Abstract
Bcr-Abl expression in leukemic cells is known to exert a potent effect against apoptosis due to antileukemic drugs, but its mechanism has not been elucidated. Recent reports have indicated that a variety of apoptotic stimuli cause the preapoptotic mitochondrial release of cytochrome c (cyt c) into cytosol, which mediates the cleavage and activity of caspase-3 involved in the execution of apoptosis. Whether Bcr-Abl exerts its antiapoptotic effect upstream to the cleavage and activation of caspase-3 or acts downstream by blocking the ensuing degradation of substrates resulting in apoptosis, has been the focus of the present studies. In these, we used (1) the human acute myelogenous leukemia (AML) HL-60 cells that are stably transfected with thebcr-abl gene (HL-60/Bcr-Abl) and express p185 Bcr-Abl; and (2) the chronic myelogenous leukemia (CML)-blast crisis K562 cells, which have endogenous expression of p210 Bcr-Abl. Exposure of the control AML HL-60 cells to high-dose Ara-C (HIDAC), etoposide, or sphingoid bases (including C2 ceramide, sphingosine, or sphinganine) caused the accumulation of cyt c in the cytosol, loss of mitochondrial membrane potential (MMP), and increase in the reactive oxygen species (ROS). These preapoptotic events were associated with the cleavage and activity of caspase-3, resulting in the degradation of poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) and DNA fragmentation factor (DFF), internucleosomal DNA fragmentation, and morphologic features of apoptosis. In contrast, in HL-60/Bcr-Abl and K562 cells, these apoptotic stimuli failed to cause the cytosolic accumulation of cyt c and other associated mitochondrial perturbations, as well as the failure to induce the activation of caspase-3 and apoptosis. While the control HL-60 cells showed high levels of Bcl-2 and barely detectable Bcl-xL, HL-60/Bcr-Abl cells expressed high levels of Bcl-xL and undetectable levels of Bcl-2, a pattern of expression similar to the one in K562 cells. Bax and caspase-3 expressions were not significantly different between HL-60/Bcr-Abl or K562 versus HL-60 cells. These findings indicate that Bcr-Abl expression blocks apoptosis due to diverse apoptotic stimuli upstream by preventing the cytosolic accumulation of cyt c and other preapoptotic mitochondrial perturbations, thereby inhibiting the activation of caspase-3 and execution of apoptosis.
CHRONIC MYELOGENOUS leukemia (CML) is a malignancy of pluripotent hematopoietic cells caused by the dysregulated activity of the tyrosine kinase (TK) encoded by the chimeric bcr-abl gene.1 This fusion gene either encodes for the p210 or p185 TK.1 These are implicated in the pathogenesis of CML and approximately 25% of the adult lymphoblastic leukemia (ALL), respectively.2Bcr-Abl–expressing leukemic blasts are highly resistant to different classes of chemotherapeutic drugs.3,4 Consistent with this, cells derived from patients with CML in blast crisis (CML-BC), eg, K562 cells, which express p210 Bcr-Abl, have also been shown to be highly resistant to antileukemic drug-induced apoptosis.5,6Additionally, these cells are known to overexpress the antiapoptotic Bcl-xL, but not Bcl-2.7 This may partly contribute toward the resistance of these cells to high doses of antileukemic drugs such as Ara-C or etoposide.6
Previous studies from our laboratory have shown that treatment of human acute myelogenous leukemia (AML) HL-60 cells with high-dose Ara-C (HIDAC) and etoposide causes cleavage and activity of caspase-3, which results in the degradation of a number of substrates, including poly (adenine diphosphate [ADP]-ribose) polymerase (PARP) and lamins, producing the morphologic features of apoptosis.8,9Caspase-3 activity has also been shown to cleave and activate a recently cloned DNA fragmentation factor (DFF), which results in the DNA fragmentation of apoptosis.10 More recent reports have also shown that after treatment with antileukemic drugs, the cleavage and activity of caspase-3 is promoted by the mitochondrial release and accumulation of cyt c into the cytosol.11-13 This is associated with the mitochondrial permeability transition, resulting in the loss of membrane potential and increase in reactive oxygen species (ROS).11,14 The three-dimensional structure of Bcl-2 and Bcl-xL has similarity to the pore-forming domain of the bacterial toxins, suggesting that Bcl-2 and Bcl-xL may be channel proteins that regulate the transport of ions and small proteins, like cyt c, across the outer mitochondrial membrane.15 Overexpression of Bcl-2 or Bcl-xLblocks the mitochondrial release of cyt c, thereby preventing the activation of caspase-3 and apoptosis.11 13 Whether the antiapoptotic effect of Bcr-Abl is due to the inhibition of the upstream preapoptotic mitochondrial events or is exerted downstream through the inhibition of the activity of caspase-3 has not been elucidated. In the present studies using HL-60 cells with enforced expression of p185 Bcr-Abl, as well as K562 cells, we show that Bcr-Abl inhibits apoptosis due to diverse stimuli, including HIDAC, etoposide, and sphingoid bases (ie, C2 ceramide, sphingosine, and sphinganine) by blocking the mitochondrial release of cyt c and other preapoptotic mitochondrial events, thus inhibiting the cleavage and activity of caspase-3 and execution of apoptosis.
MATERIALS AND METHODS
Reagants.
Ara-C and etoposide were purchased from Sigma Chemicals (St Louis, MO). Fas agonist CH11 (IgM) antibody was purchased from Kamiya Corp (Seattle, WA). The tetrapeptide caspase inhibitor, zVAD-fmk, was purchased from Bachem, Inc (Torrance, CA). C2 ceramide (N-acetyl sphingosine), D-erythrosphingosine (SO), and d,l-erythro-dihydrosphingosine (Sphinganine, SA) were purchased from Matreya, Inc (Pleasant Gap, PA). γ-Interferon was purchased from GIBCO (Grand Island, NY). Drugs were stored and reconstituted for experiments as previously described.9 Rabbit anti–Bcl-x and anti-Bax antisera and anti–Bcr-Abl antibody were purchased from Pharmingen (San Diego, CA). Monoclonal anti–Bcl-2 (No. 124) was obtained from DAKO Corp (Carpinteria, CA). Dr Ronald Jemmerson of the University of Minnesota Medical School (Minneapolis, MN) kindly provided the monoclonal antibody (MoAb) to cyt.12Anti–caspase-3 and anti-Fas ligand (FasL) antibodies were purchased from Transduction Laboratories (Lexington, KY). Anti-Fas antibody was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Rabbit anti-DFF antisera was kindly provided by Dr Xiaodong Wang of the University of Texas Southwestern School of Medicine (Dallas, TX).
Cells and transfection of the bcr-abl gene.
Human acute myeloid leukemia HL-60/Bcr-Abl and HL-60/neo cells were created by transfection of the bcr-abl gene encoding the p185 Bcr-Abl and/or neomycin-resistant gene and passaged twice per week, as previously described.16 K562 cells were passaged as previously reported.6 Logarithmically growing cells were used for the studies described below.
Western analyses of proteins.
Western analyses of Bcl-2, Bcl-xL, Bax, Bcr-Abl, DFF, and β-actin were performed using specific antisera or MoAbs (see above), as previously described.17 Briefly, protein was extracted from cells with lysis buffer (142.5 mmol/L KCl, 5 mmol/L MgCl2, 10 mmol/L HEPES (pH 7.2), 1 mmol/L EGTA, 0.2% NP40, and 0.2 mmol/L phenylmethylsulfonly fluoride [PMSF]) supplemented with 0.2 trypsin inhibitory units/mL aprotinin, 0.7 μg/mL pepstatin, and 1 μg/mL leupeptin. Appropriate protein amounts (20 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% gel). After electrophoresis, proteins were transferred to nitrocellulose sheets (0.5 A at 100 V; 4°C) for 1 to 3 hours. The blots were blocked in 5% nonfat dry milk solution for 3 hours at room temperature with gentle shaking (5% nonfat milk [wt/vol]/phosphate-buffered saline [PBS]/0.02% sodium azide [pH 7.4]). This was followed by incubation with the respective antibody (1:1,000 dilution) at room temperature and then with antirabbit or antimouse peroxidase-conjugated secondary IgG antibodies. Immune complexes were detected with an enhanced chemiluminescence detection method by immersing the blot for 1 minute in a 1:1 mixture of chemiluminescence reagents A and B (Amersham UK, Little Chalfont, UK) and then exposing to Kodak X-OMAT film (Eastman Kodak Co, Rochester, NY) for a few seconds. Horizontal scanning densitometry was performed on Western blots by using acquisition into Adobe Photo Shop (Apple, Inc, Cupertino, CA) and analysis by the NIH Image Program (US National Institutes of Health, Bethesda, MD).
Preparation of S-100 fraction and Western analysis for cytochrome c.
Untreated and drug-treated cells were harvested by centrifugation at 1,000g for 10 minutes at 4°C. The cell pellets were washed once with ice-cold PBS and resuspended with 5 vol of buffer (20 mmol/L HEPES-KOH, pH 7.5, 10 mmol/L KCl, 1.5 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L sodium EDTA, 1 mmol/L sodium EGTA, 1 mmol/L dithiothreitol, and 0.1 mmol/L PMSF), containing 250 mmol/L sucrose. The cells were homogenized with a 22-gauge needle, and the homogenates were centrifuged at 100,000g for 15 minutes at 4°C (S-100 fraction). The supernatants were collected and the protein concentrations of S-100 were determined by Bradford method (Bio-Rad, Hercules, CA). A total of 20 to 30 μg of S-100 was used for Western blot analysis of cyt c, as described previously.9 17
Measurement of mitochondrial potential and ROS.
In the untreated and drug-treated HL-60/neo, HL-60/Bcr-Abl, and K562 cells, to assess the changes in mitochondrial potential and ROS, 5 × 105 cells were incubated for 15 minutes at 37°C with 40 nmol/L 3,3′ dihexyloxacarbocyanine iodide (DiOC6[3]) and 5 μmol/L dichlorodihydrofluorescein diacetate (DCFH-DA), respectively and analyzed by fluorescence-activated cell sorting (FACS) as described previously.11,14 18
In vitro PARP cleavage activity of caspase-3.
Detection of internucleosomal fragmentation of genomic DNA by agarose gel electrophoresis.
Morphology of apoptotic cells.
After treatment with or without drug, 50 × 103 cells were washed with PBS (pH 7.3) and resuspended in the same buffer. Cytospin preparations of the cell suspensions were fixed and stained with Wright stain. Cell morphology was determined by light microscopy. In all, five different fields were randomly selected for counting of at least 500 cells. The percentage of apoptotic cells was calculated for each experiment, as previously described.20
RESULTS
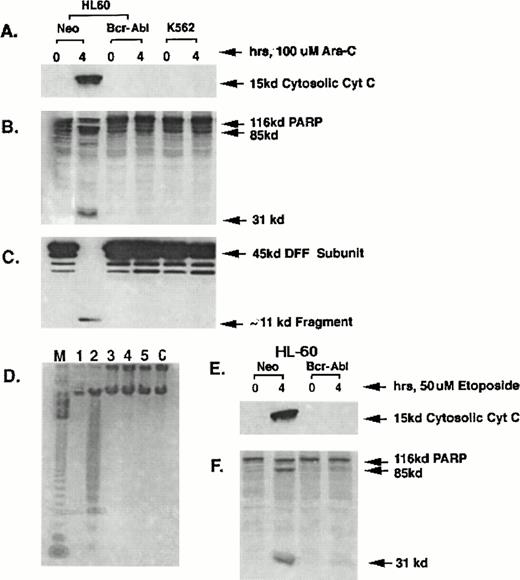
Effect of HIDAC or etoposide on Bcr-Abl expression, cytosolic accumulation of cyt c, caspase-3 activity and apoptosis.
The effects of HIDAC and etoposide treatment on the mitochondrial release of cyt c, with resultant activation of the caspase-3 activity, DNA fragmentation, and the morphologic features of apoptosis were compared in HL-60/Bcr-Abl and K562 cells versus the control HL-60/neo cells. Figure 1A shows that following an exposure to 100 μmol/L Ara-C for 4 hours, a marked increase in the cytosolic cyt c level was observed in HL-60/neo cells, but not in either the HL-60/Bcr-Abl or K562 cells. Although not shown here, after exposure to apoptotic stimuli, the cytosolic accumulation of the cyt c is due to its release from the mitochondria.11,13 The ability of HIDAC-mediated accumulation of cytosolic cyt c to trigger the protease activity of caspase-3 was determined by examining (1) whether the S-100 fraction from the untreated or HIDAC-treated cells would promote the degradation of the in vitro-translated PARP into its 85-kD and 31-kD cleaved products; or (2) whether the S-100 fraction would show the cleavage of the 45-kD subunit of DFF into 30-kD and, later, completely into 11-kD fragments.10 Figure 1B and C show that the S-100 fraction of only the HIDAC-treated HL-60/neo cells produced the degradation of the in vitro-translated PARP (Fig 1B) and showed the complete cleavage of the 45-kD subunit of DFF into its 11-kD fragment (Fig 1C), which is known to promote the DNA fragmentation of apoptosis.10 Neither the PARP nor DFF cleavage was observed in S-100 fractions of the HIDAC-treated HL-60/Bcr-Abl or K562 cells or of the untreated three cell-types (Fig 1B and C). Correspondingly, HIDAC treatment caused internucleosomal DNA fragmentation in HL-60/neo cells (Fig 1D, lane 2), but not in HL-60/Bcr-Abl and K562 cells (Fig1D, lanes 4 and 6). Lanes 1, 3, and 5 contain DNA from untreated HL-60/neo, HL-60/Bcr-Abl, and K562 cells, respectively. Figure 1E and F show that after the exposure to 50 μmol/L etoposide for 4 hours, only the HL-60/neo cells showed the cytosolic accumulation of cyt c and the degradation of the in vitro-translated PARP into its cleaved products. Again, this was not observed in the etoposide-treated HL-60/Bcr-Abl (Fig 1E and F) or K562 cells (not shown). The S-100 fractions of etoposide-treated HL-60/neo cells also showed the cleavage of the 45-kD DFF subunit into its 11-kD fragment, which was not observed in HL-60/Bcr-Abl or K562 cells (data not shown). The concentrations of Ara-C and etoposide selected for these experiments have been previously shown to be potent inducers of DNA fragmentation and the morphologic features of apoptosis in the control HL-60 cells.6,8,11 20The results presented in Fig 1A through F are representative of three separate experiments.
Western analysis of cytosolic levels of cyt c (A); 116-kD35S-labeled, in vitro–translated PARP or its cleaved products (B); 45-kD DFF subunit or its 11-kD cleavage fragment (C); as well as the internucleosomal DNA fragmentation in the untreated and HIDAC-treated HL-60/neo (D, lanes 1 and 2), HL-60/Bcr-Abl (lanes 3 and 4), or K562 cells (lanes 5 and 6). Lane M in (D) represents the 123-bp marker DNA ladder. (E and F) Show Western analyses of the levels of cytosolic cyt c (E) and PARP or its cleaved products (F) in the untreated or etoposide-treated HL-60/neo or HL-60/Bcr-Abl cells.
Western analysis of cytosolic levels of cyt c (A); 116-kD35S-labeled, in vitro–translated PARP or its cleaved products (B); 45-kD DFF subunit or its 11-kD cleavage fragment (C); as well as the internucleosomal DNA fragmentation in the untreated and HIDAC-treated HL-60/neo (D, lanes 1 and 2), HL-60/Bcr-Abl (lanes 3 and 4), or K562 cells (lanes 5 and 6). Lane M in (D) represents the 123-bp marker DNA ladder. (E and F) Show Western analyses of the levels of cytosolic cyt c (E) and PARP or its cleaved products (F) in the untreated or etoposide-treated HL-60/neo or HL-60/Bcr-Abl cells.
Loss of mitochondrial membrane potential (MMP) and increase in ROS during apoptosis.
We have recently reported that HIDAC- or etoposide-induced cytosolic accumulation of cyt c is followed by a decrease in the inner MMP and an increase in the ROS, while these preapoptotic mitochondrial perturbations are blocked by overexpression of Bcl-xL and Bcl-2.11 13 Figure 2 shows the effect of HIDAC treatment (100 μmol/L for 4 hours) on the percentage of HL-60/neo, HL-60/Bcr-Abl, or K562 cells that displayed either low MMP (ΔΨM) or increased ROS, as detected by the cellular uptake of the fluorochrome, DiOC6 (for MMP), or the staining by DCFH-DA (for ROS), respectively. As shown, HIDAC treatment caused a significant increase in the percentage of cells (39%; control, < 3.5%) showing low MMP and significantly higher levels of ROS ( 33%; control, < 2.0%) in the HL-60/neo cells, but not in the HL-60/Bcr-Abl or K562 cell populations (P < .001, pairedt-test). Similar results were obtained in etoposide-treated HL-60/neo versus HL-60/Bcr-Abl or K562 cells (data not shown). These findings are representative of three separate experiments.
Reduction of the inner mitochondrial membrane potential (▵ΨM, A) and production of ROS (B) in untreated control or Ara-C–treated (4 hours) HL-60/neo, HL-60/Bcr-Abl, and K562 cells. Ara-C treatment increased the percentage of HL-60/neo, but not HL-60/Bcr-Abl or K562 cells, which displayed either low ▵ΨM (DiOC6[3] uptake) or high ROS production (staining by DCFH-DA). The open peak (indicated by the arrow) represents the positive control after treatment with 10 mmol/L H2O2.
Reduction of the inner mitochondrial membrane potential (▵ΨM, A) and production of ROS (B) in untreated control or Ara-C–treated (4 hours) HL-60/neo, HL-60/Bcr-Abl, and K562 cells. Ara-C treatment increased the percentage of HL-60/neo, but not HL-60/Bcr-Abl or K562 cells, which displayed either low ▵ΨM (DiOC6[3] uptake) or high ROS production (staining by DCFH-DA). The open peak (indicated by the arrow) represents the positive control after treatment with 10 mmol/L H2O2.
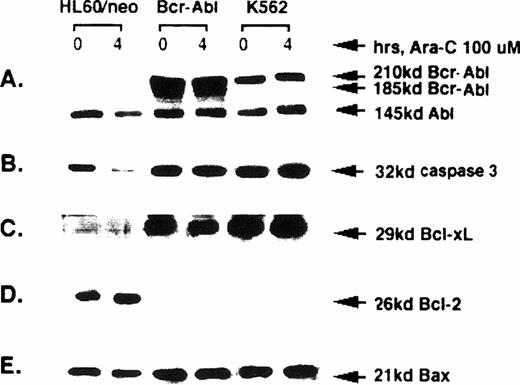
Expression of Bcr-Abl, caspase-3, Bcl-xL, Bcl-2, and Bax.
The levels of Bcr-Abl, Abl, caspase-3, Bcl-xL, Bcl-2, and Bax were determined by Western analyses of the protein extracts of untreated or HIDAC-treated HL-60/neo, HL-60/Bcr-Abl, and K562 cells. Figure 3A shows that HL-60/Bcr-Abl and K562, but not HL-60/neo, cells express p185 and p210 Bcr-Abl, respectively. In contrast, all of the cell-types express p145 Abl. Following Ara-C treatment for 4 hours, neither Bcr-Abl nor Abl levels were altered in any of the cell-types (Fig 3A). In contrast, exposure to Ara-C resulted in a significant decline in the level of 32-kD caspase-3 levels due to its cleavage and activation in HL-60/neo, but not in HL-60/Bcr-Abl or K562 cells (Fig 3B). It is noteworthy that although the latter two cell types are resistant to apoptosis, their 32-kD caspase-3 levels are not significantly different from those in HL-60/neo cells (Fig 3B), when normalized for the levels of β actin serving as the control. We have previously reported that HIDAC-induced downregulation of the 32-kD caspase-3 levels in HL-60/neo cells is due to its cleavage into 20- and 12-kD fragments.8 As previously noted for HL-60 cells, Fig 3C and D show that HL-60/neo cells have high Bcl-2, but barely detectable Bcl-xLlevels.6 Confirming our previous findings,38the enforced expression of p185 Bcr-Abl in HL-60 cells resulted in a dramatic downregulation of Bcl-2 and an associated upregulation of Bcl-xL. It is noteworthy that a similar pattern of expression of these proteins has been observed in K562 cells.6 There was no significant difference in Bax levels among the various cell types, when normalized to the intracellular β actin levels serving as the control. It is also noteworthy that, except for the caspase-3 levels, HIDAC treatment did not affect the levels of these proteins in any of the cell types (Fig 3A, C, D, and E).
Western analyses of Bcr-Abl, Abl, 32 kD caspase-3, Bcl-xL, Bcl-2, and Bax levels in the untreated or Ara-C–treated HL-60/neo, HL-60/Bcr-Abl or K562 cells.
Western analyses of Bcr-Abl, Abl, 32 kD caspase-3, Bcl-xL, Bcl-2, and Bax levels in the untreated or Ara-C–treated HL-60/neo, HL-60/Bcr-Abl or K562 cells.
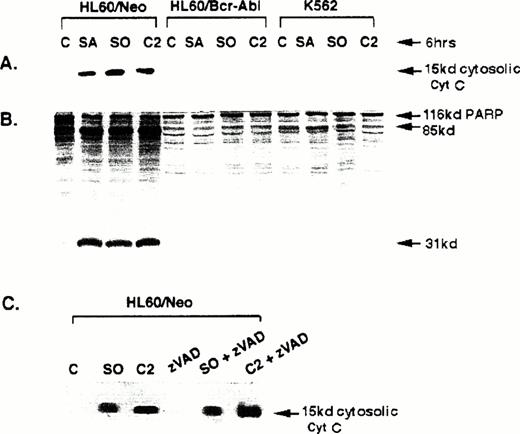
Cytosolic cyt c accumulation and caspase-3 activity caused by sphingolipids.
We next determined whether apoptotic stimuli other than the antileukemic drugs Ara-C and etoposide cause cyt c accumulation in the cytosol and trigger caspase-3 activity in HL-60/neo cells, as well as whether these are affected by the expression of Bcr-Abl in HL-60/Bcr-Abl or K562 cells. Exposure of HL-60/neo cells to the sphingoid bases C2 ceramide (50 μmol/L), sphingosine (10 μmol/L), or sphinganine (10 μmol/L) for 6 hours, which have been previously shown to induce apoptosis in HL-60 cells,21 clearly caused significant cytosolic accumulation of cyt c (Fig 4A) and the generation of the in vitro-translated PARP cleavage activity of caspase-3 (Fig 4B). These molecular events were blocked in HL-60/Bcr-Abl and K562 cells. Figure 4C shows that in HL-60/neo cells cotreatment with zVAD-fmk, a tetrapeptide inhibitor of caspases including caspase-3,22 did not affect the cytosolic accumulation of cyt c due to C2 ceramide, sphingosine (Fig4, lanes 5 and 6), and sphinganine (not shown); but prevented the PARP cleavage activity of caspase-3 and the DNA fragmentation of apoptosis due to these sphingoid bases (data not shown).
Western analyses of the levels of cytosolic cyt c (A) and 116 kD 35S-labeled, in vitro-translated PARP or its cleaved products (B) in untreated and sphingosine (SO), sphinganine (SA), or C2 ceramide-treated HL-60/neo, HL-60/Bcr-Abl, and K562 cells. (C) Shows Western analysis of the cytosolic cyt c levels in HL-60/neo cells after exposure to SO, C2 ceramide, or zVAD alone; or zVAD plus SO or C2 ceramide (C) (see text).
Western analyses of the levels of cytosolic cyt c (A) and 116 kD 35S-labeled, in vitro-translated PARP or its cleaved products (B) in untreated and sphingosine (SO), sphinganine (SA), or C2 ceramide-treated HL-60/neo, HL-60/Bcr-Abl, and K562 cells. (C) Shows Western analysis of the cytosolic cyt c levels in HL-60/neo cells after exposure to SO, C2 ceramide, or zVAD alone; or zVAD plus SO or C2 ceramide (C) (see text).
DISCUSSION
Although previous reports have shown that Bcr-Abl expression confers resistance against the antileukemic drug-induced DNA fragmentation and morphologic features of apoptosis, the mechanism or the step at which this antiapoptotic effect is exerted has not been clearly defined.23 Results of present studies using the human AML HL-60 cells have highlighted several novel findings. We show for the first time that (1) treatment with the antileukemia drugs, Ara-C or etoposide, as well as exposure to sphingoid bases (C2ceramide, sphingosine, or sphinganine) cause the mitochondrial release and cytosolic accumulation of cyt c; (2) Ara-C or etoposide treatment also induces the cleavage and activity of DFF, which promotes the DNA fragmentation of apoptosis; and (3) Bcr-Abl expression results in the inhibition of the preapoptotic mitochondrial perturbations, thereby blocking the generation of caspase activity and apoptosis.
The precise mechanism by which apoptotic stimuli cause the egress of cyt c from the inner mitochondrial membrane to the cytosol, followed by the loss of MMP and increase in ROS, has not been elucidated.24 The mechanism by which the cytosolic cyt c brings about the cleavage and activation of caspase-3 in the presence of deoxy adenosine triphosphate (dATP) (or deoxy ADP [dADP]) and two additional apoptotic protease activation factors (APAF-1 and APAF-3) has also not been determined.12 More recently, however, a clearer picture of this has emerged. Human APAF-1 has been shown to have nucleotide binding sites and to bind cyt c. APAF-1 also possesses in its N terminus domains sequence homology to the proapoptotic ced 4 and ced 3 genes of Caenorhabditis elegans; the latter gene is homologous to caspase-3.25 Nonetheless, it remains unclear how the TK encoded by Bcr-Abl either blocks the stimulus for the release of cyt c from mitochondria or prevents its accumulation in the cytosol. Phosphorylation of Bcl-2 and Bcl-xL has also been shown to affect their antiapoptotic effects, perhaps through an alteration of their function as the channel proteins residing in the outer mitochondrial membrane and regulating the trafficking of ions and small molecular weight proteins such as cyt c.15,23,26-30 But, there is no evidence that Bcr-Abl causes a posttranslational modification of Bcl-2, Bcl-xL, or Bax by affecting their phosphorylation status (Fig 3). Phosphorylation of Bad, a proapoptotic member that heterodimerizes with Bcl-xLor Bcl-2, has been shown to be a potential mechanism through which interleukin (IL)-3 mediates its antiapoptotic effect on the bone marrow progenitor cells.31,32 Whether Bcr-Abl initiates a cascade of events that results in phosphorylation of Bad is currently under investigation. It seems unlikely that Bcr-Abl would directly phosphorylate Bad, as this was shown to occur on the serine residues. However, it is possible that a serine/threonine kinase such as Raf-1 could be activated by Bcr-Abl and be responsible for at least part of its antiapoptotic effect. The ectopic expression of p210 Bcr-Abl into MO7, 32Dc13, and FDC-P1 cells has been shown to result in a constitutively hyperphosphorylated and activated Raf-1.33Raf-1 is known to phosphorylate Bad, although on a different residue from the one phosphorylated by IL-3–initiated signalling pathway.34 A previous report had indicated that in the mouse hematopoietic Ba/F3 cells, Bcr-Abl expression induces Bcl-2 mRNA expression, thereby suppressing apoptosis of these cells resulting from IL-3 withdrawal.35 Again, this is not the case in the Bcr-Abl–expressing human HL-60/Bcr-Abl or K562 cells. In HL-60/Bcr-Abl cells, Bcl-2 protein levels are almost completely downregulated by the enforced expression of Bcr-Abl, while in K562 cells, the endogenous expression of Bcr-Abl is associated with barely detectable levels of Bcl-2 (Ray et al6 and Fig 3). However, both in HL-60Bcr-Abl and K562 cells, Bcl-xL is upregulated (Fig 3). Because marked suppression of Bcl-2, which like Bcl-xL has also been shown to inhibit the mitochondrial release of cyt c, occurred concomitantly with Bcr-Abl–mediated induction of Bcl-xL, the latter is unlikely to be the sole explanation for the inhibition of cytosolic accumulation of cyt c due to various apoptotic stimuli. This is also supported by our previously reported observation that the enforced expression of the proapoptotic Bcl-xS in K562 cells, which cancels the antiapoptotic activity of Bcl-xL, only partially sensitizes K562 cells to drug-induced apoptosis.6 Also, other cell types that possess high intracellular levels of both Bcl-xL and Bcl-2 still retain the ability to undergo apoptosis after exposure to chemotherapeutic drugs.36 Taken together, the alterations in the levels of Bcl-2 and Bcl-xL observed in association with Bcr-Abl expression do not appear to be the sole mechanisms underlying the blockage of the preapoptotic mitochondrial perturbations observed in the HL-60/Bcr-Abl and K562 cells.
A previous report had indicated that Bcr-Abl expression in myeloid leukemia cells prevents Fas-induced apoptosis,37,38 but does not prevent apoptotic death mediated by cytotoxic T cells, human natural killer, or lymphokine-activated cells.39,40Recently, we have confirmed that Bcr-Abl expression confers resistance against Fas-induced apoptosis (data not shown). Treatment of HL-60 cells by γ-interferon (400 U for 48 hours) followed by CH11 anti-Fas agonist antibody (1 μg/mL for 24 hours), which triggers FasR-mediated signalling, caused the cytosolic accumulation of cyt c and induced the PARP cleavage activity of caspase-3. These cytosolic events were blocked in HL-60/Bcr-Abl cells (data not shown). During apoptosis due to treatment with sphingoid bases or the triggering of Fas death-receptor signalling, the direct cleavage and activation of caspase-3 had been previously reported.41-43 Our findings highlight the contribution of the cytosolic accumulation of cyt c due to these apoptotic stimuli in promoting the cleavage and activity of caspase-3. These data are also consistent with the previous observations showing that Bcl-2 or Bcl-xL overexpression inhibits cytosolic accumulation of cyt c, thereby inhibiting apoptosis due to Fas signalling or sphingoid bases.44-46 In this context, the upstream inhibitory effect of Bcr-Abl on the molecular cascade of apoptosis indicates an additional mechanism of resistance to sphingoid bases or Fas-induced apoptosis.47
G.P.A.-M. has been a Brazilian Research Council (CNPq) Fellow.
Address reprint requests to Kapil Bhalla, MD, Division of Hematology/Oncology, Emory University School of Medicine, Winship Cancer Center, 1365-B Clifton Rd, NE, Atlanta, GA 30322.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 2. Reduction of the inner mitochondrial membrane potential (▵ΨM, A) and production of ROS (B) in untreated control or Ara-C–treated (4 hours) HL-60/neo, HL-60/Bcr-Abl, and K562 cells. Ara-C treatment increased the percentage of HL-60/neo, but not HL-60/Bcr-Abl or K562 cells, which displayed either low ▵ΨM (DiOC6[3] uptake) or high ROS production (staining by DCFH-DA). The open peak (indicated by the arrow) represents the positive control after treatment with 10 mmol/L H2O2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1700/3/m_blod4050302.jpeg?Expires=1767781742&Signature=rlrDidU3UK~fMozHyXFdlnuSMvSudnmf5uVRQQsSnNuUh1bT~agI050J5r7mk7KK8-2sm3W5S2g5C9ReSidEoR~Ql2pZYWPNK6v0zXhFIYL6lg43XIvCzlcJI-~pZZFIYEfoOJJRP9GMcuUb4OM8bl1keCbed~NMoT~2nYDnQufN3jgnAhUdM1v2Vz8j37Dsykh1KlgluUbyAAj6u0~ihoGp4Eh6-V6h8TZqyWsEr6wrT-xE8AUvDJNVYKg-9~YgSZjXu34y~XT7dnPAZKIfsZBHbNPY9xWE2OWCHWeUe37TpCyrPd3ZhwVyy0leqj-vrnOIc8FPk4XmjJ9inhN8fg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal