Abstract
Chemokine receptors are coupled to G-proteins and their activation results in prominent changes in cell migration and growth. The downstream signaling pathways that mediate these effects of chemokines are largely uncharacterized. Macrophage inflammatory protein 1β (MIP 1β) binding to its cognate receptor CCR5 resulted in activation of the related adhesion focal tyrosine kinase (RAFTK), with subsequent activation of the cytoskeletal protein paxillin and the downstream transcriptional activators, c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK) and p38 mitogen-activated protein (MAP) kinase. Inhibition of RAFTK by a dominant-negative kinase mutant markedly attenuated JNK/SAPK activity. Thus, RAFTK appears to provide a functional “bridge” for the transmission of CCR5 receptor signaling to the cytoskeleton and nucleus, primary sites of chemotaxis and growth regulation.
CONSIDERABLE ATTENTION has recently focused on chemokines and their receptors as important mediators of the inflammatory response1-5 and of human immunodeficiency virus (HIV) pathogenesis.6-9 As inflammatory mediators, chemokines act to direct cell migration10 and to modulate cell proliferation as part of the host response to microbial and allergic stimuli.4-11 In HIV pathogenesis, certain chemokine receptors bind to HIV strains and facilitate target cell infection.6-9 Recent observations indicate that a human herpes virus called Kaposi's sarcoma herpes virus type 8 (KSHV/HHV-8) encodes functional homologues of certain chemokines and chemokine receptors, suggesting that chemokines may contribute to the growth and spread of neoplasms seen in acquired immunodeficiency syndrome (AIDS).12-16 Despite the prominent roles of chemokines in inflammatory and infectious processes, relatively limited information is available about chemokine receptor signaling.2,3 17-23
The chemokine superfamily has been subdivided into the α (C-X-C), β (C-C), γ (C), and recently identified delta (C-X-X-X-C) groups based on the arrangement of the first two of four conserved cysteine residues.5,18 Both α- and β-chemokine receptors are members of the G-protein–coupled receptor superfamily.1-5
Previous reports have shown that chemokine receptors transmit information through G-proteins, resulting in intracellular changes in adenylate cyclase and phosphoinositol lipid metabolism and in the ras/raf/map kinase pathway.2,5,20,21,23-27 It has also been suggested that G-protein–coupled receptors or receptor protein tyrosine kinases (RTKs) may result in a common signaling pathway leading to the activation of nuclear transcription factors.25-27 Recently, JNK/SAPK has been shown to be activated by transforming G-protein–coupled receptors.26 27 Importantly, the mechanisms by which the effects of chemokines on cell migration and proliferation may be functionally linked through signaling pathways have not yet been elucidated.
The CCR5 receptor binds the β-chemokines macrophage inflammatory protein 1α (MIP1α), MIP1β, and RANTES, and is a major attachment protein for the macrophage-tropic strains of HIV-1.1,6-9 We now report that signaling via the CCR5 receptor leads to the phosphorylation and activation of a recently discovered protein kinase, termed RAFTK (also known as Pyk2 or CAK-β), of the focal adhesion kinase (FAK) family.28-32 Chemokine activation of RAFTK resulted in the downstream modulation of the JNK/SAPK kinase system. Chemokine stimulation via the CCR5 receptor also resulted in the phosphorylation of the cytoskeletal protein paxillin and its association with RAFTK. Our observations provide a possible mechanism by which intracellular tyrosine kinases may coordinate chemokine receptor signaling to alter chemotaxis and cell growth.
MATERIALS AND METHODS
Reagents and materials.
RAFTK antibodies were generated using GST-fusion proteins by immunizing New Zealand rabbits as previously described.31 Serum R-4250 was chosen for further studies based on its titer in enzyme-linked immunosorbent assay (ELISA). This antisera did not crossreact with FAK and recognized both human and murine forms of RAFTK. Antibodies to paxillin, JNK, p38 kinase and recombinant GST-c-Jun amino-terminal protein (1-79 amino acids) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antiphosphotyrosine antibody (4G10) was a generous gift from Dr Brian Druker (Oregon Health Sciences University, Portland). Electrophoresis reagents were obtained from Bio-Rad Laboratories (Hercules, CA). The protease inhibitors leupeptin and α 1 antitrypsin and all other reagents were obtained from Sigma Chemical Co (St Louis, MO). The nitrocellulose membrane was obtained from Bio-Rad Laboratories. Indo-1 AM was purchased from Molecular Probes (Eugene, OR).
Construction of CCR5 stable transfectants.
We used a murine pre-B lymphoma cell line, L1.2, for transfection studies. CCR5 cDNA, tagged at the N-terminus with a Flag epitope (Asp.Tyr.Lys. Asp.Asp.Asp.Asp.Lys), was subcloned to theHindIII-Xba I site of the expression vector pMRB101 (kindly provided by Martin Robinson, CellTech, Slough, UK), in which the inserted gene was driven by a CMV promoter. The DNA was stably transfected into L1.2 cells as described33-35 except that the mycophenolic acid-selective medium, instead of G418-selective medium, was used to select for transfectants. The cell-surface expression of CCR5 was monitored by FACS analysis. These cells express CCR5 at a high level (80,000 sites/cell) and bind the β-chemokines MIP1α, MIP1β, and RANTES with high affinity (kd = 0.2 to 1.0 nmol/L). Additionally, these cells were shown to bind M-tropic HIV-1 gp120 in the presence of soluble human CD4, a characteristic of the native CCR5 receptor.35
Cell culture.
The L1.2 cells were grown at 37°C in 5% CO2 in RPMI-1640 with 10% fetal calf serum (FCS), 2 mmol/L glutamine, 1 mmol/L sodium pyruvate, 50 μg/mL penicillin, 50 μg/mL streptomycin, and 55 μmol/L 2-mercaptoethanol. CCR5 transfectants were grown in RPMI-1640 media containing HT supplements (100 nmol/L sodium hypoxanthine and 16 nmol/L thymidine), 2.5 μg/mL mycophenolic acid, and 125 μg/mL xanthine. For selection of RAFTK mutants, 0.8 mg/mL Geneticin (G418) (GIBCO-BRL, Grand Island, NY) was used in the media.
Generation of activated T cells.
Peripheral blood mononuclear cells (PBMCs) were isolated, and activated T cells were generated as described.33 36 Briefly, 2 x 106 PBMCs/mL in RPMI containing 10% fetal bovine serum (FBS) were added to tissue culture plates coated with anti-CD3 antibody TR77. T cells were removed to fresh media supplemented with recombinant human interleukin-2 (IL-2) after 4 to 6 days. Three- to 4-week-old activated T cells were used for chemokine stimulation.
Calcium flux assay.
L1.2 or CCR5 transfectants were washed with RPMI-1640 and resuspended at 10 × 106 cells/mL in RPMI. The cells were loaded with Indo-1 acetoxymethyl esters (Indo-1 AM; Molecular Probes) by adding 5 μL of working Indo-1 solution to 10 × 106 cells suspended in 1 mL of RPMI solution and incubated for 45 minutes at 37°C. Cells were diluted to 1 × 106/mL, treated with MIP1β, and analyzed for calcium mobilization by flow cytometry (Coulter Electronics, Hialeah, FL) as described.34 Calcium flux assay and all other subsequent signaling assays were repeated at least three times.
RAFTK transfectants.
Wild-type and kinase dead RAFTK mutants were produced by transfection of the CCR5-L1.2 cells. Controls consisted of a pcDNA vector without an RAFTK construct. Plasmids carrying the control vector, wild-type RAFTK (RAFTKwt), or dominant-negative mutant (RAFTKm457) were transfected by electroporation into the CCR5-L1.2 cells using Bio-Rad electroporation equipment. The dominant-negative kinase mutant RAFTKm457 was generated by replacing Lys-(457) with Ala by site-directed mutagenesis.37 38
Stimulation of cells.
Cells were washed twice with RPMI-1640 (GIBCO-BRL) and resuspended at 10 × 106 cells/mL in RPMI-1640 medium. Cells were starved for 4 hours at 37°C and were stimulated with different concentrations of MIP1β at 37°C for various time periods. After stimulation, cells were lysed in modified RIPA buffer (50 mmol/L Tris-HC1, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL of aprotinin, leupeptin and pepstatin, 10 mmol/L sodium vanadate, 10 mmol/L sodium fluoride, and 10 mmol/L sodium pyrophosphate). Total cell lysates (TCL) were clarified by centrifugation at 10,000g for 10 minutes. Protein concentrations were determined by protein assay (Bio-Rad Laboratories). Cell lysis, RAFTK immunoprecipitation, immunoblotting, kinase assays, and autophosphorylation assays were performed as described below.
Immunoprecipitation and Western blot analysis.
For immunoprecipitation studies, identical amounts of protein from each sample were clarified by incubation with protein A-Sepharose CL-4B (Pharmacia Biotech) for 1 hour at 4°C. After the removal of protein A-Sepharose by brief centrifugation, the solution was incubated with different primary antibodies as detailed below for each experiment for 4 hours or overnight at 4°C. Immunoprecipitations of the antibody-antigen complexes were performed by incubation for 2 hours at 4°C with 50 μL of protein A-Sepharose (10% suspension). Nonspecific bound proteins were removed by washing the Sepharose beads three times with modified RIPA buffer and one time with phosphate-buffered saline (PBS). Bound proteins were solubilized in 40 μL of 2× Laemmli buffer and further analyzed by immunoblotting. Samples were separated on 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk protein and probed with primary antibody for 3 hours at room temperature (RT) for 4°C overnight. Immunoreactive bands were visualized using horseradish peroxidase (HRP)-conjugated secondary antibody and the enhanced chemiluminescent (ECL) system (Amersham Corp, Arlington Heights, IL). Monoclonal antibody (4G10, IgG2a) was used for Western blot analysis of phosphotyrosine protein.
Kinase assays.
In vitro kinase assays were performed as described earlier.39 The cell lysates immunoprecipitated with RAFTK antiserum were washed twice with RIPA buffer and once in kinase buffer (20 mmol/L HEPES, pH 7.4; 50 mmol/L NaCl; 5 mmol/L MgCl2; 5 mmol/L MnCl2; 100 mmol/L Na3VO4. For the in vitro kinase assays, the immune complex was incubated in kinase buffer containing 25 μg of poly (Glu:Tyr) (4:1); 20 to 50 kD; Sigma and 5 μCi γ32P-ATP at RT for 30 minutes. The reaction was stopped by adding 2× SDS sample buffer and boiling the sample for 5 minutes at 100°C. Proteins were then separated on 7.5% SDS-PAGE and detected by autoradiography. Normal rabbit serum was used as a negative control. An autophosphorylation assay was performed by incubating the immune complex in a kinase buffer containing 5 μCi γ32P-ATP at RT for 30 minutes. The reaction was stopped by adding 4× SDS sample buffer and by boiling the sample for 5 minutes. Proteins were then separated on SDS-PAGE and detected by autoradiography.39
JNK and p38 MAP kinase assays.
The JNK assay was performed as has been described earlier.40 Briefly, cell lysates were immunoprecipitated with JNK antibody (Santa Cruz Biotechnology). The immune complexes were washed twice with RIPA buffer and once in kinase buffer (50 mmol/L HEPES, pH 7.4, 10 mmol/L MgCl2, 20 μmol/L ATP). The complex was then incubated in kinase buffer containing recombinant GST c-jun 0.2 μg/μL (1-79 amino acids) (Santa Cruz Biotechnology) and 5 μCi γ32P-ATP for 10 minutes at RT. The reaction was terminated by adding 2× SDS sample buffer and boiling the sample for 5 minutes at 100°C. Proteins were separated on 12% SDS-PAGE and detected by autoradiography. Rabbit IgG was used as a negative control. For the p38 MAP kinase assay, cell lysates from unstimulated or stimulated cells were immunoprecipitated with anti-p38 MAP kinase antibody (Santa Cruz Biotechnology). The immune complexes were washed twice with RIPA buffer and once in kinase buffer (50 mmol/L HEPES, pH 7.4; 10 mmol/L MgCl2; and 20 μmol/L ATP). The complex was incubated in kinase buffer containing 7 μg myelin basic protein (MBP; Upstate Biotechnology, Lake Placid, NY) and 5 μCi γ32P-ATP for 20 minutes at 30°C. Proteins were separated on 15% SDS-PAGE and detected by autoradiography. Rabbit IgG was used as a negative control.
RESULTS
MIP1β stimulates Ca2+ flux in CCR5 transfectants.
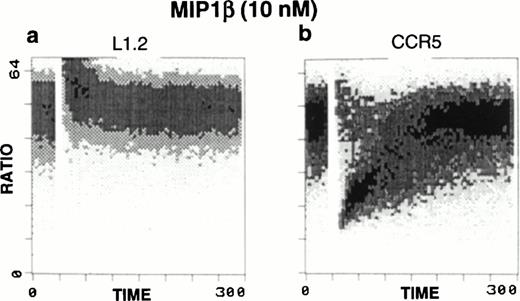
Ligand binding to chemokine receptors causes characteristic fluxes in intracellular calcium. To verify that the expressed human CCR5 receptor in the CCR5-L1.2 cells retained this fundamental signaling property, we treated these cells with MIP1β. Calcium fluxes were observed only in the CCR5-L1.2 cells and not in the untransfected L1.2 cells (Fig1).
Calcium mobilization in response to MIP1β stimuli. Ca2+ flux was performed in (a) untransfected L1.2 or (b) CCR5-transfected L1.2 cells in the presence or absence of chemokine treatment. Treatment of CCR5-L1.2 cells with 10 nmol/L MIP1β resulted in an increase in Ca2+ flux. No response was observed in the untransfected L1.2 cells.
Calcium mobilization in response to MIP1β stimuli. Ca2+ flux was performed in (a) untransfected L1.2 or (b) CCR5-transfected L1.2 cells in the presence or absence of chemokine treatment. Treatment of CCR5-L1.2 cells with 10 nmol/L MIP1β resulted in an increase in Ca2+ flux. No response was observed in the untransfected L1.2 cells.
RAFTK is phosphorylated and activated upon MIP1β stimulation of CCR5 transfectants.
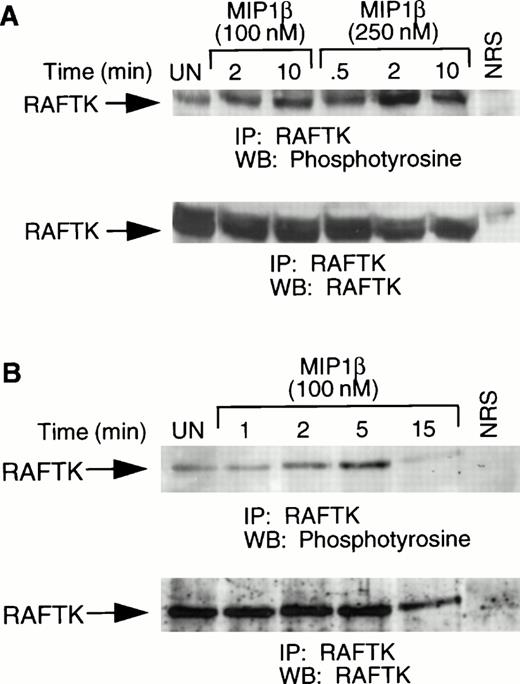
To characterize the signaling pathways in the cells expressing the chemokine receptor CCR5, the CCR5 transfectants were stimulated with MIP1β and lysates were analyzed for RAFTK phosphorylation. We observed rapid phosphorylation of endogenous murine RAFTK under these conditions (Fig 2A). This observation showed that the transfected human CCR5 receptor could signal to downstream endogenous murine substrates in response to its specific ligand, MIP1β, similar to native murine chemokine receptors. We also observed an increase in the tyrosine phosphorylation of RAFTK in activated T cells upon MIP1β stimulation (Fig 2B). Activated T cells have been shown to express high levels of CCR5.35 36 The slower activation in T cells may be due to differences in cell type compared with L1.2 cells and/or differences in numbers of cell-surface receptors.
Tyrosine phosphorylation of RAFTK in CCR5-L1.2 cells and activated T cells in response to chemokines. (A) CCR5-L1.2 cells or (B) T cells were unstimulated (UN) or stimulated with 100 nmol/L or 250 nmol/L MIP1β for varying time intervals. Cells were lysed and immunoprecipitated with anti-RAFTK, and analyzed by immunoblotting with antiphosphotyrosine (anti-pTyr). The same immunoblot was stripped and immunoblotted with anti-RAFTK (lower panel).
Tyrosine phosphorylation of RAFTK in CCR5-L1.2 cells and activated T cells in response to chemokines. (A) CCR5-L1.2 cells or (B) T cells were unstimulated (UN) or stimulated with 100 nmol/L or 250 nmol/L MIP1β for varying time intervals. Cells were lysed and immunoprecipitated with anti-RAFTK, and analyzed by immunoblotting with antiphosphotyrosine (anti-pTyr). The same immunoblot was stripped and immunoblotted with anti-RAFTK (lower panel).
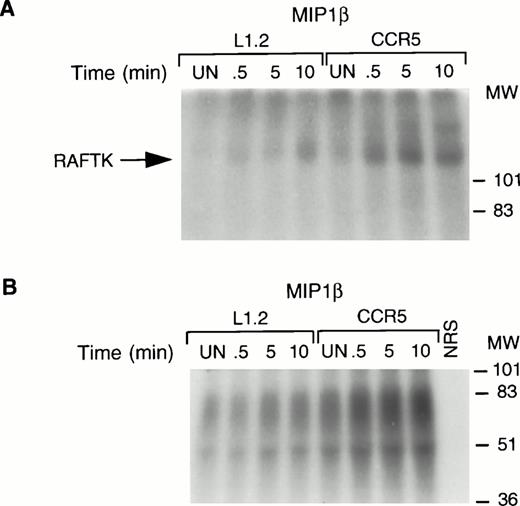
The autophosphorylation and kinase activities of protein tyrosine kinases can be activated upon their tyrosine phosphorylation, which is essential for their role in signal transduction. Therefore, we performed an autophosphorylation assay and an in vitro kinase assay in which poly (Glu:Tyr) (4:1) was used as an exogenous substrate to determine the intrinsic tyrosine kinase activity of RAFTK. As shown, the stimulation of CCR5 cells with MIP1β resulted in an increase in the autophosphorylating (Fig 3A) as well as the kinase activity of RAFTK (Fig 3B). The in vitro kinase activity observed in the RAFTK immune complexes using exogenous substrate could be the result of RAFTK and other co-associated kinases. The background levels observed in untransfected L1.2 cells likely reflect low levels of the endogenous CCR5 receptor.
RAFTK activation upon chemokine stimulation. Total cell lysates from L1.2 or CCR5-L1.2 cells unstimulated (UN) or stimulated with MIP1β (25 nmol/L) were immunoprecipitated with RAFTK antibody. The immune complexes were subjected to (A) autophosphorylating activity or (B) in vitro kinase assays using poly (Glu/Tyr, 4:1) substrate. The32P-incorporated proteins were resolved on 7.5% SDS-PAGE followed by autoradiography. Both autokinase and total kinase activity were increased upon stimulation of the CCR5-L1.2 cells with MIP1β.
RAFTK activation upon chemokine stimulation. Total cell lysates from L1.2 or CCR5-L1.2 cells unstimulated (UN) or stimulated with MIP1β (25 nmol/L) were immunoprecipitated with RAFTK antibody. The immune complexes were subjected to (A) autophosphorylating activity or (B) in vitro kinase assays using poly (Glu/Tyr, 4:1) substrate. The32P-incorporated proteins were resolved on 7.5% SDS-PAGE followed by autoradiography. Both autokinase and total kinase activity were increased upon stimulation of the CCR5-L1.2 cells with MIP1β.
RAFTK acts as a mediator of MIP1β-stimulated JNK/SAPK activity.
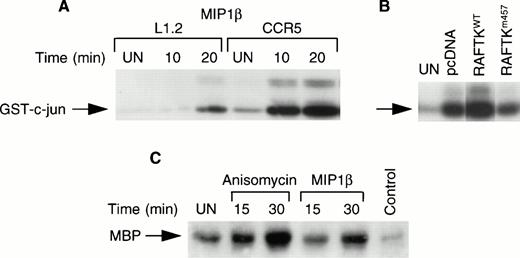
Treatment of CCR5-L1.2 cells with MIP1β resulted in the activation of JNK/SAPK activity compared with untransfected L1.2 cells (Fig4A). To address whether RAFTK participates in this process, we created double transfected L1.2 cells that expressed human CCR5 and wild-type RAFTK (CCR5-RAFTKwt) or human dominant-negative RAFTK lacking kinase activity (CCR5-RAFTKm457). An increase of ∼80% to 90% was observed in JNK/SAPK activation in chemokine-treated CCR5-RAFTKwt cells compared with pcDNA-transfected control CCR5-L1.2 cells. This activity in the dominant-negative CCR5-RAFTKm457 cells was reduced by 40% to 50% compared with the pcDNA control (Fig 4B). Similar results were observed in three different clones of each transfectant and thus do not reflect clonal variation.
Activation of JNK and p38 kinase upon chemokine stimulation. (A) L1.2 and CCR5-L1.2 cells were stimulated with MIP1β and were immunoprecipitated with JNK antibody and subjected to in vitro kinase assay using GST–c-jun (1-79 amino acids) as substrates. The experiment was repeated three times with similar results. (B) CCR5-L1.2 cells were stably transfected with vector control pcDNA, RAFTKwt or RAFTKm457. The transfectants were stimulated with MIP1β (25 nmol/L) for 15 minutes and the cells were lysed, immunoprecipitated with JNK antibody, and subjected to kinase assay. (C) CCR5-L1.2 cells were stimulated with MIP1β or anisomycin and cell lysates were immunoprecipitated with p38 kinase antibody. The immune complexes were subjected to kinase assay using MBP as a substrate.
Activation of JNK and p38 kinase upon chemokine stimulation. (A) L1.2 and CCR5-L1.2 cells were stimulated with MIP1β and were immunoprecipitated with JNK antibody and subjected to in vitro kinase assay using GST–c-jun (1-79 amino acids) as substrates. The experiment was repeated three times with similar results. (B) CCR5-L1.2 cells were stably transfected with vector control pcDNA, RAFTKwt or RAFTKm457. The transfectants were stimulated with MIP1β (25 nmol/L) for 15 minutes and the cells were lysed, immunoprecipitated with JNK antibody, and subjected to kinase assay. (C) CCR5-L1.2 cells were stimulated with MIP1β or anisomycin and cell lysates were immunoprecipitated with p38 kinase antibody. The immune complexes were subjected to kinase assay using MBP as a substrate.
Chemokine stimulation of CCR5-transfected cells enhances their p38 kinase activity.
To assess whether p38 MAP kinase also participates in β-chemokine signaling, lysates from MIP1β-stimulated CCR5-L1.2 cells were immunoprecipitated with anti-p38 MAP kinase and subjected to kinase assay. As shown in Fig 4C, MIP1β treatment resulted in an increase in p38 MAP kinase activity. Anisomycin which was used as a positive control also stimulated p38 MAP kinase activity (Fig 4C).
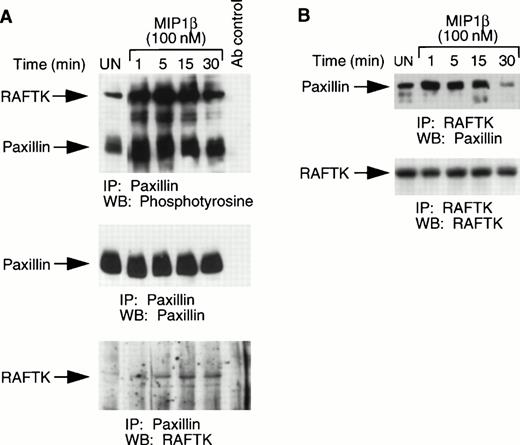
Paxillin is phosphorylated and associated with RAFTK upon MIP1β stimulation.
Since chemokines potently effect cell migration which involves alterations in cytoskeletal elements, we assessed changes in paxillin, a major cytoskeletal component of focal adhesions. As shown in Fig5A, MIP1β treatment of CCR5-L1.2 cells resulted in the tyrosine phosphorylation of paxillin. In these immunoprecipitates, we also observed a tyrosine phosphorylated protein of ∼115 kd which associated with paxillin and was shown to be RAFTK (Fig 5A). However, this complex may also contain other tyrosine phosphorylated proteins of a similar molecular weight. To further confirm the association between RAFTK and paxillin, CCR5-L1.2 cells were stimulated with MIP1β and the cell lysates were immunoprecipitated with RAFTK antibodies and subjected to Western blotting with anti-paxillin antibodies. As shown, paxillin was observed to be associated with RAFTK (Fig 5B).
Phosphorylation of paxillin and its association with RAFTK. (A) CCR5-L1.2 cells were stimulated with 100 nmol/L MIP1β for varying time intervals, and stimulated or unstimulated cell lysates were immunoprecipitated with anti-paxillin antibody. The immune precipitates were then run on SDS-PAGE and subjected to Western blotting with phosphotyrosine antibody, followed by antipaxillin and anti-RAFTK antibodies. (B) Stimulated or unstimulated cell lysates were immunoprecipitated with RAFTK antibody and blotted with paxillin antibody, followed by RAFTK antibody.
Phosphorylation of paxillin and its association with RAFTK. (A) CCR5-L1.2 cells were stimulated with 100 nmol/L MIP1β for varying time intervals, and stimulated or unstimulated cell lysates were immunoprecipitated with anti-paxillin antibody. The immune precipitates were then run on SDS-PAGE and subjected to Western blotting with phosphotyrosine antibody, followed by antipaxillin and anti-RAFTK antibodies. (B) Stimulated or unstimulated cell lysates were immunoprecipitated with RAFTK antibody and blotted with paxillin antibody, followed by RAFTK antibody.
DISCUSSION
Although chemokines have been shown to play important biological roles, relatively little information is available regarding their signaling mechanisms.1,5,19-22 Because chemokine stimulation has prominent effects on both chemotaxis and proliferation,1,2,5,10 we focused on RAFTK, a recently identified member of the focal adhesion kinase family, that has been found to link growth factors and stress signals (such as UV and osmotic shock) to the cytoskeleton and to the nucleus in hematopoietic and neuronal cells.28-32 37-39
We have observed that the chemokine MIP1β, which binds specifically to the CCR5 receptor, brings about an increase in intracellular calcium in cells overexpressing the cognate CCR5 receptor (CCR5-L1.2). Stimulation of CCR5-L1.2 cells with MIP1β resulted in an increased tyrosine phosphorylation of the RAFTK molecule. MIP1β stimulation of activated T cells also led to the increased tyrosine phosphorylation of RAFTK. The observed increase in phosphorylation was associated with RAFTK activation, a result apparent both in the enhanced autophosphorylation of RAFTK as well as in the increase in its in vitro kinase activity. Chemokine stimulation also enhanced JNK/SAPK activity, which is known to activate the AP-1 transcriptional complex.41,42 Furthermore, we observed that overexpression of wild-type RAFTK (CCR5-RAFTKwt) enhanced JNK/SAPK activity, whereas overexpression of a dominant-negative form of RAFTK lacking kinase activity (CCR5-RAFTKm457) reduced JNK/SAPK activity. This suggests that RAFTK may mediate chemokine-stimulated JNK/SAPK activation. Pyk2/RAFTK has previously been shown to mediate stress-induced JNK/SAPK activation in neuronal cells.32
Another recently discovered pathway mediating transcriptional activation is via the p38 MAP kinases. These kinases are activated by physical and chemical stresses as well as bacterial lipopolysaccharides and various cytokines.43-48 The p38 MAP kinase plays an important role in the phosphorylation and activation of transcription factors including CHOP, ELK-1, and ATF-2.46,47 JNK/SAPK and p38 kinases are known to be activated by SPRK, a mixed-lineage kinase.49 We also observed an increase in p38 MAP kinase activity upon MIP1β stimulation. Recently, it has been shown that signaling via the α-chemokine receptor for IL-8 appeared to involve p38 MAP kinase, but not JNK/SAPK.50 Our results suggest that there may be important differences in the pathways of transcriptional activation used by α- and β-chemokines.
Because chemokines potently affect cell migration which involves alterations in cytoskeletal elements, we analyzed the phosphorylation of paxillin, a major cytoskeletal component of focal adhesions. We observed that MIP1β stimulation results in the tyrosine phosphorylation of paxillin. Furthermore, after paxillin was phosphorylated by chemokines, there was an enhanced association of this protein with RAFTK. RAFTK has previously been shown to be associated with paxillin in hematopoietic cells following activation by growth factors.51 Activation and association of RAFTK with paxillin may result in the formation of a cytoskeletal complex, which contributes to enhanced chemotaxis. Interestingly, treatment of T cells with RANTES resulted in the phosphorylation of FAK and its association with paxillin.52
Our findings provide new information on the signal transduction pathways used by the β-chemokine receptor CCR5 and show how chemokines may modulate both chemotaxis and cell proliferation. We have observed that β-chemokine stimulation results in activation of the recently identified signaling molecule RAFTK, the cytoskeletal protein paxillin and JNK/SAPK and p38 MAP kinase activities. Furthermore, JNK/SAPK activities were enhanced in CCR5-RAFTKwt cells and decreased in CCR5-RAFTKm457 dominant-negative mutants. These data suggest that RAFTK may mediate CCR5 signaling to cytoskeletal elements such as paxillin and downstream to the nuclear activating protein via JNK/SAPK and p38 MAP kinase pathways.
ACKNOWLEDGMENT
We thank our colleagues William C. Hatch, Zhong-Ying Liu, Jian-Feng Wang, and Mel Ona for their help and technical assistance. We are grateful to Janet Delahanty for editing and preparation of figures as well as Evelyn Gould for her assistance with the figures. Finally, we appreciate Youngsun Jung and Tee Trac for typing the manuscript. This manuscript is submitted in honor of Ronald Ansin for his ongoing support of our research program.
The first two and last two authors of this paper contributed equally to the work.
Supported in part by National Institutes of Health Grants No. HL 55187, HL 53745, HL 43510, and HL55445.
Address reprint requests to Jerome E. Groopman, MD, Chief, Division of Experimental Medicine, Harvard Institutes of Medicine-BIDMC, 4 Blackfan Circle, Boston, MA 02115.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal