Abstract
A new factor V mutation associated with resistance to activated protein C and thrombosis (factor V Cambridge, Arg306→Thr) was found in one patient from a carefully selected group of 17 patients with venous thrombosis and confirmed APC resistance in the absence of the common Gln506 mutation. The Arg306 mutation was also present in a first degree relative who also had APC resistance. Other potential causes of APC resistance, such as a mutation at the Arg679 site and the factor V HR2 haplotype, were excluded. Subsequent screening of 585 patients with venous thromboembolism and 226 blood donors did not show any other individual with this mutation. Factor VThr306 is the first description of a mutation affecting the Arg306 APC cleavage site and is the only mutation, other than factor V Leiden (Arg506→Gln), that has been found in association with APC resistance. This finding confirms the physiologic importance of the Arg306 APC-cleavage site in the regulation of the prothrombinase complex. It also supports the concept that APC resistance and venous thrombosis can result from a variety of genetic mutations affecting critical sites in the factor V cofactor.
IN 1993, A POOR ANTICOAGULANT response to activated protein C (APC) was reported as a common cause of familial thrombophilia.1-3 APC resistance is present in 3% to 5% of asymptomatic Caucasians and is found in approximately 20% of unselected patients with venous thrombosis.3,4 In at least 95% of cases, resistance to APC is caused by a single point mutation in the factor V gene.5 A transition (G to A) at nucleotide 1691 in exon 10 results in the synthesis of a variant factor V molecule (factor V Leiden) with the substitution Arg→Gln at amino acid position 506.6-8 Factor V is converted to the active cofactor, Va, during assembly of the prothrombinase complex. Thrombin generation is limited by cleavage of factor Va by APC at Arg506, followed by cleavage at Arg306 and Arg679. In vitro experiments have shown that cleavage at Arg506 has no effect on cofactor activity but is necessary for exposure of the inactivating cleavage sites, primarily at Arg306.9-12 The rate of inactivation of factor VaGln506 is slower than that of factor VaArg506, resulting in an increased thrombin potential in vitro13 and an increased risk of thrombosis in vivo.14
In the 5% to 10% of patients with deep vein thrombosis and APC resistance without the FVGln506 mutation, APC resistance can be due to pregnancy,15 lupus anticoagulant activity,16 or high factor VIII levels.17Patients with the FVGln506 mutation in trans with the HR2 haplotype may have lower APC sensitivity ratios,18 but no mutation, other than FVGln506, has been identified as a cause of an APC-resistant factor V protein. The aim of this study was to identify mutations in the factor V gene sequence encoding the primary inactivation site at Arg306 in patients with venous thromboembolism and APC resistance. Patients were selected from a large cohort with venous thromboembolism on the basis of having confirmed APC resistance in the absence of the FVGln506 mutation.
MATERIALS AND METHODS
Patients.
APC sensitivity ratios were measured on plasma samples from 602 patients consecutively investigated after a diagnosis of deep vein thrombosis or pulmonary embolus. The patients were not taking warfarin at the time of blood sampling. Deep vein thrombosis was diagnosed by ultrasound and venography and pulmonary embolus by ventilation-perfusion lung scanning. Four hundred twenty-four patients had a diagnosis of deep vein thrombosis only and 178 had symptomatic pulmonary embolism.
Standard APC sensitivity ratio.
Samples were centrifuged twice at 2,500g for 10 minutes and aliquots of platelet-poor plasma frozen at −80°C until assay. The APC sensitivity ratios were determined after filtration of plasma through a 0.2-μm syringe filter (Gelman Sciences, Northampton, UK) with Coatest APC Resistance-C kit (Chromogenix, Molndal, Sweden). Plasma was incubated with an equal volume of activated partial thromboplastin time (APTT) reagent for 5 minutes. Clotting was initiated by the addition of CaCl2. Clotting times were expressed as a ratio of the clotting time in the presence of APC divided by the clotting time in the absence of APC. The plasma filtration method greatly reduces resistance to APC as a result of platelet phospholipid contamination and increases the specificity of the assay for true APC resistance from 32% to 98%.19 In healthy controls, APC ratios determined by this method are greater than 2.2.19
Modified APC resistance assay.
APC resistance in the presence of factor V-depleted plasma was assessed using the Coatest APC Resistance-C kit and factor V-depleted plasma (Chromogenix).20 Plasma was prediluted 1 in 5 with factor V-depleted plasma and APC sensitivity ratios were determined as in the standard assay. Modified APC sensitivity ratios were less than 2.0 in 40 patients with the Gln506 mutation who were tested (range, 1.29 to 1.96) and greater than 2.2 in 40 unselected patients without the mutation (range, 2.23 to 4.64).
Extended APC resistance assay.
An extended APC resistance assay was performed as initially described by Dahlback et al.1 Platelet-poor plasma was incubated with an equal volume of APTT reagent for 5 minutes. Clotting times were recorded after the addition of CaCl2 supplemented with APC over a final concentration range of 0 to 100 nmol/L (Diagnostica Stago, Asnières, France).
Natural anticoagulants, lupus anticoagulant activity, and the FII20210 mutation.
Levels of natural anticoagulants and lupus anticoagulant activity were measured as previously described, with normal ranges as previously determined.21 Restriction enzyme analysis withHindIII after DNA amplification using a mutagenic primer was used to screen samples for the recently reported G to A transition at position 20210 in the 3′-untranslated region of the prothrombin gene.22 23
Plasma factor V coagulant activity.
Plasma factor V coagulant activity was measured in a one-stage APTT assay on an MDA180 coagulometer (Organon Teknika, Cambridge, UK) using factor V-deficient plasma and Platelin LS phospholipid substitute (Organon Teknika). In healthy controls, the normal range is 70% to 170%.
Factor VGln506.
Restriction enzyme analysis for detection of the FVGln506mutation (factor V Leiden) was performed as previously described.24 A 147-bp fragment encoding the APC cleavage site was amplified by polymerase chain reaction (PCR), and the DNA product was digested with Mnl I and analyzed by agarose gel electrophoresis.
Factor V exon 7 amplification.
A 228-bp DNA fragment containing exon 7 of the factor V gene was amplified from genomic DNA by PCR. The amplification primers were synthesized corresponding to intronic sequences upstream (5′-TGTCTTTCTGTCCTAAC-3′) and downstream (5′-TCTTGAACCTTTGCCCA-3′) of the exon 7 sequence. Each amplification reaction (50 μL) contained 0.5 μg of genomic DNA, 200 μmol/L of each deoxynucleotide triphosphate, 25 pmol of each amplification primer, and 1.25 U of AmpliTaq DNA polymerase (Perkin Elmer Cetus, Norwalk, CT) in 10 mmol/L Tris-HCl, 50 mmol/L KCl, pH 8.3, and 1.5 mmol/L MgCl2. The amplification reactions were performed in a Perkin Elmer Cetus DNA thermal cycler with an initial denaturation step for 10 minutes at 94°C, followed by 35 cycles including denaturation at 94°C for 20 seconds, annealing at 42°C for 20 seconds, and extension at 74°C for 20 seconds, with a final extension for 10 minutes at 74°C. At the end of the reaction, 5 μL of the reaction mixture was analyzed by electrophoresis on a 1% agarose gel.
DNA sequencing.
The amplified FV exon 7 DNA was directly sequenced by dideoxy sequencing using the ThermoSequenase radiolabeled terminator cycle sequencing kit and 33P-labeled dideoxynucleotide terminators (Amersham Life Science, Bucks, UK). The amplified DNA (5 μL) was pretreated by incubation at 37°C for 15 minutes with 10 U of exonuclease I and 2 U of shrimp alkaline phosphatase (Amersham Life Science) before sequencing according to the kit manufacturer's protocol. A nested sequencing primer was used, corresponding to the noncoding strand sequence 5′-TGGTATGAACCCCAACAA-3′.
Restriction enzyme analysis for the factor V Cambridge (Arg→Thr) mutation.
Restriction enzyme digestion of the PCR products was performed by incubating an aliquot of the amplified DNA overnight at 60°C with 10 U of the enzyme BstNI (New England Biolabs [UK] Ltd, Herts, UK) in the presence of 100 μg/mL bovine serum albumin. The digestion products were analyzed by electrophoresis on a 2% agarose gel. The normal exon 7 amplified product has one BstNI restriction site that produces fragments of 66 and 162 bp after digestion. The factor V Thr306mutation results in the loss of this site, which results in an uncut 228-bp fragment after digestion.
Factor V exon 13 amplification and DNA sequencing.
A 400-bp fragment of the 3′ end of exon 13 encoding the Arg679 site was amplified with an intronic upstream primer (5′-TGCCACAATGGATTATTGTG-3′) and a downstream exonic primer (5′-AGAAACGAATTCAGTGCCAT-3′). With the exception of an annealing temperature of 50°C, PCR conditions were the same as for exon 7 amplification. A nested sequencing primer, corresponding to the noncoding strand sequence 5′-TAGGGCAGTAAGATTGAACT-3′, was used to sequence codons 631 through 727.
Factor V HR1/HR2 haplotype analysis.
A 420-bp fragment was amplified using exon 13 exonic primers 5′-CAGACCTCAGCCATACAA-3′ and 5′-CTGACTGAGTTTCTGGAGA-3′. Each amplification reaction (50 μL) contained 0.5 μg genomic DNA. A total of 200 μmol/L of each deoxynucleotide triphosphate, 50 pmol of each amplification primer, and 1.25 U AmpliTaq in 10 mmol/L Tris-HCl, 50 mmol/L KCl, pH 8.3, and 1.5 mmol/L MgCl2. Amplification was performed with an initial denaturation step at 94°C for 10 minutes, followed by 35 cycles of 94°C for 20 seconds, 50°C for 20 seconds, and 74°C for 20 seconds followed by a final extension step at 74°C for 10 minutes. After amplification, 5 μL of the reaction mixture was analyzed by electrophoresis in a 1% agarose gel.
Restriction enzyme digestion of the PCR product was performed by overnight incubation at 37°C with 10 U of Rsa I (New England Biolabs [UK] Ltd). The digestion products were analyzed by electrophoresis in a 2% agarose gel. The HR1 haplotype (His1299) is represented by an undigested fragment of 420 bp. The HR2 haplotype (Arg1299) is represented by fragments of 121 and 299 bp representing the presence of an Rsa I site.
RESULTS
Determination of APC resistance and selection of patients.
Samples for sequencing of the factor VArg306 APC-cleavage site were selected from 602 patients with venous thromboembolism. Four of these patients were homozygous for the Gln506 mutation and had APC sensitivity ratios between 1.2 and 1.9. A total of 112 patients were heterozygous with APC sensitivity ratios from 1.5 to 3.3 (median, 2.2). Four hundred eighty-six patients had a normal genotype and ratios of 1.3 to 6.0 (median, 3.4), including 22 who had sensitivity ratios of less than 2.2. These 22 patients were investigated further.
Three of the 22 patients had detectable lupus anticoagulant activity and free protein S levels were low in 2 others (9% and 25%; normal, >55%). The remaining 17 patients had normal levels of antithrombin, protein C, and free protein S; no detectable lupus anticoagulant activity; and the prothrombin gene 20210G/A mutation was not present. Plasma from these patients was therefore subjected to the modified APC resistance assay after dilution in factor V-deficient plasma and exon 7 of the factor V gene sequenced to identify any mutations affecting the Arg306 APC-cleavage site.
Of the 17 selected patients, 6 had a modified APC sensitivity ratio of less than 2.0, despite the absence of the Gln506 mutation.
DNA sequencing and restriction enzyme analysis.
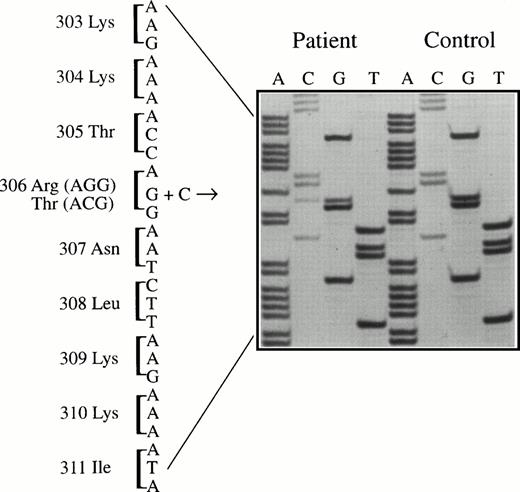
One of the 17 selected patients was found to be heterozygous for a mutation (G to C) changing the codon for amino acid 306 from AGG (arginine) to ACG (threonine) (Fig 1).
Sequencing of amplified factor V exon 7 DNA from the patient and a normal control. The mutation (G to C) is present at codon 306, resulting in an arginine306→threonine substitution in the factor V protein (factor V Cambridge).
Sequencing of amplified factor V exon 7 DNA from the patient and a normal control. The mutation (G to C) is present at codon 306, resulting in an arginine306→threonine substitution in the factor V protein (factor V Cambridge).
The new G to C mutation at codon 306 removes a recognition site for the restriction enzyme BstNI. The 228-bp amplified fragment from a normal FV gene has a single cutting site, resulting in fragments of 162 and 66 bp after enzyme digestion. As expected, digestion of exon 7 DNA from the patient with the Thr306 mutation showed bands of 228, 162, and 66 bp arising from the products of the mutant and normal alleles (patient II.1 in Fig 2).
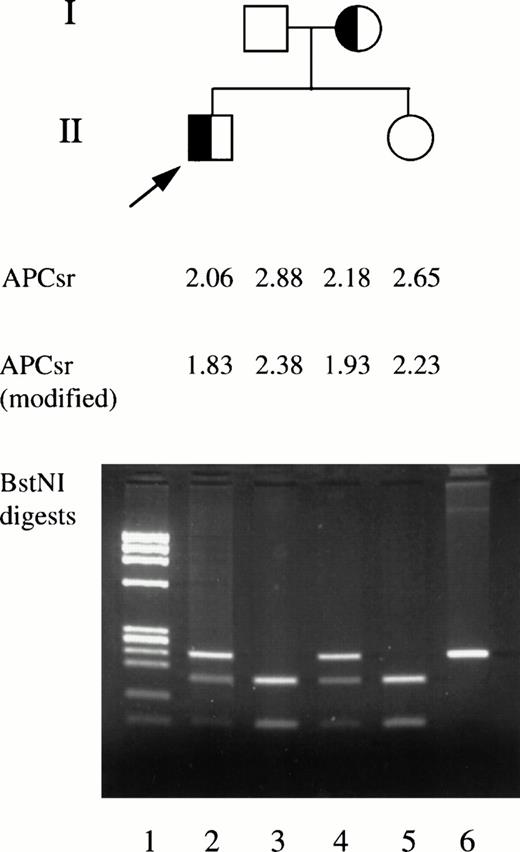
Family study of factor V Cambridge pedigree. The standard and modified APC sensitivity ratios and BstNI restriction enzyme digestion of exon 7 DNA are shown. Lane 1, DNA size markers. Lanes 2 through 5, BstNI digestion of DNA from family members. Lane 6, undigested exon 7 DNA fragment.
Family study of factor V Cambridge pedigree. The standard and modified APC sensitivity ratios and BstNI restriction enzyme digestion of exon 7 DNA are shown. Lane 1, DNA size markers. Lanes 2 through 5, BstNI digestion of DNA from family members. Lane 6, undigested exon 7 DNA fragment.
Restriction enzyme analysis using BstNI was performed on the remaining patient population. A normal digest pattern was detected in all 601 patients, including the 16 selected patients with APC resistance in the absence of the Gln506 mutation. In addition, BstNI digestion of DNA from 226 anonymous blood donors was normal.
Family study.
The index patient (II.1, Fig 2) with the factor V Cambridge mutation (Arg306→Thr) is a 49-year-old man who developed a spontaneous proximal deep vein thrombosis at the age of 47 years. He was treated with unfractionated heparin for 5 days and warfarin (target INR 2.5) for 6 months. He has now been off anticoagulant therapy without recurrence of venous thromboembolism for 2 years. His only living relatives are a 44-year-old sister and his mother and father, aged 73 and 74 years, respectively, none of whom have suffered a thrombotic event. Blood samples were obtained from the family members for DNA analysis and phenotypic study.
The patient's mother was also found to have the Arg306→Thr mutation (I.2, Fig 2). His father and sister did not have the mutation. The Arg679 site was sequenced in all four family members and was normal. Plasma factor V levels were normal in all four (94% to 134%) and all were homozygous for the HR1 haplotype.
The patient and his affected sister had APC resistance in both standard and modified APC resistance assays, whereas the unaffected father and sister were not APC resistant (Fig 2). The APC resistance phenotype of the patient and his affected mother was the same as that found in patients with the Gln506 mutation (Fig 3).
(Top panel) The effect of added APC on the APTT of plasma from the index patient with factor V Cambridge (Thr306; ▵) compared with patients heterozygous (○) and homozygous (•) for the Gln506 mutation and a normal control (Arg506; □). (Bottom panel) APC resistance of family members with (I.2 [▵] and II.1 [▴]) and without (I.1 [□] and II.2 [▪]) the Thr306 mutation.
(Top panel) The effect of added APC on the APTT of plasma from the index patient with factor V Cambridge (Thr306; ▵) compared with patients heterozygous (○) and homozygous (•) for the Gln506 mutation and a normal control (Arg506; □). (Bottom panel) APC resistance of family members with (I.2 [▵] and II.1 [▴]) and without (I.1 [□] and II.2 [▪]) the Thr306 mutation.
The patient's mother has not been exposed to any high-risk situtation for thrombosis other than two pregnancies.
DISCUSSION
We have described a new factor V mutant (factor V Cambridge, Arg306→Thr) associated with APC resistance and thrombosis. It is, to date, the only mutation other than factor V Leiden associated with this phenomenon and the first description of a mutation affecting the Arg306 APC cleavage site. The degree of APC resistance associated with Thr306 is the same as that due to the Gln506 mutation.
Thrombin generation is dependent on assembly of the prothrombinase complex on a phospholipid surface. The activated form of factor V (factor Va) is a component of the complex and enhances the rate of formation of thrombin several thousand fold.25 Cleavage of factor Va by activated protein C limits prothrombinase activity and hence thrombin generation. Inactivation of factor Va occurs via sequential cleavage by APC at Arg506, followed by Arg306 and Arg679 with loss of cofactor activity. Cleavage at Arg306 is responsible for the loss of approximately 70% of this activity, whereas cleavage at Arg679 is responsible for the loss of approximately 30%.9,10,12,26 In vitro, cleavage at Arg506has no direct effect on cofactor activity, but cleavage at this site is necessary for exposure of the inactivating cleavage sites at Arg306 and Arg679. The rate of inactivation of factor VaGln506 (factor V Leiden) is slower than that of normal factor VaArg506. On incubation with APC, factor VaArg506 is completely inactivated after 5 minutes, whereas factor VaGln506 retains approximately 50% of its initial activity.11 At low concentrations, the difference in APC inactivation of the mutant and wild-type proteins is even more pronounced.27 The mutant protein is associated with an increased thrombin potential in vitro,13 an eightfold increased risk of deep vein thrombosis in heterozygotes, and a 50- to 100-fold increased risk in homozygotes.14
Egan et al28 have recently studied factor V inactivation using site-directed mutagenesis to produce mutant recombinant factor V proteins. They have confirmed the importance of the Arg306site by demonstrating that a recombinant factor V molecule with a mutated 306 site (replacement of arginine by alanine or glutamine) is inactivated at a slower rate than plasma or recombinant wild-type factor V and at a similar rate to recombinant FVaGln506.28 However, APC cleavage patterns of factor V with a threonine substitution at the 306 site have not yet been reported. Despite the presumed physiologic importance of cleavage at Arg306, no mutation affecting this site has been identified until now. We used a rigorous screening strategy to identify patients with venous thromboembolism who showed APC resistance in the absence of the factor V Leiden mutation. In a large cohort of patients, we used an optimized standard APC sensitivity assay and a ratio of less than 2.2 as a high predictive value for the Gln506mutation.19 Patients with this degree of APC resistance in the absence of the Leiden mutation were therefore identified as candidates for other mutations. Because APC resistance has been demonstrated in the presence of lupus anticoagulant activity and thrombotic events may have been due to other forms of thrombophilia, only patients with isolated APC resistance were investigated further. Sequencing exon 7 of the factor V gene showed a normal sequence in all but one of the selected patients. This patient was found to have a G to C mutation altering the amino acid codon 306 from AGG (arginine) to ACG (threonine). This mutation would remove the primary APC cleavage-inactivation site from the mutant protein. Other potential causes of APC resistance, such as a mutation at the Arg679site and the factor V HR2 haplotype, were excluded. APC resistance was also present in the modified APC resistance assay after dilution in factor V-deficient plasma, confirming that the phenotype is due to a defect in factor V and not in another protein such as factor VIII. Conclusive evidence of the relationship between the Thr306mutation and APC resistance will require purification of the mutant protein and analysis of the APC cleavage pattern. Nevertheless, the presence of this mutation in a family with APC resistance is further evidence of the physiologic importance of the Arg306cleavage site. It seems likely in the absence of any other form of thrombophilia or predisposing high-risk situation that the mutation and the associated APC resistance was the cause of deep vein thrombosis in the index patient.
In selecting patients, the APC sensitivity ratio of 2.2 was chosen for its high specificity for the Gln506 mutation, but the sensitivity associated with this cut-off is only 50%. It is possible that some patients with a ratio greater than 2.2, who were not selected by our screening strategy, could have the Thr306 mutation and it is possible that patients with the Gln506 mutation could also have the Thr306 mutation, particularly those with very low APC sensitivity ratios. However, we screened the whole patient cohort and did not find any other affected patient. Similarly, the mutation was absent in 226 blood donors. Therefore, the Thr306 mutation is not a common polymorphism and it is a rare cause of APC resistance. However, the finding confirms the physiologic importance of the Arg306 APC-cleavage site in the regulation of the prothrombinase complex. It also supports the concept that APC resistance and venous thrombosis can result from a variety of genetic mutations affecting critical sites in the factor V cofactor.
ACKNOWLEDGMENT
The authors are grateful to Prof Robin Carrell for review of this manuscript.
Address reprint requests to Trevor Baglin, FRCP, Department of Haematology, Addenbrooke's NHS Trust, Cambridge CB2 2QQ, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 3. (Top panel) The effect of added APC on the APTT of plasma from the index patient with factor V Cambridge (Thr306; ▵) compared with patients heterozygous (○) and homozygous (•) for the Gln506 mutation and a normal control (Arg506; □). (Bottom panel) APC resistance of family members with (I.2 [▵] and II.1 [▴]) and without (I.1 [□] and II.2 [▪]) the Thr306 mutation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/4/10.1182_blood.v91.4.1140/4/m_blod4043803.jpeg?Expires=1767745915&Signature=XyuanIt24AT~Q4cqo7t7uGPBR2HLrAuKdXaK1xe4Tm6JMu0oUd2rZX3hkqU-fvnsmmh3jaBeSOnCQjPNsK4I-wsy7uKfDUABr7UOItufK9FUzSmbA3tvjOfxipVJ2iiPuxO4-GVZrIFiurMQ0q5j0cCK93WC3a8b0eO6C0Yrq7nZSLh-hh4LR4sgSQgLZqKL97M9NYA1GoCS257yxwMhU6LxePr5etkqJGmPEAuMfZCEpFmGdMNXPqNcpEaGGtoBMnIlVWkhGDabtu9kfClSnygzPMLyBCCdpwuCzBttYZ~Hngl4yh3SXElQBv1FXOCMFgFssMSjk84yApELH2Lq9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal