Abstract
A potent platelet glycoprotein Ib (GPIb) antagonist, crotalin, with a molecular weight of 30 kD was purified from the snake venom ofCrotalus atrox. Crotalin specifically and dose dependently inhibited aggregation of human washed platelets induced by ristocetin with IC50 of 2.4 μg/mL (83 nmol/L). It was also active in inhibiting ristocetin-induced platelet aggregation of platelet-rich plasma (IC50, 6.3 μg/mL). 125I-crotalin bound to human platelets in a saturable and dose-dependent manner with a kd value of 3.2 ± 0.1 × 10−7 mol/L, and its binding site was estimated to be 58,632 ± 3,152 per platelet. Its binding was specifically inhibited by a monoclonal antibody, AP1 raised against platelet GPIb. Crotalin significantly prolonged the latent period in triggering platelet aggregation caused by low concentration of thrombin (0.03 U/mL), and inhibited thromboxane B2formation of platelets stimulated either by ristocetin plus von Willebrand factor (vWF), or by thrombin (0.03 U/mL). When crotalin was intravenously (IV) administered to mice at 100 to 300 μg/kg, a dose-dependent prolongation on tail bleeding time was observed. The duration of crotalin in prolonging tail bleeding time lasted for 4 hours as crotalin was given at 300 μg/kg. In addition, its in vivo antithrombotic activity was evidenced by prolonging the latent period in inducing platelet-rich thrombus formation by irradiating the mesenteric venules of the fluorescein sodium-treated mice. When administered IV at 100 to 300 μg/kg, crotalin dose dependently prolonged the time lapse in inducing platelet-rich thrombus formation. In conclusion, crotalin specifically inhibited vWF-induced platelet agglutination in the presence of ristocetin because crotalin selectively bound to platelet surface receptor-glycoprotein Ib, resulting in the blockade of the interaction of vWF with platelet membrane GPIb. In addition, crotalin is a potent antithrombotic agent because it pronouncedly blocked platelet plug formation in vivo.
PLATELET ADHESION and aggregation play important roles in hemostasis and thrombosis. Physiologically, platelets arrest bleeding from damaged blood vessels by sealing wound and initiating the repairing processes. Thrombin-mediated platelet activation and aggregation is partially related to glycoprotein Ib (GPIb)1,2 and this membrane glycoprotein also plays a role in regulating the interactions of platelets with their environment via linking with membrane-associated cytoskeleton.2Additionally, the interaction of platelet GPIb with von Willebrand factor (vWF) is a critical event, allowing platelet adhesion, aggregation, and subsequent thrombus formation in the vessels either with high shear rates or with damaged endothelium.3-5 The role of GPIb in the adhesion of platelets to endothelial cell matrix has been studied with monoclonal antibodies (MoAbs). An MoAb against GPIb inhibited the platelet adhesion to endothelial cell matrix, whereas an MoAb against vWF which inhibits the interaction of vWF with platelets has a less pronounced effect, indicating that GPIb has another role in platelet adhesion, apart from serving as the binding site for vWF.6
Many of the currently investigated antithrombotic drugs interfere with the process of platelet aggregation.7-9 However, the inhibition of platelet adhesion may be an important and efficient process for preventing the platelet-rich thrombus formation in the damaged vessels with high shear rate. In addition, thrombin is an important stimulator for platelet aggregation under normal physiological and pathological conditions.10-12 Thrombin is an important mediator of platelet aggregation in stenosed canine coronary arteries.13 Therefore, an inhibitor of thrombin and vWF platelet interaction may be used in the prevention of thrombosis. Several snake venom constituents affecting blood coagulation and platelet aggregation, especially Arg-Gly-Asp (RGD)–containing peptides, have been extensively studied.14,15 In addition, GPIb agonists as well as GPIb antagonists have been isolated and characterized.16-20 We report here the purification of a protein, crotalin, from Crotalus atrox venom that specifically inhibits ristocetin-induced agglutination or aggregation without affecting the aggregation induced by adenosine diphosphate (ADP) or collagen. Of particular interest is that crotalin, when administered intravenously (IV), markedly delays platelet-rich thrombus formation of the irradiated mesenteric venules in a fluorescein sodium-treated mice model. Among the antithrombotic agents tested, crotalin appears not only to be the most efficacious agent in prolonging the time lapse for inducing platelet-rich thrombus formation in this model, but also exhibits this antiplatelet activity with a longer duration.
MATERIALS AND METHODS
Materials.
C atrox venom was obtained from LATOXAN (Rosans, France). All of DEAE-Sephadex A-50, Sephadex G-75, Mono-S, and Superose columns were purchased from Pharmacia (Uppsala, Sweden). Halysin was purified fromAgkistrodon halys venom as previously described.21ADP, U46619, collagen (type I, bovine achilles tendon), and fluorescein sodium were purchased from Sigma Chemical Co (St Louis, MO).
MoAb AP1 raised against platelet GPIb was generously provided by Dr Robert Montgomery (Blood Center of Southeastern Wisconsin Milwaukee) and 7E3, an MoAb raised against platelet GPIIb/IIIa complex, was supplied from Dr Barry Coller (The Mount Sinai Medical Center, New York, NY).
Purification of vWF.
According to the modified method of a previous report,22vWF was purified from human plasma through cryoprecipitation, polyethylene glycol (PEG) sedimentation, and gel filtration with a Sepharose CL-4B column, and the purity of vWF was assessed by 5% sodium dodecyl sulfate (SDS)-gel electrophoresis.
Human platelet aggregation.
Blood was collected from healthy human volunteers, who did not take any medication within the 2 weeks before the study, and anticoagulated with 3.8% sodium citrate (9:1, vol/vol). Citrated blood was immediately centrifuged for 10 minutes at 120g and 25°C, and the supernatant (platelet-rich plasma) was obtained. Human washed platelet suspension was prepared as previously described.22 41Washed platelets were suspended in modified Tyrodes' solution (pH 7.3) (in mmol/L: NaCl, 136.9; CaCl2, 2; KCl, 2.7; MgCl2, 1.0; NaH2PO4, 0.4; NaHCO3, 11.9; glucose, 11.1) containing bovine serum albumin (3.5 mg/mL) and adjusted to about 3 × 108platelets/mL.
The turbidimetric method,23 using a Lumi-Aggregometer (Chrono-Log, Havertown, PA), was used to measure platelet aggregation. The extent of aggregation was expressed in light transmission unit.
Assay of thromboxane B2 formation.
EDTA (2 mmol/L) and indomethacin (50 μmol/L) were added to platelet suspension at 6 minutes after the addition of thrombin (0.03 U/mL). After centrifugation with an Eppendorf Centrifuge (Model 5414; Hamburg, Germany) at 14,000 cpm for 2 minutes, thromboxane B2 level of the supernatant was determined by thromboxane B2 EIA Kit (Amersham, Buckinghamshire, UK).
Radiolabeling of crotalin using enzymobead.
The vial of enzymobead reagent (Bio-Rad, San Francisco, CA) was hydrated with 50 μL distilled water at 4°C for 1 hour, followed by adding 50 μL phosphate buffer, 50 μg crotalin, 0.5 mCi Na125I, and 25 μL β-D-glucose (1%). The mixture was incubated at room temperature for 25 minutes.125I-conjugated crotalin was eluted through a Sephadex G-10 column (0.7 × 23 cm; bed volume, 10 mL) with phosphate buffer. The radioactivity of 125I-labeled crotalin was detected with radiocounter (Capintec, CRC-7) continuously.
Binding of 125I-crotalin to platelets.
It was performed according to previously described methods24,25 with slight modification. Aliquot of 0.4 mL of platelet suspension (4.5 × 108/mL) were incubated in the presence of Tyrode solution (control), AP1, or excess unlabeled crotalin at room temperature for 2 minutes. Then,125I-crotalin in various concentrations was added, and the mixture was gently shaken and incubated for 6 minutes. Platelet suspension mixture, 0.4 mL, was overlayed on sucrose solution (20%, wt/vol) and centrifuged for 5 minutes at 14,000 rpm (Eppendorf centrifuge, 5415). The radioactivities of supernatant (200 μL) and the cut-off tips containing platelet pellet were separately measured by using a γ-counter (Beckman, Fullerton, CA). The specific 125I-crotalin binding to the platelet pellet was calculated by subtracting the nonspecific radioactivity bound from the total radioactivity bound. The number of crotalin binding sites per platelet and dissociation constant (kd) were calculated by the method of Scatchard.26
Measurement of bleeding time in mice.
Bleeding time of mice was measured by a modification of the method described by Dejana et al.27 Saline or various concentrations of crotalin was injected intravenously through a lateral vein of the mouse (ICR, with an average body weight of 20 g). A sharp cut of 2 to 3 mm from tail tip of mouse was made 10 minutes (except as noted) after injection. The tail was then immediately placed into a tube filled with saline, and kept at 37°C for measuring bleeding time.
Platelet-rich thrombosis model in mice.
Normal male mice (ICR) were used. The mice weighing between 18 and 20 g were anesthetized with sodium pentobarbital (50 mg/kg) by intraperitoneal injection. The method of this thrombogenic animal model has been described in detail.21,28 29 In brief, an external jugular vein was cannulated for the administration of dye and crotalin, and a mesenteric preparation was then mounted on a transilluminator and continuously rinsed with warm saline. The room temperature around microscope was kept at 37°C. Mesenteric venules with a diameter around 20 μm were selected for thrombogenic assay.
Fluorescein sodium (100, 150, or 200 μg/mL) was first injected through a cannula, and microvessels were irradiated by filtered light 10 minutes later. Simultaneously, a video timer was started. Platelet adhesion and aggregation were observed through a television monitor and time lapse for inducing the formation of platelet-rich thrombus, as reflected by cessation of blood flow, was taken. In the epi-illumination system, the light from a 100-W mercury lamp was excited by a filter (B-2A; Nikon) with a dichronic mirror (DM510; Nikon, Tokyo, Japan). The filtered light then irradiated a microvessel through an objective lens (20×). In this experimental model, platelet thrombus formation was induced and found to consist exclusively of platelets with pseudopod formation and degranulated appearance, accompanied with moderately damaged endothelial cells.21
Statistics.
All data are presented as mean ± SEM (n). Student's t-test was used to assess the statistical differences.
RESULTS
Purification and physicochemical characterization of crotalin.
Crotalin was purified from C atrox venom through columns of DEAE-Sephadex A-50 and Sephadex G-75 and refractionated by fast protein liquid chromatography using Mono-S (Fig 1A)and Superose columns (Fig 1B). The purified fraction migrated as a single band and the apparent molecular weight was estimated to be 30 kD under nonreducing or reducing conditions by SDS-polyacrylamide (15%) gel electrophoresis (Fig 1B, inlet) and named crotalin.
(A) Rechromatography of crotalin on Mono-S column. The active fraction was dissolved in 0.02 N ammonium acetate, pH 5.0, and loaded on Mono-S column. Elution was carried out with a gradient from 0 to 0.75 mol/L NaCl as indicated (---) at a flow rate of 0.5 mL/min. The active fraction (*) was collected. (B) Gel filtration chromatography on Superose HR 10/30 column. The active fraction above was applied (5 mg) to this column equilibrated with 0.05 mol/L phosphate buffer (pH 7.2). The column was eluted at a flow rate of 0.25 mL/min with the same buffer. The absorbance profile monitored at 280 nm was shown. The active fraction (*) which was eluted at 70 minutes was named crotalin.
(A) Rechromatography of crotalin on Mono-S column. The active fraction was dissolved in 0.02 N ammonium acetate, pH 5.0, and loaded on Mono-S column. Elution was carried out with a gradient from 0 to 0.75 mol/L NaCl as indicated (---) at a flow rate of 0.5 mL/min. The active fraction (*) was collected. (B) Gel filtration chromatography on Superose HR 10/30 column. The active fraction above was applied (5 mg) to this column equilibrated with 0.05 mol/L phosphate buffer (pH 7.2). The column was eluted at a flow rate of 0.25 mL/min with the same buffer. The absorbance profile monitored at 280 nm was shown. The active fraction (*) which was eluted at 70 minutes was named crotalin.
Amino acid analysis showed that crotalin is a polypeptide, consisting of about 260 amino acid residues (Asp/Asn 36, Glu/Gln 25, Ser 14, Gly 21, His 9, Thr 8, Ala 14, Arg 22, Pro 10, Tyr 6, Val 16, Ile 18, Leu 28, Cys 7, Phe 10, and Lys 16). However, the N-terminal amino acid sequence of crotalin was found to be blocked.
Inhibition of ristocetin-induced platelet aggregation.
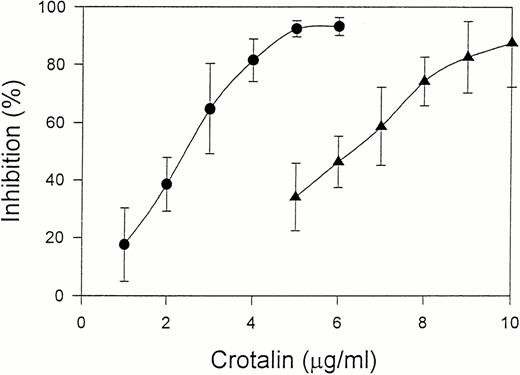
Crotalin showed a marked inhibitory effect on ristocetin (1.0 mg/mL)-induced human washed platelet aggregation with IC50of 2.4 μg/mL (Fig 2). This inhibitory effect was independent of the incubation time of crotalin with platelets. Furthermore, crotalin was specific for ristocetin-induced platelet aggregation because it had little effect on the platelet aggregations caused by ADP (20 μmol/L) plus 200 μg/mL of fibrinogen, U46619 (1 μmol/L), or collagen (10 μg/mL). Crotalin also inhibited ristocetin (1 mg/mL)-induced platelet aggregation of platelet-rich plasma with IC50 value of 6.3 μg/mL (Fig2), indicating that it might have an antithrombotic effect in vivo.
Dose-response relationship of crotalin on platelet aggregation induced by 1 mg/mL of ristocetin in human washed platelets in the presence of 10 μg/mL of vWF (•) or in platelet-rich plasma (▴). Values are presented as mean ± SEM (n = 4).
Dose-response relationship of crotalin on platelet aggregation induced by 1 mg/mL of ristocetin in human washed platelets in the presence of 10 μg/mL of vWF (•) or in platelet-rich plasma (▴). Values are presented as mean ± SEM (n = 4).
Similarly, crotalin (10 μg/mL) apparently did not affect platelet aggregation caused by high concentration of thrombin (>0.05 U/mL). However, crotalin (20 to 100 μg/mL) prolonged the latent period in triggering platelet aggregation in a dose-dependent manner, with a slight inhibition on the maximal aggregation (<30%) inhibition). The MoAb AP1 (40 to 80 μg/mL) showed a similar effect to that of crotalin (data not shown).
Effect of crotalin on thromboxane B2 formation of platelets caused by thrombin and other agonists.
As shown in Table 1, crotalin at a lower concentration (5 to 10 μg/mL) inhibited thromboxane B2formation of platelets stimulated by ristocetin and vWF. At higher concentration (20 μg/mL), crotalin significantly inhibited thromboxane B2 formation of platelets caused by thrombin (0.03 U/mL), whereas it did not affect thromboxane B2formation stimulated by collagen.
Effects of Crotalin on Thromboxane B2Formation of Washed Platelets Stimulated by Thrombin and Other Agonist
| . | Thromboxane B2(ng/mL) . | ||
|---|---|---|---|
| Ristocetin + vWF . | Thrombin . | Collagen . | |
| Control | 12.4 ± 2.6 | 53.3 ± 3.2 | 211.3 ± 12.5 |
| Crotalin | |||
| 5 μg/mL | 3.4 ± 0.6-150 | ND | ND |
| 10 μg/mL | 3.5 ± 0.4-150 | 40.9 ± 3.5 | ND |
| 20 μg/mL | ND | 27.3 ± 3.9-151 | 198.1 ± 10.3 |
| 40 μg/mL | ND | ND | 189.3 ± 9.2 |
| . | Thromboxane B2(ng/mL) . | ||
|---|---|---|---|
| Ristocetin + vWF . | Thrombin . | Collagen . | |
| Control | 12.4 ± 2.6 | 53.3 ± 3.2 | 211.3 ± 12.5 |
| Crotalin | |||
| 5 μg/mL | 3.4 ± 0.6-150 | ND | ND |
| 10 μg/mL | 3.5 ± 0.4-150 | 40.9 ± 3.5 | ND |
| 20 μg/mL | ND | 27.3 ± 3.9-151 | 198.1 ± 10.3 |
| 40 μg/mL | ND | ND | 189.3 ± 9.2 |
Human washed platelets were preincubated with saline or various concentrations of crotalin at 37°C for 5 minutes before the addition of ristocetin (1 mg/mL) plus vWF (10 μg/mL), thrombin (0.03 U/mL), and collagen (10 μg/mL). Six minutes after stimulation, the reaction was terminated by the addition of EDTA (2 mmol/L) and indomethacin (50 μmol/L). The basal value of resting platelets was 2.4 ± 0.5 ng/mL. Values are presented as mean ± SEM.
Abbreviation: ND, not determined.
P < .01 as compared with the respective control, n = 5.
P < .05.
Characterization of the binding of 125I-crotalin to human platelets.
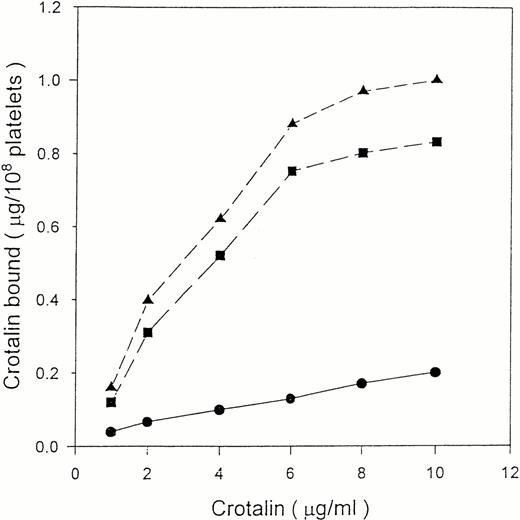
125I-crotalin bound to platelets in a dose-dependent manner, reaching a saturated binding at 10 μg/mL (0.33 μmol/L) (Fig3). The Scatchard analysis of125I-crotalin binding data showed that the binding sites of crotalin were 58,632 ± 3,152 per platelet with a kd value of 3.2 ± 0.1 × 10−7 mol/L (Fig 4).125I-crotalin binding to platelets was blocked either by unlabeled crotalin (100 μg/mL, >85%) or AP1 (20 μg/mL, >90%), but not by the MoAb against GPIIb/IIIa, 7E3 (40 μg/mL), or 5 mmol/L EDTA (data not shown).
Binding isotherm of 125I-crotalin on human platelet suspension. Platelets were incubated with various concentrations of 125I-crotalin. Total binding (▴) and nonspecific binding (•) in the presence of unlabeled crotalin (200 μg/mL) were determined, respectively. Specific binding (▪) was calculated by subtracting the nonspecific binding from total binding. This is a representative one of four similar experiments.
Binding isotherm of 125I-crotalin on human platelet suspension. Platelets were incubated with various concentrations of 125I-crotalin. Total binding (▴) and nonspecific binding (•) in the presence of unlabeled crotalin (200 μg/mL) were determined, respectively. Specific binding (▪) was calculated by subtracting the nonspecific binding from total binding. This is a representative one of four similar experiments.
Scatchard plot of the 125I-crotalin binding to human washed platelets. This plot is a representative one of four experiments.
Scatchard plot of the 125I-crotalin binding to human washed platelets. This plot is a representative one of four experiments.
Effect of crotalin on bleeding time of mice.
IV administration of crotalin to mice significantly prolonged the bleeding time in a dose-dependent manner (Fig5). Crotalin pronouncedly prolonged the bleeding time (>10 minutes) as measured 1 hour after a bolus injection of crotalin (300 μg/kg), and this effect slowly ran down thereafter (Fig 6). The bleeding time almost returned to the baseline level within 4 hours after the administration of crotalin. However, the platelet count, as measured at 10 minutes after the administration of crotalin (300 μg/kg), did not show a major change, although about 20% decreasement was observed (128 ± 10 × 104, n = 4, control v104 ± 13 × 104 platelets/μL, n = 4, experimental; P > .05). Even when the dose of crotalin was increased to 600 μg/kg, a transient slight decrease of platelet count (about 20%) was observed at the first 5 minutes after the administration of crotalin, and the platelet count returned to control level within 20 minutes.
Effects of crotalin on tail bleeding time in mice. Bleeding time was measured 10 minutes after the IV administration of saline or various doses of crotalin. Bleeding time longer than 10 minutes was expressed as >10 min. Bar represents the mean value (n = 6). Each different symbol represents the bleeding time of the individual mouse.
Effects of crotalin on tail bleeding time in mice. Bleeding time was measured 10 minutes after the IV administration of saline or various doses of crotalin. Bleeding time longer than 10 minutes was expressed as >10 min. Bar represents the mean value (n = 6). Each different symbol represents the bleeding time of the individual mouse.
The effects of crotalin on the bleeding time after the IV administration of crotalin (300 μg/kg). This study was performed as described in Fig 5 and bleeding time was measured at 10 minutes, 1 hour, 2 hours or 4 hours after the administration of crotalin. Bar represents the mean value (n = 6). Each different symbol represents the bleeding time of the individual mouse.
The effects of crotalin on the bleeding time after the IV administration of crotalin (300 μg/kg). This study was performed as described in Fig 5 and bleeding time was measured at 10 minutes, 1 hour, 2 hours or 4 hours after the administration of crotalin. Bar represents the mean value (n = 6). Each different symbol represents the bleeding time of the individual mouse.
Platelet-rich thrombus formation in the microvessels.
As the given dose of fluorescein sodium was increased, the latent period in inducing platelet plug formation was shortened (Table 2). IV administration of crotalin at 300 μg/kg pronouncedly delayed platelet-rich thrombus formation and significantly prolonged the occlusion time in mice receiving different doses of fluoresein sodium (Table 2). The occlusion time was lengthened from 100 ± 14 to 311 ± 25 seconds in fluorescein sodium (150 μg per mouse)-treated mice after IV administration of crotalin. Crotalin dose-dependently prolonged the occlusion time in causing platelet plug formation, and administration of 300 μg/kg of crotalin resulted in the maximal lengthening of occlusion time (Table3). This antithrombotic effect lasted at least for 2 hours (Fig 7). On the other hand, a continuous infusion of prostaglandin I2(PGI2) at 0.5 μg/kg/min, as in our previous study,29 showed a maximal lengthening effect on occlusion time. Higher dose (2 μg/kg/min) of PGI2 did not further increase its antithrombotic activity. Halysin, an RGD-containing peptide purified from venom of A halys, inhibited platelet aggregation via a competitive inhibition of fibrinogen binding to platelet GPIIb/IIIa.30 It completely inhibited ex vivo platelet aggregation of platelet-rich plasma induced by collagen (15 μg/mL) 20 minutes after the IV administration of halysin at the dose of 10 mg/kg (data not shown). In comparing the maximal effect of PGI2, halysin, ancrod,29 and crotalin on the occlusion time in the same in vivo model, crotalin appears to be the most efficacious agent in prolonging the occlusion time (Table4).
Effect of Fluorescein Sodium and Crotalin on the Occlusion Time in Causing Platelet-Rich Thrombus Formation in Mesenteric Venules of Mice
| . | Dose of Fluorescein Sodium per Mouse . | ||
|---|---|---|---|
| 100 μg . | 150 μg . | 200 μg . | |
| Normal saline | 180 ± 23 (4) | 100 ± 14 (4) | 63 ± 7 (3) |
| Crotalin (300 μg/kg) | 310 ± 36 (4)* | 311 ± 25 (5)* | 155 ± 13 (3)* |
| . | Dose of Fluorescein Sodium per Mouse . | ||
|---|---|---|---|
| 100 μg . | 150 μg . | 200 μg . | |
| Normal saline | 180 ± 23 (4) | 100 ± 14 (4) | 63 ± 7 (3) |
| Crotalin (300 μg/kg) | 310 ± 36 (4)* | 311 ± 25 (5)* | 155 ± 13 (3)* |
Values are expressed as elapsed time (in seconds) in causing platelet plug formation on the irradiation of venules and presented as mean ± SEM (n).
P < .001 as compared with the respective control.
Dose-Response Relationship of Crotalin on the Elapsed Time of Inducing Thrombus Formation on Light Irradiation of Venules of Mice Pretreated With Fluorescein Sodium (150 μg per mouse)
| Dose of Crotalin (μg/kg) . | Occlusion Time (s) . | |
|---|---|---|
| Saline . | Crotalin . | |
| 100 | 94 ± 7 (4) | 161 ± 28 (4)* |
| 200 | 97 ± 20 (4) | 273 ± 15 (4)† |
| 300 | 99 ± 19 (4) | 317 ± 31 (5)† |
| Dose of Crotalin (μg/kg) . | Occlusion Time (s) . | |
|---|---|---|
| Saline . | Crotalin . | |
| 100 | 94 ± 7 (4) | 161 ± 28 (4)* |
| 200 | 97 ± 20 (4) | 273 ± 15 (4)† |
| 300 | 99 ± 19 (4) | 317 ± 31 (5)† |
The occlusion time was expressed (in seconds) as the time lapse for inducing platelet-rich thrombus formation on the irradiation of the venules in fluorescein sodium-treated mice. The significant difference of elapsed time was compared between groups before and after administration of crotalin. Values are presented as mean ± SEM (n).
P < .05.
P < .001.
The effects of crotalin on the elapsed time in causing platelet plug formation upon the irradiation of venules in mice. Fluorescein sodium (150 μg per mouse) was intravenously injected 10 minutes before the irradiation, and the irradiation was then started for inducing the formation of thrombus at the indicated time intervals after the IV administration of crotalin (300 μg/kg). Values are presented as mean ± SEM (n = 5-6). BL indicates baseline value. (▿) Represents the occlusion time of each mouse measured at the indicated time. *P < .05, **P< .01 as compared with basal value.
The effects of crotalin on the elapsed time in causing platelet plug formation upon the irradiation of venules in mice. Fluorescein sodium (150 μg per mouse) was intravenously injected 10 minutes before the irradiation, and the irradiation was then started for inducing the formation of thrombus at the indicated time intervals after the IV administration of crotalin (300 μg/kg). Values are presented as mean ± SEM (n = 5-6). BL indicates baseline value. (▿) Represents the occlusion time of each mouse measured at the indicated time. *P < .05, **P< .01 as compared with basal value.
Effect of Crotalin, PGI2, Halysin and Ancrod on the Lapsed Time in Inducing Thrombus Formation Caused by Irradiation of Mesenteric Venules of Fluorescein Sodium-Treated Mice
| Administration . | Occlusion Time (s) . |
|---|---|
| Saline | 105 ± 12 (6) |
| PGI2, 2 μg/kg/min | 148 ± 16 (6)3-150,3-151 |
| Halysin, 10 mg/kg | 155 ± 20 (6)3-150,3-151 |
| Ancrod, 1 U/kg | 176 ± 31 (6)3-150,3-151 |
| Crotalin, 300 μg/kg | 293 ± 38 (6)3-150 |
| Administration . | Occlusion Time (s) . |
|---|---|
| Saline | 105 ± 12 (6) |
| PGI2, 2 μg/kg/min | 148 ± 16 (6)3-150,3-151 |
| Halysin, 10 mg/kg | 155 ± 20 (6)3-150,3-151 |
| Ancrod, 1 U/kg | 176 ± 31 (6)3-150,3-151 |
| Crotalin, 300 μg/kg | 293 ± 38 (6)3-150 |
Platelet-rich thrombus formation was induced as described in Table2. The occlusion time was measured 10 to 20 minutes after the administration of halysin, ancrod, crotalin, and PGI2. Values are presented as mean ± SEM (n = 6).
P < .05 as compared with the control group (receiving normal saline).
P < .05 as compared with crotalin group.
DISCUSSION
Crotalin, a newly purified protein from the venom of C atrox,specifically inhibited ristocetin-induced platelet aggregation in vitro and exhibited the antithrombotic activity in vivo. Crotalin specifically inhibited platelet aggregation induced by ristocetin either in platelet suspension supplemented with vWF or in platelet-rich plasma with an IC50 of 2.4 and 6.3 μg/mL, respectively. In contrast to RGD-containing peptides, crotalin at 40 μg/mL did not affect collagen- and U46619-induced platelet aggregation. Ristocetin-induced platelet aggregation was mediated through the initial binding of vWF to platelet GPIb, and subsequently resulted in the exposure of the fibriongen receptor.31,32 The125I-crotalin binding site was 58,632 per platelet with a kd value of 3.2 × 10−7 mol/L. 125I-crotalin binding to platelets was selectively inhibited by AP1, an MoAb raised against platelet GPIb, but not by 7E3, an MoAb raised against GPIIb/IIIa. The binding was not affected by EDTA, indicating that the binding process is divalent-cation independent. Through the analysis of binding data and its inhibitory activity on ristocetin-induced platelet aggregation, crotalin appears to be a selective antagonist of platelet membrane GPIb. Regarding the binding sites of crotalin, the estimation of 58,632 per platelet is higher than that estimated with GPIb MoAb (around 30,000). It has been reported that the binding sites of other venom GPIb antagonists ranged from 21,500 to 47,440.20 We also obtained a similar value of 60,000 per platelet as probed with another GPIb antagonist purified from A acutus (unpublished data, December 1996). Therefore, the varied number of binding sites among GPIb antagonists and MoAbs may be explained as following: the smaller molecular size of venom GPIb antagonists may get a better access to the surface canalicular system of platelets as compared to the bulky, bivalent structure of the MoAb. Earlier studies indicate that low concentration of thrombin exerts its effect through the binding of platelet membrane GPIb, a high-affinity binding site of thrombin.42 Kroll et al33suggested that the binding of vWF to GPIb leads to the activation of phospholipase A2 and subsequent formation of thromboxane. In this study, crotalin as well as AP1 prolonged the latent period in triggering platelet aggregation caused by low concentrations of thrombin (0.03 U/mL), with a slight inhibition on platelet aggregation. Crotalin blocked thromboxane B2 formation of platelets challenged by ristocetin plus vWF or low concentration of thrombin, confirming the hypothesis that the ligation of GPIb may lead to the activation of endogenous phospholipase A2. Regarding the molecular characterization of crotalin, a platelet GPIb antagonist, the preliminary data show that crotalin is unique as a single chain polypeptide that is quite different from the known venom GPIb-binding proteins because they are heterodimer in nature, sharing a highly homologous sequence with C-type lectins.20 Whether it exists as a monomer or dimer under physiological condition is unknown. However, the detailed characterization of the physiocochemical properties of crotalin is in progress.
An IV infusion of crotalin lengthened bleeding time of mice in a dose-dependent manner. Maximal prolongation was observed during a period of 10 to 60 minutes after injection of crotalin (300 μg/kg), and bleeding time progressively returned to control values over a 4-hour period. However, the platelet count after the administration of crotalin did not show a major change. It has been reported that echicetin and jararaca GPIb-BP caused a transient thrombocytopenia in mice,18 19 and therefore it may be an advantage of crotalin when considering its potential use as antithrombotic agent. From its in vivo experiment, we suggest that crotalin may inhibit platelet aggregation as well as platelet adhesion to subendothelium in vivo through blocking the interaction of vWF with platelet GPIb.
As the first step in hemostasis or thrombosis, the binding of vWF to platelet GPIb is essential for platelet adhesion at high-shear blood flow.34 The platelets from patients with Bernard-Soulier Syndrome were defective in expression of functional GPIb-V-IX complex, and poorly adhered to subendothelium at all shear rates.2Much effort has recently been devoted to characterize the interaction of vWF and platelet GPIb at the molecular level with an aim of developing inhibitors that could be useful in the prevention of thrombosis.35-38 Recently, it has been indicated that inhibition of vWF-platelet GPIb interaction is effective in preventing acute restenosis after thrombolytic therapy.39
In the present study we evaluated the antithrombotic effect of crotalin in a mouse model. Surprisingly, crotalin apparently delayed platelet-rich thrombus formation in mesenteric microvessels and its antithrombotic activity was dose dependent. The time course of its antithrombotic effect was consistent with that of its effect on bleeding time in mice (Figs 6 and 7).
Table 4 shows the minimal dose of PGI2, halysin, ancrod, and crotalin in causing the maximal prolongation of occlusion time in this platelet-rich thrombus animal model. Halysin, an RGD-containing venom peptide, completely inhibited ex vivo platelet aggregation for 20 minutes after the administration of halysin at 10 mg/kg. Ancrod (1 U/kg), a thrombin-like enzyme, caused defibrinogenation and exhibited antiplatelet activity for 60 minutes.29 Of the compounds tested, crotalin showed the most pronounced effect in prolonging the occlusion time of the irradiated vessels in inducing platelet-rich thrombus formation as compared with PGI2, halysin, and ancrod, indicating that blockade of the interaction between vWF and platelet GPIb may be a potential strategy in causing a marked antithrombotic effect. In addition, crotalin exhibits a antithrombotic activity with a longer duration as compared with short duration of PGI2, halysin or another disintegrin, triflavin.40
Considering the pharmacokinetic of crotalin, crotalin may be more active as an antithrombotic agent in mice than in human beings because the effective dosage of crotalin in mice ranged from 100 to 300 μg/kg, equivalent to 1.3 to 3.8 μg/mL (assuming 2.0 mL plasma per mouse), even if protein binding is neglected. However, the IC50 of crotalin was about 6.3 μg/mL (in human platelet-rich plasma), five times higher than the effective dosage of 100 μg/kg in mice. On the other hand, the in vivo antithrombotic effect of crotalin in mice may result from both the antiplatelet and anticoagulant activities because the preliminary results show that crotalin prolonged the whole blood clotting time and activated partial thromboplastin time but not the prothrombin time as crotalin was administered IV (unpublished data, December 1996). However, its anticoagulant activity is under investigation.
In conclusion, crotalin specifically inhibited ristocetin-induced platelet agglutination in the presence of vWF through a selective binding of platelet membrane GPIb, resulting in a blockade of interaction between vWF and GPIb. Furthermore, crotalin markedly prolonged the bleeding time when administered IV into mice and was efficious in blocking platelet plug formation in vivo experimental model. Therefore, crotalin may be a valuable tool for developing a new class of antithrombotic drugs for clinic use through the study of its structure-activity relationship.
ACKNOWLEDGMENT
We appreciate the secretarial work of I.S. Peng.
Supported by Grant No. NSC84-2331-B-002-217 from the National Science Council of Taiwan.
Address reprint requests to Tur-Fu Huang, PhD, Pharmacological Institute, College of Medicine, National Taiwan University, No 1, Jen-Ai Rd, 1st Section, Taipei, 10018, Taiwan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal