Abstract
We have evaluated the performance of a new analyzer using high shear stress, the PFA-100 (Platelet Function Analyzer, Dade International, Massy, France), for screening of patients with von Willebrand disease (vWD). Whole citrated blood is aspirated through a capillary to the central aperture of a membrane coated with collagen and with a platelet agonist (either epinephrine or adenosine diphosphate [ADP]). The time required to obtain occlusion of the aperture by a platelet plug is defined as the closure time (CT). We studied 60 patients with different types of vWD and 96 normal subjects. Fourteen subjects with hemophilia and 15 patients with a platelet disorder were also analyzed. When omitting results from two patients with type 2N, the 58 other patients with type 1, type 2A, type 2B, type 3, or acquired vWD all exhibited an abnormal occlusion with collagen-ADP (sensitivity, 100%) and 56 of 58 had an abnormal CT with collagen-epinephrine (sensitivity, 96.5%). Only two patients with mild type 1 were not detected with collagen-epinephrine. In comparison, the bleeding time (BT) was normal in 20 patients: 17 with type 1, two with type 2A, and one with acquired vWD (sensitivity, 65.5%). The specificity of the PFA-100 was over 95% with both types of cartridges. Thus, the analyzer is well adapted to routine testing, as it has the advantages of simplicity and ease of execution, and demonstrates a high sensitivity, clearly superior to that of BT, for the screening of patients with vWD.
VON WILLEBRAND DISEASE (vWD), which is the most common inherited bleeding disorder with an incidence as high as 1%,1 results from quantitative or qualitative defects of von Willebrand factor (vWF).2 vWF is a large multimeric glycoprotein and its degree of polymerization and integrity of specific domains are essential for function of the protein.3,4 vWF is involved in platelet adhesion to the injured vessel wall, platelet spreading, and platelet-platelet interactions under conditions of high shear.3,4 These functions require interaction of vWF with two platelet receptors, glycoprotein (GP) Ib/IX, and GPIIb/IIIa.5-8 vWF also serves to transport factor VIII (F.VIII) and to protect it from proteolytic degradation.3 4
Recently, a pathophysiologic classification for vWD was introduced by Sadler.9 Quantitative defects of vWF may either be classified as partial (type 1) or total (type 3). vWD type 2 (subtypes 2A, 2M, 2B, and 2N) corresponds to a qualitative defect, and distinct mutations have been found in the vWF gene corresponding to different functional domains of vWF.10 11 Type 2A is characterized by both a decreased interaction of vWF with platelets and lack of the highest sized multimers. In contrast, type 2M corresponds to a decreased interaction of vWF with platelets, which is not associated with a loss of high molecular weight multimers. Type 2B is characterized by an increased affinity of vWF for GPIb/IX. Type 2N is due to a qualitative abnormality of vWF causing defective interaction with F.VIII, which is not anymore protected from rapid proteolysis.
At present, the diagnosis of vWD relies on the determination of the bleeding time (BT) and assessment of vWF antigen (vWFAg), vWF ristocetin cofactor activity (vWFRCo), and F. VIII activity. Ristocetin-induced platelet agglutination (RIPA) and distribution of vWF multimers allow for further diagnosis and classification.
Historically, the BT has been considered as an essential screening test for primary hemostasis disorders and particularly for vWD, except in type 2N, probably because of the lack of a better alternative. However, the accuracy, validity, predictibility, and reproducibility of the BT have frequently been questioned.12 As a result, the BT is usually replaced by assays such as vWFRCo and/or vWFAg that require more time and skill to perform. In this study, we tested a new instrument, the PFA-100 (Platelet Function Analyzer, Dade International, Massy, France), that allows a rapid and simple determination of platelet function in primary hemostasis. We used the PFA-100 to screen 60 patients with different types of vWD. The present study shows that the PFA-100 is strikingly more sensitive than the BT in screening patients with vWD type 1, type 2A, type 2B or type 3, and acquired forms of the disease.
MATERIALS AND METHODS
Patients.
Sixty patients with vWD were included in the study after obtaining appropriate consent. Twenty-six men and thirty-four women, with a mean age of 32.4 years (range, 2 to 77) were investigated. These patients had not received vWF concentrates or DDAVP for at least 1 month. vWD had been previously diagnosed by a personal or familial bleeding history and results of laboratory tests such as vWFAg, vWFRCo, F.VIII, RIPA, and multimeric composition of vWF. For this study, all parameters were retested and patients were classified according to the revised classification of vWD.9 The following vWD types were represented in the 60 patients: type 1, n = 36; type 2A, n = 10; type 2B, n = 3; type 3, n = 4; and type 2N, n = 2. Among the 36 patients with type 1, 32 of them exhibited all criteria for “definite” type 1 vWD, ie, a personal history of significant mucocutaneous bleeding, results of vWFRCo and vWFAg <2 standard deviation (SD) below the mean of the normal population when adjusted to the blood type and a positive family history of vWD. The four other patients had “possible” type 1 vWD, ie, positive personal history and laboratory criteria, but no evidence of familial vWD. We also studied five patients with acquired vWD associated with benign (n = 3) or malignant (n = 2) B-cell disorders.
Fifteen patients with a previously well-defined platelet disorder were also investigated: two with Glanzmann thrombasthenia, three with pseudo- or platelet-type vWD, four with congenital storage pool disease, and six with aspirin-like defect.
Control group.
One hundred and ten subjects devoid of any primary hemostasis disorder were used as controls. They had not taken any medication for at least 2 weeks. Ninety-six healthy volunteers (31 men and 65 women) served as the first control group. Their mean age was 34.5 years (range, 15 to 65). These individuals had no history of bleeding. Fourteen subjects with hemophilia A (n = 12) or B (n = 2) were used as the second control group. Informed consent was obtained from each of them.
Laboratory tests.
Hematocrit and platelet counts were performed with a Coulter STKS cell counter (Coulter Corporation, Coultronics, France). Blood samples were also collected for blood group. Citrated whole blood (1 vol of 3.8% sodium citrate mixed with 9 vol of blood) was obtained by clean venipuncture. The activated partial thromboplastin time (APTT) was measured with PTT-LT (Diagnostica Stago, Asnieres, France) using an STA analyzer (Diagnostica Stago). F.VIII activity was performed by a one-stage clotting assay based on the APTT using F.VIII-deficient plasma (Diagnostica Stago) on the STA. vWFAg was measured by enzyme-linked immunosorbent assay (ELISA) with a commercial kit (Asserachrom vWF, Diagnostica Stago). vWFRCo was assayed by aggregometry using a commercially available kit from Behring (Marburg, Germany), which consists of lyophilized platelets and ristocetin A. All results are the mean of two determinations and expressed as IU/dL of plasma. The Second International Reference Preparation for Factor VIII-related activities (National Institute for Biological Standards and Control, London, UK) was used as a standard. Multimeric composition of plasma vWF was estimated by sodium dodecyl sulfate gel electrophoresis as previously described.13 RIPA was performed in platelet-rich plasma on an aggregometer (Thrombo-Agregametre, Regulest, France) with ristocetin (Diagnostica Stago) at three final concentrations: 0.5, 1.0, and 1.5 mg/mL. Platelet aggregation was performed at 37°C in platelet-rich plasma on the same aggregometer using 2.5 and 5 μmol/L of adenosine-5′-diphosphate (ADP), 5 μmol/L of epinephrine from Diagnostica Stago, 1.0 and 4.0 μg/mL of equine collagen from Hormon Chemie, Munich, Germany, or 1.5 mmol/L of arachidonic acid from Helena Laboratories, Beaumont, TX.
BT was determined by a modified Ivy technique using the Simplate sterile disposable device (Organon Teknika Corp, Durham, NC) according to the instructions of the manufacturer.
PFA-100 system.
The PFA-100 is a high shear-inducing device that simulates primary hemostasis after injury to a small vessel.14-16 The system consists of a microprocessor-controlled instrument and a disposable test cartridge. The test cartridge contains a reservoir for citrated whole blood and a capillary (200 μm internal diameter) surmounted by a cup containing a biologically active membrane with a central aperture (approximately 150 μm diameter). The membrane is coated with fibrillar type I equine tendon collagen. Additionally, either epinephrine (10 μg) or ADP (50 μg) is present on the membrane. These agents provide a controlled stimulation of platelets as the blood sample passes through the aperture. The analyzer provides a constant negative pressure that aspirates whole blood (800 μL) through the capillary into the cup where it comes into contact with the membrane and then passes through the aperture. In response to stimulation by collagen, in conjunction with either epinephrine or ADP, as well as by high shear rates (5,000 to 6,000 second-1), platelets adhere and aggregate on the membrane surface at the area surrounding the aperture. During the course of measurement, a platelet plug forms that ultimately occludes the aperture and blood flow is stopped. The time required to obtain occlusion of the aperture is defined as the closure time (CT).
All normal subjects and patients were tested with both types of cartridges (collagen/epinephrine or collagen/ADP). For each cartridge type, results were based on the mean of duplicate testing. If results of duplicate tests deviated by more than 20%, a third test was performed. Citrated whole blood was stored at room temperature less than 4 hours before testing.
Statistical analysis.
Mean, SD, and reference ranges were determined for all parameters. The normal range for the PFA-100 was calculated as mean ± 2 SD of the healthy volunteers group. Sensitivity was calculated as the percentage of correctly detected vWD patients, using the formula: sensitivity (%) = true positive × 100/true positive + false negative. Specificity was calculated as the percentage of values within the normal range among the total control group (healthy volunteers and hemophiliacs), using the formula: specificity (%) = true negative × 100/true negative + false positive. Positive predictive value (PPV) was estimated as the percentage of patients with vWD among the subjects whose CT is prolonged, using the formula: PPV = true positive × 100/true positive + false positive. Negative predictive value (NPV) was estimated as the percentage of subjects devoid of any primary hemostasis disorder among the subjects whose CT is in the normal range, using the formula: NPV = true negative × 100/true negative + false negative. The analysis of correlations was performed with statgraphics (Statview; Abacus Concepts, Berkeley, CA) as simple regression.
RESULTS
Blood group, hematocrit, and platelet count.
Blood group type O was found in 32 healthy volunteers (33.3%), non-O in 64 (66.7%). Blood group O was found in seven subjects with hemophilia (50%), non-O in three (21.4%), and was unknown in four (28.6%). In vWD patients, 27 (45%) were of group O, 30 (50%) of group non-O, the blood group being unknown for three (5%). In all controls and patients, the hematocrit was > 35% and platelet count was > 150 × 109/L, except in one patient with type 2B vWD (90 × 109/L).
vWF assays.
Table 1 shows the extreme values, mean, and SD of vWF levels in the 96 healthy volunteers. Table 2 indicates the results in the 14 hemophiliacs. Table 3 shows vWFAg and vWFRCo levels in the 36 patients with type 1 vWD. Among these patients, 18 had vWFRCo levels between 26 and 39 UI/dL, 10 between 11 and 25 IU/dL, and eight between 1 and 10 IU/dL. Results in patients with type 2 (2A, 2B, 2N), type 3, and acquired vWD are reported in Table 4. One patient with type 2B was tested during pregnancy, with a normal vWFRCo level (88 IU/dL) and a high vWFAg level (208 IU/dL).
Mean and SD of Laboratory Parameters in 96 Healthy Volunteers
| . | vWFAg (IU/dL) . | vWFRCo (IU/dL) . | CT (sec) . | |
|---|---|---|---|---|
| ADP . | Epi . | |||
| Extreme values | 56-207 | 58-209 | 66-126 | 77-186 |
| Mean | 102.8 | 101.7 | 89 | 120 |
| SD | 31.5 | 32 | 15.2 | 19.9 |
| Mean ± 2 SD | 40-166 | 38-140 | 59-120 | 80-160 |
| . | vWFAg (IU/dL) . | vWFRCo (IU/dL) . | CT (sec) . | |
|---|---|---|---|---|
| ADP . | Epi . | |||
| Extreme values | 56-207 | 58-209 | 66-126 | 77-186 |
| Mean | 102.8 | 101.7 | 89 | 120 |
| SD | 31.5 | 32 | 15.2 | 19.9 |
| Mean ± 2 SD | 40-166 | 38-140 | 59-120 | 80-160 |
Abbreviation: CT, closure time.
Laboratory Parameters in 14 Patients With Hemophilia
| Patients . | F.VIII (IU/dL) . | vWFAg (IU/dL) . | vWFRCo (IU/dL) . | CT (sec) . | |
|---|---|---|---|---|---|
| ADP . | Epi . | ||||
| Hemophilia A | |||||
| 1 | <1 | 66 | 54 | 97 | 108 |
| 2 | <1 | 200 | 164 | 63 | 87 |
| 3 | <1 | 60 | 60 | 119 | 138 |
| 4 | <1 | 73 | 68 | 119 | 117 |
| 5 | 3 | 157 | 157 | 91 | 98 |
| 6 | 3 | 57 | 54 | 103 | 123 |
| 7 | 5 | 68 | 56 | 99 | 133 |
| 8 | 11 | 147 | 125 | 101 | 108 |
| 9 | 14 | 110 | 101 | 89 | 115 |
| 10 | 16 | 76 | 71 | 92 | 126 |
| 11 | 25 | 148 | 137 | 101 | 120 |
| 12 | 27 | 87 | 84 | 87 | 132 |
| Hemophilia B | |||||
| 1 | 1.5 | 74 | 58 | 109 | 128 |
| 2 | 3 | 190 | 186 | 60 | 77 |
| Patients . | F.VIII (IU/dL) . | vWFAg (IU/dL) . | vWFRCo (IU/dL) . | CT (sec) . | |
|---|---|---|---|---|---|
| ADP . | Epi . | ||||
| Hemophilia A | |||||
| 1 | <1 | 66 | 54 | 97 | 108 |
| 2 | <1 | 200 | 164 | 63 | 87 |
| 3 | <1 | 60 | 60 | 119 | 138 |
| 4 | <1 | 73 | 68 | 119 | 117 |
| 5 | 3 | 157 | 157 | 91 | 98 |
| 6 | 3 | 57 | 54 | 103 | 123 |
| 7 | 5 | 68 | 56 | 99 | 133 |
| 8 | 11 | 147 | 125 | 101 | 108 |
| 9 | 14 | 110 | 101 | 89 | 115 |
| 10 | 16 | 76 | 71 | 92 | 126 |
| 11 | 25 | 148 | 137 | 101 | 120 |
| 12 | 27 | 87 | 84 | 87 | 132 |
| Hemophilia B | |||||
| 1 | 1.5 | 74 | 58 | 109 | 128 |
| 2 | 3 | 190 | 186 | 60 | 77 |
Laboratory Parameters in 36 Patients With Type 1 vWD
| Patients . | vWFAg (IU/dL) . | vWFRCo (IU/dL) . | BT (min) . | CT (sec) . | |
|---|---|---|---|---|---|
| ADP . | Epi . | ||||
| 1 | 48 | 39 | 10 | 136 | 137 |
| 2 | 40 | 39 | 8 | 147 | 173 |
| 3 | 34 | 38 | 6 | 154 | 202 |
| 4 | 62 | 38 | 8.5 | 148 | 164 |
| 5 | 39 | 36 | 18 | 193 | 159 |
| 6 | 45 | 35 | 6 | 141 | 184 |
| 7 | 37 | 34 | 6 | 144 | 183 |
| 8 | 35 | 34 | 16 | 210 | >250 |
| 9 | 38 | 34 | 5 | 145 | 218 |
| 10 | 45 | 34 | 6 | 138 | 180 |
| 11 | 42 | 31 | 5.5 | 127 | 174 |
| 12 | 38 | 30 | 8 | 129 | 191 |
| 13 | 35 | 28 | 18.5 | 197 | >250 |
| 14 | 29 | 28 | 6 | 170 | >250 |
| 15 | 59 | 27 | >20 | >250 | >250 |
| 16 | 48 | 27 | 3.5 | 141 | 219 |
| 17 | 31 | 26 | 7 | >250 | >250 |
| 18 | 38 | 26 | 17 | >250 | >250 |
| 19 | 28 | 22 | 15 | >250 | >250 |
| 20 | 23 | 22 | 12 | >250 | >250 |
| 21 | 39 | 22 | 17 | 181 | >250 |
| 22 | 30 | 22 | 15 | 181 | >250 |
| 23 | 38 | 16 | 8.5 | 197 | >250 |
| 24 | 24 | 15 | 6.5 | >250 | >250 |
| 25 | 24 | 14 | >20 | >250 | >250 |
| 26 | 44 | 13 | >20 | >250 | >250 |
| 27 | 26 | 12 | >20 | >250 | >250 |
| 28 | 15 | 11 | >20 | >250 | >250 |
| 29 | 23 | 10 | 11.5 | >250 | >250 |
| 30 | 12 | 10 | 9 | >250 | >250 |
| 31 | 15 | 10 | >20 | >250 | >250 |
| 32 | 27 | 10 | 11 | >250 | >250 |
| 33 | 12 | 9 | 4 | >250 | >250 |
| 34 | 29 | 9 | 8.5 | >250 | >250 |
| 35 | 9 | 9 | >20 | >250 | >250 |
| 36 | 9 | 5 | 7.5 | >250 | >250 |
| Patients . | vWFAg (IU/dL) . | vWFRCo (IU/dL) . | BT (min) . | CT (sec) . | |
|---|---|---|---|---|---|
| ADP . | Epi . | ||||
| 1 | 48 | 39 | 10 | 136 | 137 |
| 2 | 40 | 39 | 8 | 147 | 173 |
| 3 | 34 | 38 | 6 | 154 | 202 |
| 4 | 62 | 38 | 8.5 | 148 | 164 |
| 5 | 39 | 36 | 18 | 193 | 159 |
| 6 | 45 | 35 | 6 | 141 | 184 |
| 7 | 37 | 34 | 6 | 144 | 183 |
| 8 | 35 | 34 | 16 | 210 | >250 |
| 9 | 38 | 34 | 5 | 145 | 218 |
| 10 | 45 | 34 | 6 | 138 | 180 |
| 11 | 42 | 31 | 5.5 | 127 | 174 |
| 12 | 38 | 30 | 8 | 129 | 191 |
| 13 | 35 | 28 | 18.5 | 197 | >250 |
| 14 | 29 | 28 | 6 | 170 | >250 |
| 15 | 59 | 27 | >20 | >250 | >250 |
| 16 | 48 | 27 | 3.5 | 141 | 219 |
| 17 | 31 | 26 | 7 | >250 | >250 |
| 18 | 38 | 26 | 17 | >250 | >250 |
| 19 | 28 | 22 | 15 | >250 | >250 |
| 20 | 23 | 22 | 12 | >250 | >250 |
| 21 | 39 | 22 | 17 | 181 | >250 |
| 22 | 30 | 22 | 15 | 181 | >250 |
| 23 | 38 | 16 | 8.5 | 197 | >250 |
| 24 | 24 | 15 | 6.5 | >250 | >250 |
| 25 | 24 | 14 | >20 | >250 | >250 |
| 26 | 44 | 13 | >20 | >250 | >250 |
| 27 | 26 | 12 | >20 | >250 | >250 |
| 28 | 15 | 11 | >20 | >250 | >250 |
| 29 | 23 | 10 | 11.5 | >250 | >250 |
| 30 | 12 | 10 | 9 | >250 | >250 |
| 31 | 15 | 10 | >20 | >250 | >250 |
| 32 | 27 | 10 | 11 | >250 | >250 |
| 33 | 12 | 9 | 4 | >250 | >250 |
| 34 | 29 | 9 | 8.5 | >250 | >250 |
| 35 | 9 | 9 | >20 | >250 | >250 |
| 36 | 9 | 5 | 7.5 | >250 | >250 |
Laboratory Parameters in 24 Patients With Types 2A, 2B, 2N, Type 3, and Acquired vWD
| Patients . | vWFAg IU/dL . | vWFRCo IU/dL . | BT (min) . | CT (sec) . | |
|---|---|---|---|---|---|
| ADP . | Epi . | ||||
| Type 2A | |||||
| 1 | 75 | 43 | 8.5 | >250 | >250 |
| 2 | 92 | 24 | 8.5 | >250 | >250 |
| 3 | 85 | 23 | 18 | >250 | >250 |
| 4 | 79 | 22 | >20 | >250 | >250 |
| 5 | 69 | 22 | >20 | >250 | >250 |
| 6 | 30 | 14 | >20 | >250 | >250 |
| 7 | 72 | 14 | >20 | >250 | >250 |
| 8 | 45 | <3 | >20 | >250 | >250 |
| 9 | 92 | <3 | >20 | >250 | >250 |
| 10 | 50 | <3 | >20 | >250 | >250 |
| Type 2B | |||||
| 1* | 208 | 88 | 9 | 3-151 | 184 |
| 2 | 51 | 17 | >20 | >250 | >250 |
| 3 | 61 | 15 | 14 | >250 | >250 |
| Type 2N | |||||
| 1 | 83 | 78 | 5.5 | 81 | 135 |
| 2 | 67 | 54 | 4 | 94 | 141 |
| Type 3 | |||||
| 1 | <1 | <3 | >20 | >250 | >250 |
| 2 | <1 | <3 | >20 | >250 | >250 |
| 3 | <1 | <3 | >20 | >250 | >250 |
| 4 | <1 | <3 | >20 | >250 | >250 |
| Acquired form | |||||
| 1 | 49 | 40 | 12 | 140 | 166 |
| 2 | 42 | 36 | 7.5 | 179 | >250 |
| 3 | 48 | 33 | 9 | >250 | >250 |
| 4 | 27 | 21 | 18 | >250 | >250 |
| 5 | 14 | 3 | 10 | >250 | >250 |
| Patients . | vWFAg IU/dL . | vWFRCo IU/dL . | BT (min) . | CT (sec) . | |
|---|---|---|---|---|---|
| ADP . | Epi . | ||||
| Type 2A | |||||
| 1 | 75 | 43 | 8.5 | >250 | >250 |
| 2 | 92 | 24 | 8.5 | >250 | >250 |
| 3 | 85 | 23 | 18 | >250 | >250 |
| 4 | 79 | 22 | >20 | >250 | >250 |
| 5 | 69 | 22 | >20 | >250 | >250 |
| 6 | 30 | 14 | >20 | >250 | >250 |
| 7 | 72 | 14 | >20 | >250 | >250 |
| 8 | 45 | <3 | >20 | >250 | >250 |
| 9 | 92 | <3 | >20 | >250 | >250 |
| 10 | 50 | <3 | >20 | >250 | >250 |
| Type 2B | |||||
| 1* | 208 | 88 | 9 | 3-151 | 184 |
| 2 | 51 | 17 | >20 | >250 | >250 |
| 3 | 61 | 15 | 14 | >250 | >250 |
| Type 2N | |||||
| 1 | 83 | 78 | 5.5 | 81 | 135 |
| 2 | 67 | 54 | 4 | 94 | 141 |
| Type 3 | |||||
| 1 | <1 | <3 | >20 | >250 | >250 |
| 2 | <1 | <3 | >20 | >250 | >250 |
| 3 | <1 | <3 | >20 | >250 | >250 |
| 4 | <1 | <3 | >20 | >250 | >250 |
| Acquired form | |||||
| 1 | 49 | 40 | 12 | 140 | 166 |
| 2 | 42 | 36 | 7.5 | 179 | >250 |
| 3 | 48 | 33 | 9 | >250 | >250 |
| 4 | 27 | 21 | 18 | >250 | >250 |
| 5 | 14 | 3 | 10 | >250 | >250 |
*During pregnancy (5 months).
Obstruction of the capillary by platelet aggregates.
BT.
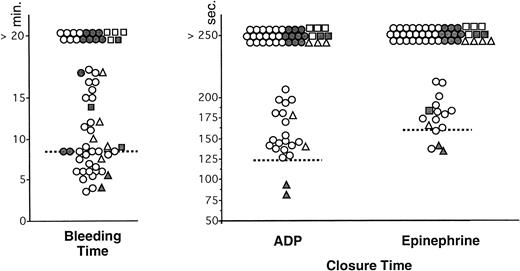
For evident ethical reasons, BT was not performed in the control group. The values in vWD patients are indicated in Tables 3 and 4. The BT was normal in the two patients with type 2N vWD. Only 38 of 58 patients with other types of vWD showed a prolonged BT (> 8.5 minutes) (Fig 1). Twenty patients had a normal BT: two with type 2A, one with an acquired form, and 17 with type 1 (Fig1). When excluding results in type 2N vWD, the sensitivity of the BT, calculated from these results, was 65.5%.
Comparison of BT (Simplate) and measurement of CT with collagen-ADP and collagen-epinephrine cartridges in the PFA-100 in patients with von Willebrand disease (n = 60). Each type of vWD is identified by the following symbol: (○) type 1, (•) type 2A, (▪) type 2B, (▴) type 2N, (□) type 3, and (▵) acquired vWD. The interrupted lines represent the upper limit of the normal range (8.5 minutes) for the BT and the mean value + 2 SD of the control group for CT (120 seconds with collagen-ADP and 160 seconds with collagen-epinephrine).
Comparison of BT (Simplate) and measurement of CT with collagen-ADP and collagen-epinephrine cartridges in the PFA-100 in patients with von Willebrand disease (n = 60). Each type of vWD is identified by the following symbol: (○) type 1, (•) type 2A, (▪) type 2B, (▴) type 2N, (□) type 3, and (▵) acquired vWD. The interrupted lines represent the upper limit of the normal range (8.5 minutes) for the BT and the mean value + 2 SD of the control group for CT (120 seconds with collagen-ADP and 160 seconds with collagen-epinephrine).
CT with collagen-epinephrine test cartridges.
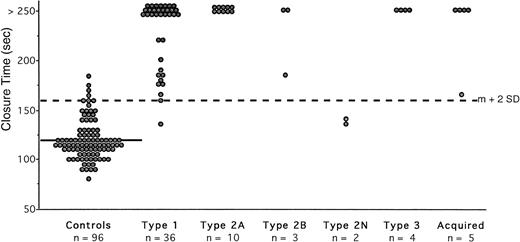
The extreme values of CT obtained in 96 normal subjects (Table 1) were 77 to 186 seconds, with a mean value of 120 seconds and a mean CV of 5.4%. This control group was used to calculate the normal range, indicating an upper limit of 160 seconds (mean + 2 SD). Four healthy volunteers had a slightly prolonged CT (167, 169, 174, and 186 seconds, respectively). The 14 hemophiliacs had a normal CT (Table 2). Among the 110 controls without any primary hemostasis disorder, the frequency of prolonged CT was 3.6%, indicating a specificity of 96.4%. Type 2N patients exhibited normal occlusion ( Fig 2). Fifty-six of the remaining 58 patients showed abnormal occlusion (Tables 3 and4). All patients with type 3 and type 2A vWD had infinite CT, that is greater than 250 seconds (Fig 2). All patients with type 2B had a prolonged CT, even the patient with a normal vWFRCo level. Among the 41 patients with acquired or type 1 vWD, 28 showed an infinite CT (Fig 2). Only two patients with type 1 had a normal CT (137 and 159 seconds) and their level of vWFRCo was 39 and 36 IU/dL, respectively (Table 3). When excluding results from type 2N vWD, the sensitivity of the test with collagen-epinephrine cartridges was 96.5%. The CT with collagen-epinephrine exhibited a PPV of 93.3% and an NPV of 98.2%.
Measurement of CT with collagen-epinephrine cartridges in the PFA-100 in normal subjects and in patients with vWD disease. Each closed circle is the mean of duplicate testing in an individual. The solid line represents the mean value in the normal subjects (120 seconds); the interrupted line across the figure represents the upper limit of normal (160 seconds), which was calculated as the mean (m) + 2 SD.
Measurement of CT with collagen-epinephrine cartridges in the PFA-100 in normal subjects and in patients with vWD disease. Each closed circle is the mean of duplicate testing in an individual. The solid line represents the mean value in the normal subjects (120 seconds); the interrupted line across the figure represents the upper limit of normal (160 seconds), which was calculated as the mean (m) + 2 SD.
CT with collagen-ADP test cartridges.
In the normal subjects (Table 1), the extreme values of CT were 66 to 126 seconds with a mean value of 89 seconds (mean CV of 4.5%), indicating an upper limit of 120 seconds (mean + 2 SD). Only one healthy volunteer had a slightly prolonged CT (126 seconds). All of the hemophiliacs had a normal CT (Table 2). Thus, less than 1% of the control group showed a prolonged CT, indicating a specificity of 99%. The CT was normal in type 2N vWD. All other patients exhibited abnormal occlusion (Fig 3). The CT was infinite (> 250 seconds) in all patients with type 3 and type 2A vWD, in two with type 2B, in 18 with type 1, and in three with acquired vWD (Fig 3). In the pregnant patient with type 2B, the CT could not be estimated because of rapid flow obstruction of the capillary. When omitting results from type 2N patients, the sensitivity of the test with collagen-ADP cartridges was 100%. The CT collagen-ADP exhibited a PPV of 98.3% and an NPV of 100%.
Measurement of CT with collagen-ADP cartridges in the PFA-100 in normal subjects and in patients with vWD. Each closed circle is the mean of duplicate testing in an individual. The solid line represents the mean value in the normal subjects (89 seconds); the interrupted line across the figure represents the upper limit of normal (120 seconds), which was calculated as the mean (m) + 2 SD.
Measurement of CT with collagen-ADP cartridges in the PFA-100 in normal subjects and in patients with vWD. Each closed circle is the mean of duplicate testing in an individual. The solid line represents the mean value in the normal subjects (89 seconds); the interrupted line across the figure represents the upper limit of normal (120 seconds), which was calculated as the mean (m) + 2 SD.
Correlations.
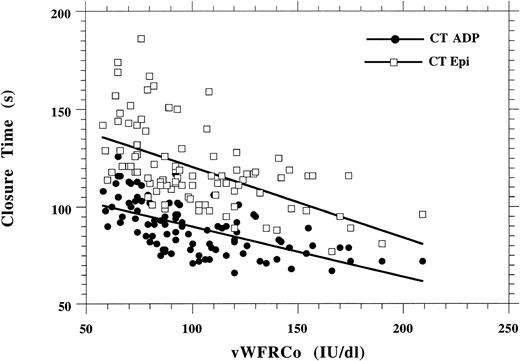
Figure 4 shows the correlation between CT and vWFRCo levels in the 96 healthy volunteers: R = 0.62 with ADP and R = 0.56 with epinephrine. The correlation between CT and vWFRCo levels in patients with vWD could not be calculated because a large number of patients had an infinite CT (> 250 seconds): n = 37 with collagen-ADP cartridges and n = 44 with collagen-epinephrine cartridges (Tables 3and 4). However, results in type 1 vWD indicated that on the whole the prolongation of the CT was inversely proportional to the level of vWFRCo (Table 3).
Correlation between vWFRCo levels and CT with either collagen-epinephrine or collagen-ADP in 96 normal subjects.
Correlation between vWFRCo levels and CT with either collagen-epinephrine or collagen-ADP in 96 normal subjects.
DISCUSSION
The detection of vWD is still a challenge, especially in the mild forms. Because there is an abnormal interaction of platelets with the subendothelium in all types of vWD except type 2N,17 the BT has been considered in the past as a useful screening tool to detect these patients. Due to poor sensitivity, lack of reproducibility and large variability, the BT is however not a suitable method for screening of vWD. The poor sensitivity of the BT has been illustrated by the Italian working group,12 showing that about 20% of patients with moderate vWD have a normal BT. Our study confirms these findings and indicates that 47% of patients with type 1 vWD, 20% with type 2A, and 20% with acquired vWD, have a normal BT.
An optimal screening procedure to detect patients with a primary hemostasis disorder needs to be reproducible, sensitive, and specific. The PFA-100 shows excellent reproducibility as results of duplicate tests deviated by more than 20% in only 2% of controls and 0% of patients.
The sensitivity of the PFA-100 is also highly satisfactory. When omitting results in type 2N vWD (showing as expected a normal CT), the PFA-100 detected all patients with vWD using the collagen-ADP cartridges and all but two patients using the collagen-epinephrine cartridges. The latter two patients had a positive history of mild bleeding and laboratory criteria (vWFRCo levels of 39 and 36 IU/dL) in favor of vWD, but no evidence for an inherited form. According to the recent recommendations of the Standardization and Scientific Committee (SSC) of the International Society on Thrombosis and Haemostasis (ISTH), both patients had “possible” and not “definite” type 1 vWD. In addition, it is well known that individuals with group O have lower levels of vWF18 than those of other groups and may have a very mild bleeding tendency or none at all. Knowing that these two subjects were of group O, they may also be considered as borderline normal subjects and do not really limit the excellent sensitivity of the PFA-100 in detecting patients with vWD.
The specificity of the PFA-100 was assessed by studying a large control group devoid of any primary hemostasis disorder (healthy volunteers and hemophiliacs); we detected four of 96 normal subjects whose CT with epinephrine was either slightly prolonged or prolonged, and one whose CT was borderline with ADP. A forgotten medication with any drug interacting with primary hemostasis may have explained the four prolonged CT with epinephrine, although the platelet aggregation tests were found to be normal. As expected, results in hemophilia showed that the CT was not affected by coagulation abnormalities. The good specificity of CT was confirmed by the estimation of predictive values. Positive and negative predictive values were excellent, the latter being essential for a screening test used to exclude the diagnosis of vWD.
The relationship between BT and plasma vWFRCo levels has been analyzed by several investigators and Weiss,19 in particular, reported a good correlation between both tests. In the present study, we analyzed the correlation between the CT measured in the PFA-100 and the plasma vWFRCo levels. The correlation was fair in normal subjects, but could not be calculated in vWD patients because a large number exhibited an infinite CT due to the high sensitivity of the method.
A recent study16 has shown the involvement of vWF in platelet adhesion and aggregation under the high shear stress conditions of the PFA-100 by demonstrating that monoclonal antibodies to vWF, specific for the collagen-, GPIb/IX-, or GPIIb/IIIa-binding sites of vWF, caused a dose-dependent prolongation of the CT. In contrast, a polyclonal antibody to fibrinogen did not affect the CT.16 In fact, we tested a patient with afibrinogenemia and found a normal CT with both types of cartridges (results not shown). These results are in agreement with those of perfusion studies6 indicating that under high shear stress flow conditions, vWF is the only protein to play a role in platelet adhesion and aggregation. This has been corroborated by experiments of shear-induced platelet aggregation.20
Interestingly, the PFA-100 was capable of detecting patients with type 2B vWD. This is in agreement with our results of perfusion studies21 and with experiments using the blood filtration test showing defective platelet retention and aggregation in all vWD patients tested.22 On the contrary, 2B variants are not detected by shear-induced platelet aggregation measured in the cone-and-plate viscometer23 where there is an increased aggregation, paradoxically masking the defective platelet adhesion and thrombus formation in these patients. In vivo, binding of abnormal vWF to platelet GPIb results in occupation of the receptor so that it is no longer available for mediating platelet adhesion. The PFA-100 system appears to detect a decreased ability of platelets to adhere rather than an increased tendency of platelets to aggregate. Therefore, this system may mirror the defective hemostatic function of vWF in type 2B as well as in all other types of vWD, except type 2N. Thus, the PFA-100 reflects vWF-dependent adhesive interactions as they occur in vivo and is a global predictor of vWF-dependent platelet function under high shear stress.
The PFA-100 may be used in a more general setting to predict the bleeding tendency resulting from functional platelet alterations in patients with defects other than vWD and to monitor vWD patients treated by deamino-8-D-arginine vasopressin (DDAVP). Studies using monoclonal antibodies to GPIb or to GPIIb/IIIa, and aspirin14-16 have shown the usefulness of the the PFA-100 for detecting patients with inherited or acquired platelet dysfunction. Indeed, we tested patients with various platelet disorders and found that the PFA-100 is an excellent analyzer for their screening. All patients showed prolonged CT using epinephrine and ADP, except three with an aspirin-like defect whose CT was normal with ADP (Table 5). However, ADP is known not to be discriminating for the diagnosis of aspirin-like defects.15In regard to the therapeutic monitoring of vWD patients, we found that DDAVP completely corrected the CT with both types of cartridges in the 11 cases with type 1 vWD that we tested (results not shown), emphasizing the usefulness of the PFA-100 as compared with the other tests (BT, vWFRCo, and vWFAg).
CT in 15 Patients With Platelet Disorders
| Platelet Disorder . | Patients . | CT (sec) . | |
|---|---|---|---|
| ADP . | Epi . | ||
| Glanzmann thrombasthenia (n = 2) | 1 | >250 | >250 |
| Pseudo (“platelet”)-vWD (n = 3) | 2 1 2 3 | >250 >250 >250 >250 | >250 >250 >250 >250 |
| Storage pool disease (n = 4) | 1 2 3 | >250 >250 >250 | >250 >250 >250 |
| 4 | >250 | >250 | |
| Aspirin-like defect (n = 6) | 1 2 3 | 150 130 121 | >250 >250 >250 |
| 4 | 115 | 228 | |
| 5 | 104 | 216 | |
| 6 | 81 | 210 | |
| Normal range (mean ± 2 SD) | 59-120 | 80-160 | |
| Platelet Disorder . | Patients . | CT (sec) . | |
|---|---|---|---|
| ADP . | Epi . | ||
| Glanzmann thrombasthenia (n = 2) | 1 | >250 | >250 |
| Pseudo (“platelet”)-vWD (n = 3) | 2 1 2 3 | >250 >250 >250 >250 | >250 >250 >250 >250 |
| Storage pool disease (n = 4) | 1 2 3 | >250 >250 >250 | >250 >250 >250 |
| 4 | >250 | >250 | |
| Aspirin-like defect (n = 6) | 1 2 3 | 150 130 121 | >250 >250 >250 |
| 4 | 115 | 228 | |
| 5 | 104 | 216 | |
| 6 | 81 | 210 | |
| Normal range (mean ± 2 SD) | 59-120 | 80-160 | |
In conclusion, the PFA-100 is well adapted to routine testing, as it has the advantage of simplicity and ease of execution. It provides fast results and uses the same citrated blood that is routinely drawn for other coagulation testing; the latter is particularly useful in emergency situations, especially before surgery. The test can be safely delayed for up to 4 hours from the time of blood sampling, provided that the blood is kept at room temperature. When expressed by its sensitivity, specificity, and predictive values, the clinical performance of the test is excellent in vWD with both types of cartridges, possibly slightly better in the presence of ADP. The high sensitivity demonstrated by the PFA-100 analyzer is clearly superior to that of BT for the detection of patients with vWD. This leads to the proposal of the following strategy: if the CT is within the normal range, one may exclude the diagnosis of vWD (except type 2N) and probably of a hereditary or acquired platelet disorder; if the CT is abnormal, a medical questioning should first search for any medication capable of interacting with platelet aggregation. Secondly, the CT should be retested before measuring vWFRCo and/or vWFAg levels and investigating for platelet function defects.
ACKNOWLEDGMENT
We are most grateful to Marlies Ledford for her helpful comments. We also thank Christine Euzen and Anne-Lise Marville-Gigot for expert secretarial assistance.
Address reprint requests to Prof Dominique Meyer, MD, INSERM U143, Hôpital Bicêtre, Secteur Violet, Porte 19, 94275 Le Kremlin-Bicêtre Cedex, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal