Abstract
The fibrinogen αC domain (Aα 220-610) is one of the earliest targets attacked by plasmin following fibrinolytic system activation. Monoclonal antibodies (MoAbs) to defined sequences within the αC domain provide the opportunity to explore the structure-function relationships involved in plasmin's interaction with its Aα chain substrate at greater resolution and can serve as reagents with potential clinical use for detecting fibrinogenolysis in vivo. The MoAb F-104 was raised against a multiple antigenic peptide derivative modelled after the hydrophilic 12-residue sequence corresponding to Aα 487-498 within the αC domain. A sensitive solution phase competitive enzyme-linked immunosorbent assay (ELISA) was developed for MoAb F-104 that can be applied for the direct measurement of intact fibrinogen (purified or plasma; ED50%≈5 pmol Aα chain equivalents/mL), with negligible cross-reactive interference from peptide cleavage products released by plasmin from the COOH-terminal end of the Aα chain (<3%). Immunoblotting and ELISA studies to characterize the fate of the F-104 epitope during fibrinogenolysis in vitro indicated a rapid loss of fibrinogen-associated immunoreactivity that reflected the heterogeneity of plasmin cleavage sites within the αC domain; cleavage at the 493-494 arg-his bond destroyed the F-104 epitope, while cleavage at other sites released it in an altered, inaccessible, conformation within the structure of 35- to 40-kD and 17.5- to 18-kD Aα chain degradation products. Application of the F-104 ELISA to monitor the course of Aα chain proteolysis in a small study population of patients undergoing thrombolytic therapy for myocardial infarction (n = 14) showed that the loss of fibrinogen-associated F-104 immunoreactivity was a very early marker (within 15 to 30 minutes) of in vivo fibrinogenolysis. Additional data obtained suggest that MoAb F-104 may have promise as a reagent for evaluating the creation of an effective lytic state early during therapy, information that could help determine the need for further clinical intervention. Thus, these studies illustrate a rational, targeted, approach towards the development of a novel antifibrinogen MoAb whose application as a structural probe for the region Aα 487-498 in vitro and in vivo can provide new insights into the various molecular forms of fibrinogen that circulate under physiologic conditions and in disease.
THE PAST DECADE has witnessed a growing interest in the role of fibrinogen as a risk factor in cardiovascular disease. Elevated plasma fibrinogen levels are associated with a number of pathologic conditions, including myocardial infarction, stroke, and peripheral vascular disease.1-5 Circulating fibrinogen concentrations are also increased during menopause, correlating with the enhanced risk of heart disease in older women.6,7Relatively little is known about the mechanism by which elevated fibrinogen levels exert their deleterious effect and, in part, this is due to the limited number of assays available for probing the various structure-function relationships associated with fibrinogen's role in maintaining hemostasis. Indeed, current methods for the measurement of circulating fibrinogen rely, primarily, on clottability-based functional assays.8 While the ease and speed of clottability determinations account for their routine use in the clinical setting, the limitations of this methodology are also well documented.9
The COOH-terminal two thirds of the fibrinogen Aα chain (Aα 220-610), also referred to as the αC domain10 represents a rich source of structural markers for probing the various functionalities associated with fibrinogen's role in maintaining hemostasis. This region is involved in fibrin polymerization,11 it serves as a substrate for Factor XIIIa12and plasmin,13 and it binds to endothelial cells.14 Monoclonal antibodies (MoAbs) that recognize defined sequences within the αC domain have been previously applied to explore several of these processes at greater resolution15-17 and to measure various forms of fibrinogen based on the detection of novel epitopes.18 19
In the case of the two enzyme-linked immunosorbent assays (ELISAs) reported for the specific quantitation of “intact” plasma fibrinogen based on epitopes within the αC domain,18,19the immunogens and the antigens used for antibody selection were large (A)α chain derivatives. The respective epitopes involved in antibody binding were subsequently localized using smaller proteolytic fragments originating from the COOH-terminal two thirds of the Aα chain. The alternative approach, the development of antipeptide MoAbs that target small, defined, sequences within the fibrinogen molecule, has not yet been explored although there is ample precedent for the success of this methodology in the development of fibrin-specific antibodies.20 21
These studies illustrate this targeted approach to MoAb development and focus on the role of the fibrinogen αC domain as a plasmin substrate. The antibody produced, MoAb F-104, specifically recognizes an epitope within the region Aα 487-498 of the dimeric fibrinogen molecule that is not available for binding once plasmin action has occurred. In view of this specificity, MoAb F-104 represents a unique reagent that can be applied not only to learn more about basic aspects of fibrinogen biochemistry, but also to monitor fibrinogenolysis in vivo and consider how disturbances in this process might contribute to the development and management of cardiovascular disease.
MATERIALS AND METHODS
Synthetic Peptides
Aα 487-498 multiple antigenic peptide.
The 12-residue sequence, L-D-G-F-R-H-R-H-P-D-E-A, was synthesized on a Model 431A synthesizer using standard t-Boc chemistry and the resin, [α-N-t-Boc-lys(ε-N-t-Boc)]4-lys2-lys-ala-OCH2-PAM (Applied Biosystems, Foster City, CA). The resulting multiple antigenic peptide, or TAM peptide,22 was comprised of eight copies of the 12-residue sequence attached to a seven-member polylysine core. The crude peptide was used as an immunogen and as an antigen for MoAb characterization (see below) without further purification. Amino acid analysis indicated the following molar composition: Asp, 15.04; Glu, 8.28; Pro, 7.23; Gly, 8.20; Ala, 9.81; Leu, 7.81; Phe, 7.56; His, 15.66; Lys, 7.35; Arg, 16.40.
Aα 487-498 linear peptide.
The monomeric form of the TAM peptide was synthesized as described above except that the starting resin was t-Boc-L-ala-OCH2-PAM (Applied BioSystems). The crude, linear peptide was purified by reversed phase high performance liquid chromatography (HPLC) using a Dynamax preparative C-8 column (2.1 × 25 cm; Rainin, Woburn, MA) and an increasing gradient of acetonitrile for elution (4% to 60% in 0.1% trifluoroacetic acid [TFA]). Analytic HPLC (Dynamax C-8; 0.46 × 25 cm) of the purified material indicated a single, homogenous peak eluting at 31.1% acetonitrile. Results of amino acid analysis gave the following molar composition: Asp, 1.91; Glu, 1.05; Pro, 0.95; Ala, 0.73; Leu, 0.83; Phe, 0.95; His, 1.89; Arg, 2.01.
Monoclonal Antibody Development
Immunization.
Balb/c mice were immunized weekly for 1 month with multiple, intraperitoneal injections of the TAM peptide (50 μg/dose). Serum titers were determined in a direct binding screening ELISA that evaluated comparative binding to fibrinogen and its COOH-terminal Aα chain plasmin derivatives, Aα FDPs (see below). One mouse whose antiserum exhibited preferential binding to intact fibrinogen was taken for fusion.
Hybridoma selection.
Screening assay.
Hybridoma lines secreting immunoglobulins that recognized the region, Aα 487-498, in fibrinogen and/or in Aα FDPs were identified in a comparative direct binding ELISA on antigen-coated plates (purified fibrinogen, 0.86 μg; 5.1 pmol Aα chain equivalents/well; Aα FDPs, 0.73 μg; 24.5 pmol Aα chain equivalents/well), with horseradish peroxidase-conjugated goat antimouse IgG (GAM IgG-HRP) (Kierkegaard & Perry, Gaithersburg, MD) used to detect bound antibody.25 Of the four cell lines selected for cloning based on their strong binding to fibrinogen on the solid phase, one proved stable and was taken for further study.
Antibody production and characterization.
The cell line of interest was expanded in pristane-primed mice26 and its immunoglobulin, designated MoAb F-104 IgG, then purified from ascites on Protein A Sepharose (Bio-Rad, Richmond, CA). The protein concentration of a standard solution of the purified IgG was determined by amino acid analysis. Isotyping was performed with the Mouse Monoclonal Sub-Isotyping Kit (Hyclone, Logan, UT).
Purified Antigens for Epitope Characterization
Human fibrinogen.
Kabi fibrinogen (Chromogenix/Pharmacia-Hepar, Franklin, OH) was further purified by sequential affinity and ion-exchange chromatography on lysine-Sepharose and DEAE-Sephacel.27 The concentration of a standard solution of the purified fibrinogen preparation was determined by amino acid analysis and expressed as molar Aα chain equivalents, assuming a molecular weight of 340 kD and 2 moles of Aα chain per mole of fibrinogen.
Aα FDP preparations.
COOH-terminal Aα chain fibrinogenolytic derivatives, Aα FDPs, were released from purified fibrinogen by mild plasmin digestion and then isolated by affinity chromatography on Concanavalin A Sepharose; the single Aα FDP preparation used for the studies here contained 6,137 ± 1,640 pmol Aα chain equivalents/mL (± standard error of mean [SEM]; n = 3) and a concentration of 182 μg/mL.19
Aα 477-517.
The cyanogen bromide (CNBr) fragment corresponding to the Aα chain region 477-517, referred to here as CNBr IX (to indicate its relative position from the Aα chain's NH2-terminal CNBr fragment, CNBr I), was obtained in partially purified form as reported.28 The CNBr IX-enriched pool isolated by gel filtration was further purified by reversed phase semipreparative HPLC (C-18 column; 0.78 × 30 cm; Waters, Milford, MA), using a mobile phase comprised of 0.1% TFA (Buffer A) and 60% acetonitrile (Buffer B) and an increasing gradient of acetonitrile concentration (30% to 55% B) for elution. The fragment eluting at 35.8% acetonitrile was identified as CNBr IX based on its molar composition, determined by amino acid analysis, and the reported primary structure of the Aα chain.29 30
Proteolysis of Aα 487-498–containing peptides.
The octavalent and linear synthetic peptides (100 μg) were each digested with trypsin (TPCK; Sigma, St Louis, MO) in 0.2 mol/L NH4HCO3, pH 8.2, at a peptide concentration of 0.1% and an enzyme:substrate ratio of 1:100. Incubation was conducted for 1½ hours at 37°C and proteolysis interrupted by addition of acetic acid to 20%, final, followed by vacuum centrifugation. The peptides and the Aα chain fragment, CNBr IX, were also digested with plasmin (0.009 CU/mg substrate) (Kabi/Chromogenix, Pharmacia-Hepar) in 0.05 mol/L Tris-0.15 mol/L NaCl, pH 7.6, (TBS) for 1½ hours at 37°C. Plasmin activity was inhibited by addition of Trasylol (Mobay Pharmaceuticals, New York, NY) to 250 U/mL, final.
Animal fibrinogens for species cross-reactivity studies.
Purified bovine, murine, and rabbit fibrinogens were used as supplied (90% to 94% clottable; Sigma). Equine fibrinogen (82% clottable; Sigma) was further purified essentially as described for human fibrinogen (see above) except that a final affinity chromatography step on Protein A Sepharose was included to remove contaminating IgG from the purified equine fibrinogen pool. The molar concentration of standard solutions of each of the animal fibrinogens was determined by amino acid analysis and expressed as Aα chain equivalents.
Plasmin Proteolysis of Fibrinogen
Purified system.
Fibrinogen, 2.05 mg/mL in TBS 1 mmol/L CaCl2, was digested with plasmin (0.009 CU/mg fibrinogen) for increasing periods of time (T1' to T18h) at 37°C. Aliquots were removed at each of nine time points and digestion interrupted by the addition of Trasylol to 250 U/mL, final. Plasmin digestion of purified fibrinogen was also performed following an initial preincubation step with MoAb F-104; the MoAb F-102 (anti-Aα 563-578)19 31was used as a control in parallel incubations to evaluate the effect of IgG binding to a different epitope within the αC domain. Antibody binding was conducted at a twofold molar excess of IgG, relative to Aα chain epitope equivalents, for 1 1/2 hours at 37°C. Plasmin and calcium were then added and samples processed as described above. Control digests, ie, the MoAbs treated with plasmin in the absence of fibrinogen, were assayed at each time point for residual binding to fibrinogen-coated wells (see ELISA screening assay, above), using horseradish peroxidase-conjugated rabbit antimouse immunoglobulins (RAM Ig-HRP; Dako, Carpenteria, CA) for detection. MoAb F-104 IgG was unaffected by plasmin treatment during the first 35 minutes of digestion, with 50% of its initial fibrinogen binding capacity still retained at 1 hour. MoAb F-102 IgG binding was fully preserved, even after 1 hour of plasmin digestion.
Plasma system.
Activation of endogenous plasminogen in a single donor normal plasma was initiated by the addition of streptokinase (SK) (Streptase, Astra Pharmaceutical, Westborough, MA) to a final concentration of 67 U/mL plasma. Fibrinogenolysis was interrupted at seven time points (T1' to T18h) following removal of aliquots and addition of Trasylol to 350 U/mL, final.
Immunoblotting
Discontinuous sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was conducted on Laemmli gels32 under nonreducing and/or reducing conditions. Prestained molecular weight markers were included on each run (Amersham, Arlington Heights, IL; GIBCO-BRL, Gaithersburg, MD; 200 kD, myosin; 97 kD, phosphorylase B; 68 kD, bovine serum albumin; 43 kD, ovalbumin; 28 kD, carbonic anhydrase, 18 kD, B-lactoglobulin; 15 kD, lysozyme). Electrophoresed components were transferred to nitrocellulose (Schleicher and Schuell, Keene, NH) or polyvinylidene difluoride (PVDF) (Millipore, Bedford, MA) membranes.33,34 In some cases, transfers were stained for total protein with Amido Black. MoAbs F-104 (anti-Aα 487-498) and F-102 (anti-Aα 563-578) were used for immunoblotting at 1 to 2 μg IgG/mL and bound immunoglobulins then detected with RAM Ig-HRP, following reaction with 4-chloro-1 naphthol (Bio-Rad).25
Amino Acid Analysis
Samples were hydrolyzed in 6 N HCl for 24 hours under vacuum at 110°C in a Pico Tag workstation (Waters) and amino acid analysis conducted on a Beckman Model 6300 amino acid analyzer (Beckman, Palo Alto, CA).
MoAb F-104 ELISA
This assay takes advantage of the fact that solution phase antigens that include an expressed F-104 epitope can compete with fibrinogen on the solid phase for MoAb F-104 binding. Details of the F-104 solution phase competitive ELISA have been recently reported.25Briefly, equal volumes of serially diluted competitor and MoAb F-104 IgG (13.6 ng/mL) were preincubated for 1 1/2 hours at room temperature and then incubated for 18 hours at 4°C on fibrinogen-coated wells (0.86 μg/100 μL). Bound MoAb F-104 was detected as 414 nm absorbance following successive incubations with RAM Ig-HRP and its substrate, o-phenylenediamine (OPD), on the solid phase. Dose-response curves were generated using logit transformation to extend the effective linear portion of the curve, with logit-transformed %B (the ratio of fibrinogen-bound MoAb F-104, in the presence and in the absence of solution phase competitor) plotted against the molar concentration of purified competitor, determined by amino acid analysis. Plasma fibrinogen Aα chain concentrations were derived using purified fibrinogen as the assay standard for quantitation.19
Data Analysis
ELISA data are expressed as the mean ± SEM of at least two separate experiments (each performed in duplicate), unless otherwise indicated. The Student's t-test was used for statistical analysis, with a difference at the P < .05 level considered significant.
Plasma Samples
Blood from an individual normal donor was collected into 3.8% sodium citrate (9 parts blood: 1 part anticoagulant) and Trasylol added to 250 U/mL, final. Platelet poor plasma was harvested following centrifugation at 2,100g for 10 minutes at 4°C and then aliquoted and stored frozen at −80°C for single use. Clottable fibrinogen determinations for this plasma were performed at the Columbia Presbyterian Medical Center (CPMC) Coagulation Laboratory.19 Plasma aliquots from patients undergoing thrombolytic therapy with either recombinant tissue plasminogen activator (rt-PA) or streptokinase (SK) were archival and originated from the TIMI (Phase I) Trial at CPMC. Blood collection and plasma processing for these samples have been detailed elsewhere.35
RESULTS
Species specificity of the antifibrinogen Aα chain MoAb, F-104 (IgG1).
Figure 1 illustrates the SDS-PAGE and F-104 immunoblotting profiles obtained for human, equine, bovine, rabbit, and murine fibrinogens. Specific F-104 immunoreactivity was detected in the human fibrinogen lanes only. The results obtained under reducing conditions showed that the epitope responsible for antibody binding was localized within the Aα chain. The faint band of immunoreactivity at the B chain position is consistent with the comigration of a 56-kD Aα chain remnant (degraded Aα chain), and reflects the known structural heterogeneity of the COOH-terminal portion of the Aα chain.36 These findings show that although MoAb F-104 was raised against a small peptide derivative corresponding to the sequence Aα 487-498 in human fibrinogen, it recognizes its Aα chain epitope within the immunogen's large, parent protein.
MoAb F-104 species specificity. Nonreducing and reducing SDS-PAGE (8% and 10% gels, respectively) and F-104 immunoblotting (PVDF transfers) were conducted as described in the text. Approximately 5 μg fibrinogen were applied to each lane for Amido Black-stained transfers and 2.5 μg for F-104 epitope localization, as follows: HU (human), EQ (equine), BO (bovine), RA (rabbit), and MU (murine). The migration of standard molecular weight markers is indicated at the left of each panel. The weak band of immunoreactivity observed for murine fibrinogen (top) is nonspecific and reflects binding of the HRP conjugate to contaminating IgG in this preparation; the same profile was obtained when the F-104 incubation step was omitted.
MoAb F-104 species specificity. Nonreducing and reducing SDS-PAGE (8% and 10% gels, respectively) and F-104 immunoblotting (PVDF transfers) were conducted as described in the text. Approximately 5 μg fibrinogen were applied to each lane for Amido Black-stained transfers and 2.5 μg for F-104 epitope localization, as follows: HU (human), EQ (equine), BO (bovine), RA (rabbit), and MU (murine). The migration of standard molecular weight markers is indicated at the left of each panel. The weak band of immunoreactivity observed for murine fibrinogen (top) is nonspecific and reflects binding of the HRP conjugate to contaminating IgG in this preparation; the same profile was obtained when the F-104 incubation step was omitted.
The immunoblotting findings in Fig 1 also suggest that the determinant responsible for the generation of MoAb F-104 is not conserved within the Aα chains of a number of animal fibrinogens. Epitope denaturation was probably not a contributing factor to these negative findings, as the four animal fibrinogens characterized here also failed to compete with human fibrinogen for antibody binding in the MoAb F-104 solution phase competitive ELISA (see below); (data not shown). As shown in Table 1, available sequence data for the region corresponding to (human) Aα 487-498 in nine animal species (including equine, bovine, and murine),24 37-39 identify several nonconserved residues, particularly in the segment Aα 489-492, which may account for the antihuman fibrinogen specificity of MoAb F-104 and for the antibody's generation in a murine host.
Interspecies Sequence Homology (Aα 487-498)
| Aα Chain . | 487493498 . |
|---|---|
| Human | LDGFRHRHPDEA |
| Rhesus | LDEFRHRHPDAA |
| Equine | LDGLFHRHPDEA |
| Bovine | LDDFFHR__DKD |
| Porcine | LDEFFHRHRDE_ |
| Canine | LDDLHARHPDLE |
| Chicken | FHKLDRLLPDLE |
| Hamster | FDEFAHRFPDQA |
| Rat | LDELSRMHPELG |
| Murine | LDELSERHPDLS |
| Aα Chain . | 487493498 . |
|---|---|
| Human | LDGFRHRHPDEA |
| Rhesus | LDEFRHRHPDAA |
| Equine | LDGLFHRHPDEA |
| Bovine | LDDFFHR__DKD |
| Porcine | LDEFFHRHRDE_ |
| Canine | LDDLHARHPDLE |
| Chicken | FHKLDRLLPDLE |
| Hamster | FDEFAHRFPDQA |
| Rat | LDELSRMHPELG |
| Murine | LDELSERHPDLS |
Immunologic characterization of the MoAb F-104 epitope.
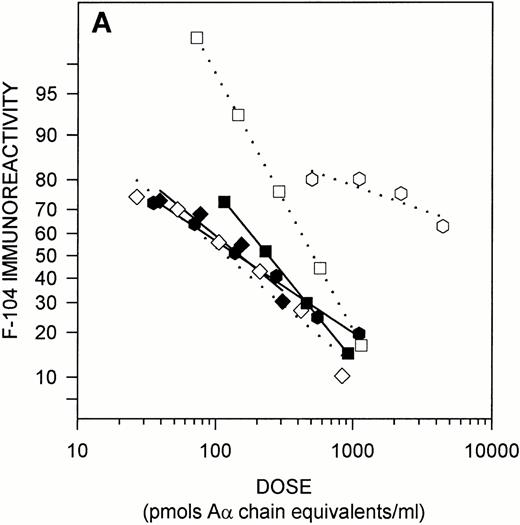
Figure 2 illustrates the results of solution phase competitive ELISAs designed to characterize the native structure of the epitope recognized by MoAb F-104. In these assays, various antigens were tested for their ability to compete with fibrinogen on the solid phase for antibody binding. As shown in Fig 2A, the octavalent immunogen and its 12-residue, linear, counterpart were effective solution-phase competitors, each exhibiting nearly superimposable dose response curves in the F-104 ELISA (ED50%B 155 ± 25.5 and 137 ± 25.9, respectively). The 41-residue CNBr fragment, Aα 477-517, also competed with fibrinogen for MoAb F-104 binding, but the slope of its dose response curve differed from that of the peptides and approximately twice the dose of CNBr IX was required to achieve 50% binding (239 ± 30.8). Plasmin treatment destroyed the F-104 epitope in the TAM peptide and, to a lesser extent, in the CNBr fragment (46% preserved), but had no effect on the immunoreactivity of the small, linear peptide. Trypsin treatment, however, did result in destruction of the epitope in this derivative (data not shown). Collectively, these findings suggest that one or both arginine residues within the sequence Aα 487-498 are involved in MoAb F-104 binding (see Table 1, human sequence) and that steric factors may render these residues somewhat inaccessible within the structure of CNBr IX. The absence of lysines in the linear peptide, but not in the octavalent derivative whose structure includes a polylysine core, implies that the F-104 epitope may have been “protected” from plasmin cleavage within the smaller peptide because of the enzyme's inability to bind to this substrate via ‘pseudo’ requisite lysine binding sites.
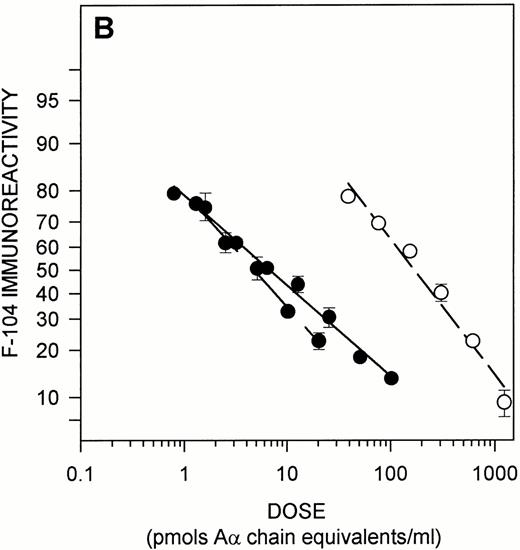
Immunochemical characterization of the MoAb F-104 epitope. (A) Epitope expression in purified Aα chain derivatives. The following antigens whose structures include Aα 487-498 were tested as solution phase competitors in the MoAb F-104 ELISA: TAM peptide (•); linear peptide (⧫); CNBr IX (Aα 477-517) (▪); plasmin-digested peptides (open symbols, dotted lines). The molar concentration of the octavalent TAM peptide was normalized to the number of moles of Aα 487-498/mole of peptide. Immunoreactivity is expressed as logit %B (see text). (B) Epitope expression in fibrinogen and its Aα chain plasmin derivatives. Purified fibrinogen (solid symbols and line), plasma fibrinogen from a single normal donor (solid symbols, dashed line), and Aα FDPs obtained from plasmin-treated purified fibrinogen (open symbols, dashed line) were assayed for F-104 immunoreactivity. Data are presented as in (A). The molar concentration of fibrinogen in the single donor plasma was derived from clottability measurements (3.45 mg/mL) and expressed as Aα chain equivalents (20,294 pmol/mL).
Immunochemical characterization of the MoAb F-104 epitope. (A) Epitope expression in purified Aα chain derivatives. The following antigens whose structures include Aα 487-498 were tested as solution phase competitors in the MoAb F-104 ELISA: TAM peptide (•); linear peptide (⧫); CNBr IX (Aα 477-517) (▪); plasmin-digested peptides (open symbols, dotted lines). The molar concentration of the octavalent TAM peptide was normalized to the number of moles of Aα 487-498/mole of peptide. Immunoreactivity is expressed as logit %B (see text). (B) Epitope expression in fibrinogen and its Aα chain plasmin derivatives. Purified fibrinogen (solid symbols and line), plasma fibrinogen from a single normal donor (solid symbols, dashed line), and Aα FDPs obtained from plasmin-treated purified fibrinogen (open symbols, dashed line) were assayed for F-104 immunoreactivity. Data are presented as in (A). The molar concentration of fibrinogen in the single donor plasma was derived from clottability measurements (3.45 mg/mL) and expressed as Aα chain equivalents (20,294 pmol/mL).
As shown in Fig 2B, both purified and plasma fibrinogen were potent solution phase competitors in the F-104 ELISA, with similar dose response curves characterized by ED50%B levels of 6.0 ± 0.31 and 4.7 ± 0.65 pmol/mL, respectively. Aα FDPs exhibited less than 3% cross-reactivity in this assay. These findings indicate that the F-104 epitope is expressed in native, intact fibrinogen and that plasmin cleavage either destroys it and/or releases it within Aα chain derivatives whose solution phase structures include the region Aα 487-498 in a buried or altered conformation.
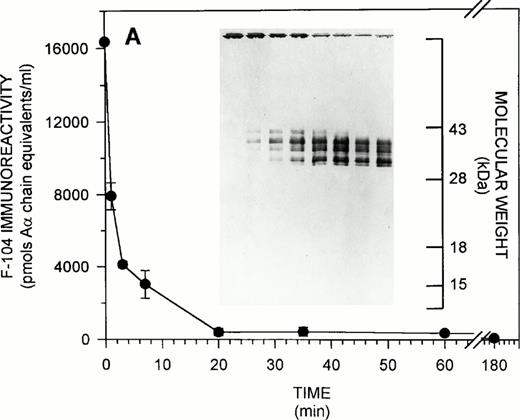
To extend the findings in Fig 2B, solution phase competitive ELISAs, in parallel with immunoblotting studies, were conducted to characterize the fate of the F-104 epitope during the course of fibrinogen proteolysis by plasmin. Figure 3illustrates the results obtained for both purified (Fig 3A) and plasma (Fig 3B) systems. As shown in Fig 3A, F-104 solution phase immunoreactivity decreased to 27.8% of its T0 level within the first 3 minutes of plasmin digestion, with less than 10% remaining after 20 minutes, under the experimental conditions used. As visualized by immunoblotting, this solution phase immunoreactivity profile paralleled both the disappearance of intact fibrinogen and the appearance of 35- to 40-kD Aα FDPs, which increased in intensity as digestion progressed. (Smaller Aα FDPS, 17 to 18.5 kD, were occasionally visualized in other F-104 immunoblots of plasmin-treated fibrinogen, but these fragments always represented a minor component of the total F-104-immunoreactive Aα FDP population released over the entire course of proteolysis). As shown in Fig 3B, similar findings were observed for the plasma fibrinogenolytic system. The collective findings in Figs 2B and 3 confirm the specificity of the MoAb F-104 ELISA for intact fibrinogen and show that for at least one group of Aα FDPs the Aα chain region 487-498 is present, but inaccessible for antibody binding in solution.
F-104 epitope expression during fibrinogenolysis. (A) Purified system. Purified fibrinogen was digested with plasmin for increasing periods of time and residual F-104 immunoreactivity quantified by solution phase competitive ELISA. The T0F-104 immunoreactivity observed (16,346 ± 47 pmol Aα chain equivalents/mL) was 136% of the expected value based on amino acid analysis. Inset. SDS-PAGE was conducted on 12.5% gels under nonreducing conditions followed by transfer to nitrocellulose and F-104 immunoblotting as described in Materials and Methods. A total of 10 μg (74.1 pmol Aα chain equivalents, determined immunologically) of intact or plasmin-treated fibrinogen was applied to each lane. The migration of standard molecular weight markers is indicated at the extreme right for reference. (B) Plasma system. Fibrinogenolysis was initiated in a single donor normal plasma and F-104 immunoreactivity monitored over the course of digestion by solution phase competitive ELISA. Results are expressed as for (A). F-104 immunoreactivity in the T0 plasma (31,671 ± 2,063 pmol Aα chain equivalents/mL) was 156% of the expected value based on clottability determination. Inset. SDS-PAGE and immunoblotting were conducted as for (A). Approximately 3.5 μg clottable plasma fibrinogen (31.7 pmol Aα chain equivalents, determined immunologically) were applied to each lane.
F-104 epitope expression during fibrinogenolysis. (A) Purified system. Purified fibrinogen was digested with plasmin for increasing periods of time and residual F-104 immunoreactivity quantified by solution phase competitive ELISA. The T0F-104 immunoreactivity observed (16,346 ± 47 pmol Aα chain equivalents/mL) was 136% of the expected value based on amino acid analysis. Inset. SDS-PAGE was conducted on 12.5% gels under nonreducing conditions followed by transfer to nitrocellulose and F-104 immunoblotting as described in Materials and Methods. A total of 10 μg (74.1 pmol Aα chain equivalents, determined immunologically) of intact or plasmin-treated fibrinogen was applied to each lane. The migration of standard molecular weight markers is indicated at the extreme right for reference. (B) Plasma system. Fibrinogenolysis was initiated in a single donor normal plasma and F-104 immunoreactivity monitored over the course of digestion by solution phase competitive ELISA. Results are expressed as for (A). F-104 immunoreactivity in the T0 plasma (31,671 ± 2,063 pmol Aα chain equivalents/mL) was 156% of the expected value based on clottability determination. Inset. SDS-PAGE and immunoblotting were conducted as for (A). Approximately 3.5 μg clottable plasma fibrinogen (31.7 pmol Aα chain equivalents, determined immunologically) were applied to each lane.
Because the immunoblotting studies described in Fig 3 are blind to COOH-terminal Aα chain degradation products that are released by plasmin cleavage within the F-104 epitope per se (which leads to epitope destruction and loss of F-104 immunoreactivity), evidence for this second group of Aα FDPs was sought by using MoAb F-102 (anti-Aα 563-578) as an immunoblotting probe. As shown in Fig 4 (left panel), plasmin digestion of purified fibrinogen resulted in the release of three major fragments, 37 kD, 17.5 kD, and 9.9 kD, each of which included the region Aα 563-578 within their respective structures. As shown in the right panel of Fig 4, when plasmin digestion was conducted using fibrinogen that had been preincubated with MoAb F-104 to block plasmin's access to the F-104 epitope (within Aα 487-498), only two of the three Aα FDPs were detected (in contrast, there was no change in the pattern of F-104 immunoreactive fragments released, compared with control incubations, when MoAb F-102 was used as the blocking antibody before plasmin cleavage; data not shown). The observed molecular weight of the “missing” Aα FDP is most consistent with that predicted for a fragment corresponding to Aα 494-583, ie, 9.8 kD (and/or possibly Aα 492-583; 10.1 kD). The findings in Fig 4 thus provide strong evidence implicating the arginine residue at Aα 493 (and/or 491), within the F-104 epitope, as a major fibrinogen Aα chain plasmin cleavage site.
The F-104 epitope in fibrinogen includes a plasmin cleavage site as visualized by immunoblotting with MoAb F-102 (anti-Aα 563-578). Purified fibrinogen was digested with plasmin (see Fig 3 legend) either without (left) or with (right) an initial MoAb F-104 preincubation step. PVDF transfers from 12.5% SDS-PAGE (nonreduced) were subjected to immunoblotting with MoAb F-102; 9.6 μg (56 pmol Aα chain equivalents) of intact or plasmin-treated fibrinogen were applied to each lane. At this load application, COOH-terminal Aα chain degradation products present at low level in the starting fibrinogen preparation are visualized in the T0 lanes. The high molecular weight material at the top of the lanes in the right panel reflects the F-104 IgG added during the preincubation step (16.8 μg, 113 pmol IgG/lane).
The F-104 epitope in fibrinogen includes a plasmin cleavage site as visualized by immunoblotting with MoAb F-102 (anti-Aα 563-578). Purified fibrinogen was digested with plasmin (see Fig 3 legend) either without (left) or with (right) an initial MoAb F-104 preincubation step. PVDF transfers from 12.5% SDS-PAGE (nonreduced) were subjected to immunoblotting with MoAb F-102; 9.6 μg (56 pmol Aα chain equivalents) of intact or plasmin-treated fibrinogen were applied to each lane. At this load application, COOH-terminal Aα chain degradation products present at low level in the starting fibrinogen preparation are visualized in the T0 lanes. The high molecular weight material at the top of the lanes in the right panel reflects the F-104 IgG added during the preincubation step (16.8 μg, 113 pmol IgG/lane).
Application of the F-104 ELISA for the detection of fibrinogenolysis during thrombolytic therapy.
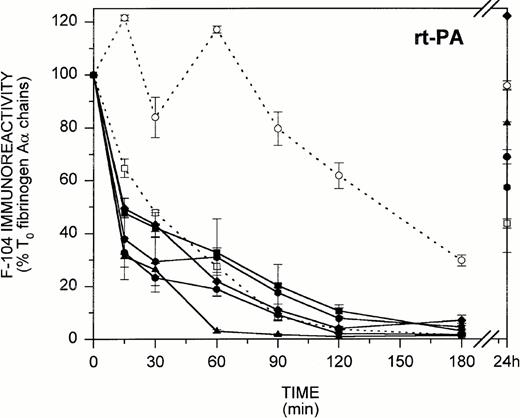
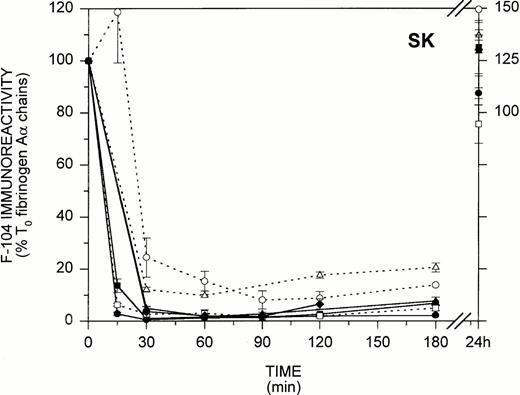
In view of the findings in Figs 3 and 4 showing that during in vitro fibrinogenolysis the loss of fibrinogen-associated F-104 immunoreactivity parallels COOH-terminal Aα chain degradation, we next considered whether MoAb F-104 could be applied to characterize fibrinogenolysis as it occurs in vivo. Figure 5 illustrates the F-104 ELISA profiles obtained for 14 patients undergoing thrombolytic therapy for myocardial infarction, where rt-PA (n = 7) and SK (n = 7) were both used as thrombolytic agents. As shown in Fig 5 for the rt-PA-treated group (left), five patients exhibited an early loss of F-104 immunoreactivity with less than 50% (31.4% to 49.5%) of the T0 circulating fibrinogen remaining after 15 minutes of therapy. Fibrinogenolysis was less efficient in the two other patients (>60% to 100% of the T0 fibrinogen was still detected at 15 minutes), both of whom failed to show radiographic evidence of successful reperfusion at the end of treatment. As shown in Fig 5 for the SK-treated group (right), less than 5% (0.5% to 4.8%) of the T0 F-104 immunoreactivity remained after 30 minutes of therapy in five of the seven patients examined. Aα chain proteolysis appeared to proceed more slowly in the two other patients, both of whom failed to reperfuse, with 10% to 20% of the T0 F-104 immunoreactivity persisting over the entire course of treatment.
In vivo fibrinogenolysis during thrombolytic therapy. Plasmas from 14 patients undergoing thrombolytic therapy with either rt-PA (left) or SK (right) were assayed for fibrinogen-associated F-104 immunoreactivity by ELISA. Eight time points, including pretreatment and 24-hour posttreatment samples, were examined (plasmas for one 24-hour point, one 90-minute point, and three 15-minute time points were not available). Patients who exhibited radiographic evidence of reperfusion are identified by solid lines and symbols, while those who did not are identified by dashed lines and open symbols. Results are expressed as the percent of pretreatment F-104 immunoreactivity.
In vivo fibrinogenolysis during thrombolytic therapy. Plasmas from 14 patients undergoing thrombolytic therapy with either rt-PA (left) or SK (right) were assayed for fibrinogen-associated F-104 immunoreactivity by ELISA. Eight time points, including pretreatment and 24-hour posttreatment samples, were examined (plasmas for one 24-hour point, one 90-minute point, and three 15-minute time points were not available). Patients who exhibited radiographic evidence of reperfusion are identified by solid lines and symbols, while those who did not are identified by dashed lines and open symbols. Results are expressed as the percent of pretreatment F-104 immunoreactivity.
F-104 immunoblotting profiles obtained for two representative patients from each group showed that the Aα FDPs released during thrombolytic therapy were the same as those shown in Fig 3 for fibrinogenolysis in vitro (data not shown). Collectively, the findings in Fig 5 indicate that Aα chain proteolysis in vivo and in vitro proceed by a common mechanism and that plasmin cleavage at the F-104 epitope is an early marker of fibrinolytic system activation.
DISCUSSION
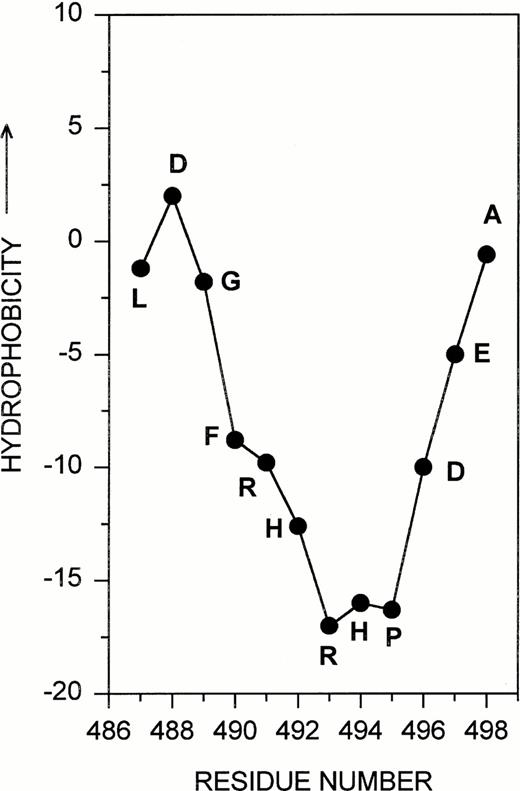
The studies in this report are concerned with the development and application of a new structural probe (MoAb F-104) for the detection of fibrinogenolysis. The studies focus on the 12-residue sequence Aα 487-498 and were prompted by several observations related to the unique structure of this localized region within the αC domain. First, hydropathy calculations40 predicted that the region Aα 487-498 was extremely hydrophilic and, therefore, likely to assume an exposed conformation on the surface of the fibrinogen (Aα chain) molecule (Fig 6). The residues arg-his-arg-his within this sequence appeared to form a particularly vulnerable site for potential interaction with other proteins, possibly plasmin, based on its complement of positively charged amino acids lying at the apex of the hydrophilic peak. Only five other Aα chain segments share this unique hydrophilicity, as well as a prominently accessible arginine (or lysine) residue. Because three of these localized regions correspond to sequences known to be involved in the plasmin-mediated release of Fragments E and D,41 core fragments viewed as the primary hallmarks of fibrinogenolysis,42 we considered the possibility that Aα 487-498, within the αC domain, might also play a significant and as yet unrecognized role in fibrinogen's proteolysis by plasmin. This argument was strengthened by interspecies structural comparisons for the Aα chain region corresponding to human Aα 487-498 (Table 1). These data indicated that several amino acids in the vicinity of, and including, arg 493 were highly conserved, thus suggesting an important structure-function relationship for this localized region of the fibrinogen molecule.
Aα 487-498. The 12-residue sequence targeted for antibody development is shown as part of the Kyte Doolitle hydropathy plot derived for the human fibrinogen Aα chain.
Aα 487-498. The 12-residue sequence targeted for antibody development is shown as part of the Kyte Doolitle hydropathy plot derived for the human fibrinogen Aα chain.
Several important findings that support this rationale emerged from these studies as a consequence of having successfully developed a MoAb to the targeted sequence, Aα 487-498. First, arg Aα 493 (and/or possibly arg Aα 491) is an early plasmin cleavage site within the αC domain. Second, the sequence Aα 487-498 (as represented by the F-104 epitope) is exposed on the surface of intact fibrinogen, but this accessibility is altered once plasmin attacks its multiple cleavage sites within αC domain; cleavage at the 493-494 bond severs the structural integrity of this localized sequence, while cleavage at other sites within the αC domain releases it in a buried conformation within the structure of moderately sized Aα FDPs. Third, MoAb F-104 can be used as a structural probe for early fibrinogenolysis because the loss of fibrinogen-associated F-104 immunoreactivity is a hallmark of plasmin activity at the Aα 493-494 bond (and/or the 491-492 bond). Finally, MoAb F-104 can detect fibrinogenolysis in vivo, as illustrated here for the Aα chain proteolysis that accompanies thrombolytic therapy. (Parenthetically, MoAb F-104 represents an unusual example where immunization with a TAM peptide derivative produced a highly specific, antifibrinogen antibody. Although TAM peptides, by virtue of their epitope density, offer enhanced antigenicity relative to their monomeric counterparts, their use as immunogens for the development of antipeptide antibodies that cross-react with their cognate proteins in solution has met with limited success).43
Previous studies from several different laboratories have documented the presence of multiple cleavage sites within the COOH-terminal two thirds of the Aα chain13,42,44 and implicated specific sites that are likely to function during fibrinogenolysis in vivo, based on the structural characterization of Aα FDPs isolated from plasmin-treated fibrinogen.45-47 The identification, in this report, of an additional in vivo Aα chain plasmin cleavage site, ie, 493/491, illustrates the value of anti-Aα chain peptide antibodies when applied as structural probes for elucidating aspects of fibrinogen's proteolysis by plasmin. It should also be possible to use these reagents in similar immunochemical studies to learn, for example, more about the Aα chain heterogeneity that is a well-known feature of normal fibrinogen populations,36 to define the sequence of plasmin attack sites within the αC domain, and to elucidate how the released products differ when cross-linked fibrin, as distinct from fibrinogen, serves as the plasmin substrate.
Two other MoAbs directed at epitopes in the vicinity of Aα 476 and Aα 575, within the αC domain, have been previously used for the clinical detection of fibrinogenolysis.19,48 MoAb F-104, ie, anti-Aα 487-498, represents a new structural probe for monitoring the initial stages of fibrinogenolysis in vivo, based on the findings obtained here for 14 patients undergoing thrombolytic therapy for myocardial infarction. Recognizing that this small sample population renders the findings and inferences drawn from them somewhat speculative, one aspect of the results obtained seems worth highlighting. Data for the rt-PA treatment group suggest that there is a significant difference in the levels of intact fibrinogen circulating at 15 to 30 minutes (P = .022; .038) between patients who show clinical evidence of reperfusion at the end of treatment and those who do not. (This same trend was not observed for the SK-treated patients [P = .109], although one of the three who failed to reperfuse exhibited the slowest rate of fibrinogenolysis in this group.) While these findings will require confirmation from a larger study population, the underlying implication is that cleavage at the F-104 epitope may be a sensitive marker of the systemic lytic state that is considered to be a prerequisite for effective thrombolysis.49 Currently, the identification of high risk patients undergoing thrombolytic therapy relies on the clinical evaluation of resolution in ST segment elevation and on biochemical markers such as troponin T.50 MoAb F-104's potential clinical use as a vehicle for monitoring the creation of a systemic lytic state early during treatment (and thus providing information about whether or not additional intervention may be warranted) would obviously require a more rapid detection system than the one developed here, but there is ample precedent in the literature for assay technologies, including latex agglutination,51 which emphasize adequate speed. In light of the recent interest in thrombolytic therapy as a treatment modality for stroke52and for thrombotic disease in children,53 markers such as the F-104 epitope could provide important information for the development of appropriate dosing regimens in these relatively new therapeutic arenas.
ACKNOWLEDGMENT
The authors thank Andy Pound (Howard Hughes Protein Core Facility of Columbia University) for performing the amino acid analyses and Ay-Chin Huang (CPMC Coagulation Laboratory) for performing the clottable plasma fibrinogen determinations required over the course of this study.
Address reprint requests to Joan H. Sobel, PhD, Columbia University College of Physicians & Surgeons, Department of Medicine/Black Building 1011/Box 104, 630 W 168th St, New York, NY 10032.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal