Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV or HHV8) sequences are present in primary effusion lymphomas (PEL). KSHV+cell lines have been established from such lymphomas. Here we report the first description of the establishment of a KSHV+, EBV− cell line (BCP-1) from the peripheral blood of a patient with PEL. Using this cell line and a KSHV+, EBV+ PEL cell line (HBL-6) previously established from ascitic fluid, we investigated whether in nonobese diabetic/severe combined immunodeficiency disease (Nod/SCID) mice tumors representing PEL can be established. When injected intravenously (IV) into Nod/SCID mice, BCP-1 and HBL-6 infiltrated organs, with only occasional macroscopic tumor formation. Intraperitoneal injections (ip) led to the development of ascites and diffuse infiltration of organs, without obviously solid lymphoma formation, resembling the diffuse nature of human PEL. To investigate a possible mechanism for the peculiar phenotype of PEL, we examine the presence of adhesion molecules and homing markers on PEL cells before and after growing in mice. Both BCP-1 and HBL-6 cells lack expression of important cytoadhesion molecules including CD11a and CD18 (LFA1 α and β chains), CD29, CD31, CD44, CD54 (ICAM-1), and CD62L and E (L and E selectins).
KAPOSI'S sarcoma-associated virus (KSHV or HHV-8) is a gamma herpesvirus first documented in Kaposi's sarcoma.1 KSHV is also present in a rare form of B-cell lymphoma, primary effusion lymphoma (PEL), also designated body cavity–based lymphoma.2 PEL occurs predominantly in human immunodeficiency virus (HIV) infected patients but is also occasionally seen in HIV seronegative individuals. These lymphomas manifest with effusions, usually without lymph node involvement or solid tumor formation.3 PEL is usually, but not always, coinfected with Epstein-Barr virus (EBV).4,5 In contrast to EBV+ and EBV− Burkitt's lymphoma (BL) cells, KSHV+ PELs lack c-myc rearrangements.3Other lymphomas with effusion phenotypes, such as pyothorax-associated lymphoma, do not contain KSHV but are frequently infected with EBV and also have c-myc rearrangements. KSHV+ PELs therefore represent a distinct group of non-Hodgkin's lymphomas (NHL) with distinct clinical, morphologic, immunophenotypic, and genetic characteristics.6 Cell lines have been established from ascitic fluid associated with PEL, most with and some without EBV coinfection.7-11 These lines are latently infected with KSHV and can be induced with the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) to produce KSHV virions.8,12 Previously established cell lines from PEL lack T-cell lineage, most B-cell lineage, and some activation markers including CD19, CD20, CD22, and CD23.2,9 10 The reason for the unusual phenotype of PEL is not known.
The human herpesvirus most closely related to KSHV is EBV.12 In vitro infection of human B lymphocytes by EBV induces proliferation of these cells13 and activates the cells to the stage of lymphoblast differentiation with the expression of B-cell activation markers, including CD23 and CD38. These B cells enter a state of continuous proliferation (immortalization) leading to the establishment of a lymphoblastoid cell line (LCL).14 In LCLs, the virus expresses nine latent proteins (EBNA1-6 and LMP-1, -2A, and -2B) and two EBV-encoded RNAs (EBER-1 and -2); these cell lines closely resemble the EBV+lymphoproliferations that occur in immunosuppressed individuals.
At least two latent proteins of EBV, EBNA-2 and LMP-1, are essential for the immortalization potential of the virus. EBNA-2 acts as a transcriptional activator of cellular and viral genes including LMP-1, which upregulates B-cell activation markers such as CD23, CD40, and the adhesion molecules ICAM-1 (CD54) and LFA-1 (CD11). EBNA-1 binds to the origin of viral replication (oriP) and is expressed in all latently infected cells.13 EBNA-1 is the only EBV latent gene product expressed in BL and representative cell lines, although constitutive activation of the cellular proto-oncogene c-myc by chromosomal translocation to an immunoglobulin locus is invariably also present.15 EBV infection and c-myc activation, either of which alone is normally sufficient for immortalization, can synergize to establish truly transformed cells that are capable of growth in soft agar and tumor formation in mice.16EBV-infected human lymphoblastoid cells derived from newborn cord blood or peripheral blood of patients with aquired immune deficiency syndrome (AIDS) can also be transformed in vitro when transfected with c-myc.16
Here we describe the establishment of a KSHV+, EBV− cell line (called BCP-1) from the peripheral blood of an HIV seronegative patient with a PEL. The characteristics of the primary tumor were previously described.17 BCP-1, and also another KSHV+ cell line dually infected with EBV (HBL-6), form colonies in soft agar and tumors in nonobese diabetic/severe combined immunodeficiency disease (Nod/SCID) mice. Both BCP-1 and HBL-6 lack most adhesion molecules and homing markers, which may help to explain the peculiar body distribution of PEL.
MATERIALS AND METHODS
Nod/SCID mice.
All animal experiments were approved by the British Home Office and the Ethical Committee for animal procedures of the Institute of Cancer Research (ICR). Nod/SCID mice, bred at the ICR, were kept in containment level 2 cabinets, four mice per cage, with all food and water autoclaved.
Isolation of human peripheral-blood mononuclear cells and generation of KSHV+ cell line in vitro.
The cell line BCP-1 was derived from the peripheral-blood mononuclear cells (PBLs) of a HIV seronegative patient with a PEL who previously had Kaposi's sarcoma. The lymphoma was KSHV+ and EBV−, with clinical and morphologic features as previously reported.17 Blood was collected during the course of standard diagnostic procedures under sterile conditions. PBLs were isolated from heparinized blood by isopycnic centrifugation on Ficoll-Hypaque (Pharmacia Fine Chemicals, Piscataway, NJ). The cells were plated at a concentration of 5 × 105 cells per mL in 96-well plates without cyclosporin. Cells were grown in RPMI 1640 supplemented with 20% fetal bovine serum (FBS) and 20% T-stim media (Collaborative Biomedical Products, Bedford, MA) at 37°C in the presence of 5% CO2 and 95% humidity. After 45 days a single clone, BCP-1, was visible. This clone was amplified and cultured without T-stim media.
HBL-6 is a cell line established from the ascitic effusion of an HIV seropositive patient with PEL. This cell line contains both KSHV and EBV. The histology of the primary tumor was previously described.18 Both lines are negative for cytomegalovirus, HHV-6, and HIV by polymerase chain reaction (PCR).
CB33 is an EBV-immortalized, but not transformed, LCL (gift from Ricardo Dalla-Favera16).
Soft agar assays.
Cells (1 × 104) were suspended in a 0.35% agar solution in RPMI 1640 supplemented with 10% FBS and overlaid onto a 0.5% agar solution in RPMI 1640 containing 10% FBS in 35-mm plates prepared the previous day. After incubation for 1 day, 2 mL of RPMI 1640 supplemented with 10% FBS was added. Colonies in soft agar were counted 12 days after plating. Cloning efficiency is the number of colonies ×100 divided by the number of cells plated. Each determination was repeated three times in separate experiments.
Establishment of mouse tumors and ascites.
Four- to 6-week-old Nod/SCID mice were injected intravenously (iv; 107 cells/tail vein injection), intraperitoneally (ip; 5 to 6 × 106 cells/injection), and subcutaneously (sc; 5 to 6 × 106 cells/injection) with either cell line (see Table 2). A total of 32 mice were injected. If animals developed ascites or solid tumors greater than 2 cm or became ill they were killed. Tumors and other organs were fixed in 10% buffered formalin for histology and immunohistochemistry or snap frozen in liquid nitrogen for DNA or RNA extraction. Tumors and ascites were also used to prepare viable cell suspensions for cell-surface phenotype analysis and cell culture.
Injection Sites and Results of KSHV Cell Lines in Nod/SCID Mice
| . | No. of Mice Injected . | No. of Mice With Ascites . | No. of Mice With Tumors . |
|---|---|---|---|
| ip Injections | |||
| BCP-1 | 6 | 6 (100%) | 0 |
| HBL-6 | 6 | 6 (100%) | 0 |
| sc Injections | |||
| BCP-1 | 4 | 0 | 1 (25%) |
| HBL-6 | 4 | 0 | 4 (100%) |
| iv Injections | |||
| BCP-1 | 6 | 0 | 0 |
| HBL-6 | 6 | 0 | 2 (33%) |
| . | No. of Mice Injected . | No. of Mice With Ascites . | No. of Mice With Tumors . |
|---|---|---|---|
| ip Injections | |||
| BCP-1 | 6 | 6 (100%) | 0 |
| HBL-6 | 6 | 6 (100%) | 0 |
| sc Injections | |||
| BCP-1 | 4 | 0 | 1 (25%) |
| HBL-6 | 4 | 0 | 4 (100%) |
| iv Injections | |||
| BCP-1 | 6 | 0 | 0 |
| HBL-6 | 6 | 0 | 2 (33%) |
Histopathology and immunohistochemistry.
Formalin-fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Immunohistochemistry was performed with antibodies to the following antigens: epithelial membrane antigen (EMA; Dako, Glostrup, Denmark), CD3 (Dako), CD20 (L26; Dako), CD30 (Ber H2; Dako), CD43 (Leu 22; Becton Dickinson, Mountain View, CA), and an antibody specific to the interleukin-6 (IL-6) homolog encoded by KSHV (vIL-6).19 In situ hybridization (ISH) for EBV (EBER) was performed as previously described.20
Cell surface immunofluorescence (FACS analysis) of cell lines, tumor cell suspensions, and ascites.
Cell suspensions from tumors were established as previously described.21 Cell lines, cell suspensions, and ascites were stained with unconjugated and fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (MoAbs) specific for cell-surface or cytoplasmic antigens, followed by direct or indirect fluorescence analysis on a FACS 440 (Becton Dickinson) as previously described.21 The MoAbs used were CD10 (Serotec, Kidlington, Oxford, UK), CD19 (FMC 63; Selinus, Hawthorne, Victoria, Australia), CD20 (Dako), CD23 (Dako), CD22 (SeraLab, Crawley, Sussex, UK), CD79a (Dako), CD79b (SN8; gift from B.K. Seon, Roswell Park Cancer Institute, Buffalo, NY), CD39 (AC2), CD72 (BU40), CD25 (Dako), CD30 (Dako), CD38 (BA-6; Serotec), CD3 (Dako), CD5 (Dako), CD7 (Dako), CD13 (Dako), CD14 (UCHM1; Sigma, St Louis, MO), CD11a (Becton Dickinson), CD11b (Serotec), CD11c (Dako), CD18, CD29 (8A2 recognizes β1-subunit of integrin receptors; gift from Nick Kovach, Harbor View Medical Centre, Seattle, WA), CD49d (HP2/1; gift from Sanchez-Madrid, Hospital de la Princesa, Madrid, Spain), 9EG7 (this antibody recognizes an epitope on integrin chain β1, which is only accessible after activation of this integrin; gift from Dietmar Vestweber, WestFalische, Munster, Germany), CD31 (HC1/6; Serotec), CD44, CD49e (Serotec), CD54 (ICAM-1; Becton Dickinson), CD58 (LFA-3; ATCC Hybridoma, ATCC, Bethesda, MD), CD62L (L-selectin; Becton Dickinson), CD62E (R & D Systems, Minneapolis, MN), EMA (ICR-1; gift from C. Dean, ICR, London), terminal deoxynucleotidal transferase (TdT; SeraLab), CD34 (QBend-10; Quantum Biosciences, Cambridge, UK), CD43 (DFT-1; Serotec), CD45 (Sigma), and HLA-DR (L243; Becton Dickinson).
Daudi cells (an EBV+ BL cell line) were used in the FACS analyses as a control EBV+ BL cell line.
EBV latent gene expression.
EBV latent and lytic gene expression in HBL-6 cells (EBV+; KSHV+) was investigated by immunocytochemistry on acetone-ethanol–fixed cytocentrifuge preparations and by immunoblotting of protein extracts. BCP-1 (KSHV+; EBV−) and B958 (EBV+; KSHV−) cell lines were used as negative and positive controls, respectively. For phenotyping, EBV-latent antigens were detected by anticomplement immunocytochemistry22 using polyclonal human antisera with high titre activity to EBV nuclear antigens EBNA1-6 (CP) and EBNA-2 (JT), and by standard three layer immunoalkaline phosphatase antialkaline phosphatase (APAAP; Dako) technique using MoAbs to EBNA-2 (PE2) and to the latent membrane proteins LMP-1 and -2 (CS1 and 4; from L. Young, Department of Cancer Studies, Birmingham University, UK).
PCR and Southern blotting for KSHV.
Genomic DNA was extracted by phenol/chloroform from the two cell lines, ascitic fluid, mouse tumors, and from other mouse organs of interest. PCR for KSHV and EBV were performed with primers and conditions as previously described.23,24 Southern blotting was done with a plasmid containing the terminal repeat (TR) sequence of KSHV as previously described.25 DNA samples were digested withNdell, which cuts only once in each 801-bp TR unit.25
Clonality and Ig gene rearrangement analyses.
Ig heavy chain gene rearrangement analysis was performed by PCR as previously described26 on the patients PEL cells (from ascites) and the BCP-1 cells from the peripheral blood to confirm that BCP-1 was derived from the original PEL. PCR products were TA cloned into PCRScript (Invitrogen, Leek, The Netherlands), and sequence was bidirectionally confirmed on a 377 ABI Sequenator (Perkin Elmer, Norwalk, CT).
Cytogenetic studies.
Cytogenetic analysis was performed using standard techniques. Briefly, colcemid was added to the BCP-1 cells for 90 minutes, and following lysis with 0.075 mmol/L potassium chloride hypotonic solution, the cell pellet was fixed in methanol:acetic acid (3 V:1 V). Slides were allowed to age for 1 week, and metaphase spreads were analyzed after Trypsin-Giemsa staining.
Chromosome painting was performed with biotin-labeled paint probes for chromosomes X, 12, and 14 and visualized with fluorescein conjugated (FITC) antibodies as described elsewhere.27
RESULTS
Tumorigenic capacity of HBL-6 and BCP-1.
Normal B cells and the EBV+ LCL CB33 do not grow in soft agar.16 HBL-6 and BCP-1 were compared with CB33 and NIH3T3 cells for soft agar colony forming ability. As shown in Table1, the KSHV-infected cell lines (Fig1) had high colony-forming efficiency relative to CB33 and NIH3T3. This indicates that both KSHV+cell lines represent truly transformed phenotypes, and EBV is not essential to establish this.
Cloning Efficiencies of Experimental Cell Lines
| Cell Line . | Cloning Efficiency (%) . |
|---|---|
| BCP-1 | 11.5 (2.0) |
| HBL-6 | 9 (1.2) |
| CB33 | 0 |
| NIH3T3 | 0.8 (0.3) |
| Cell Line . | Cloning Efficiency (%) . |
|---|---|
| BCP-1 | 11.5 (2.0) |
| HBL-6 | 9 (1.2) |
| CB33 | 0 |
| NIH3T3 | 0.8 (0.3) |
BCP-1 (A) and HBL-6 (B) cells grow in soft agar and form loose disaggregated colonies.
BCP-1 (A) and HBL-6 (B) cells grow in soft agar and form loose disaggregated colonies.
All mice injected ip with either cell line (BCP-1 or HBL-6) developed lymphomatous effusions similar to PEL, as well as tumor diffusion into various organ sites (Fig 2; Table2). Microscopic lymphoma infiltration was seen in kidneys, liver, pancreas spleen, lungs, and heart, but not in brain. The lymphoma infiltration was confirmed by PCR for a human marker (erv-3) and PCR and Southern hybridization evidence for KSHV. In none of the mice injected ip was there evidence of macroscopic lymphoma.
BCP-1 cells circulating through and forming microscopic tumor deposits in kidney (A) and liver (B) after ip inoculations in Nod/SCID mice. Mitotic figures are prominent.
BCP-1 cells circulating through and forming microscopic tumor deposits in kidney (A) and liver (B) after ip inoculations in Nod/SCID mice. Mitotic figures are prominent.
All mice injected sc with the dually infected HBL-6 cells developed solid lymphomas, but only 1 of 4 injected sc with BCP-1 developed such tumors. These solid lymphomas were surrounded by a prominent vascularity of mouse origin, suggesting that the KSHV-infected lymphoma cells induce angiogenesis through paracrine mechanisms. Such vascularity surrounding KSHV+ lymphomas induced in SCID mice was also recently reported by another group.28
Two of the six mice injected iv with HBL-6 developed solid tumors, but none of the BCP-1 iv-injected mice developed visible tumors or ascites (Table 2).
Histologic examination of the SCID mice xenograft tumors showed a diffuse infiltrate of neoplastic lymphoid cells. The constituent cells were medium-to-large and contained nuclei with unevenly distributed chromatin and prominent nucleoli. Many cells showed plasmacytoid features characterized by eccentrically placed nuclei and amphophilic cytoplasm. Immunohistochemical studies showed the neoplastic lymphoid cells to express the activation antigen CD30 (Ki-1), the hematolymphoid antigen CD43, as well as EMA (Fig 3). The cells lacked CD20 and CD3 reactivity. These immunohistochemical findings are identical to those described in PEL. Although tumor masses developed after sc injections, these could be caused by tumor cell restriction within the tissue plane, rather than tumor aggregation.
Solid lymphoma established in Nod/SCID mouse after iv injection with HBL-6 cells. (A) EBER ISH showing intense localization of signal (black chromagen) in nuclei of tumor cells indicating coinfection by EBV (60× magnification). (B) Polyclonal rabbit antiserum against KSHV vIL-6. A minority of cells express detectable protein (red chromagen), which is restricted to the cytoplasmic compartment (with exclusion of nuclei). (60× magnification; Mayer's hematoxylin counterstain). (C) EMA is strongly expressed in anaplastic tumor cells characterized by large pleomorphic nuclei and abundant mitotic activity (60× magnification; Mayer's hematoxylin counterstain). (D) Leukocyte common antigen (LCA) is not generally expressed in tumor cells consistent with the null immunophenotype association with PEL and derived cell lines (60× magnification; Mayer's hematoxylin counterstain).
Solid lymphoma established in Nod/SCID mouse after iv injection with HBL-6 cells. (A) EBER ISH showing intense localization of signal (black chromagen) in nuclei of tumor cells indicating coinfection by EBV (60× magnification). (B) Polyclonal rabbit antiserum against KSHV vIL-6. A minority of cells express detectable protein (red chromagen), which is restricted to the cytoplasmic compartment (with exclusion of nuclei). (60× magnification; Mayer's hematoxylin counterstain). (C) EMA is strongly expressed in anaplastic tumor cells characterized by large pleomorphic nuclei and abundant mitotic activity (60× magnification; Mayer's hematoxylin counterstain). (D) Leukocyte common antigen (LCA) is not generally expressed in tumor cells consistent with the null immunophenotype association with PEL and derived cell lines (60× magnification; Mayer's hematoxylin counterstain).
The majority of mice injected iv with either BCP-1 or HBL-6 did not develop visible macroscopic tumors, but postmortem examination of several tissues (sampled up to 16 weeks after inoculations; Fig4 and 5) showed the presence of diffuse infiltrates of single or small clumps of KSHV-infected cells, as determined by histology and vIL-6 immunohistochemistry (Fig 4). This was confirmed by PCR and Southern blot analyses (Fig 5) in which various organs were shown to contain KSHV. The human origin of the infiltrating infected cells was confirmed by PCR amplification of human ERV-3 sequences from KSHV+tissues (ERV-3 is an endogenous human retrovirus present at two copies per genome in humans). Tissues from those mice injected with HBL-6 also contained EBV as evidenced by EBER ISH and EBV-specific PCR.
KSHV+ cells are present in various mouse organs after iv injections of KSHV+ cells. (A) Mouse kidney with rare vIL-6+ (BCP-1) cells infiltrating parenchyma. (B) KSHV vIL6+ BCP-1 cells in retroperitoneal fat. (C) Mouse thymus showing numerous small mouse lymphocytes with a small population of larger BCP-1 cell infiltrate, one stained positive for vIL-6.
KSHV+ cells are present in various mouse organs after iv injections of KSHV+ cells. (A) Mouse kidney with rare vIL-6+ (BCP-1) cells infiltrating parenchyma. (B) KSHV vIL6+ BCP-1 cells in retroperitoneal fat. (C) Mouse thymus showing numerous small mouse lymphocytes with a small population of larger BCP-1 cell infiltrate, one stained positive for vIL-6.
Southern blot (A) and PCR (B) for KSHV in various mouse tissues. (Note Southern blots were done independently of PCR and do not represent blotting of PCR products.) MW, molecular markers. Lane 1: Southern blot and PCR-positive ascitic cells from HBL-6 ip-injected mouse. Lane 2: Southern blot and PCR-positive tumor cells from HBL-6 iv-injected mouse. Lane 3: PCR-positive small intestine from HBL-6 iv-injected mouse. Lane 4: Southern blot and PCR-positive testis from HBL-6 iv-injected mouse. Lane 5: Southern blot and PCR-positive kidney from HBL-6 iv-injected mouse. Lane 6: PCR-positive heart from HBL-6 iv-injected mouse. Lane 7: Southern blot and PCR-positive lungs from HBL-6 iv-injected mouse. Lane 8: PCR-positive spleen from HBL-6 iv-injected mouse. Lane 9: Blank. Lane 10: PCR-positive kidney from BCP-1 iv-injected mouse. Lane 11: PCR-positive (weakly) lung from BCP-1 iv-injected mouse. Lane 12: PCR-positive (weakly) liver from BCP-1 iv-injected mouse. Lane 13: PCR-positive thymus from BCP-1 iv-injected mouse.
Southern blot (A) and PCR (B) for KSHV in various mouse tissues. (Note Southern blots were done independently of PCR and do not represent blotting of PCR products.) MW, molecular markers. Lane 1: Southern blot and PCR-positive ascitic cells from HBL-6 ip-injected mouse. Lane 2: Southern blot and PCR-positive tumor cells from HBL-6 iv-injected mouse. Lane 3: PCR-positive small intestine from HBL-6 iv-injected mouse. Lane 4: Southern blot and PCR-positive testis from HBL-6 iv-injected mouse. Lane 5: Southern blot and PCR-positive kidney from HBL-6 iv-injected mouse. Lane 6: PCR-positive heart from HBL-6 iv-injected mouse. Lane 7: Southern blot and PCR-positive lungs from HBL-6 iv-injected mouse. Lane 8: PCR-positive spleen from HBL-6 iv-injected mouse. Lane 9: Blank. Lane 10: PCR-positive kidney from BCP-1 iv-injected mouse. Lane 11: PCR-positive (weakly) lung from BCP-1 iv-injected mouse. Lane 12: PCR-positive (weakly) liver from BCP-1 iv-injected mouse. Lane 13: PCR-positive thymus from BCP-1 iv-injected mouse.
These data show that both cell lines have the capacity to form tumors in Nod/SCID mice and that ip injection gives rise to an effusion tumor similar to that observed in human PEL, including ascites and diffusion of lymphoma cells in various organ systems, but seldom macroscopic lymphoma formation.
Cell surface immunophenotype.
The immunophenotype profile was essentially similar for the cell line established from peripheral blood (BCP-1) and did not change upon xenotransplantation in Nod/SCID mice (Table 3; Fig6). For the coinfected cell line HBL-6 surface phenotype also did not change after xenotransplantation.
FACS Immunophenotype of KSHV and Control Cell Lines
| Cell Lines . | Daudi . | HBL-6 . | BCP-1 . |
|---|---|---|---|
| B-cell markers | |||
| CD10 | + | − | − |
| CD19 | + | − | − |
| CD20 | + | − | − |
| CD22 | + | − | − |
| CD79a | + | + | − |
| CD79b | ND | − | − |
| CD39 | ND | − | − |
| CD72 | ND | − | − |
| Activation markers | |||
| CD23 | + | + | + |
| CD25 | + | + | + |
| CD30 | + | − | − |
| CD38 | + | + | +* |
| Cytoadhesins | |||
| CD11a | + | − | − |
| CD11b | − | + | + |
| CD11c | ND | − | − |
| CD18 | + | − | − |
| CD29 | + | + | + |
| 8A2 | + | + | + |
| CD31 | + | − | − |
| CD44 | + | − | − |
| CD49d | + | + | − |
| CD49e | − | + | − |
| CD54 | + | − | − |
| CD58 | − | + | − |
| CD62L | + | − | − |
| CD62E | + | − | − |
| EMA | − | + | + |
| Miscellaneous markers | |||
| Tdt | ND | − | − |
| CD34 | − | − | − |
| CD43 | + | + | + |
| CD45 | + | − | − |
| HLA-DR | + | − | − |
| Cell Lines . | Daudi . | HBL-6 . | BCP-1 . |
|---|---|---|---|
| B-cell markers | |||
| CD10 | + | − | − |
| CD19 | + | − | − |
| CD20 | + | − | − |
| CD22 | + | − | − |
| CD79a | + | + | − |
| CD79b | ND | − | − |
| CD39 | ND | − | − |
| CD72 | ND | − | − |
| Activation markers | |||
| CD23 | + | + | + |
| CD25 | + | + | + |
| CD30 | + | − | − |
| CD38 | + | + | +* |
| Cytoadhesins | |||
| CD11a | + | − | − |
| CD11b | − | + | + |
| CD11c | ND | − | − |
| CD18 | + | − | − |
| CD29 | + | + | + |
| 8A2 | + | + | + |
| CD31 | + | − | − |
| CD44 | + | − | − |
| CD49d | + | + | − |
| CD49e | − | + | − |
| CD54 | + | − | − |
| CD58 | − | + | − |
| CD62L | + | − | − |
| CD62E | + | − | − |
| EMA | − | + | + |
| Miscellaneous markers | |||
| Tdt | ND | − | − |
| CD34 | − | − | − |
| CD43 | + | + | + |
| CD45 | + | − | − |
| HLA-DR | + | − | − |
Cells from all three lines were negative for T-cell (CD3, CD5 and CD7) and myelymonocytic (CD13 and CD14) markers.
Abbreviation: ND, not determined.
The activation marker CD38 was absent on native BCP-1 cells, but repeatedly became upregulated after growth in the peritoneal cavity of mice.
Representative example of FACS profiles for BCP-1 and HBL-6 cells and cells obtained from Nod/SCID ascitic fluid. EBV+ Daudi cells were used as a control. Fluorescence intensity is expressed on an arbitrary logarithmic scale. Black histograms represent antibody stained cells, and white histograms are isotype-specific controls for each antibody used. As previously shown, KSHV+ PEL cells lack B-cell markers including CD19, but express EMA. ICAM-1 or CD54 is an important adhesion molecule present on most lymphoma cells and upregulated by EBV LMP-1.
Representative example of FACS profiles for BCP-1 and HBL-6 cells and cells obtained from Nod/SCID ascitic fluid. EBV+ Daudi cells were used as a control. Fluorescence intensity is expressed on an arbitrary logarithmic scale. Black histograms represent antibody stained cells, and white histograms are isotype-specific controls for each antibody used. As previously shown, KSHV+ PEL cells lack B-cell markers including CD19, but express EMA. ICAM-1 or CD54 is an important adhesion molecule present on most lymphoma cells and upregulated by EBV LMP-1.
Both lines lacked T-cell and the majority of B-cell markers, although HBL-6 cells expressed CD79a, a marker which identifies the α-chain of the B-cell receptor.29 Both lines expressed B-cell activation markers including CD23, CD25, and CD38 and were, as the previously described PEL lines, EMA+.2 9 Both lines lacked expression of several important adhesion and homing molecules including ICAM-1 (CD54), L- and E-selectin (CD62L and E), CD31, CD44, and CD11a and CD11c. The activation marker CD38 was absent on native BCP-1 cells, but repeatedly became upregulated after growth in the peritoneal cavity of mice.
Normal plasma cells also lack expression of most B-cell antigens and CD11a, CD45, CD62L, CD49e while expressing CD38. By FACS analyses, there is no surface Ig expression on BCP-1 cells; however, heavy chain IgG is expressed as seen on Western blotting (data not shown), indicating internal rather than surface expression.
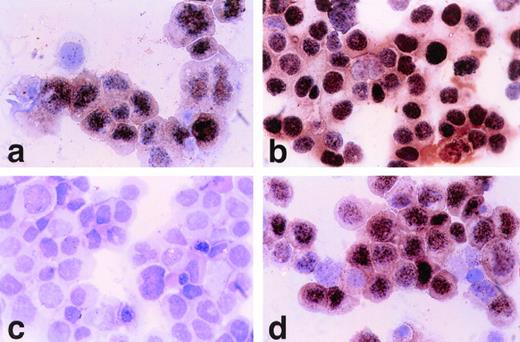
HBL-6 cells displayed a restricted EBV antigen profile limited to EBNA-1 expression, similar to BL. With polyclonal antisera (CP), strong granular staining similar to that seen on the EBV+ B958 cell line was present in all nuclei (Fig 7a and b, see page 1675). HBL-6 cells were negative with MoAbs to EBNA-2 and LMP-1 and -2 (Fig 7c and d).
(a) Polyclonal anti–EBNA1-6 human antisera (CP). Nuclear positive staining on HBL-6 (KSHV+, EBV+) cells. (630× magnification). (b) Polyclonal anti–EBNA1-6 (CP) shows all EBV+ B958 cells have nuclear reactivity. (c) Monoclonal anti–EBNA-2 shows no staining of HBL-6 cells, but B958 cells are positive (d).
(a) Polyclonal anti–EBNA1-6 human antisera (CP). Nuclear positive staining on HBL-6 (KSHV+, EBV+) cells. (630× magnification). (b) Polyclonal anti–EBNA1-6 (CP) shows all EBV+ B958 cells have nuclear reactivity. (c) Monoclonal anti–EBNA-2 shows no staining of HBL-6 cells, but B958 cells are positive (d).
Clonality of BCP-1 cells.
To investigate the relationship, at a molecular level, between the KSHV+ primary ascites cells and the BCP-1 cell line derived from the peripheral blood, we analyzed the VDJ segments of the Ig heavy chain gene from the KSHV+ PEL cells and that from the BCP-1 cell line. PCR amplification showed VDJ products of similar length when analyzed on ethidium bromide–stained polyacrylimide gel. Subsequent DNA sequence analysis showed identical sequences (Fig 8) of both 97-bp amplicons, indicating a clonal identity between the PEL cell line and the BCP-1 cells derived from peripheral blood.
Cytogenetic analysis of BCP-1.
Previous analyses have shown that both the HBL-6 cell line and its parental tumor11 have a normal c-myc allele configuration. Cytogenetic analysis of BCP-1 also revealed no c-myc rearrangements, although this cell line does possess a complex karyotype involving chromosomes X, 12, and 14. Chromosome painting showed that the der(x) correspond to t(x;14,12) (q12; q11:q22-23; q23-24) (Fig 9, see page 1675).
Chromosome painting for chromosomes X, 12, and 14 showed that the der(x) correspond to t(x;14,12) (q12; q11:q22-23; q23-24).
Chromosome painting for chromosomes X, 12, and 14 showed that the der(x) correspond to t(x;14,12) (q12; q11:q22-23; q23-24).
DISCUSSION
We describe the establishment of a KSHV+, EBV− cell line (BCP-1) from the peripheral blood of a patient with PEL. BCP-1 and a KSHV/EBV dually infected cell line (HBL-6), established from an effusion of another patient with PEL, both grow in soft agar and form tumors in mice, indicating transformed phenotypes.16
Clonal analysis of the KSHV+ PEL and the BCP-1 cell line showed identical IgH VDJ segments, providing molecular evidence for the derivation of the BCP-1 cells from the original effusion lymphoma. This finding indicates that PEL cells circulate in the peripheral blood and that cell lines can be derived from these; furthermore, they circulate and infiltrate numerous organs in Nod/SCID mice.
BCP-1 has a complex karyotype concordant with that previously shown in PEL cell lines derived from malignant effusions, but no breakpoints common to other PELs have been identified.9 HBL-6 cells show certain similarity to EBV+ BL cells in that they have a restricted expression of EBV latent antigens (only EBNA-1), but, unlike the latter, they lack c-myc rearrangements. However, HBL-6 cells show evidence of transformation, and this suggests that KSHV can replace c-myc activation as a further step toward a transformed phenotype in the presence of EBNA-1 expression.16 KSHV+ BCP-1 cells also lack c-myc rearrangements, are EBV−, and show evidence of transformation, indicating that KSHV by itself is potentially sufficient to transform B cells.
HBL-6 and BCP-1 have the capacity to generate tumors after injection into Nod/SCID mice, providing further evidence that these cells are transformed (Table 2). Differences were apparent, however, between the two cell lines in tumor-forming capacity and resulting tumor phenotypes, which were dependent on the site of injection.
When injected iv into immunodeficient mice, BCP-1 and HBL-6 infiltrated organs, with only occasional macroscopic tumor formation, whereas ip injections led to the development of ascites without solid lymphoma formation, resembling the diffuse nature of human PEL.30
The major difference in cell surface antigen expression between the HBL-6 and BCP-1 cell lines is the presence of the integrins CD49d, CD49e, and CD58 on HBL-6 but not on BCP-1. CD49e, a component of the fibronectin receptor very late activation antigen 5 (VLA-5; CD49e/CD29 or a5b1), has been shown to correlate with the capacity of malignant human B lymphocytes to disseminate in SCID mice.31 VLA-4 (CD49d/CD29 or α4β1) is also involved in B-cell migration, especially on activated endothelium, through its ligand VCAM-1. The expression of these α-integrins on HBL-6 cells only is consistent with the higher capacity of this line to form solid tumors distal to the site of inoculation in mice.
The presence of Ig gene rearrangements along with the immunophenotype described suggest that these PEL cell lines (and mouse-induced neoplasms) belong to the B-cell lineage with plasmacytic differentiation. The only true “aberrancies” regarding normal plasma cells seem to be the expression of CD23 and lack of CD49d and surface Ig expression. KSHV infection either arrests cells at this stage of differentiation or infects and transforms these mature B cells.
Similar to EBV, KSHV might also transactivate cellular genes involved in B-cell activation32 because KSHV+ PEL cells and cell lines derived from them also express activation markers CD23, CD25, and CD38. Interestingly, CD38 was negative on the BCP-1 cell line, but upregulated after culturing in a different environment, ie, the peritoneal cavity of immunodeficient mice.
The unusual propensity of PEL to involve predominantly body cavity surfaces could be the result of a peculiar homing pattern induced by KSHV infection.33 The traffic of lymphocytes to lymphoid and other tissues is controlled in part by the interaction of lymphocyte adhesion molecules called homing receptors with tissue-selective endothelial ligands known as vascular addressins. LFA-1 α- and β-chains (CD11a/CD18), α4β1 integrin (CD49d/CD29), ICAM-1 (CD54), and L-selectin (CD62L) have all been shown to be involved in the homing of lymphocytes to lymphoid tissues.34 KSHV+PELs do not express these molecules in contrast to the EBV+BLs, EBV+ LCLs, anaplastic large cell lymphomas, and primary central nervous system lymphomas.35 36
The absence of B-cell markers CD10, CD19, CD20, and CD22 on PEL cell lines has already been described.2,10 The lack of CD19 expression may also contribute to the effusion phenotype of these lymphomas, as this B-cell antigen has been shown to be involved in the homing of B cells to lymph nodes and in germinal center formation.37 CD19 is also involved in the spontaneous homotypic adhesion seen in culture,37 and notably BCP-1 and HBL-6 do not grow in clumps in culture. We further observed that both cell lines lack CD79b, a specific and functional B-cell marker identifying the β-chain of the B-cell receptor,29 whereas only HBL-6 expresses CD79a.
The absence of CD44 on PEL cells is also noteworthy. CD44 (Pgp-1 also known as Hermes lymph node homing receptor) is a glycoprotein proposed to be the principal cell surface receptor for hyaluronan and involved in lymphocyte trafficking from blood to lymphatic tissues.38 Different isoforms of CD44 expressed in lymphoid tumor cells have distinct effects on their ability to attach to hyaluronan surfaces and consequently their capacity to form solid tumors in vivo.39 EBV+ LCLs express CD44, as do most primary lymphomas.40 In contrast, EBV+ BLs and Burkitt's cell lines are negative for CD44.41
Both lines express EMA (also known as MUC-1 or eposialin), which is expressed on a variety of epithelial cells and also on anaplastic large lymphoma cells.42 It has been suggested that EMA is an “antiadhesion molecule” involved in the metastatic process (in release of cells from a primary tumor) and in escape from immune surveillance.43
It has been postulated that the absence of certain adhesion molecules on neoplastic cells could contribute to lymphomagenesis by conferring on them some degree of protection from immunosurveillance.32 CD54 and CD58 expressed on B cells have been proposed to interact directly with T cells,44 and EBV+ cells lacking CD11a/CD18 are poor stimulators of T-cell responses.45 Notably, CD54, CD58, CD11a, and CD18 are absent on BCP-1 cells, and HBL-6 cells only express CD58.
Apart from EBV and human T-cell leukemia virus (HTLV)-positive cell lines, this is the only other report of a presumably viral-driven and maintained cell line established from human peripheral blood. These cells grow in soft agar and in immunodeficient mice confirming their transformed phenotype and supporting an oncogenic role for KSHV.
ACKNOWLEDGMENT
We thank J.P. Parry for technical assistance.
Supported by grants from the Cancer Research Campaign, NIH NCI CA 67391, and the James S McDonnel foundation.
Address reprint requests to Chris Boshoff, Chester Beatty, Laboratories, 237 Fulham Rd, London, SW3 6JB, UK or to Yuan Chang, Department of Pathology, College of Physicians and Surgeons of Columbia University, 630 W 168th St, New York, NY.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.









This feature is available to Subscribers Only
Sign In or Create an Account Close Modal