Abstract
We studied the expression and function of the granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor in the human prostate carcinoma cell line LNCaP and looked for its presence in normal and neoplastic human prostatic tissue. The GM-CSF receptor is composed of two subunits, α and β. While the isolated α subunit binds GM-CSF at low-affinity, the isolated β subunit does not bind GM-CSF by itself; but complexes with the α subunit to form a high-affinity receptor. Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) showed expression of mRNAs encoding the α and β subunits of the GM-CSF receptor in LNCaP cells, and the presence of the α and β proteins was confirmed by immunolocalization with anti-α and anti-β antibodies. Receptor binding studies using radiolabeled GM-CSF showed that LNCaP cells have about 150 high-affinity sites with a kd of 40 pmol/L and approximately 750 low-affinity sites with a kd of 2 nmol/L. GM-CSF signaled, in a time- and dose-dependent manner, for protein tyrosine phosphorylation and induced the proliferation of the LNCaP cells. Immunolocalization studies showed low level expression of GM-CSF α and β subunits in normal prostate tissue, with substantial expression in benign prostatic hyperplasia and prominent expression in neoplastic prostate tissue. Maximal expression of both subunits was observed in prostatic carcinomas metastatic to lymph node and bone. Tumor cells that stained positively with anti-α subunit antibodies were also reactive with anti-β subunit antibodies, indicating that they express high-affinity GM-CSF receptors. Our data show that the LNCaP cells express functional GM-CSF receptors and that prostatic carcinomas have prominent GM-CSF receptor expression. These findings imply that both hyperplastic and neoplastic prostatic tissues may be responsive to GM-CSF.
GRANULOCYTE-MACROPHAGE colony-stimulating factor (GM-CSF) is an important regulator of the proliferation and differentiation of myeloid precursor cells and enhances the function of mature granulocytes and mononuclear phagocytes.1 Receptors for GM-CSF are expressed on myeloid progenitors and mature mononuclear phagocytes, monocytes, eosinophils and neutrophils.1-4 The GM-CSF receptor is composed of two subunits, α and β.5,6 The isolated α subunit binds GM-CSF at low affinity (kd, 1 to 7 nmol/L). The isolated β subunit does not bind GM-CSF by itself; however, in a complex with the α subunit forms a high-affinity receptor (kd, 20 to 100 pmol/L). The binding of GM-CSF to its cognate receptor triggers a number of cellular responses.7-12 In human neutrophils, GM-CSF induces increased protein tyrosine phosphorylation through the activation of specific protein tyrosine cascades9,13-15 and transcriptional activation of early genes such as c-fos, c-jun, and c-myc.16 Signaling through the GM-CSF receptor may occur via participation of both α and β subunits5,10,17,18 and also through the isolated α subunit.19 20
Receptors for GM-CSF are also present in nonhematopoietic cells such as placental trophoblasts, endothelial cells, and oligodendrocytes in the central nervous system.21-24 Some primary neoplasms and tumor cell lines such as melanoma, small cell lung cancer, colon, pancreatic, renal, and ovarian carcinomas have also been reported to express GM-CSF receptors.25-29
Although there is evidence indicating that GM-CSF may stimulate the growth of some nonhematopoietic tumor cell lines,29-34 the physiologic role of the GM-CSF receptors expressed in normal or neoplastic nonhematopoietic tissue is unknown. In these studies, we provide evidence that human LNCaP prostate cancer cells express functional high-affinity GM-CSF receptors that transduce signals involving protein tyrosine phosphorylation and cell proliferation. We also show that the α and β subunits of the GM-CSF receptor are expressed at low level in normal human prostatic tissue, with substantial expression in benign prostatic hyperplasia and prominent expression in prostatic carcinoma.
MATERIALS AND METHODS
Cell culture.
The human prostatic cell line LNCaP35 was cultured in RPMI-1640 supplemented with 6% heat-inactivated fetal bovine serum, 1% L-glutamine, and antibiotics. The human leukemia cell line HL-6036 was maintained in Iscove's modified Dulbecco's medium (IMDM) supplemented with 10% heat-inactivated fetal bovine serum, 1% L-glutamine, and antibiotics.
Proliferation assay.
Cell proliferation was assessed by [3H]-thymidine37 and [125I]-deoxyuridine incorporation. Briefly, 40,000 cells per well were plated in sextuplicate in 6-well plates (Costar, Cambridge, MA), and bacterial recombinant human GM-CSF (0.01 to 100 mmol/L) (a gift from Amgen, Thousand Oaks, CA) was added at the beginning of the culture. Every day for 6 days of culture, 2 μCi of [3H]-thymidine (DuPont NEN, Boston, MA) or 0.45 μCi of [125I]-dUridine (DuPont NEN) was added to each well. The cells were harvested after 20 hours using a cell harvester (Skatron, Sterling, VA) or extracted with 1 N NaOH. The radioactivity incorporated into DNA was quantitated by liquid scintillation or γ spectrometry.
Reverse transcription-polymerase chain reaction (RT-PCR).
Total RNA was isolated from LNCaP and HL-60 cells using guanidinium thiocyanate (RNAzol B, Cinna/Biotecx Laboratories, Houston, TX). Single-stranded cDNA synthesis and quantitative PCR were performed as previously described.38 The primers used were: α-subunit primer: 5′:AGCCCGAGCAAAACACA, position 1009-1026 and 3′:CCATGCCA TTCCTACACCCT, position 1360-1379; β-subunit primer: 5′:CTACAAGCCCAGCCC AGATGC, position 859-879 and 3′:ACCCGTAGATGCCACAGAAGC, position 1390-1410. The PCR conditions were 94°C for 1 minute and 65°C for 2 minutes for 35 cycles. For quantitation of the β subunit, the GM-CSF receptor β cDNA subcloned into pBluescript (Stratagene, La Jolla, CA) was transcribed in vitro (Megascript, Ambion, Austin, TX) and the RNA was digested with DNase and quantitated spectrophotometrically and by the addition of trace levels of [α32P] uridine triphosphate (UTP) (DuPont NEN). RT-PCR of serial dilutions was performed in the presence of 1 μg of RNA derived from HeLa cells, a cell line that does not express the β subunit.
Binding assays.
For binding assays, 7 × 106 cells were suspended in RPMI-1640 containing 0.2% bovine serum albumin (BSA) and increasing concentrations of 125I-labeled human GM-CSF (DuPont NEN) with or without excess unlabeled human GM-CSF. After incubation for 20 hours at 4°C, the cells were centrifuged for 5 minutes at 4°C through a cushion of fetal bovine serum and the cell pellets were washed with cold phosphate-buffered saline (PBS) pH 7.4. Bound GM-CSF was quantitated by γ spectrometry.
Immunoblotting.
Cells were serum starved in RPMI-1640 containing 0.2% BSA for 18 hours, washed, resuspended at 1 × 107 cells/mL and incubated in the absence or in the presence of increasing concentrations of GM-CSF (0.01 nmol/L to 1 μmol/L) for different periods of time (2 seconds to 20 minutes) at 37°C. The cells were washed with cold PBS and the cell pellets were resuspended in 100 μL of lysis buffer14 and disrupted by sonication. The soluble proteins were resolved by SDS-PAGE (100 μg of cell lysate per lane) in a 10% polyacrylamide gel and transferred to immobilon (Millipore, Bedford, MA). Proteins phosphorylated on tyrosine residues were detected using an antiphosphotyrosine antibody (UBI, Lake Placid, NY). Phosphorylated mitogen-activated protein (MAP) kinase was localized using an antiphosphoMAP kinase antibody (New England Biolabs, Beverly, MA). Anti-JAK2 antibody was purchased from UBI. The antibody blots were developed by chemiluminescence (New England Biolabs).
Immunocytochemistry.
For immunoperoxidase localization,39 LNCaP cells cultured in 6-chamber microscopic slides were fixed in buffered paraformaldehyde-acetone, treated with 0.3% H2O2 for 5 minutes and incubated for 30 minutes at room temperature in 4% BSA-PBS pH 7.8, followed by incubation overnight at 4°C in 1% BSA-PBS pH 7.8 and anti-α or anti-β GM-CSF receptor subunit antibodies (1:500) (Alpha Diagnostics, San Antonio, TX). Cells were washed and incubated with antirabbit IgG-horseradish peroxidase (1:100) (Amersham, Arlington Heights, IL) for 2.5 hours at room temperature. Immunostaining was developed using 0.05% diaminobenzidine and 0.03% H2O2. As controls, cells were incubated with antibodies preabsorbed with the respective peptide used to generate the antibodies. Cells were counterstained with hematoxylin.
Archived, formalin fixed, and paraffin embedded human prostate tissues were obtained from the Department of Pathology at Memorial Sloan-Kettering Cancer Center. For immunostaining,39 tissue sections were rehydrated, treated with 3% hydrogen peroxide for 15 minutes at room temperature, and blocked with 4% BSA-PBS pH 7.8, followed by incubation in a humid chamber overnight at 4°C with anti-α or anti-β subunit GM-CSF receptor antibodies (1:500) in 1% BSA-PBS pH 7.8. After extensive washing, sections were incubated for 2.5 hours at room temperature with antirabbit IgG-horseradish peroxidase (1:100, Amersham). The peroxidase activity was developed with 0.05% diaminobenzidine and 0.03% H2O2. Tissues were counterstained with hematoxylin.
RESULTS
LNCaP cells express GM-CSF receptors.
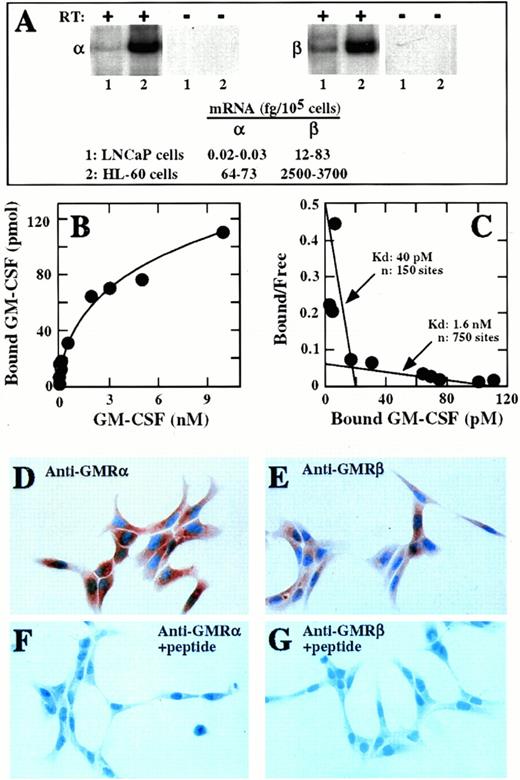
A band of approximately 370 nucleotides, the expected size of the amplification product for the membrane-bound form of the α-subunit mRNA, was amplified by RT-PCR from LNCaP cells RNA (Fig 1A). Primers specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as an internal standard for the efficacy of the RT-PCR procedure. A similar approach using primers complementary to the β subunit of the GM-CSF receptor showed the expected amplification product of approximately 570 nucleotides (Fig 1A). The size of the amplified bands corresponded exactly to the size of the respective bands amplified from RNA obtained from HL-60 cells, which express abundant mRNA for the α and the β subunits of the GM-CSF receptor (Fig 1A).38 No amplification products were observed in parallel reactions in which the reverse transcriptase was omitted (Fig 1A), confirming the absence of contaminating DNA in the RNA preparations. The content of α and β subunit mRNAs in LNCaP cells was quantitated by interpolation on a standard curve generated by performing RT-PCR with known amounts of either α or β subunit RNA in parallel with LNCaP cell-derived RNA. LNCaP cells expressed 0.02 to 0.33 fg of α subunit mRNA per 105 cells (0.24 to 4 copies per 103 cells), a value that is 60 to 200 times lower than the α subunit mRNA levels expressed in HL-60 cells (Fig 1A). LNCaP cells expressed 12 to 83 fg of β subunit mRNA per 105 cells (80 to 550 copies per 103 cells), which is 30 to 200 times lower than the β subunit mRNA levels expressed in HL-60 cells (Fig 1A). LNCaP cells also expressed low levels of mRNA encoding the soluble isoform of the α subunit (data not shown).
Expression of GM-CSF receptors in LNCaP cells. (A) RT-PCR analysis. Total RNA was isolated from LNCaP (lane 1) or HL-60 (lane 2) cells and subjected to RT-PCR using radiolabeled primers specific for the α (left panel) or the β (right panel) subunits of the GM-CSF receptor. PCR products were size-fractionated on 5% acrylamide gels and autoradiographed. Shown are the PCR products corresponding to the α (370 bp) and the β (570 bp) subunits of the GM-CSF receptor obtained in the presence (+) or in the absence (-) of reverse transcriptase (RT). (B) Binding analysis. Cells were incubated with radiolabeled GM-CSF at concentrations that ranged from 5 pmol/L to 10 nmol/L. GM-CSF binding was dose dependent and saturable approximately at 10 nmol/L. (C) Scatchard analysis of data from (B) showing the presence of two classes of binding sites in the LNCaP cells. (D through G) Immunostaining with antihuman GM-CSF receptor antibodies. Cells were cultured, fixed, and incubated with anti-α (D and F) or anti-β subunit (E and G) antibodies in the absence (D and E) or the presence (F and G) of the peptides used to elicit them (original magnification × 160).
Expression of GM-CSF receptors in LNCaP cells. (A) RT-PCR analysis. Total RNA was isolated from LNCaP (lane 1) or HL-60 (lane 2) cells and subjected to RT-PCR using radiolabeled primers specific for the α (left panel) or the β (right panel) subunits of the GM-CSF receptor. PCR products were size-fractionated on 5% acrylamide gels and autoradiographed. Shown are the PCR products corresponding to the α (370 bp) and the β (570 bp) subunits of the GM-CSF receptor obtained in the presence (+) or in the absence (-) of reverse transcriptase (RT). (B) Binding analysis. Cells were incubated with radiolabeled GM-CSF at concentrations that ranged from 5 pmol/L to 10 nmol/L. GM-CSF binding was dose dependent and saturable approximately at 10 nmol/L. (C) Scatchard analysis of data from (B) showing the presence of two classes of binding sites in the LNCaP cells. (D through G) Immunostaining with antihuman GM-CSF receptor antibodies. Cells were cultured, fixed, and incubated with anti-α (D and F) or anti-β subunit (E and G) antibodies in the absence (D and E) or the presence (F and G) of the peptides used to elicit them (original magnification × 160).
Radiolabeled GM-CSF bound to LNCaP cells in a dose-dependent and saturable manner (Fig 1B). Scatchard analysis of the binding data (Fig1C) showed that the LNCaP cells expressed approximately 150 high-affinity binding sites for GM-CSF with a kd of 40 pmol/L, and 750 low-affinity binding sites. The presence of the α and β subunits of the GM-CSF receptor in the LNCaP cells was confirmed by immunolocalization with antibodies specific for each subunit. The LNCaP cells were immunoreactive with both antibodies, with intense immunostaining in both the cytoplasm and the plasma membrane (Fig 1D and F). The LNCaP cells consistently showed enhanced immunoreactivity with the anti-α antibody compared with the anti-β antibody. No immunoreactivity was observed when the primary antibodies were preabsorbed with the peptides used to generate them (Fig 1E and G).
GM-CSF signaling in LNCaP cells.
Proliferation assays, measuring the incorporation of [3H]-thymidine or [125I]-deoxyuridine in DNA, showed that GM-CSF stimulated proliferation of the LNCaP cells in a dose- and time-dependent manner (Fig 2B and C; only [3H]-thymidine incorporation is shown). An increase in [3H]-thymidine incorporation of 20% to 40% was observed after 3 or 4 days of culture in the presence of 0.3 or 100 nmol/L GM-CSF (Fig 2B). The effect of GM-CSF on [3H]-thymidine incorporation was evident during the exponential phase of cell growth and decreased at latter stages (Fig2A and B). Dose-response studies indicated a biphasic effect of GM-CSF on [3H]-thymidine incorporation (Fig 2C). GM-CSF induced a measurable increase in [3H]-thymidine at a concentration of 0.03 nmol/L, an effect that reached saturation at 1 nmol/L GM-CSF, with no further increase observed at concentrations of GM-CSF from 1 to 30 nmol/L. However, 100 nmol/L GM-CSF induced an additional increase in [3H]-thymidine incorporation, which was also evident in the time-course experiments (Fig 2B and C).
GM-CSF signals for proliferation in LNCaP cells. (A) Growth curve. LNCaP cells were maintained in continuous culture with no stimulation and the cell number was determined by counting the cells every day for 6 days and the cell viability was assessed by exclusion of trypan blue. (B) Time course. Cells were incubated with 0.3 (•) or 100 nmol/L (○) GM-CSF for 1 to 6 days and parallel cultures were pulse-labeled with [3H]-thymidine for 20 hours every day. (C) Dose response. Cells were incubated with increased amounts of GM-CSF (0.01 to 100 nmol/L) for 3 (•) or 4 days (○) and pulse-labeled with [3H]-thymidine for the last 20 hours.
GM-CSF signals for proliferation in LNCaP cells. (A) Growth curve. LNCaP cells were maintained in continuous culture with no stimulation and the cell number was determined by counting the cells every day for 6 days and the cell viability was assessed by exclusion of trypan blue. (B) Time course. Cells were incubated with 0.3 (•) or 100 nmol/L (○) GM-CSF for 1 to 6 days and parallel cultures were pulse-labeled with [3H]-thymidine for 20 hours every day. (C) Dose response. Cells were incubated with increased amounts of GM-CSF (0.01 to 100 nmol/L) for 3 (•) or 4 days (○) and pulse-labeled with [3H]-thymidine for the last 20 hours.
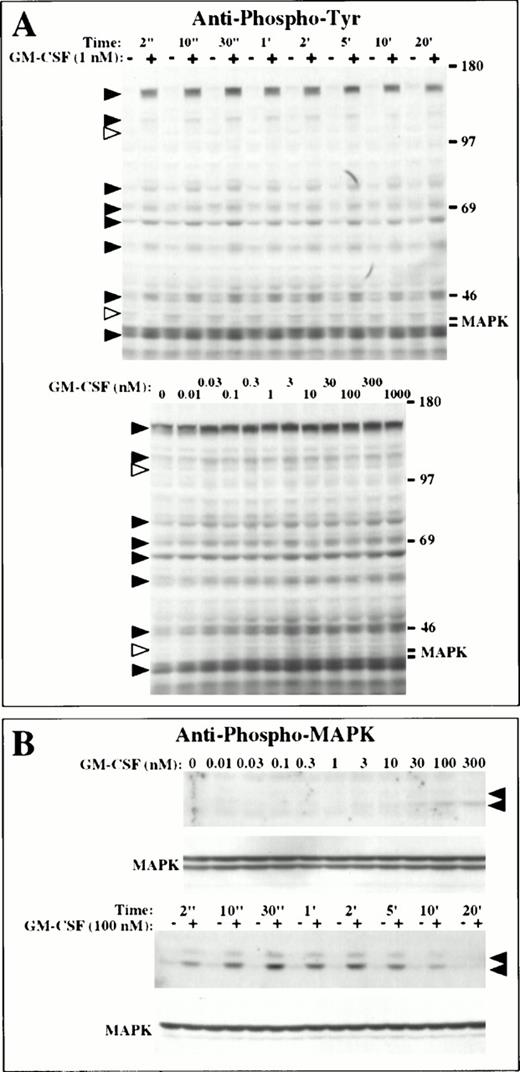
We next analyzed whether GM-CSF induced protein tyrosine phosphorylation in the LNCaP cells. A transient increase in tyrosine phosphorylation was observed in several proteins when LNCaP cells were treated with GM-CSF (Fig 3A). Proteins with apparent molecular weights of 160, 130, 75 to 80, 68 to 70, 60, 55, 47, and 40 kD (black arrowheads, Fig 3A) showed maximal phosphorylation during the first 30 seconds of treatment with 1 nmol/L GM-CSF and remained phosphorylated for 20 minutes, with phosphorylation returning to basal levels after 1 to 2 hours (Fig 3A). Phosphorylation of these proteins was induced at concentrations of GM-CSF ranging from 0.03 nmol/L to 1 μmol/L (Fig 3A). Interestingly, we observed the rapid dephosphorylation of proteins with an apparent molecular weight of 45 and 110 kD after incubating the cells with 1 nmol/L GM-CSF (white arrowheads, Fig 3A). These proteins were dephosphorylated after treating the cells with GM-CSF at concentrations from 0.03 nmol/L to 1 μmol/L and remained dephosphorylated for at least 18 hours (Fig 3A).
GM-CSF signals for protein tyrosine phosphorylation in LNCaP cells. (A) Protein tyrosine phosphorylation. Cells were incubated in the absence (−) or in the presence (+) of 1 nmol/L GM-CSF from 2 seconds to 20 minutes at 37°C (upper panel), or with 0.01 nmol/L to 1 μmol/L GM-CSF for 2 minutes at 37°C (bottom panel). Tyrosine phosphoproteins were identified by immunoblotting with antiphosphotyrosine antibodies. Black and white arrowheads indicate proteins phosphorylated and dephosphorylated in response to GM-CSF, respectively. (B) Phosphorylation of MAP kinase. Cells were incubated with 10 pmol/L to 0.3 μmol/L GM-CSF for 1 minute at 37°C, or were incubated in the absence (−) or in the presence (+) of 100 nmol/L GM-CSF from 2 seconds to 20 minutes at 37°C. Phosphorylation of MAP kinase was assessed by immunoblotting with an antiphospho MAP kinase antibody. MAP kinase was identified with an anti-MAP kinase antibody. The arrowheads indicate the positions of the p42 and p44 MAP kinases.
GM-CSF signals for protein tyrosine phosphorylation in LNCaP cells. (A) Protein tyrosine phosphorylation. Cells were incubated in the absence (−) or in the presence (+) of 1 nmol/L GM-CSF from 2 seconds to 20 minutes at 37°C (upper panel), or with 0.01 nmol/L to 1 μmol/L GM-CSF for 2 minutes at 37°C (bottom panel). Tyrosine phosphoproteins were identified by immunoblotting with antiphosphotyrosine antibodies. Black and white arrowheads indicate proteins phosphorylated and dephosphorylated in response to GM-CSF, respectively. (B) Phosphorylation of MAP kinase. Cells were incubated with 10 pmol/L to 0.3 μmol/L GM-CSF for 1 minute at 37°C, or were incubated in the absence (−) or in the presence (+) of 100 nmol/L GM-CSF from 2 seconds to 20 minutes at 37°C. Phosphorylation of MAP kinase was assessed by immunoblotting with an antiphospho MAP kinase antibody. MAP kinase was identified with an anti-MAP kinase antibody. The arrowheads indicate the positions of the p42 and p44 MAP kinases.
We did not detect tyrosine phosphorylation of MAP kinase in LNCaP cells treated with GM-CSF using antiphosphotyrosine antibodies (Fig 3A). The p42 MAP kinase was identified by reprobing the membrane with an anti-MAP kinase antibody and was found to be present at a constant level at the expected position in the blots (Fig 3B). Phosphorylation of MAP kinase, however, was evident when using a monoclonal antibody specific for tyrosine phosphorylated MAP kinase (Fig 3B). Phosphorylation of MAP kinase occurred only at concentrations of GM-CSF of 3 nmol/L or higher and reached a maximal level at 100 nmol/L GM-CSF. Maximal phosphorylation was observed at 30 seconds, with phosphorylation returning to basal levels after 20 minutes (Fig 3B). No phosphorylation of JAK2 was evident in cells treated with GM-CSF.
Increased expression of GM-CSF receptors in human prostate tumors.
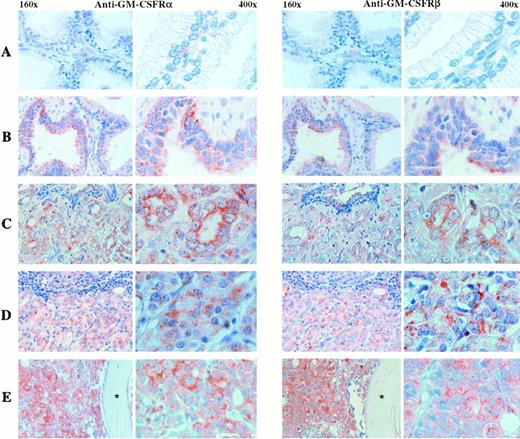
We examined whether the α and β subunits of the GM-CSF receptor were expressed in normal prostate, benign prostatic hyperplasia, and prostatic carcinomas, including primary tumors and metastatic lesions to lymph node and bone (Fig 4 and Table 1). Very weak to undetectable immunostaining was observed in the luminal epithelial cells of the acini of the normal prostate, although weak to moderate reactivity was evident in basal cells (Fig 4). Ducts exhibited a more intense immunoreactivity than acini for both antibodies. Moderate immunostaining was also found in the epithelial cells of most of the samples of benign prostatic hyperplasia analyzed with both antibodies, although moderate to strong reactivity was observed in two specimens (Fig 4). In addition, basal cell hyperplasia, a histologically different form of hyperplasia, was seen in one sample and displayed strong reactivity with both antibodies. All primary tumors analyzed, which exhibited Gleason scores from 5 to 9, were positive for both antibodies, with homogeneous and heterogeneous staining patterns of moderate to strong intensity (Fig 4and Table 1). The metastatic tumors displayed homogeneity, as well as heterogeneity in immunostaining profiles, with moderate to strong reactivity to both antibodies and had increased staining compared with the primary tumors (Fig 4). One sample from a bone metastasis was negative for both antibodies. Tumor cells that were immunopositive for the anti-α antibody were also positive for the anti-β antibody, as immunohistochemistry was conducted in consecutive sections and numerous lesions showed homogeneous staining. In addition, most of the cases studied showed a more intense anti-α immunoreactivity than the anti-β immunoreaction.
Immunolocalization of GM-CSF receptors in benign and malignant human prostate tissues. Consecutive tissue sections were incubated with anti-α (left panel) or anti-β–subunit (right panel) antibodies. (A) Low to undetectable levels in the epithelial cells of the normal gland. (B) Moderate levels of expression in the epithelial cells of benign prostatic hyperplasia. (C) Increased immunoreactive pattern in a primary tumor. (D) High levels of expression in a lymph node and a bone (E) metastases. Stars in bone metastases images indicate bone location. Two different magnifications of each immunostained tissue section are shown (160× and 400×).
Immunolocalization of GM-CSF receptors in benign and malignant human prostate tissues. Consecutive tissue sections were incubated with anti-α (left panel) or anti-β–subunit (right panel) antibodies. (A) Low to undetectable levels in the epithelial cells of the normal gland. (B) Moderate levels of expression in the epithelial cells of benign prostatic hyperplasia. (C) Increased immunoreactive pattern in a primary tumor. (D) High levels of expression in a lymph node and a bone (E) metastases. Stars in bone metastases images indicate bone location. Two different magnifications of each immunostained tissue section are shown (160× and 400×).
Expression of GM-CSF Receptors in Human Prostatic Tissue
| . | GMRα . | GMRβ . | ||
|---|---|---|---|---|
| Positive Cases* . | Intensity-151 . | Positive Cases . | Intensity . | |
| Normal prostate | 4/7-152 | ±/+ | 4/7 | ±/+ |
| Benign hyperplasia | 7/7-152 | +/++ | 7/7 | +/++ |
| Prostatic carcinoma Grade (Gleason score) | ||||
| 5 | 3/3 | ++ | 3/3 | ++ |
| 7 | 3/3 | ++/+++ | 3/3 | ++/+++ |
| 8 | 4/4 | ++/+++ | 4/4 | ++/+++ |
| 9 | 1/1 | +++ | 1/1 | +++ |
| Metastases | ||||
| Bone | 4/5 | +++ | 4/5 | ++/+++ |
| Lymph node | 4/4 | ++/+++ | 4/4 | +++ |
| . | GMRα . | GMRβ . | ||
|---|---|---|---|---|
| Positive Cases* . | Intensity-151 . | Positive Cases . | Intensity . | |
| Normal prostate | 4/7-152 | ±/+ | 4/7 | ±/+ |
| Benign hyperplasia | 7/7-152 | +/++ | 7/7 | +/++ |
| Prostatic carcinoma Grade (Gleason score) | ||||
| 5 | 3/3 | ++ | 3/3 | ++ |
| 7 | 3/3 | ++/+++ | 3/3 | ++/+++ |
| 8 | 4/4 | ++/+++ | 4/4 | ++/+++ |
| 9 | 1/1 | +++ | 1/1 | +++ |
| Metastases | ||||
| Bone | 4/5 | +++ | 4/5 | ++/+++ |
| Lymph node | 4/4 | ++/+++ | 4/4 | +++ |
*No. of positives cases/total no. of cases studied.
± , very weak; +, weak; ++, moderate; +++, strong.
Epithelial cells.
DISCUSSION
GM-CSF is a hematopoietic growth factor and a host defense regulator used clinically to stimulate hematopoietic cell proliferation after chemotherapy, as well as autologous or allogeneic bone marrow transplantation.40-42 The physiologic role of GM-CSF receptors in nonhematopoietic tissue is unknown. Even more problematic are the implications and consequences of GM-CSF receptor expression on malignant, nonhematopoietic tissue. Distinct types of neoplastic cells have been shown to have functional GM-CSF receptors.25,30 31 Whether or not therapeutically administered hematopoietic growth factors can stimulate nonhematopoietic cell growth, including solid tumor cells, has remained a controversial issue.
We report here a detailed study addressing the issue of GM-CSF receptor expression in human prostate cancer. The presence of GM-CSF receptors in the LNCaP cells was defined by quantitative RT-PCR, ligand binding, immunolocalization, and functional assays. Quantitative RT-PCR showed that the LNCaP cells expressed mRNAs for the α and β subunits of the GM-CSF receptor. The immunolocalization experiments confirmed the presence of the α and β proteins in the LNCaP cells and the ligand-binding studies showed that LNCaP expressed both high- and low-affinity GM-CSF receptors. The number of high-affinity receptors present in the LNCaP cells was similar to the number present in cells of hematopoietic origin in which GM-CSF induces proliferation and differentiation43 and in nonhematopoietic cells such as mouse fibroblasts expressing the human high-affinity receptors, which respond to GM-CSF with cell proliferation and protein phosphorylation.10 44 The identification of approximately 750 low-affinity GM-CSF receptors in the LNCaP cells, compared with about 150 high-affinity sites, indicates the presence of an excess of α as compared with β subunit in these cells. Consistent with these findings, the immunolocalization experiments showed greater immunoreactivity with the anti-α subunit antibodies than with the anti-β subunit antibodies in the LNCaP cells. The RT-PCR experiments, however, indicated that LNCaP cells express a higher number of mRNA molecules per cell encoding the β than the α subunit of the GM-CSF receptor. These data suggest that, in the LNCaP cells, the expression of the α and β subunit proteins is regulated at the level of translation or protein stability.
Biologic response analyses confirmed the presence of functionally active GM-CSF receptors in the LNCaP cells. A previous study34 reported a 2.8-fold increase in LNCaP cell proliferation in the presence of suprapharmacologic concentrations of GM-CSF (>1 μmol/L). These concentrations of GM-CSF are at least four orders of magnitude higher than the concentrations we used here (≈100 pmol/L) and are not compatible with the expression of high-affinity GM-CSF receptors in the LNCaP cells. On the other hand, although our data indicated that GM-CSF increased LNCaP cell proliferation at concentrations consistent with the presence of high-affinity receptors in these cells, these concentrations of GM-CSF (≈100 pmol/L) were at least one order of magnitude higher than that necessary in cells such as HL-60 (≈10 pmol/L), which express a similar number of high-affinity GM-CSF receptors. The origin of these discrepancies is not evident from these studies; however, the data suggest the existence of cell-specific effects that modulate the functional activity of the GM-CSF receptor.
Tyrosine phosphorylation of p42 and p44 MAP kinases is an early step in GM-CSF signal transduction.9,10,13-15 45 We found that GM-CSF induced time- and dose-dependent tyrosine phosphorylation of several proteins in the LNCaP cells and induced tyrosine phosphorylation of the p42 and p44 MAP kinases. Phosphorylation of proteins other than MAP kinase was observed at GM-CSF concentrations of 0.1 nmol/L or less, which is consistent with the presence of high-affinity GM-CSF receptors in the LNCaP cells. On the other hand, phosphorylation of MAP kinase was triggered only at GM-CSF concentrations of at least 3 nmol/L. Furthermore, we failed to observe JAK2 phosphorylation in these cells. Because the LNCaP cells express about 750 low-affinity sites that likely correspond to excess of α subunits (in addition to approximately 150 high-affinity sites), the data raise the intriguing possibility that excess of α-subunit expression may modulate signaling through the high-affinity receptor.
The immunohistochemical analysis of human prostatic tissue using anti-α and -β antibodies confirmed the presence of the α and the β subunits of the GM-CSF receptor in prostate tumors. The lack of immunoreactivity or weak pattern of staining observed in normal epithelial cells, contrasts with the substantial immunoreactivity with anti- α and -β antibodies observed in all cases of benign prostatic hyperplasia. Although primary tumors showed higher levels of expression compared with benign prostatic hyperplasia, we observed a further increase in the immunoreactivity of metastases to lymph node and bone compared with that of primary tumors. These data are compatible with increased expression of the GM-CSF receptor as the disease progresses from localized tumors to the development of metastatic disease.
Although the action of growth factors and their receptors in normal prostate physiology and progression of prostate cancer are not well understood, our results suggest that GM-CSF may have a role in maintenance of function in the normal prostate, as well as in prostate cancer progression. The presence of both subunits of the GM-CSF receptor in the tumor cells indicate that they express functional high-affinity GM-CSF receptors and therefore this hematopoietic growth factor may have an effect on prostate carcinoma cells, which have a proclivity to metastasize to bone. The increased expression of GM-CSF receptors in prostatic hypertrophy and neoplastic prostate ephitelium suggest a relationship between prostatic epithelial cell growth and GM-CSF.
Supported in part by Grants No. R01 CA30388, R01 HL42107, CA-DK-47650, and P30 CA08748 from the National Institutes of Health, Bethesda, MD; by Memorial Sloan-Kettering Institutional funds; by the Schultz Foundation, Verona, NJ; by the PepsiCo Foundation, Purchase, NY; and by the David H. Koch Charitable Foundation, Wichita, KS.
Address reprint requests to David W. Golde, MD, Program in Molecular Pharmacology and Therapeutics, Box 451, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 2. GM-CSF signals for proliferation in LNCaP cells. (A) Growth curve. LNCaP cells were maintained in continuous culture with no stimulation and the cell number was determined by counting the cells every day for 6 days and the cell viability was assessed by exclusion of trypan blue. (B) Time course. Cells were incubated with 0.3 (•) or 100 nmol/L (○) GM-CSF for 1 to 6 days and parallel cultures were pulse-labeled with [3H]-thymidine for 20 hours every day. (C) Dose response. Cells were incubated with increased amounts of GM-CSF (0.01 to 100 nmol/L) for 3 (•) or 4 days (○) and pulse-labeled with [3H]-thymidine for the last 20 hours.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/3/10.1182_blood.v91.3.1037/3/m_blod4032602.jpeg?Expires=1767703088&Signature=Zi7Z~ZOBzG1renbIUQRJ4Fc6VEU3NBNDgXAi-KlOlbqjCjp2CoeRJ9MyTvqZZpvzMHQ3AhofjH6frcXMYcbV84-bfz8XhUPO1veZ35m41Pe12Rp7Yy~yJFIi8WAH2J4Or3A9VO4RmkA1utY-5qs6KrSiPpiO~B2lTnS8lLf1xP2z-ZcsdxFH98RLFToIUkUdvMVsA19QE6IGpgeZyiZf0isqTAu3YpYUkY4AFqnmQWiADp8V84etubGHX2obS~cVvhwlVETKoxY5NDnG-s62SnvrPzaI0fUt1BLJfNClY8QC7CDFN8Jeo9~QKCIjye6pH8aMsl3RJ-lUDJiY7hDbYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal