Abstract
Thirty-one consecutive patients with acute myelogenous leukemia (AML) in first complete remission and 8 with AML in second complete remission received T cell–depleted allogeneic bone marrow transplants from HLA-identical sibling donors. Patients received myeloablative cytoreduction consisting of hyperfractionated total body irradiation, thiotepa, and cyclophosphamide. Those patients at risk for immune-mediated graft rejection received additional immune suppression with antithymocyte globulin and methylprednisolone in the early peritransplant period. Patients with AML who underwent allogeneic T-cell–depleted bone marrow transplantations (BMT) in first or second remission have achieved respective disease-free survival (DFS) probabilities of 77% (median follow-up at approximately 56 months) and 50% (median follow-up at approximately 48 months). Ten of 31 patients transplanted in first remission were ≥ 40 years old and have attained a DFS at 4 years of 70%. For patients with AML transplanted in first or second remission, the respective cause-specific probabilities of relapse were 3.2% or 12.5%, and those of nonleukemic mortality were 19.4% or 37.5%. There were no cases of immune-mediated graft rejection and no cases of grade II to IV acute graft-versus-host disease (GVHD). All survivors enjoy Karnofsky performance scores (KPS) of 100%, except 2 patients with KPS of 80% to 90%. T-cell–depleted allogeneic BMT can provide durable DFS together with an excellent performance status in the majority of patients with de novo AML. In addition, GVHD is not an obligatory correlate of the graft-versus-leukemia benefit or freedom from relapse afforded by allogeneic BMT administered as postremission therapy for AML. This study provides a basis for prospective comparison with other postremission therapies considered standard in the management of patients with this disease.
THE MAJORITY of adults with de novo acute myelogenous leukemia (AML) achieve remission after initial induction with cytarabine and an anthracycline.1,2 Unfortunately, most of these patients relapse. Postremission treatment options designed to ensure long-term disease-free survival (DFS) include chemotherapy alone, high-dose chemotherapy with autologous stem cell rescue, or allogeneic bone marrow transplantation (BMT). Although the relative merits of these approaches continue to be debated and are the subjects of other comparative trials, recipients of allogeneic bone marrow transplants have consistently shown reductions in the risk of relapse.3-12 The greater curative potential of an allogeneic BMT has often been mitigated, however, by the risks of transplant-associated morbidity and mortality, particularly those caused by graft-versus-host disease (GVHD) and its complications. This is especially true in older patients, who constitute the majority of patients with AML.
In an earlier study we examined the efficacy of allogeneic bone marrow transplants, depleted of T cells by lectin agglutination and sheep erythrocyte rosetting, as postremission therapy in patients with AML in first remission.8 Although that study showed an almost complete elimination of acute and chronic GVHD without compromising the antileukemic efficacy of the allograft, immune-mediated graft rejection was a significant cause of treatment failure. As a consequence, the 3-year DFS was 45%. The pretransplant cytoreductive regimen and peritransplant supportive care were therefore modified to reduce or eliminate the incidence of graft failure and immune rejection, without increasing regimen-related toxicity. These modifications included the introduction of thiotepa between total body irradiation and cyclophosphamide, based on murine studies13 and clinical data14,15 that had shown the engraftment-potentiating, myeloablative, and immunosuppressive activity of thiotepa. Antithymocyte globulin and steroids were also added to the peritransplant regimen to prevent immune-mediated rejection caused by residual host T cells.16-18 We now report the results of T-cell–depleted allogeneic bone marrow transplants using this regimen for 31 consecutive patients with AML in first remission and 8 in second remission who have achieved a median follow-up duration of approximately 4.8 years and 4 years, respectively.
MATERIALS AND METHODS
Patient characteristics.
Thirty-one consecutive patients with primary AML in first remission and 8 consecutive patients in second remission underwent a T-cell–depleted, allogeneic bone marrow transplant during the period of October 31, 1991 to October 12, 1995 and were analyzed as of August 1, 1997. This trial was conducted in accordance with an Institutional Review Board–approved clinical research protocol. Eligibility criteria for enrollment and analysis in this study included a diagnosis of AML in first or second remission, in the absence of a preexisting myelodysplastic syndrome or treatment-related secondary leukemia; age less than 55 years; availability of an HLA-identical related donor; absence of active infection; and lack of coexisting cardiac, hepatic, or renal dysfunction that would preclude administration of the cytoreductive regimen. Patients who were seropositive for hepatitis A, B, or C were not excluded unless there was coexisting active hepatitis. HLA matching was established by serological identity for HLA-A, HLA-B, and HLA-DR loci, and where indicated by isoelectric focusing for subtype matching at the A and B loci and by DNA sequence–specific oligonucleotide typing for HLA-DR. Details of the patient characteristics before transplant are presented in Table 1. Of the 31 patients with AML in first complete remission (CR), the median age was 36.7 years; 10 patients (32%) were ≥40 years old at the time of transplant. Eight of the 31 patients with AML in first CR required two inductions to achieve remission, and 9 had karyotypes at diagnosis that have now been associated with favorable prognoses after chemotherapy alone.10 19 No patient received high-dose cytarabine during induction, and only 9 (13%) patients received high-dose cytarabine during consolidation.
Pretransplant Patient Characteristics
| . | AML-1°CR (n = 31) . | AML-2°CR (n = 8) . |
|---|---|---|
| Age [yr] | ||
| Median [range] | 36.7 [16.5-51.5] | 32.9 [14.8-54.8] |
| 14-19 | 1 | 1 |
| 20-29 | 7 | 2 |
| 30-39 | 13 | 3 |
| ≥40 | 10 | 2 |
| FAB classification | ||
| M0 | 1 | |
| M1 | 5 | 1 |
| M2 | 12 | 1 |
| M3 | 1 | 3 |
| M4 | 5 | 1 |
| M5 | 4 | |
| M6 | 1 | |
| Unknown/indeterminate | 2 | 2 |
| Karyotype | ||
| Normal | 7 | |
| t[8;21]-150 | 7 | 1 |
| t[15;17]-150 | 1 | 3 |
| inv[16]-150 | 1 | |
| trisomy 8 | 4 | |
| Other pseudodiploid | 2 | 1 |
| Other hyperdiploid | 1 | |
| Not available | 8 | 3 |
| No. of induction courses to achieve CR | ||
| 1 | 23 | n/a |
| 2 | 8 | n/a |
| No. of consolidation courses | ||
| 0 | 5 | n/a |
| 1 | 21 | n/a |
| 2 | 5 | n/a |
| Elapsed months to BMT, median [range] | ||
| From diagnosis | 3.9 [2.4-8.8] | n/a |
| From CR | 2.3 [0.6-7.8] | n/a |
| Duration of first CR, months, median [range] | n/a | 11.44 [1.87-109] |
| . | AML-1°CR (n = 31) . | AML-2°CR (n = 8) . |
|---|---|---|
| Age [yr] | ||
| Median [range] | 36.7 [16.5-51.5] | 32.9 [14.8-54.8] |
| 14-19 | 1 | 1 |
| 20-29 | 7 | 2 |
| 30-39 | 13 | 3 |
| ≥40 | 10 | 2 |
| FAB classification | ||
| M0 | 1 | |
| M1 | 5 | 1 |
| M2 | 12 | 1 |
| M3 | 1 | 3 |
| M4 | 5 | 1 |
| M5 | 4 | |
| M6 | 1 | |
| Unknown/indeterminate | 2 | 2 |
| Karyotype | ||
| Normal | 7 | |
| t[8;21]-150 | 7 | 1 |
| t[15;17]-150 | 1 | 3 |
| inv[16]-150 | 1 | |
| trisomy 8 | 4 | |
| Other pseudodiploid | 2 | 1 |
| Other hyperdiploid | 1 | |
| Not available | 8 | 3 |
| No. of induction courses to achieve CR | ||
| 1 | 23 | n/a |
| 2 | 8 | n/a |
| No. of consolidation courses | ||
| 0 | 5 | n/a |
| 1 | 21 | n/a |
| 2 | 5 | n/a |
| Elapsed months to BMT, median [range] | ||
| From diagnosis | 3.9 [2.4-8.8] | n/a |
| From CR | 2.3 [0.6-7.8] | n/a |
| Duration of first CR, months, median [range] | n/a | 11.44 [1.87-109] |
Abbreviation: n/a, not applicable.
Associated with favorable outcomes after chemotherapy alone.19
Preparative regimen.
All patients received myeloablative cytoreduction consisting of hyperfractionated total body irradiation (HFTBI) followed by thiotepa and high-dose cyclophosphamide. HFTBI was administered in fractions of 125 cGy at a dose rate of 8 to 20 cGy/min, three fractions per day, at 5- to 7-hour intervals for 4 days, to a total dose of 1,500 cGy. All patients had protective lung shielding to reduce the effective dose to the lung parenchyma to approximately 800 to 900 cGy. Overlying ribs received an additional 600 cGy boost using high-energy electrons to increase the total dose to the chest wall to approximately 1,500 cGy. Male patients received an additional 400 cGy testicular boost with electrons in a single fraction on the first day of HFTBI.20
After completion of HFTBI, thiotepa 5 mg/kg/dose was administered over 4 hours on each of 2 consecutive days, with no adjustment for obesity. This was followed by high-dose cyclophosphamide 60 mg/kg/dose on each of 2 consecutive days. Cyclophosphamide was dosed according to the lesser of actual or ideal body weight. In patients whose actual weight exceeded ideal body weight by more than 25%, an adjusted ideal body weight was used (adjusted ideal body weight = [{actual total body weight − ideal body weight} × 40%] +[ideal body weight]).
Bone marrow collection, T-cell–depletion, and transplantation.
Normal bone marrow was aspirated from the iliac crests under general anesthesia. T cells were removed from the bone marrow by sequential soybean lectin agglutination (SBA) and sheep red blood cell (sRBC)-rosette depletion.8,21 This method achieves a 2.8- to 3-log10 depletion of clonable T lymphocytes.22 Marrow was infused through central venous access 24 to 48 hours after the completion of high-dose cyclophosphamide.
Rejection prophylaxis.
Published analyses from this institution have shown that all patients aged 30 years or older, and any aged recipient of a male donor graft, are at risk for immune-mediated graft failure after T-cell–depleted allogeneic BMT.16 17 These patients therefore received antithymocyte globulin (ATG) and methylprednisolone for graft rejection prophylaxis. Of the 31 first remission patients, 21 received ATG at a dose of 30 mg/kg/d and methylprednisolone 2 mg/kg/d on days −5 and −4; 5 patients received ATG 15 mg/kg every other day from day +5 through day +13 along with methylprednisolone 2 mg/kg/d until day +13 followed by a rapid taper over the ensuing 14 days; 3 patients received steroids alone posttransplant because of anaphylactic reactions to ATG; and 2 patients required no graft rejection prophylaxis. Among the 8 second remission patients, 3 patients did not require prophylaxis; 2 patients received two doses of ATG and methylprednisolone as above on days −5 and −4; and 3 patients received ATG and methylprednisolone beginning day +5, as above.
GVHD prophylaxis.
No additional prophylaxis against GVHD was administered.
GVHD evaluation and management.
GVHD was diagnosed clinically, confirmed pathologically by skin or mucosal biopsy, and classified according to standard critieria.23 24 Only patients who engrafted and survived ≥30 days were evaluable for acute GVHD, unless it had already been diagnosed before a terminal event. Patients who developed acute GVHD were treated with either topical steroids or methylprednisolone 2 mg/kg/d. Patients surviving 100 days or longer were evaluable for chronic GVHD.
Supportive care.
All patients were hospitalized in single rooms with filtered air and reverse isolation. Standard prophylaxis against opportunistic infections included sulfamethoxazole/trimethoprim prophylaxis againstPneumocystis carinii pneumonia (PCP) pretransplant. Those patients who had received ATG and steroids as rejection prophylaxis were given aerosolized pentamidine after transplant to prevent PCP. Once stable engraftment was achieved with a platelet count ≥100,000, all patients resumed prophylaxis with sulfamethoxazole/trimethoprim prophylaxis. Acyclovir was administered as prophylaxis against DNA herpesvirus infections. All cytomegalovirus (CMV)-seronegative patients received CMV-seronegative blood products regardless of the CMV serological status of the marrow donor. Ten of 31 first remission patients and 6 of 8 second remission patients received fluconazole antifungal prophylaxis. Prophylactic antibacterials were not used. Of the 39 total patients, 16 (11/31 transplanted in first CR and 5/8 transplanted in second CR) received granulocyte colony-stimulating factor (G-CSF) in the early posttransplant period; representative clinical indications included BMT donor cell dose <2 × 107 mononuclear cells (MNC)/kg of recipient weight or persistent fever or infection despite appropriate antibiotics. No other cytokines were administered.
Engraftment and donor chimerism.
Myeloid engraftment was defined as an absolute neutrophil count (ANC) ≥500/μL on 3 consecutive days posttransplant. Platelet engraftment was defined as an untransfused platelet count of ≥20,000/μL for at least 3 consecutive days. Bone marrow aspirates were obtained at regular intervals posttransplant to assess cellularity and presence or absence of leukemic cells. Donor chimerism was assessed in sex-mismatched donor-recipient pairs by metaphase karyotype analyses or fluorescent in situ hybridization (FISH) of the X and Y chromosomes in interphase cells; restriction fragment length DNA polymorphisms (RFLP) were compared in sex-matched pairs.
Data collection and statistical methods.
Analyses were performed as of August 1, 1997. DFS was defined as the interval from BMT to death, relapse, or last follow-up, and was estimated using the method of Kaplan-Meier.25Estimates of the probability of relapse and nonleukemic mortality were calculated after adjustments for the competing risks of treatment failure.25
RESULTS
Engraftment and donor chimerism.
The median cell dose of the infused marrow (SBA-negative, sRBC-rosette–negative fraction) was 2.77 × 107nucleated cells/kg of recipient weight (range 0.69 to 7.44). All 31 patients with AML in first CR and all 8 patients with AML in second CR achieved primary engraftment, with complete donor chimerism. This has been maintained in all survivors, except 1 patient transplanted in first CR who has evidenced mixed chimerism (approximately 50:50 by RFLP) in the bone marrow without relapse, now 5.5 years status post-BMT; this patient did not have a favorable karyotpe10 19 at diagnosis. Three additional patients, among 14 recipients of sex-mismatched allografts whose interphase cells could be analyzed by FISH, have sustained mixed chimerism with 30% to 50% host cells in the peripheral blood mononuclear fraction, despite full donor chimerism in the bone marrow. The two relapses in this series, however, were not predicted by antecedent mixed host/donor chimerism.
There were no episodes of immune-mediated graft rejection as reported in the previous series from this institution.8,16 17Patients with AML in first CR achieved stable myeloid engraftment by day +13 (median, range 11 to 19 days). Platelet engraftment occurred by day +24 (median, range 12 to 93 days). Comparable engraftment parameters characterized the patients transplanted with AML in second CR. These data are summarized in Table 2.
Outcome Parameters After Allogeneic BMT for AML
| . | AML 1st CR (n = 31) . | AML 2nd CR (n = 8) . |
|---|---|---|
| Engraftment | ||
| Median transplant cell dose ×107/kg (range) | 2.68 (0.69-7.44) | 2.9 (1.08-4.60) |
| Median days to sustained* ANC ≥500 (range) | 13 (11-19)† | 14 (11-21)‡ |
| Median days to sustained1-153 platelet count ≥20,000 (range) | 24 [12-93] | 25 (15-43) |
| Posttransplant EBV-LPD | 3 | 0 |
| GVHD | ||
| Acute, grade I | 2 | 0 |
| Acute, grade II-IV | 0 | 0 |
| Chronic | 21-155 | 0 |
| Causes of death | ||
| Relapse | 1 | 1 |
| Infectious causes | 2 CMV pneumonitis¶ | 1 CMV pneumonitis |
| 1 fungal pneumonia | 1 toxoplasmosis | |
| 1 P carinii pneumonia | ||
| GVHD and secondary complications | 1 | |
| Secondary complications related to treatment of EBV-LPD | 1 | |
| Other | 1 late IP | |
| Alive and no evidence of disease | 24 | 4 |
| Sustained complete donor chimerism in bone marrow | 23 | 4 |
| Median f/u months (range) | 56.2 (28.4-66.2) | 47.5 (37.9-47.5) |
| . | AML 1st CR (n = 31) . | AML 2nd CR (n = 8) . |
|---|---|---|
| Engraftment | ||
| Median transplant cell dose ×107/kg (range) | 2.68 (0.69-7.44) | 2.9 (1.08-4.60) |
| Median days to sustained* ANC ≥500 (range) | 13 (11-19)† | 14 (11-21)‡ |
| Median days to sustained1-153 platelet count ≥20,000 (range) | 24 [12-93] | 25 (15-43) |
| Posttransplant EBV-LPD | 3 | 0 |
| GVHD | ||
| Acute, grade I | 2 | 0 |
| Acute, grade II-IV | 0 | 0 |
| Chronic | 21-155 | 0 |
| Causes of death | ||
| Relapse | 1 | 1 |
| Infectious causes | 2 CMV pneumonitis¶ | 1 CMV pneumonitis |
| 1 fungal pneumonia | 1 toxoplasmosis | |
| 1 P carinii pneumonia | ||
| GVHD and secondary complications | 1 | |
| Secondary complications related to treatment of EBV-LPD | 1 | |
| Other | 1 late IP | |
| Alive and no evidence of disease | 24 | 4 |
| Sustained complete donor chimerism in bone marrow | 23 | 4 |
| Median f/u months (range) | 56.2 (28.4-66.2) | 47.5 (37.9-47.5) |
*Sustained denotes 3 consecutive days.
Eleven of 31 patients received G-CSF (see text).
Five of 8 patients received G-CSF (see text).
Sustained denotes 3 consecutive days and platelet transfusion independent.
One of these two cases occurred solely as a complication of treatment of EBV-LPD with donor leukocytes (see text).
¶One of these two patients also had hepatic veno-occlusive disease.
One patient with AML in first CR experienced late graft failure associated with CMV infection and its treatment. Analysis of lymphoid elements confirmed persistent donor chimerism. This patient received secondary infusions of T-cell–depleted marrow and peripheral blood progenitors from the primary donor. These secondary infusions were administered without cytoreduction and have resulted in sustained trilineage donor engraftment.
DFS and relapse.
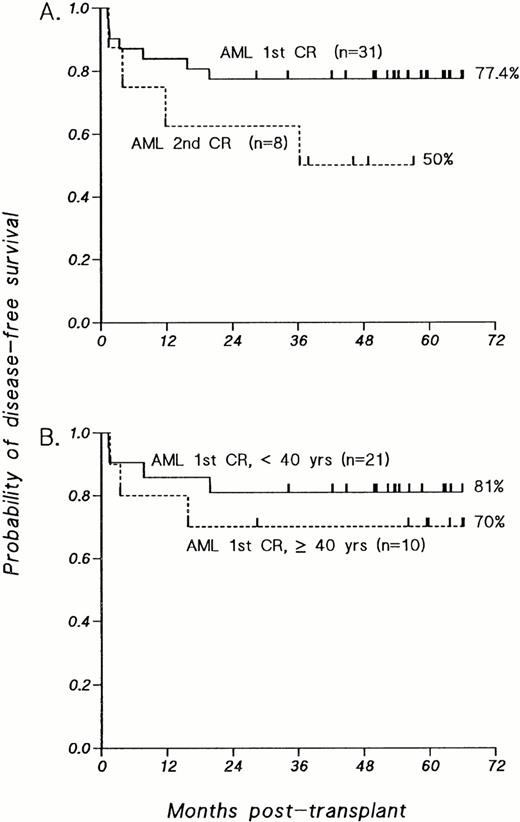
The estimated probability of DFS at 4 years for patients with AML transplanted in first remission is 77.4% (SE = 7.5%), with a median follow-up of 56.2 months (Fig 1A). For patients transplanted in second remission, the DFS probability estimate at 3 years is 50.0% (SE = 18%), with a median follow-up of 47.5 months (Fig 1A).
Kaplan-Meier estimates of probability of DFS for patients transplanted with T-cell–depleted allografts for AML. (A) Patients with AML transplanted in first CR compared with those transplanted in second CR. (B) Patients with AML transplanted in first CR, analyzed according to age at time of BMT; P value was not significant.
Kaplan-Meier estimates of probability of DFS for patients transplanted with T-cell–depleted allografts for AML. (A) Patients with AML transplanted in first CR compared with those transplanted in second CR. (B) Patients with AML transplanted in first CR, analyzed according to age at time of BMT; P value was not significant.
As shown in Fig 1B, the probability of 4-year DFS for patients with primary AML transplanted in first remission under the age of 40 years is 81%; older patients, ranging in age from 40 to 51.5 years, have achieved a 4-year DFS probability of 70% (P = .52). Comparison of the 23 patients who achieved a first CR after a single induction with the 8 patients who required two induction courses also reveals no significant differences in 4-year DFS probabilities (one induction course, 73.9%; two induction courses, 87.5%; P =.45; data not shown). Furthermore, when the overall group is segregated into “good risk” karyotypes [n = 9, t(8;21) in 7 patients and 1 each with t(15;17) or inv(16)] versus all other karyotypes (n = 22), the 4-year DFS probability estimates are the same (77.8% and 77.3%, respectively; data not shown). The inclusion of patients with good risk karyotypes therefore did not favorably influence the overall results.
Twenty-three of 24 long-term survivors transplanted in first remission and 3 of 4 long-term survivors transplanted in second remission enjoy Karnofsky performance scores of 100%. The other 2 patients have performance scores of 80% and 90%. One, transplanted in first CR, had prolonged delay in hematopoietic reconstitution but is now durably engrafted. The other, transplanted in second remission, has compensated but fixed neurological deficits that antedated and were independent of the transplant.
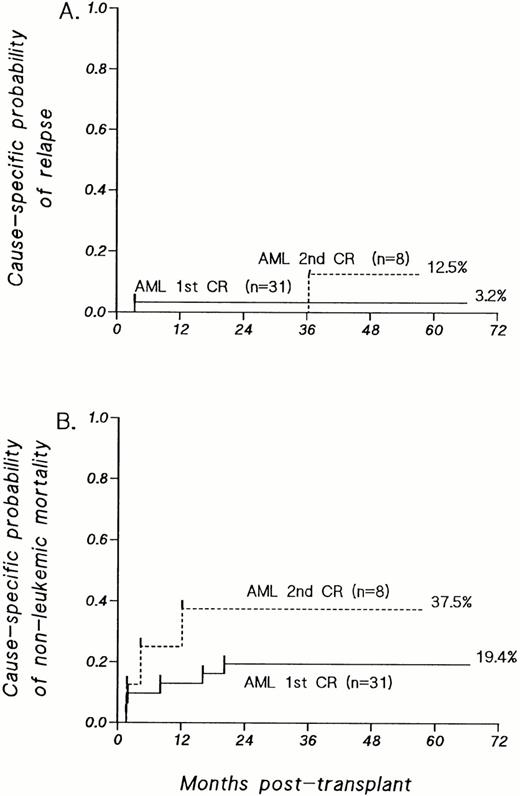
Only 2 patients in the entire series have relapsed to date. Both were over 50 years old. The sole patient who relapsed after transplantation in first remission had AML FAB-M4 with trisomy 8. The single patient who relapsed after transplantation in second remission had AML FAB-M3 with t(15;17). The cause-specific probability of relapse, controlling for the competing risks of treatment failure caused by nonleukemic causes, is 3.2% (SE = 6%) at 4 years for patients transplanted in first remission and 12.5% (SE = 20%) at 3 years for those transplanted in second remission (Fig 2A).
(A) Cause-specific probability of relapse, controlling for the competing risks of treatment failure by nonleukemic causes, among patients with AML transplanted in first or second CR. (B) Cause-specific probability of death, adjusting for the competing risk of leukemic relapse, among patients with AML transplanted in first or second CR. For patients with AML in first CR, the cause-specific probability of regimen-related mortality in the first 100 days after transplant is 9.7% ± 5%.
(A) Cause-specific probability of relapse, controlling for the competing risks of treatment failure by nonleukemic causes, among patients with AML transplanted in first or second CR. (B) Cause-specific probability of death, adjusting for the competing risk of leukemic relapse, among patients with AML transplanted in first or second CR. For patients with AML in first CR, the cause-specific probability of regimen-related mortality in the first 100 days after transplant is 9.7% ± 5%.
Posttransplant Epstein-Barr virus lymphoproliferative disorders (EBV-LPD).
Three of the 31 patients transplanted with AML in first CR developed posttransplant EBV-LPD.26 Two of these were treated with donor leukocytes and resolved. One of these 2 patients developed chronic GVHD secondary to the donor leukocyte infusion and subsequenly died (see below). The third patient had EBV-LPD limited to the tonsils, but had no evidence of additional disease after primary excisional biopsy and was not treated with donor leukocytes. None of the 8 patients transplanted in second CR developed EBV-LPD.
GVHD.
All patients were evaluable for acute GVHD. Two patients transplanted in first remission developed grade I acute GVHD involving only the skin. One was treated with topical corticosteroids, and the other was treated with a brief course of systemic methylprednisolone, both with complete resolution and without recurrence upon cessation of therapy. None of the 8 patients transplanted in second remission developed acute GVHD. Thus, the incidence of grade I GVHD in the entire group was approximately 5%; grades II to IV GVHD were not observed.
Thirty-five patients (28 in first CR, 7 in second CR) were evaluable for chronic GVHD. Of these, only 1 patient transplanted in first CR developed de novo extensive chronic GVHD involving the skin, liver, and oral mucosa. The incidence of chronic GVHD solely attributable to the T-cell–depleted marrow graft is therefore approximately 3%. One additional patient developed an EBV-LPD 127 days posttransplant in first CR. This patient developed chronic GVHD involving the oral mucosa after receiving unirradiated donor-derived leukocytes containing 1 × 106 CD3+ cells/kg26 (also see below).
Causes of death.
The causes of treatment failure and other outcome parameters are summarized in Table 2. Of the 31 patients transplanted with AML in first remission, 7 have died. Three patients died at 39, 43, and 49 days posttransplant as a result of fungal pneumonia, hepatic venoocclusive disease complicated by CMV pneumonia, and CMV pneumonia alone, respectively. One patient died late posttransplant (15.8 months) of an interstitial pneumonitis; on open lung biopsy, no cause could be established. The patient successfully treated for an EBV-LPD with donor leukocytes, who subsequently developed chronic GVHD as noted above, later died of sepsis 19.8 months after transplant. The single patient who developed de novo extensive chronic GVHD succumbed to fungal pneumonia complicating chronic immunosuppressive therapy. One patient with trisomy 8 at diagnosis relapsed at 3.5 months posttransplant and subsequently died of her disease. For patients transplanted in first remission, the cause-specific probability of regimen-related mortality, adjusted for the competing risk of leukemic relapse, is 9.7% (SE = 5%) at 100 days (Fig 2B). The cause-specific probability of death by all causes other than leukemic relapse is 19.4% (SE = 8%) overall (Fig 2B).
Of the 8 patients transplanted with AML in second remission, 4 died. Three of these deaths were caused by infectious complications (Table2). One patient relapsed 3 years after transplant. The cause-specific probability of nonleukemic mortality in this group is 12.5% (SE = 12%) at 100 days and 37.5% (SE = 18%) overall in this group.
DISCUSSION
Thirty-one consecutive patients undergoing T-cell–depleted allogeneic bone marrow transplants for de novo AML in first remission have achieved a DFS of almost 80% with a median follow-up of 4.8 years. A smaller group of 8 patients with de novo AML in second remission has achieved a DFS of 50% at a median follow-up of 4 years. Patients transplanted in first remission were somewhat older than those represented in most other series. Stratified according to age less than 40 years versus 40 years and older, the product limit estimates for DFS are nevertheless 81% and 70%, respectively, for patients in first remission. This broadening of age eligibility for BMT has been facilitated by the near total elimination of clinical GVHD afforded by T-cell–depleted allografts as well as the overall improvement in diagnosis and treatment of posttransplant complications. Accordingly, 26 of the 28 long-term survivors transplanted in either first or second remission enjoy Karnofsky performance scores of 100%.
All patients reported in this series were in complete remission at the time of allogeneic transplantation, underscoring the value of current chemotherapy regimens and supportive care standards used in the primary treatment of leukemia. The patients transplanted in first CR also represented a relatively favorable group in that all patients had primary AML and most achieved CR after a single induction course of chemotherapy. There was also no exclusion of patients with favorable karyotypes,10 19 as data supporting their positive outcome after chemotherapy alone was not widely established when accrual began. Nevertheless, segregating good risk karyotypes from all others, or patients requiring single versus more than one induction course to achieve CR, revealed no significant differences in the outcomes reported. The median time from diagnosis to transplant in this series of slightly less than 4 months also avoided the time-censoring bias that often plagues transplant studies in which patients already potentially cured by chemotherapy are sometimes overrepresented.
The largest and most current series to date using chemotherapy alone as postremission treatment is a multicenter Cancer and Leukemia Group B (CALGB) study reported by Mayer et al in 1994.27 This study randomized patients with de novo AML in first remission to three dose levels of cytarabine for four courses of consolidation, followed by four uniform monthly cycles of maintenance. The median age of the overall group was 52 years (range 16 to 86 years), but restricting analyses to those ≤60 years old showed a statistically significant advantage in DFS of 44% at 4 years for patients assigned to receive high-dose cytarabine (3 g/m2/dose × 6 doses). These results extended those previously reported from smaller series using high-dose cytarabine28-31 and were comparable to then published results of allogeneic bone marrow transplants for similar patients.3,5,8,9 12
Although comparative studies between chemotherapy and unmodified BMT have not previously shown a survival advantage to BMT, this has not been because of relapse.3,6,12,32 Rather, any potential benefits of allogeneic BMT have typically been offset by regimen-related mortality, complications of GVHD after unmodified allografts,33,34 or graft rejection after T-cell–depleted transplants.8,16 17
Relapse has never been a significant cause of treatment failure in AML patients transplanted with T-cell–depleted allografts at this institution, even when administered after total body irradiation and cyclophosphamide only.8 This cytoreductive regimen is commonly used to prepare patients for unmodified allografts where relapse rates have been higher.3,35 36 This therefore suggests that the antileukemic effect of a bone marrow allograft, applied to the treatment of AML in remission, is retained irrespective of the presence or absence of donor T lymphocytes. These results also reflect the low tumor burden with which patients with successfully remitted AML begin cytoreduction for BMT.
Hence, the preparative regimen, which now includes thiotepa in addition to HFTBI and cyclophosphamide, may alone be sufficient to eradicate residual leukemic cells. Alternatively or in addition, the enhanced leukemic resistance conferred by a bone marrow allograft is based on effectors other than the alloreactive T cells in the donor marrow that cause GVHD. Along these lines, the exceptional patient in this series with sustained mixed chimerism in blood and/or marrow has not relapsed. In addition, the two relapses reported in this series could not have been predicted by antecedent mixed chimerism. Experience at this and other institutions using donor lymphocytes as salvage therapy for AML patients relapsing after any type of allogeneic marrow graft has also shown little success37 (and unpublished observation). The immunobiology is not well defined, but it contrasts with that in patients with chronic myelogenous leukemia who have a much higher tumor burden at the start of cytoreduction, who have a lower relapse rate after unmodified compared with T-cell–depleted allografts, and who respond dramatically to infusions of donor lymphocytes for posttransplant relapse.38-40
In contrast to the previous series of similar patients reported from this institution in which immune-mediated graft rejection was the leading cause of treatment failure, affecting 16% of patients,8 there was not a single case of graft rejection in this entire group of either first or second remission AML patients. The current results cannot discriminate whether the additional chemotherapy or immune suppression is the more critical modification of the transplant regimen. However, among the patients at risk for rejection,16-18 the few who have received identical myeloablative cytoreduction but who have not received antithymocyte globulin because of anaphylactic reactions have also not suffered immune-mediated graft rejections.
The data that we have reported from this single institution, phase II series represents a substantial improvement in durable DFS for AML, especially in first remission. Improved results after allogeneic BMT have been reported with recent enhancements in diagnosis and treatment of transplant complications and when younger patients have been more numerous in the study population.4,32,35 Bunjes et al41 recently reported a group of 30 patients (median age 40 years), all but one of whom had AML in first remission, who underwent HLA identical allogeneic BMT with in vivo and in vitro T-cell depletion using monoclonal antibodies (MoAbs) Campath 1G/1M. This group also achieved a DFS of approximately 80% but with shorter follow-up (median 30 months), a higher relapse rate of 10%, and incidences of 4% acute GVHD ≥grade II and 0% chronic GVHD.
Soiffer et al have also reported encouraging results after transplantation of bone marrow allografts partially depleted of T cells with anti-CD6 MoAb and administered after cytoreduction primarily with a cyclophosphamide/TBI regimen.42 These investigators evaluated 41 patients transplanted in first remission of either acute lymphocytic (n = 13) or myelogenous leukemia (n = 28). Six of the 28 patients with AML had antecedent myelodysplastic syndrome (MDS), and one had received prior chemotherapy for another malignancy. Thirty-four of the 41 patients received matched-related allografts, whereas 7 of the allografts were from related donors mismatched for one or two HLA loci. The event-free survival for patients with AML was 63% at 4 years. However, the relapse rate among patients transplanted for AML in first CR was 31%, with a 15% incidence of grade II to IV acute GVHD in all recipients of matched-related allografts and a 14% incidence of chronic GVHD in the group overall.
The low relapse rate, the eradication of immune-mediated graft rejection, and the near complete elimination of clinically apparent GVHD largely account for the positive outcomes in this series. This study therefore supports the continued application and evaluation of T-cell–depleted allografts to patients with AML in remission, especially older patients in whom AML is more common. This study also provides a rationale for comparison with other postremission therapies considered standard in the management of this disease, and it forms the basis of a recently opened prospective randomized trial at this institution to compare T-cell–depleted with unmodified marrow allografts in the postremission management of acute leukemias.
ACKNOWLEDGMENT
We gratefully acknowledge the expert care provided to these patients by the fellows and housestaff of Memorial Sloan-Kettering Cancer Center, as well as the nursing staffs on Memorial 5, 6, and 19, led by Ann Cleary, RN, Marianne Wallace, RN, Ruth Ford, RN, and Karen Smith, RN. We also appreciate the unflagging dedication of Catherine Jagiello in assisting with the T-cell depletions of the marrow grafts. The commitment and service of our Patient Coordinator, Patricia Walka, merit special recognition.
Supported by P01 CA23766 from the National Cancer Institute, National Institutes of Health; and by the Vincent Astor Chair in Clinical Research (R.J.O.).
Address reprint requests to James W. Young, MD, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal