Abstract
The transmigration of hematopoietic progenitor cells (HPCs) across vascular endothelium is a critical step in the homing of transplanted stem cells, but the molecular basis for this is unknown. We used mobilized peripheral blood CD34+ selected cells and cultured bone marrow microvascular (BMECs) and human umbilical vein endothelial cells (HUVECs) to investigate the adhesion and transendothelial migration of HPCs. Colony-forming cells (CFCs) in freshly isolated CD34+ cells showed high levels of adhesion to both forms of endothelium (28% ± 4% and 38% ± 6% of granulocyte-macrophage colony-forming cells [GM-CFCs] adhering to HUVECs and BMECs, respectively), but were unable to migrate to any significant extent across either (1.0% ± 0.3% and 1.1% ± 0.6% of GM-CFCs migrating across HUVECs and BMECs, respectively). Greater than 95% of peripheral blood CD34+ cells are in G0/G1 of the cell cycle, but after 48 to 72 hours of stimulation with growth factors (interleukin-3 [IL-3] 12 ng/mL, stem cell factor 10 ng/mL, and IL-6 10 ng/mL), 28% ± 5% of cells were in S+G2/M. Growth factor stimulation had no effect on the adhesion of mobilized CFCs but resulted in enhanced migration of these cells (9.8% ± 1.6% and 12.6% ± 3.1% of GM-CFCs migrating across HUVECs and BMECs, respectively; P < .01, n = 6). Assessment of cell proliferation by the3H-thymidine suicide method showed that, whereas 11.7% ± 3.3% of proliferating CFCs transmigrated across endothelium, only 1.3% ± 0.3% of nonproliferating CFCs did so (P < .05, n = 5). Transmigration of growth factor-activated CFCs was inhibited by anti-CD18 monoclonal antibody (MoAb; 50% ± 18% inhibition) and by anti–platelet endothelial cell adhesion molecule-1 (PECAM-1) MoAb (70.8% ± 7.1% inhibition; P < .05, n = 3). IL-1 stimulation of HUVECs had no significant effect on CD34+cell transmigration, but caused marked enhancement of neutrophil migration. Stem cell homing may depend, in part, on the ability of local cytokines to upregulate the transmigratory ability of these cells. The transmigration of HPCs shares at least some molecular pathways with that of mature cells (CD18 and PECAM-1), but is differently affected by endothelial activation.
THE ABILITY OF hematopoietic progenitor cells (HPCs) and stem cells to selectively localize to the bone marrow, the specialized microenvironment in which such cells are able to reconstitute hematopoiesis in the host, provides the biological basis for hematopoietic stem cell transplantation. Despite much advance in our understanding of the inflammatory signals that regulate leukocyte recruitment, the molecular mechanisms responsible for the selective homing of HPCs to the bone marrow remain poorly understood. The diapedesis of circulating HPCs from the bone marrow sinuses into the marrow compartment itself is likely to occur as a multistep process, as has been proposed for leukocyte homing to high endothelial venules or to inflammatory sites.1,2 In this model, cells initially roll on vascular endothelium tethered by selectins and their respective ligands. Cell activation by signaling molecules, such as chemokines, which are released by vascular and nonvascular cells, leads to upregulation of integrin function, and the high affinity binding of activated integrins to endothelial ligands produces tight adhesion, shape change, and diapedesis to localize in extravascular foci.2 The specificity of leukocyte extravasation in inflammation, and of lymphocyte homing to peripheral tissues, is considered to lie in recognition events involving cell surface receptors, primarily the selectins, and also α4-containing integrins, which mediate the initial engagement of rolling cells. By analogy, the particular adhesion molecule phenotype of HPCs might allow specific receptor/ligand recognition events to occur between these cells and the lumenal surface of bone marrow sinusoidal endothelium. HPCs have been shown to express several adhesion molecules including lymphocyte function-associated–1 (LFA-1), very late activation antigen-4 (VLA-4), VLA-5, LFA-3, L-selectin, Sialyl Lewisx(SLex), CD44, intracellular adhesion molecule-1 (ICAM-1), and platelet endothelial cell adhesion molecule-1 (PECAM-1).3-5 These receptors bind ligands on endothelial cells, stromal cells, and extracellular matrix components. The recent development of methods to isolate and culture bone marrow microvascular endothelial cells (BMECs) has provided the opportunity for studying the properties of this specialized endothelium.6-8 Studies suggest that these cells have constitutive expression of inducible adhesion molecules such as E-selectin and vascular cell adhesion molecule-1 (VCAM-1),9which function in the binding of leukocytes to activated endothelium.2 After this initial binding step, HPCs must traverse the endothelium, which is continuous, and this step of transendothelial migration has been shown in mature cells to be precisely tuned by the coordinate expression of adhesion molecules and chemokines.10
Diapedesed cells subsequently migrate within the bone marrow compartment to localize in particular microenvironmental niches in which the molecular mechanisms mediating anchorage also impart proliferative and differentiating signals. The adhesive components in this last step have been the most studied, and various adhesion pathways, including VLA-4 binding to VCAM-1 and fibronectin,11,12 CD44 binding to hyaluronic acid,13 and β1 integrin binding to fibronectin,14 as well as the role of proteoglycans in mediating the adhesion to cytokines,15 have been implicated. Earlier studies on the adhesion of hematopoietic cells to bone marrow stroma identified a putative lectin with galactose/mannose specificities present on early progenitors,16 17 but it is unclear whether this pathway mediates the initial interactions with sinusoidal endothelium or the later step of binding to stromal elements. We now know something about the adhesive phenotype of HPCs, but it remains unclear which, if any, of these receptors are responsible for the tissue specificity of stem cell homing or at which stage such specificity is regulated.
Transendothelial migration of HPCs is a prerequisite for the establishment and maintenance of hematopoietic tissue at anatomically distinct sites in the body and for the successful reconstitution of hematopoiesis by infused stem cells. Although the extravasation of mature cells, in particular phagocytic cells, is critically dependent on the integrin family, CD11/CD18, patients suffering from the rare leukocyte adhesion deficiency (LAD) do not have major problems with hematopoiesis in contrast to the greatly impaired migration of inflammatory cells.18 This raises the possibility that the molecular basis for, as well as the regulation of, HPC transmigration is distinct from that of mature cells. Such differences might lie in the nature and function of bone marrow sinusoidal endothelium, but it is not known if and how BMECs are specialized to support the transmigration of primitive cells. Finally, cell migration involves more than the engagement of cell surface receptors, for example, control of actin assembly and disassembly is important,19as is the redistribution and polarization of membrane structures. Cell migration on vitronectin, for instance, has been shown to be critically dependent on the ability of the cell to recycle specific membrane receptors toward the moving edge of the cell in a highly regulated endocytotic cycle, thus releasing adhesions at the rear of the cell and providing a fresh source of membrane molecules for attachment at the leading edge.20 The control of cell migration, a process critical to the homing as well as the mobilization of HPCs, and the egress of maturing cells may involve molecular pathways other than the modulation of surface adhesion receptors.
In this study, we examined the ability of mobilized HPCs to migrate across both specialized BMECs and the more undifferentiated human umbilical vein endothelial cells (HUVECs) and the way in which this migratory ability is regulated.
MATERIALS AND METHODS
Materials
Cytokines.
Interleukin-1β (IL-1β) was obtained from Boehringer Mannheim (Mannheim, Germany). Recombinant human granulocyte colony-stimulating factor (rhG-CSF) was a gift from Chugai Pharma (London, UK), recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF), and rhIL-3 were obtained from Sandoz (Camberly, UK), recombinant human stem cell factor (rhSCF) was purchased from First Link (Brierly Hill, UK), rhIL-6 was obtained from Schering Plough Ltd (Welwyn Garden City, UK), and endothelial cell growth supplement (ECGS) was obtained from Sigma Chemicals (Poole, UK).
Monoclonal antibodies (MoAbs).
Purified 60.3 (anti-CD18) was a gift from Dr John Harlan (University of Washington, Seattle, WA), and 1.3 (anti–PECAM-1) was a gift from Dr P. Newman (Blood Research Institute, Milwaukee, WI). Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated MoAbs (anti-CD54, anti-CD31, anti–VLA-4, anti–L-selectin, anti-CD45, and anti-CD34) used for phenotyping were obtained from Becton Dickinson (Oxford, UK).
Reagents.
RPMI 1640, Iscove's Modified Dulbecco's Medium (IMDM), fetal calf serum (FCS), Penicillin/Streptomycin, and phosphate-buffered saline (PBS) were obtained from Life Technologies (Paisley, Scotland, UK). Trypsin/EDTA, fibronectin, and collagenase (Clostridium histolyticum, type A) were obtained from Boehringer Mannheim. 3H-thymidine (80 mCi/mL) was obtained from Amersham (Little Chalfont, UK).
Endothelial Cells
HUVECs were harvested from umbilical cords by collagenase treatment (0.1% in PBS) and grown to confluence in fibronectin (2 μg/cm2)-coated Falcon tissue culture flasks (Becton Dickinson, Plymouth, UK) in IMDM with 20% FCS, ECGS (25 μg/mL), and heparin (25 U/mL). BMECs were isolated using a modification of the methods of Rafii et al6 and Masek and Sweetenham.7 Bone marrow aspirates were obtained after informed consent from normal donors undergoing bone marrow harvest. Bone marrow spicules, obtained by filtration through a 40-micron filter were washed and digested with collagenase (0.1% in Hanks' balanced salt solution) for 20 minutes at 37°C. The digested material was washed and filtered again and both loose cells as well as residual particles were plated onto fibronectin-coated tissue culture flasks in endothelial serum free medium with 5% FCS and 50 μg/mL ECGS. Cultures took 2 to 3 weeks to reach confluence, at which point they were passaged using trypsin/EDTA and reselected for endothelial cells using Ulex europeaus–coated Dynabeads (Dynal, Wirral, UK). Only cultures staining positive for the endothelial cell marker, BMA-120, and with at least 80% von Willebrand factor (vWF) expressing cells were used in adhesion and transmigration experiments. The cells were passaged using trypsin (0.05%) EDTA (0.01%) in PBS and seeded onto fibronectin-coated 48-well tissue culture plates (Falcon) for adhesion assays, or onto 24.5-mm diameter millipore (3 micron) filter inserts placed in wells of a 6-well cluster plate (Transwell filters, Costar, UK) for transmigration experiments. The formation of confluent monolayers on Transwell filters was verified by visual inspection by using an inverted microscope. In addition, the ability of these monolayers to act as a barrier was tested by measuring the permeability to 125I-labeled human serum albumin (125I-hsa). Briefly, confluent monolayers on filters were washed, aliquots of 125I-hsa (1.8 mBq/mL, Amersham) in assay medium were placed in the upper chambers of Transwells, and the filters were incubated at 37°C in parallel with filters used for transmigration assays. Contents of the lower chamber were collected at the end of the incubation period, and radioactivity was counted. Equilibration of 125I-hsa was consistently less than 10% across endothelium-covered Transwell filters over 20 hours, whereas complete equilibration occurred across nonendothelium-covered filters.
Leukocytes
Neutrophils were isolated from heparinized venous blood by double density centrifugation (Histopaque 1077 and 1119, Sigma Chemicals) as previously described.21 Monocytes were obtained from EDTA-stabilized venous blood by sedimentation with Hespan, and centrifugation at 600g for 15 minutes over Nycoprep (S.G. 1.068; Nycomed, Oslo, Norway), as previously described.22 T lymphocytes were prepared by Ficoll centrifugation and depleted of monocytes and B cells using anti-CD14 and anti-CD19 together with goat antimouse-coated Dynabeads (Dynal Ltd, Wirral, UK). Purified cell populations were labeled with 51chromium (2 μCi/106 cells) for 60 minutes at 37°C and washed four times before use.
CD34+ Cell Purification
CD34+-selected cells were obtained from patients with relapsed or resistant lymphoma undergoing peripheral blood stem cell transplantation or from normal donors. Local ethical committee approval for the study was in place, and informed consent was obtained from all patients. All subjects were mobilized with a protocol described previously.23 Low-dose cyclophosphamide (1.5 g/m2) was given on day 1 followed by G-CSF given subcutaneously at 10 μg/kg (filgrastim) or a single vial of lenograstim (263 μg/vial) 24 hours afterwards and daily thereafter until harvesting was complete. One to three apheresis collections were performed on days 8 to 12, on a Cobe Spectra (Cobe Laboratories, Gloucester, UK) commencing when the recovery white blood cell count was around 5.0 × 109/L. Single apheresis collections were processed for clinical scale CD34+ cell purification using a Ceprate SC immunoaffinity column (Cellpro Inc, Bothell, WA) and associated microprocessor device as previously described.24 Cytocentrifuge preparations of the final product were made for blast cell morphology, and the percentage of CD34+ cells was evaluated by alkaline phosphatase-antialkaline phosphatase immunoenzymatic staining as well as by dual immunofluorescence staining with anti-CD45–FITC and anti-CD34–PE and flow cytometry. The cell preparations had a median purity of 80% (range 52% to 95%).
Immunofluorescent Flow Cytometry
Immunofluorescence using flow cytometry and specific MoAbs was used to determine the expression of various surface markers, including adhesion molecules, on CD34+ cells. Aliquots (2 to 5 × 104 cells) were incubated with saturating concentrations of directly conjugated specific MoAbs for 45 minutes at 4°C, washed with cold PBS, and analyzed on a FACScan flow cytometer (Becton Dickinson). Negative controls were stained with isotype-matched IgG, and all samples were double stained with anti-CD34 MoAb.
Clonogenic Assays in Methylcellulose
Granulocyte/monocyte colony-forming cells (GM-CFCs) and burst-forming units-erythroid (BFU-E) were simultaneously evaluated in a methylcellulose-based clonogenic assay medium that consisted of Methocult (Stem Cell Technologies, Vancouver, Canada), with 20% IMDM and supplemented with IL-3 30 ng/mL, GM-CSF 25 ng/mL, G-CSF 25 ng/mL, SCF 10 ng/mL, and erythropoietin 2 U/mL. Purified CD34+cells were set up in quadruplicate at 5.0 × 102/mL. Colonies were counted after 14 days incubation at 37°C in a humidified CO2 atmosphere.
Adhesion and Transmigration of Leukocytes and Progenitor Cells
In adhesion assays, confluent endothelial monolayers in 48-well plates were washed three times with warm medium before the addition of51Cr-labeled neutrophils or monocytes (2 × 105 cells in IMDM/20% FCS), or unlabeled CD34+cells (8 × 104 cells in IMDM/20% FCS) to each well. After an incubation of 60 minutes at 37°C, unattached cells were removed by three gentle washes of 300 μL exchanges of medium. Plates were checked by microscopy to ensure that there was no disruption of the endothelial monolayers. Adherent leukocytes were recovered by detergent lysis of the endothelium and adherent cells, and the associated cell counts were expressed as a percentage of the counts initially added to the wells. For quantification of progenitor cell adhesion nonadherent cells were counted and set up in clonogenic assays. Percentage adhesion of clonogenic cells was calculated from the clonogenic output of the cell suspension initially placed in the wells. For migration experiments, confluent endothelial monolayers on Transwell filters were washed three times with warm medium and placed in wells with fresh medium (IMDM/20% FCS). Labeled leukocytes, or CD34+ cells (8 × 105 cells per filter), were placed in the upper chamber and incubated for times as stated at 37°C in 5% CO2 in a humidified chamber. At the end of the experiment, transmigrated cells were recovered from the lower compartment. For leukocytes, percent migration was calculated from the associated counts, whereas transmigrated CD34+ cells were manually counted and set up in clonogenic assays. Percentage migration of clonogenic cells was calculated from the clonogenic output of the CD34+ cell suspension originally seeded onto the filters.
In experiments to investigate the effect of IL-1 stimulation on cell migration, confluent endothelial monolayers were preincubated with IL-1 (20 U/mL) for 6 hours, washed, and used in transmigration assays. Alternatively, IL-1 was included in the migration assay and present throughout the length of the assay. In some experiments, the effect of MoAbs against CD18 or PECAM-1 was tested on adhesion and transmigration. In these experiments, endothelial cells were preincubated with anti–PECAM-1 (10 μg/mL) for 20 minutes at 37°C before the addition of CD34+ cells, or anti-CD18 (10 μg/mL) was added to CD34+ cells 5 minutes before the start of the assay. All MoAbs were present for the duration of the assay. Negative controls were incubated with isotype-matched IgGs.
Growth Factor Stimulation
CD34+ cells were stimulated with IL-3 (12 ng/mL), SCF (10 ng/mL), and IL-6 (10 ng/mL) in IMDM/20% FCS for up to 5 days. Cells were harvested at varying time points, washed, and resuspended in fresh medium for phenotyping, adhesion, and transmigration assays as well as for cell cycle analysis.
Cell Cycle Analysis
Five to 10 × 105 cells were pelleted in a 15-mL tube and fixed in ice-cold 70% ethanol. Two-color flow cytometry of DNA (stained with propidium iodide [PI]) and total cell protein (stained with FITC) was performed as previously described.25Analysis was carried out using an Epics-Elite flow cytometer (Coulter Electronics, Louton, UK).
3H-Thymidine Suicide Assay
Proliferation of CFCs was assessed with a 3H-thymidine suicide assay, which was adapted from previously described methods.14 26 Briefly, growth factor-stimulated cells were washed twice in serum-free IMDM before being divided into two equal aliquots of 1 to 3 × 106 cells (at 1 × 106 cells/mL). Test cells were incubated with 20 μL/mL of3H-thymidine, whereas control cells were incubated with 20 μL/mL of 3H-thymidine together with excess cold thymidine (400 μg/mL), for 20 minutes at 37°C. Both aliquots of cells were subsequently washed twice with 10 mL each of IMDM containing 400 μg/mL of thymidine before being resuspended in IMDM/20%FCS for transmigration assays. Percent migration of clonogenic cells was quantified as described previously with clonogenic cultures set up for input and transmigrated cells in both the3H-thymidine-treated and control samples. The contribution of nonproliferating CFCs to the migration in control cells was calculated from the percent migration in3H-thymidine–treated (ie, nonproliferating) cells and subtracted from the overall migration in the control to give the actual contribution of proliferating CFCs to the overall transmigration in the control. This contribution was then expressed as percent migration of proliferating CFCs (number killed by 3H-thymidine suicide assay).
Statistics
Results are expressed as the mean ± standard error of the mean (SEM). Data were compared by using Student's t-test and aP value of <.05 was considered significant.
RESULTS
Adhesion of Primitive and Mature Cells to Endothelium
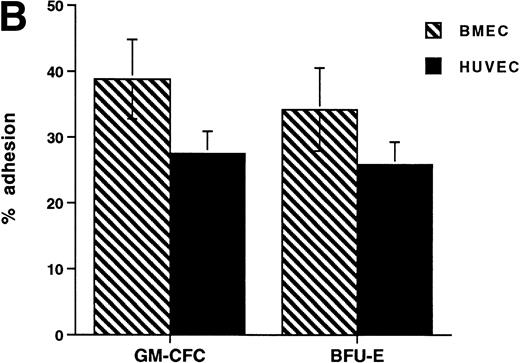
The CFCs within the population of freshly isolated mobilized CD34+ cells showed significant levels of adhesion to unstimulated HUVECs (32% ± 3% for GM-CFCs, n = 12; and 35% ± 6% for BFU-E, n = 3) over a 1-hour incubation period (Fig 1A). These levels are comparable with those seen in peripheral blood monocytes (24.3% ± 2.2%). In comparison, neutrophils showed much lower levels of adhesion (5.6% ± 1.0%). Clonogenic cells adhered to both forms of endothelium (BMECs and HUVECs). There was no significant difference in adhesion between these two types of endothelium (Fig 1B).
Adhesion of hematopoietic cells to endothelium. (A) Adhesion of neutrophils, monocytes, and CFCs in freshly isolated peripheral blood CD34+-selected cells to HUVECs. All adhesion assays were performed over 1 hour. Data are mean ± SEM of seven experiments with neutrophils, four with monocytes, and five with CD34+ selected cells. (B) Comparison of the adhesion of CFCs to BMECs and to HUVECs. Data are mean ± SEM of six paired experiments.
Adhesion of hematopoietic cells to endothelium. (A) Adhesion of neutrophils, monocytes, and CFCs in freshly isolated peripheral blood CD34+-selected cells to HUVECs. All adhesion assays were performed over 1 hour. Data are mean ± SEM of seven experiments with neutrophils, four with monocytes, and five with CD34+ selected cells. (B) Comparison of the adhesion of CFCs to BMECs and to HUVECs. Data are mean ± SEM of six paired experiments.
Transmigration of Primitive and Mature Cells Across Endothelium
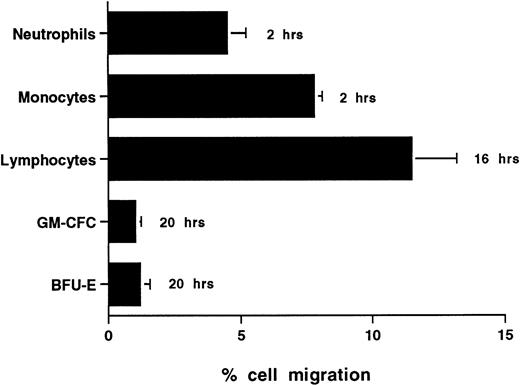
The time taken for any cell type to traverse endothelium to any significant extent depends a great deal on the type of cell under study and the conditions of activation. CFCs in freshly isolated mobilized CD34+ cells showed very low levels of migration with only 1.0% ± 0.3% (n = 6) and 1.1% ± 0.6% (n = 3) of GM-CFCs migrating across unstimulated HUVECs and BMECs, respectively, over 20 hours (Figs 2 and4D). In contrast, neutrophils and monocytes showed significant levels of transmigration over 2 hours and lymphocytes were able to transmigrate over 20 hours (Fig 2). We did not extend transmigration experiments beyond 20 hours, because there was progressive loss of barrier function of the endothelial monolayer after this time. The low levels of CFC migration contrasted with the high degree of adhesiveness of these cells.
Transmigration of mature leukocytes and CFCs in freshly isolated peripheral blood CD34+-selected cells across HUVECs. The times shown indicate the duration of the assay for each cell type. Data are mean ± SEM of six experiments with neutrophils, four with monocytes, six with lymphocytes, and nine with CD34+-selected cells.
Transmigration of mature leukocytes and CFCs in freshly isolated peripheral blood CD34+-selected cells across HUVECs. The times shown indicate the duration of the assay for each cell type. Data are mean ± SEM of six experiments with neutrophils, four with monocytes, six with lymphocytes, and nine with CD34+-selected cells.
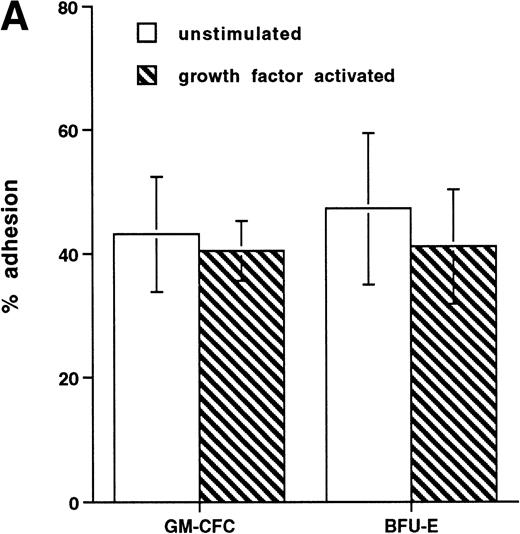
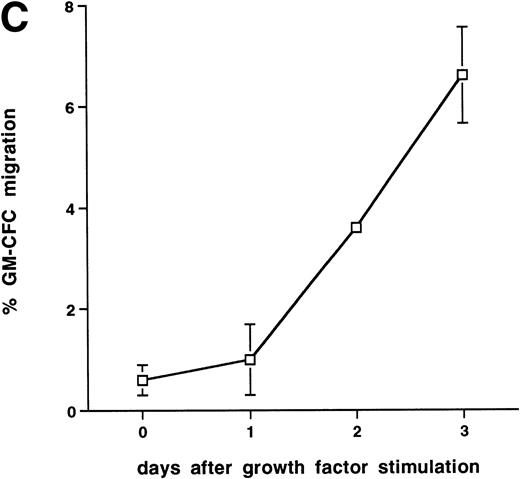
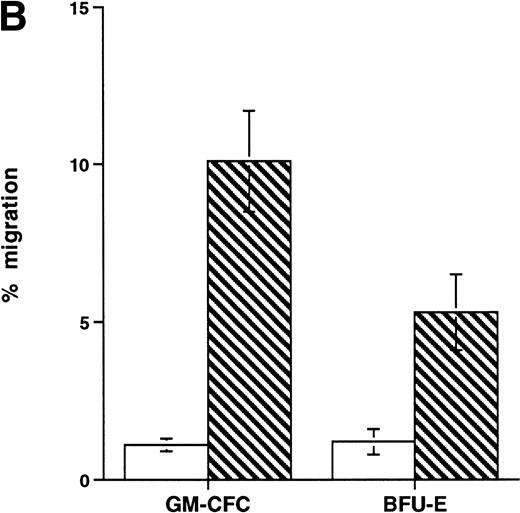
Effect of growth factor stimulation on adhesion and transmigration of CFCs in peripheral blood CD34+-selected cells. (A) Percentage adhesion of GM-CFCs to HUVECs before (unstimulated) and after 3 days of growth factor stimulation (growth factor activated), mean ± SEM of five experiments. (B) Effect of growth factor stimulation (3 days) on transmigration of GM-CFCs and BFU-E across HUVECs (mean ± SEM of eight experiments). (C) Kinetics of growth factor-induced upregulation of GM-CFC migration across HUVECs, mean ± SEM of three experiments. (D) Transmigration of unstimulated, and 3-day growth factor-activated GM-CFs across BMECs and HUVECs. Data are the mean ± SEM of the percent migration of GM-CFCs in four paired experiments.
Effect of growth factor stimulation on adhesion and transmigration of CFCs in peripheral blood CD34+-selected cells. (A) Percentage adhesion of GM-CFCs to HUVECs before (unstimulated) and after 3 days of growth factor stimulation (growth factor activated), mean ± SEM of five experiments. (B) Effect of growth factor stimulation (3 days) on transmigration of GM-CFCs and BFU-E across HUVECs (mean ± SEM of eight experiments). (C) Kinetics of growth factor-induced upregulation of GM-CFC migration across HUVECs, mean ± SEM of three experiments. (D) Transmigration of unstimulated, and 3-day growth factor-activated GM-CFs across BMECs and HUVECs. Data are the mean ± SEM of the percent migration of GM-CFCs in four paired experiments.
Comparison of Bone Marrow and Peripheral Blood CD34+ Cells Harvested Simultaneously During G-CSF Mobilization in Transmigration Experiments
In three patients undergoing G-CSF mobilization, bone marrow and peripheral blood progenitors were harvested simultaneously and set up in transmigration experiments using HUVECs. As seen in Table 1, there was no detectable difference between bone marrow and peripheral blood progenitors, both displaying very low levels of migration across endothelium.
Transmigration of Clonogenic Cells Across HUVECs: Comparison of Bone Marrow and Peripheral Blood Progenitors Harvested During Mobilization
| . | Subject 1 . | Subject 2 . | Subject 3 . | |||
|---|---|---|---|---|---|---|
| GM-CFC . | BFU-E . | GM-CFC . | BFU-E . | GM-CFC . | BFU-E . | |
| BM | 1.1 | 0.3 | 0.3 | 0.2 | 1.9 | 1.5 |
| PB | 1.2 | 0.5 | 0.1 | 0.3 | 2.2 | 0.5 |
| . | Subject 1 . | Subject 2 . | Subject 3 . | |||
|---|---|---|---|---|---|---|
| GM-CFC . | BFU-E . | GM-CFC . | BFU-E . | GM-CFC . | BFU-E . | |
| BM | 1.1 | 0.3 | 0.3 | 0.2 | 1.9 | 1.5 |
| PB | 1.2 | 0.5 | 0.1 | 0.3 | 2.2 | 0.5 |
Bone marrow- and peripheral blood-derived CD34+ cells harvested simultaneously from subjects receiving G-CSF were compared in 20-hour transmigration assays across HUVECs.
Abbreviations: BM, bone marrow; PB, peripheral blood.
Cell-Cycle Analysis of CD34+-Selected Cells and the Effect of Stimulation by Growth Factors
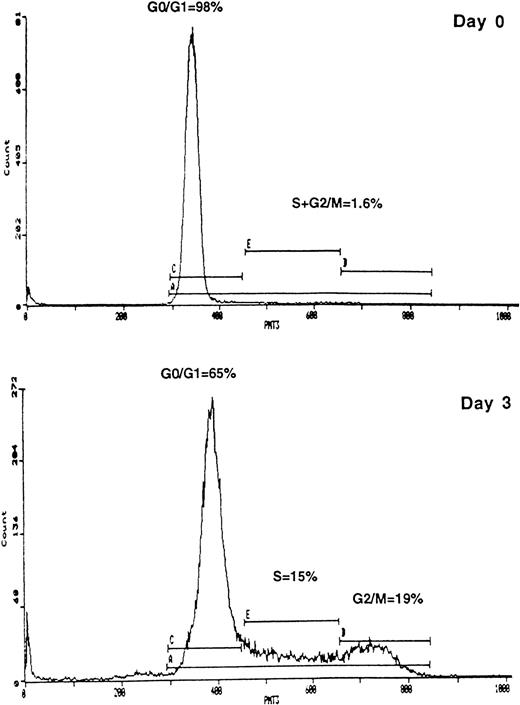
We performed cell cycle analysis on mobilized, CD34+ cells by using PI staining. Greater than 95% of freshly isolated CD34+ cells are in G0/G1 of the cell cycle (Fig 3). After incubation with growth factors (IL-3 12 ng/mL, IL-6 10 ng/mL, and SCF 10 ng/mL) for 3 days, 28% ± 5% of cells (n = 6) are in S/G2/M (Fig3). Using this growth factor combination, >90% of cells remain positive for CD34 with high levels of expression maintained at up to 4 days of stimulation (data not shown).
Cell cycle analysis of freshly isolated peripheral blood CD34+-selected cells (day 0) and the same cells after 3 days of stimulation with IL-3, IL-6, and SCF (day 3) as detailed in the Materials and Methods. Cells were stained with propidium iodide and analyzed on an Epics-Elite flow cytometer (Coulter Electronics). The percentages of cells within G0/G1, S, and G2/M phases of the cell cycle are indicated. DNA histograms are representative of five experiments.
Cell cycle analysis of freshly isolated peripheral blood CD34+-selected cells (day 0) and the same cells after 3 days of stimulation with IL-3, IL-6, and SCF (day 3) as detailed in the Materials and Methods. Cells were stained with propidium iodide and analyzed on an Epics-Elite flow cytometer (Coulter Electronics). The percentages of cells within G0/G1, S, and G2/M phases of the cell cycle are indicated. DNA histograms are representative of five experiments.
Effect of Growth Factor Stimulation on Adhesion and Transmigration of CD34+ Cells
Short-term exposure to cytokines has been reported to increase adhesion of HPCs,27 an effect that might alter the transmigratory properties of these cells. Thus, we examined the transmigration of CFCs over 24 hours after cytokine addition. We used the cytokine combination of IL-3/IL-6/SCF as detailed previously. In these experiments, the transmigration of cytokine-treated GM-CFCs was 0.4% ± 0.2%, whereas that of untreated GM-CFCs was 0.6% ± 0.3% (the corresponding data for all CFCs being 0.5% ± 0.3% and 1.0% ± 0.5%, respectively), suggesting that short-term stimulation with cytokines does not alter transmigration of clonogenic CD34+cells. We then proceeded to examine the effect of growth factor stimulation on adhesion and transmigration after 3 days of stimulation with IL-3, IL-6, and SCF. There is little difference in adhesion between freshly isolated and growth factor-activated CFCs (Fig 4A). In contrast, 3 days of stimulation with growth factors leads to a marked enhancement in the transmigration of clonogenic cells across HUVECs (9.8% ± 1.6% for GM-CFCs, P < .01; and 5.3% ± 1.2% for BFU-E, P< .05; n = 16 for both; Fig 4B). Differentiation of hematopoietic cells may be expected to lead to increased migratory capacity; however, the use of clonogenic assays helps to ensure that the cells being studied are at a similar level of differentiation.
Further experiments were performed to determine the kinetics of upregulation of migration in growth factor-activated CFCs. Figure 4C summarizes the results of three time course experiments showing that, over the first 48 hours, growth factor stimulation has little effect on the transmigration of CD34+ cells. At 48 hours transmigration of GM-CFCs increased from 0.6% ± 0.3% to 3.6% ± 0.05% (P < .05), whereas at 72 hours 6.6% ± 0.9% of GM-CFCs migrated. Cells maintained for the same period of time in SCF alone remained in G0/G1 and did not transmigrate (1.0% ± 0.3% migration of GM-CFCs compared with 8.2% ± 0.6% GM-CFC migration in parallel cultures stimulated with growth factors [IL-3/IL-6/SCF]). Growth factor-stimulated CFCs also showed enhanced migration across BMECs to levels similar to those seen with HUVECs (Fig 4D).
These data suggest that cytokine activation leads to a specific upregulation of migratory capacity independent of an effect on adhesion.
Effect of Growth Factor Stimulation on Expression of Adhesion Molecules on CD34+ Cells
Enhanced transmigration after growth factor stimulation may relate to changes in surface adhesive phenotype. To address this issue, we examined CD34+-selected cells for surface expression of adhesion molecules before and after 3 days of exposure to growth factors. All samples were double stained with anti-CD34, and gates were set on CD34high side scatterlow cells. The vast majority of freshly isolated, peripheral blood-derived CD34+-selected cells express VLA-4 and PECAM-1 (CD31), whereas expression of LFA-1, L-selectin, and ICAM-1 was characterized by more individual variability (Table 2). Growth factor stimulation did not lead to any consistent change in expression of adhesion molecules with perhaps the exception of LFA-1, which, in three out of five patients studied, was expressed at low levels on unstimulated cells and was markedly upregulated after growth factor stimulation. The data for individual patients is given in Fig 5.
Effect of Growth Factor Activation on Surface Adhesion Molecule Expression in CD34+Cells
| . | Mobilized PBPC . | Cytokine-Activated Mobilized PBPC . |
|---|---|---|
| LFA-1 | 56 (6-84) | 56 (51-89) |
| L-selectin | 57 (23-87) | 35 (22-76) |
| VLA-4 | 99 (85-99) | 98 (94-99) |
| ICAM-1 | 31 (2.3-81) | 50 (33-77) |
| PECAM-1 | 98 (95-99) | 97 (95-99) |
| . | Mobilized PBPC . | Cytokine-Activated Mobilized PBPC . |
|---|---|---|
| LFA-1 | 56 (6-84) | 56 (51-89) |
| L-selectin | 57 (23-87) | 35 (22-76) |
| VLA-4 | 99 (85-99) | 98 (94-99) |
| ICAM-1 | 31 (2.3-81) | 50 (33-77) |
| PECAM-1 | 98 (95-99) | 97 (95-99) |
Freshly isolated mobilized PBPCs were compared with cytokine-activated (3 days stimulation with IL-3 12 ng/mL, SCF 10 ng/mL, and IL-6 10 ng/mL) mobilized PBPCs for expression of each surface molecule. Data are median percentage and range (in brackets) of cells staining positive for the antigen.
Effect of growth factor stimulation on the expression of LFA-1 (CD11a) by CD34+ cells. Freshly isolated mobilized CD34+-selected PBPCs and cytokin-activated (IL-3, IL-6, and SCF for 3 days) mobilized CD34+ PBPCs were double stained for CD34 and LFA-1. Data are given for each of five patients studied and are the percentage of CD34+ cells that express LFA-1.
Effect of growth factor stimulation on the expression of LFA-1 (CD11a) by CD34+ cells. Freshly isolated mobilized CD34+-selected PBPCs and cytokin-activated (IL-3, IL-6, and SCF for 3 days) mobilized CD34+ PBPCs were double stained for CD34 and LFA-1. Data are given for each of five patients studied and are the percentage of CD34+ cells that express LFA-1.
Proliferating CFCs Preferentially Migrate Across Endothelium
The upregulation of migratory ability coincided with the onset of cell proliferation. To explore further the possible association between cell proliferation and cell transmigration, we used the3H-thymidine suicide assay to eliminate actively proliferating cells and performed transmigration assays on the remaining nonproliferating population. Control cells were incubated with 3H-thymidine but in the presence of excess unlabeled thymidine. Treatment of growth factor-activated cells with3H-thymidine led to 35.0% ± 6.9% loss of CFCs as determined by clonogenic assays. The elimination of proliferating cells by using 3H-thymidine led to a dramatic reduction in transmigration of CFCs. To determine the separate contribution of proliferating versus nonproliferating cells to the enhanced transmigration of growth factor-activated CFCs we calculated percent transmigration for each of these populations separately (see Materials and Methods). In this series of experiments, actively proliferating CFCs showed 11.7% ± 3.3% transmigration, whereas nonproliferating CFCs showed 1.3% ± 0.3% transmigration (P < .05, n = 5, Fig 6).
Comparison of transmigration of proliferating and nonproliferating CFCs in cytokine-activated peripheral blood CD34+ cells. Determination of CFC proliferation was done by 3H-thymidine assay as detailed in the Materials and Methods. Clonogenic assays were set up with both control and3H-thymidine–treated cells before and after transmigration, and percent migration of proliferating versus nonproliferating CFCs was then calculated as outlined in the Materials and Methods. Data are the mean ± standard deviation of five separate experiments.
Comparison of transmigration of proliferating and nonproliferating CFCs in cytokine-activated peripheral blood CD34+ cells. Determination of CFC proliferation was done by 3H-thymidine assay as detailed in the Materials and Methods. Clonogenic assays were set up with both control and3H-thymidine–treated cells before and after transmigration, and percent migration of proliferating versus nonproliferating CFCs was then calculated as outlined in the Materials and Methods. Data are the mean ± standard deviation of five separate experiments.
CD31 (PECAM-1) Is Required for the Transmigration of CD34+ Cells
CD31 (PECAM-1) is a member of the Ig superfamily and has been identified as playing a role in transendothelial migration without mediating cell adhesion to endothelium.28 CD34+cells express high levels of PECAM-1,5 with little difference between freshly isolated and growth factor-activated cells (Table 2). Experiments using neutrophils established that optimal inhibition of migration could be achieved with preincubating just the endothelial cells with blocking MoAb. Preincubation of HUVECs with anti-CD31 MoAb (10 μg/mL) produced marked inhibition of transmigration (70.8% ± 7.1% inhibition of GM-CFC transmigration;P < .05, n = 3). Adhesion of clonogenic cells was not inhibited by anti-CD31 (Fig 7). In contrast, anti-CD18 MoAb is able to inhibit both adhesion and transmigration (Fig 7), suggesting that this receptor is not specific for transmigration. In view of the increase in LFA-1 expression in three out of five patients studied, we tested the effect of anti–LFA-1 MoAb on transmigration of CD34+ cells. In two experiments, this MoAb (10 μg/mL) produced 81% and 51% inhibition of transmigration of GM-CFCs across HUVECs.
Effect of anti-CD18 and anti–PECAM-1 (CD31) MoAbs on adhesion and transmigration of cytokine-activated mobilized peripheral blood GM-CFCs using HUVECs. Anti–PECAM-1 MoAb was preincubated with endothelium for 20 minutes at 37°C, whereas anti-CD18 MoAb was added to CD34+ cells 5 minutes before the start of adhesion or migration assays. All MoAbs were used at 10 μg/mL and were present throughout the assay. Isotype-matched IgG was used as a control in each experiment. Data are the mean ± SEM of four experiments. *P < .05, **P < .01 when compared with control.
Effect of anti-CD18 and anti–PECAM-1 (CD31) MoAbs on adhesion and transmigration of cytokine-activated mobilized peripheral blood GM-CFCs using HUVECs. Anti–PECAM-1 MoAb was preincubated with endothelium for 20 minutes at 37°C, whereas anti-CD18 MoAb was added to CD34+ cells 5 minutes before the start of adhesion or migration assays. All MoAbs were used at 10 μg/mL and were present throughout the assay. Isotype-matched IgG was used as a control in each experiment. Data are the mean ± SEM of four experiments. *P < .05, **P < .01 when compared with control.
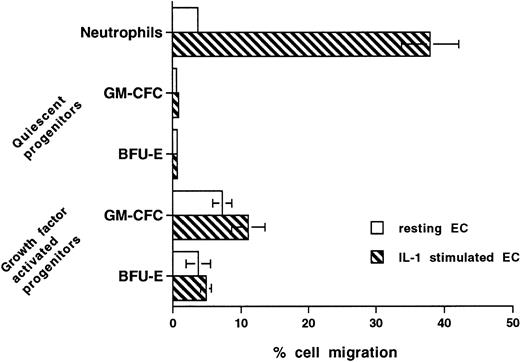
Differential Regulation of the Transmigration of CD34+ Cells and Peripheral Blood Neutrophils by IL-1
Stimulation of endothelium with cytokines such as IL-1 increases expression of adhesion receptors and the production of chemokines, thus leading to significant increase in the transmigration of inflammatory cells, including neutrophils and monocytes. Figure 8 shows that, after stimulation with IL-1 (10 U/mL for 4 hours), neutrophil transmigration is increased from 3.7% ± 0.3% to 38% ± 4.2% (P < .0001, n = 6). In contrast, IL-1 stimulation (20 U/mL for up to 18 hours) had no effect on the transmigration of unstimulated CFCs and minimal effect on the transmigration of cytokine-activated CFCs (Fig 8).
Effect of prestimulating HUVECs with IL-1 on transmigration of hematopoietic cells. Confluent HUVECs on Transwell filters were prestimulated with IL-1 (20 U/mL) for 4 hours and washed with warm medium before the start of transmigration assays. In further experiments with CD34+ cells, HUVECs were preincubated with IL-1 for up to 16 hours and/or IL-1 was present throughout the assay. The data from all experiments with CD34+ cells were similar and, hence, were pooled. Data are the mean ± SEM of seven experiments with neutrophils, four experiments with freshly isolated mobilized (quiescent) CD34+-selected cells, and four with growth factor-activated mobilized CD34+-selected cells.
Effect of prestimulating HUVECs with IL-1 on transmigration of hematopoietic cells. Confluent HUVECs on Transwell filters were prestimulated with IL-1 (20 U/mL) for 4 hours and washed with warm medium before the start of transmigration assays. In further experiments with CD34+ cells, HUVECs were preincubated with IL-1 for up to 16 hours and/or IL-1 was present throughout the assay. The data from all experiments with CD34+ cells were similar and, hence, were pooled. Data are the mean ± SEM of seven experiments with neutrophils, four experiments with freshly isolated mobilized (quiescent) CD34+-selected cells, and four with growth factor-activated mobilized CD34+-selected cells.
DISCUSSION
The main findings reported here are that, unlike their mature counterparts, and despite being highly adhesive, quiescent HPCs are unable to diapedese across endothelium, unless activated by growth factors. This dependence on growth factor activation applies to migration across both microvascular BMECs, as well as the more undifferentiated HUVECs. Growth factor stimulation leads to cell proliferation as well as increased migration. Elimination of proliferating CFCs by 3H-thymidine suicide assay results in marked reduction of transmigration in growth factor-activated cells, suggesting that proliferating CFCs migrate more efficiently than nonproliferating CFCs. The transmigration assay used in these studies takes into account the gross maturational effect of growth factor stimulation because, rather than measuring total cell migration, the assay quantifies clonogenic cells that are at similar levels of differentiation. We cannot conclude from these studies that cell cycling is a prerequisite for HPC homing/mobilization as subtle changes in activation status and maturational stage may have occurred in conjunction with entry into cell cycle. Cells measured in clonogenic assays are heterogenous and it is possible that after initial cytokine stimulation, there is a difference in growth factor requirements in the in vitro colony assays, which could influence the assessment of CFC migration. Although we cannot exclude this possibility, we have used multiple cytokine stimulation in the colony assays to minimize this problem. Initial studies of bone marrow CD34+ cells in three patients undergoing PBSC mobilization indicate that levels of transmigration are equally low in bone marrow-derived CFCs despite the fact that a proportion are known to be in cycle. This observation is unexplained but would support the notion that the link between proliferation and migration is not a direct causal one.
The extremely low levels of transendothelial migration displayed by freshly isolated CD34+ cells in this study are in agreement with data from another group29 who used BMECs. The inability of these freshly isolated progenitor cells to migrate across vascular endothelium is surprising, not only in view of the adhesiveness of these cells, but also because clinical studies have shown that these growth factor-mobilized PBPCs, when reinfused, produce more rapid engraftment than do bone marrow HPCs.23,30,31Thus, the clinical data suggest not only that these cells have an ability to “home” to the bone marrow, but also that they are somehow primed to respond to proliferative stimuli. Despite this, PBPCs show a highly quiescent phenotype,32,33 and indeed our analysis of pocket proteins-E2F complexes suggest that these cells are in G0 of the cell cycle.34 Growth factor activation induces transmigration as well as cell proliferation; however the relationship between proliferation and migration may be indirect. The upregulation of transmigration in response to growth factor activation requires 2 to 3 days and, hence, is not seen over the relatively short time frame (20 hours) of the transmigration assay used here and is only evident after cells are exposed to growth factors for at least 2 to 3 days.
Increased migration was not correlated with an increase in adhesion nor with any consistent changes in adhesion molecule expression. Upregulation of LFA-1 in response to growth factor stimulation was seen in three cases, all of which displayed low expression before cytokine stimulation. The significance of this is unclear but may reflect transient changes in expression related to the process of extravasation. One group has compared adhesion molecule expression on leukapheresis and bone marrow products harvested simultaneously from growth factor-treated patients and found higher expression of LFA-1 on bone marrow CD34+ cells.35 The contribution of changes in adhesion molecule expression and function to stem cell mobilization and homing is still unclear. It may well be that the important changes in adhesion molecule function occur independently of any changes in antigen density. Additionally, interactions with other membrane receptors can also influence integrin function, for example, glycosol-phosphatidyl-inositol–linked urokinase plasminogen activator receptor associates with and modifies the function of CD11b.36 37 These receptors colocalize at the leading edge of migrating cells suggesting that their association might be important for cell movement.
One further question is whether increased migration is a direct effect of growth factors. Growth factor effects on the adhesion and transmigration of hematopoietic cells have been shown in other systems. Stimulation with a combination of growth factors similar to the one used in our studies resulted in increased adhesion of HPCs to fibronectin over a 30-minute incubation period.38 The cytokine combinations that produced the greatest adhesive effect also had the most effect on proliferation. The same investigators have also shown that GM-CSF, IL-3, and SCF are able to induce activation of VLA-4 and VLA-5 in HPCs. They suggest that there might be a functional link between activation of proliferation and adhesion. We did not find any effect of growth factors on transmigration of clonogenic cells over 24 hours, and indeed, growth factor-induced transmigration was only evident after 48 hours of stimulation and coincided with the onset of proliferation. Furthermore growth factor stimulation over 3 days had no effect on adhesion of CFCs to endothelium. These observations, together with the lack of migration in cells exposed to SCF only (which did not induce cycling over the period tested) argue against an immediate effect of growth factors to upregulate a particular adhesive mechanism. We ourselves have shown that G-CSF–activated neutrophils display enhanced migration across HUVECs but no increased adhesion, another example in which upregulation of migration occurs independently of adhesion.39
HPCs differ from mature leukocytes in having a minimal migratory response to endothelial activation by cytokines. This difference in the regulation of HPC transmigration and the migration of mature cells would make biological sense. Cytokine activation of vascular endothelium serves to recruit inflammatory cells to peripheral tissues to affect the elimination, containment, and resolution of infection. On the other hand, primitive hematopoietic cells are destined for quite a different kind of tissue, namely one in which the microenvironment is specialized to provide anchorage as well as proliferation and differentiation signals. Transmigration of HPCs, like that of neutrophils and monocytes, is dependent on CD31 and on CD18. Whereas the role of CD18 in transmigration is attributable to its role in adhesion, that of CD31 appears to be confined to migration alone. Interestingly, transmigration of HPCs in our studies was only partially inhibited by anti-CD18 MoAb. This is in contrast to the almost complete inhibition of neutrophil transmigration by anti-CD18 MoAb.21 40 These observations together with the relatively normal hematopoiesis in patients with LAD18 would suggest that, when compared with neutrophils and monocytes, transmigration of primitive hematopoietic cells is less dependent on CD18.
The specific signals that cause circulating HPCs to localize in hematopoietic tissue remain to be elucidated, but our results here suggest an alternative to adhesion molecule-based theories. We hypothesize that stem-cell homing does not depend on the selective binding of HPCs to marrow sinusoidal endothelium, but rather that the exposure of HPCs within marrow sinuses to appropriate concentrations of growth factors leads to cell activation and the subsequent diapedesis of proliferating cells. It is not known what the minimal concentrations of growth factors required to stimulate transmigratory ability in HPCs are or whether cytokines other than the ones used in our system are able to do so. Further elucidation of such cytokines, including their preferential location in various tissues, will allow a clearer understanding of the way in which modulation of transmigratory ability contributes to tissue-specific homing of HPCs. In support of the theory of tissue-specific localization of migration-promoting cytokines, BMECs have been shown to produce higher concentrations of growth factors, including SCF, IL-6, G-CSF, and GM-CSF when compared with HUVECs.41 The role of BMEC-associated proteoglycans in presenting such growth factors to trafficking stem cells may also be important. Finally, these studies suggest that the homing of HPCs is likely to involve more than adhesion molecule binding and that other factors that can directly influence the migratory ability of these cells may also be important.
ACKNOWLEDGMENT
We thank Dr John Sweetenham and Lisa Masek, Medical Oncology Unit, Southampton Hospital, UK for help with the isolation and culture of bone marrow endothelial cells. We also thank Dr John Harlan and Dr P. Newman for generous provision of antibodies.
Supported in part by the Kay Kendall Leukaemia Fund and the Medical Research Council, UK.
Address reprint requests to Kwee L. Yong, MA, MB, BS, MRCP, Department of Haematology, Royal Free Hospital School of Medicine, Rowland Hill Street, London NW3 2PF, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal