severity. The linear regression plot of hospitalization days/yr versus fp(Fig 5) gave a correlation coefficient of 0.089 and a P value of .555 for the slope. Thus, there was no statistically significant association between these two variables. Certain trends were discernible however: (1) No patient with fp less than 0.45 had a high number of mean hospitalization days per year (Fig 5), and (2) these patients also had CSS values of 0 or 1 and none had values of 2 or 3 (Fig 6). On the other hand, patients with high fp did not necessarily have high hospitalization rates; instead, many had few mean hospital days (Fig 5) and low clinical severity scores (Fig 6). This suggested that a high fp could be a necessary, but not sufficient condition, for high vasoocclusive severity. Other factors such as membrane abnormalities or endothelial adhesion tendency probably influence severity in patients with high fp, as well.
Hospitalization days per year versus fp(r = 0.089, P = .555, indicating no association between these variables).
Hospitalization days per year versus fp(r = 0.089, P = .555, indicating no association between these variables).
Clinical severity score (derived from hospitalization frequency data [see footnote in Table 1 for details]) versus fp (no statistical analysis of these data was possible). The figure shows the distribution of fp for patients examined over the range of clinical severity observed (0 to 3). Although all 46 patients were included, some patients (n = 16) with identical CSS and fp appear as a single point. For this reason, the number of points is less than 46.
Clinical severity score (derived from hospitalization frequency data [see footnote in Table 1 for details]) versus fp (no statistical analysis of these data was possible). The figure shows the distribution of fp for patients examined over the range of clinical severity observed (0 to 3). Although all 46 patients were included, some patients (n = 16) with identical CSS and fp appear as a single point. For this reason, the number of points is less than 46.
Figure 6 shows a plot of the clinical severity score (see footnote in Table 1) versus polymer fraction. These data are not amenable to statistical analysis and are shown only to demonstrate that values of 0 and 1 for CSS encompass fpvalues throughout the range observed (0.30 to 0.59). By contrast, there were a few patients (six in all) with CSS values of 2 or 3, and these tended to have high values of fp (0.47 to 0.58).
Six of the 46 patients were lost to follow-up soon after the study. For the remaining 40 patients, the median follow-up period was 7 years (range, 3 to 10 years). Five of these patients have died of sickle cell disease complications. The polymer fractions were 0.48 in erythrocytes from three of these patients and 0.54 and 0.58 for the other two. No patient with a polymer fraction less than 0.48 died during the follow-up period.
DISCUSSION
The underlying pathophysiologic events in sickle cell anemia are chronic hemolysis and microvascular occlusion. The principal clinical manifestations arising from these events are anemia, acute painful crises, and organ dysfunction. The marked variability in severity of symptoms among SS patients has made it difficult to establish a meaningful clinical severity scoring system for this disease.41 Although a single measurable parameter for assessing clinical severity does not exist, a useful index that assigned weighted scores to the presence of a group of vasoocclusive events was devised by Hebbel et al20 for this purpose. Because painful episodes requiring hospitalization are the most frequent vasoocclusive event in this disease,42 we used only such hospitalization data to assign a clinical severity score for each patient evaluated and did not assess the contribution of organ damage to the clinical picture.
We have been able to quantitate the interaction among the five cellular variables (2,3-DPG concentration, pHi, non-S hemoglobin composition, O2 saturation, and MCHC) that modulate the polymerization tendency of sickle erythrocytes (Table 1) in a cohort of homozygous SS patients (n = 46). There was an excellent correlation between the calculated values of fp and two parameters: %Hb F and MCHC (Figs 1 and 2). Thus, these independent cellular variables influence polymerization tendency in a strong and predictable fashion: that is, fp varies inversely with Hb F concentration and directly with MCHC. We next attempted to correlate the calculated values of fp for these patients with indices of hemolytic and clinical severity. Linear regression plots of fp versus hematocrit (Fig 3), whole blood hemoglobin concentration (Fig 4), and hospitalization days/yr (Fig 5) were made. An inverse correlation was found between fp and hematocrit, which would be expected if low values of fp were associated with lower hemolytic rates and vice versa. The correlation between fp and whole blood hemoglobin concentration was not significant (Fig 4). These results are discrepant with those of Keidan et al,35 who showed a strong negative correlation between fp and hemoglobin concentration. Although we can offer no explanation for this anomaly, it may be due to variation in the number of patients evaluated (this study, n = 46; Keidan et al, n = 30).
The correlation between fp and vasoocclusive severity measured by hospitalization data, however, was not good. Whereas fp > 0.45 appeared to be necessary for a severe clinical course and perhaps also for short survival, many SS patients with high fp had a mild clinical course. The only other study in which a correlation between fp and clinical severity for a cohort of homozygous SS patients was attempted is that of Keidan et al35 in which laboratory and clinical parameters for 30 patients were evaluated. In this case, the solubility of mixtures of Hb S with non-S hemoglobins, as a function of O2 saturation, was estimated by use of theoretical assumptions regarding the thermodynamics of gelation.28,30,34 Keidan et al35 calculated values of fp at pO2= 0 (0% saturation) and pO2 = 40 mm Hg (corresponding to the oxygen saturation of the venous circulation); these values correlated well (P < .05) with a visual analogue scale used by each patient to indicate perceived disease severity. A strong inverse correlation (P < .01) was also found between fp at pO2 = 40 mm Hg and whole blood hemoglobin concentration, suggesting that polymerization tendency at the venous pO2 is a determinant of the hemolytic rate in individuals homozygous for Hb S.
We can offer several reasons for the disparity between our findings and those of Keidan et al.35 First, our calculations of fp used measured values of the cellular variables that modulate the solubility of partially liganded Hb S (2,3-DPG concentration, non-S hemoglobin composition, and MCHC). It is the interplay of these variables, along with pHi and O2 affinity, that determine csat, the solubility of monomeric Hb S inside the partially oxygenated sickle erythrocyte. Second, our patient population was skewed toward individuals with the greatest disease severity, as our clinic tends to attract such patients. Third, different criteria were used to assess disease severity.
Because the microvascular occlusion that underlies the pathophysiology of sickle cell anemia is polymerization-dependent, the polymer fraction of erythrocytes from individual patients should correlate with disease severity. Our results seem to indicate, however, that the clinical course of homozygous SS disease in individual patients cannot be predicted exclusively by fp, which assumes a homogeneous distribution of the five cellular variables that modulate intraerythrocytic polymerization. This suggests that the assumption of a uniform distribution in the cell population of the variables that modulate polymer formation (intracellular hemoglobin concentration, 2,3-DPG concentration, pHi, non-S hemoglobin composition, and O2 saturation) may not be valid.26 27
Because polymerization is far from equilibrium in the majority of cells in circulating erythrocytes,43,44 a quantitative approach to disease severity would require a kinetic analysis and knowledge of the distribution of delay times (td) for intracellular polymerization, which has not yet been done. However, the well-known supersaturation relationship45 relates td to ci and csat [(1/td = γ•(ci/csat)n, where γ is a kinetic constant and n has values of 30 to 40] so that kinetic parameters should correlate with equilibrium parameters. Distributions of fp at equilibrium would also be helpful, but such data are not available. One is left, then, with the measurement of whole cell average parameters (ie, pHi, 2,3-DPG concentration, non-S hemoglobin composition, and MCHC).
It has been amply demonstrated46,47 that the red blood cell population in sickle cell anemia is not homogeneous,43 44but contains cells of widely varying Hb F content, 2,3-DPG, and total hemoglobin concentration (MCHC). Thus, the amount of polymerized Hb S varies considerably among the cell population, and our calculated values of fp represent only a weight-mean average. Accordingly, the red blood cell heterogeneity in individual patients implies that some cells are much higher in MCHC than others; some are devoid of Hb F, while others are rich in it; and cells show considerable variability in 2,3-DPG concentration and O2saturation. The net result is that fp varies considerably and the mean fp for all cells does not provide a reliable yardstick to measure disease severity.
Address reprint requests to Oswaldo Castro, MD, Center for Sickle Cell Disease, Howard University, 2121 Georgia Ave, Washington, DC 20059.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 6. Clinical severity score (derived from hospitalization frequency data [see footnote in Table 1 for details]) versus fp (no statistical analysis of these data was possible). The figure shows the distribution of fp for patients examined over the range of clinical severity observed (0 to 3). Although all 46 patients were included, some patients (n = 16) with identical CSS and fp appear as a single point. For this reason, the number of points is less than 46.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1777/3/m_blod4050606.jpeg?Expires=1767734445&Signature=ZSYc2fkH6G7I6NNv0Thja9z8-aRG16mY~I6gTCDQt1xMwM1LAjP-s96ORmBnXxzCYTSjlv6Oi7qmqnxKlhuD-wv6JuNgU7T1RoN6YoizppvHvQK3gsuzqdZCMgRdhyqTYEqYeCKCVYxHJOBtpl-hqsgpNI6e6kZcYEdVQ~nNQrBpxeUh4yb4l-7eFYv44Ub7QFVlzVY-jWAQial0sFGkQ1axS-0eZnf1Q5pJ6pxZCSG7ZgPUbHtxJ6M~PQn0L~Z-OIutKyfr7-yftY3Ohk5FI99Hp0OF9bN3Ns9co3XD0L2gSp178CwZc59j2DlqXhz-ugxWBae5-eSdy9~8A8yGMw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal