Abstract
We have previously shown that the disruption/deletion of the MTS(MTS1-MTS2) locus due to illegitimate V(D)J recombinase activity is a genetic event characteristic of T-cell acute lymphoblastic leukemia (T-ALL). Inactivation of the p16INK4a tumor suppressor protein, encoded by MTS1, is thought to be the major functional consequence of these chromosomal rearrangements. The two other cell cycle inhibitors encoded by genes identified in the locus (p19ARF by MTS1 and p15INK4b by MTS2), also represent possible candidates for inactivating events. By analyzing p16INK4aexpression in three cases in which an identical 36-kb deletion had deleted MTS2 and disrupted the p19ARF, but spared the p16INK4aMTS1 encoding exons, we have excluded p16INK4a and pinpointed p19ARF and/or p15INK4b as the functional target(s) of this rearrangement. Moreover, by the study of the MTS genomic configuration of 149 rearranged alleles from a large series of T-ALL cases, we have shown that p19ARF encoding exons were always disrupted or deleted, whereas p16INK4a and p15INK4b encoding exons were spared in four and 21 cases, respectively. These results suggest that p19ARF may be targeted by the genetic events that occur in the MTS locus in the majority of T-ALLs.
WE SHOWED 3 YEARS ago that deletions of the MTS1/p16INK4a andMTS2/p15INK4b genes occur frequently in T-cell acute lymphoblastic leukemias (T-ALL) cells.1 This has been confirmed by a number of studies,2-5 and it is now established that chromosomal rearrangements deleting or disrupting theMTS locus represent a genetic event characteristic of T-ALL.6 In the majority of cases, the whole MTSlocus is deleted on both chromosomes. In about a third of alleles, the breakpoints fall within the locus and most of them are located in two major clusters (MTS1bcrα andMTS1bcrβ).5,7 We have recently cloned and sequenced several breakpoints falling in either cluster and have shown that these recombinations are due to V(D)J recombinase activity, which, active in thymocytes, is involved in the generation of antigen T-cell receptor diversity.7 Although the incidence and the mechanism of these rearrangements are now well-defined in T-ALL, their functional targets remain to be identified.
The MTS locus8 extends over 50-kb at the 9p21 chromosomal band and includes two genesMTS1/CDKN2/p16INK4a andMTS2/p15INK4b. MTS1 acts as a tumor suppressor.9 The gene has a complex structure, as it encodes two unrelated proteins, p16INK4a10 and p19ARF11 from two types of RNAs,11-14MTS1α and MTS1β, respectively. These RNAs are transcribed from distinct promotors located 5′ to alternative first exons (MTS1 exon 1α and exon 1β, respectively) and share the MTS1 exons 2 and 3. The MTS1 exon 2 is read in two distinct frames to code for p16INK4a and p19ARF. MTS2 includes two exons and encodes p15INK4b.15 p16INK4a and p15INK4b are two of the INK4 family members demonstrating cyclin-dependent kinase (cdk) 4 and 6 binding and inhibiting activities.16 Their expression blocks the cell cycle at late G1 by inhibiting retinoblastoma gene protein product (Rb) phosphorylation by the CyclinDs-cdk4/6 complexes. p19ARF is also a cell cycle inhibitor, but its biochemical function, which is distinct from that of the INK4 family members, remains unknown.11 A wide body of evidence supports the view that p16INK4a is a tumor suppressor protein,6 and it is thought to represent the major functional target of genetic events occurring in the MTS locus. However, there is, to date, no definitive argument for discarding p19ARF and p15INK4b as functional targets of inactivating genetic events in cancer. Moreover, the demonstration that the MTS2 promotor is hypermethylated in various malignant hematologic disorders, including T-ALL, supports the view that MTS2is a tumor suppressor gene.17-19
In this work, we have searched for evidence of p19ARFinactivation in T-ALL. First, by analyzing the consequences onMTS1 expression of a particular rearrangement in three cases, we have excluded p16INK4a as the target of the recombination and pinpointed p19ARF and p15INK4b as likely candidates. Second, by studyingMTS locus configuration in a large series of T-ALLs, we have shown that the p19ARF encoding gene is always deleted/disrupted when chromosomal rearrangements occur in the locus, whereas p15INK4b and p16INK4a encoding exons are spared in a significant number of cases, suggesting that p19ARF is targeted by the inactivating events.
MATERIALS AND METHODS
Cells.
Bone marrow or peripheral blood samples were obtained from 86 patients with T-ALL. Mononuclear cells from bone marrow or peripheral blood samples were isolated by Ficoll-Hypaque centrifugation and viably frozen in liquid nitrogen until use. A high percentage (usually more than 90%) of tumor cells was present in all samples. Patients were identified by unique numbers used in all reports from our group. Hela, RS4;11, Molt4, K562, and Saos2 cell lines were used in selected experiments.
Southern blot analysis of the MTS locus configuration.
Southern blots were performed as previously described.5Analysis was performed in the Molt4 cell line and in 87 samples from 86 T-ALLs including 58 previously studied patients (in one case, T39, theMTS locus configuration was different at presentation [T39P] and at relapse [T39R] and both samples have been included in the study). An MTS1 exon 1β probe7 was used in addition to the panel of probes used in our previous work.5Data were analyzed by comparison with the restriction map of the locus. The localization of the MTS1 exon 1β indicated in our first version of the MTS map5 was that published by Duro et al12 and has been subsequently found to be incorrect.7 We have precisely localized this exon and a corrected map is now available.7
Reverse-transcriptase-polymerase chain reaction (RT-PCR) amplification.
Total RNA was extracted from cryopreserved cells according to Chomczynski and Sacchi.20 Quality and quantity of RNA was controlled on an ethidium bromide stained 1% agarose gel containing 2.2 mol/L formaldehyde. RT-PCR analysis was performed as previously described.21 Amplification of MTS1α andMTS1β cDNA was performed with 2 or 2.5 mmol/L MgCl2, respectively, and 5% formamide. After an initial denaturation step of 3 minutes at 94°C, a limited number of cycles (the number of cycles for each RT-PCR is given in the figure legends) consisting of 1 minute at 94°C, 1 minute at 60°C, 1 minute at 72°C followed by a final extension of 10 minutes at 72°C were performed on a DNA thermal cycler (Stratagene, La Jolla, CA). For all RNA samples, amplification of Abelson mRNA was performed as a control from a common cDNA. MTS1αtranscripts were amplified using primers OL187: 5′-GCCCAACGCACCGAATAGT-3′ and OL29: 5′-GCTACCGGGTCGAGGAGTC-3′19 located on exon 1α and exon 2, respectively. MTS1β transcripts were amplified using primers OL27: 5′-ATGGTGCGCAGGTTCTTGGT-3′12 and OL28: 5′-TGCACGGGTCGGGTGAGAGT-3′ located on exon 1β and exon 2, respectively. Abelson transcripts: abl1 (2nd exon): 5′-CCTTCAGCGGCCAGTAGCATCTGAC-3′ and abl2 (3rd exon): 5′-GGACACAGGCCCATGGTACCAGGAG-3′.
Analysis of PCR products.
PCR products were run on 2% NuSieve (FMC BioProducts, Rockland, ME) 1% agarose gel, transferred to a Hybond N+ membrane (Amersham International plc, Les Ulis, France) and hybridized to 30 ng of a specific oligonucleotide probe labeled with 10 μCi γ-32P–adenosine triphosphate (ATP) by T4 Polynucleotide Kinase (New England Biolabs Inc, Beverly, MA). MTS1αtranscripts: 5′-CGATCCAGGTCATGATGAT-3′. MTS1βtranscripts: 5′-GAAGACCAGGTCATGATGAT-3′. Abl transcripts: Abl3: 5′-TCCCAATTGTGATTATAGCC-3′.
Western blotting analysis of p16INK4a and Rb expression.
Cell proteins were extracted with Triple Detergent Lysis Buffer, quantified using the BCA kit (Pierce, Rockford, IL), size fractionated on either 15% Tris-glycine sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) for p16INK4a, or on 8% Tris-glycine SDS-PAGE for Rb. After electrotransfer onto nitrocellulose, the membranes were blocked 1 hour in 10% nonfat dry milk in PBST (phosphate-buffered saline; Tween 20, 0.1%) and probed with antibodies. After a 1-hour incubation, the membranes were washed with PBST and incubated with peroxidase-conjugated secondary antibodies for 1 hour. After washes in PBST, membranes were revealed by chemiluminescence according to the manufacturer's instructions (ECL, Amersham). An antiactin antibody (Oncogene Science, Uniondale, NY) was used to verify protein loading and integrity. The RS4.11 cell line (which demonstrates a biallelicMTS1 deletion, unpublished data, 1995) and the Hela cell line (which demonstrates a high level of MTS1 protein expression) were used as negative and positive controls forMTS1 protein expression, respectively. The K562 myeloid leukemia cells and SAOS2 osteosarcoma cells were used as positive and negative controls for Rb protein expression, respectively. Antibodies used and their sources were as follows: anti-MTS1/p16INK4a monoclonal antibody (Pharmingen, San Diego, CA), antibodies against Rb (Santa Cruz Biotechnology, Santa Cruz, CA), goat antirabbit IgG-peroxidase conjugate and goat antimouse IgG-peroxidase conjugate (Boehringer Mannheim, Reylan, France).
Single-strand conformation polymorphism (SSCP) analysis.
SSCP analysis of MTS1 exon 1α and MTS1 exon 2 was performed as described previously.5 Oligonucleotides used for MTS1 exon 3 SSCP analysis were: OL30: 5′-TTTTCTTTCTGCCCTCTGC-3′ located immediately upstream of the splice acceptor site, and OL31: 5′-TTGTGGCCCTGTAGGACCTT-3′ located 73 nucleotides downstream of the stop codon. SSCP analysis of MTS1exon 1β was performed on PCR products amplified using the following oligonucleotides: OL2: 5′-GGGGTGGGGGTGAAGGTG-3′ located 113 nt upstream of the putative transcriptional start site and OL4: 5′-ACGATTGAGGGCTGTGTGAAG-3′ located 204 nt downstream of the donor splice site. PCR products were digested by the NarI restriction endonuclease resulting in two fragments of 281 and 303 bp. Running conditions were identical to those described in Cayuela et al.5
RESULTS AND DISCUSSION
A recurrent interstitial deletion inactivates the p15INK4b/MTS2 gene, disrupts the p19ARFencoding exons, but spares the p16INK4a encoding MTS1 exons.
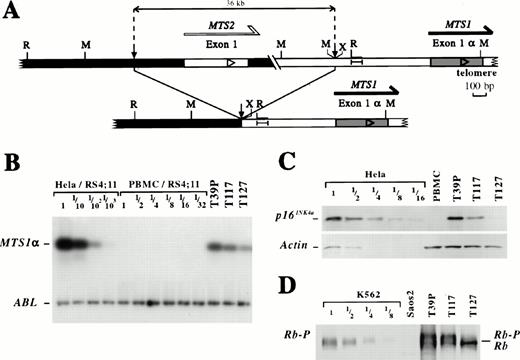
In four cases, a rearrangement sparing MTS1 exon 1α, exon 2, and exon 3 had occurred and was characterized in three cases (T39P, T117, and T127). An identical 36-kb interstitial deletion due to illegitimate V(D)J recombinase activity had occurred, juxtaposing the 5′ MTS2 exon 1 and 5′ MTS1 exon 1α regions. All MTS2 exons and the MTS1 exon 1β had been deleted.7 A schematic representation of the rearrangement is shown in Fig 1A and relevant clinical and hematologic data are summarized in Table 1.
Genomic configuration, MTS1 product expression and Rb phosphorylation in three cases with a short interstitial deletion. (A) Schematic representation of the 36-kb interstitial deletion occurring in the MTS locus in T39p, T117, and T127. Small vertical arrows indicate breakpoints. Large horizontal arrows indicate the transcriptional orientation. The DNA sequences involved in transcriptional activation of the promotor located 5′ to theMTS1 exon 1α in cells with physical or functional Rb inactivation24 are shown: I—-I. R: EcoRI; M:Mbo I, X: Xba I restriction sites. The Xba I site has been deleted by the V(D)J process in the T39p sample.7 (B) RT-PCR detection of ABL (30 PCR cycles) andMTS1α (26 PCR cycles) transcripts in T39p, T117, and T127 samples and in dilutions of Hela and PBMC RNAs into RS4;11 RNAs (results observed in this experiment for other T-ALL cases are shown in Fig 3A). PCR products were electrophoresed on agarose gels, transferred to filters and hybridized with specific radiolabeled oligoprobes. The Hela cell line expresses high level MTS1α; MTS1 is deleted on both alleles in the RS4;11 cell line. (C) Western blot analysis of p16INK4a and actin proteins in T39p, T117, T127, and in a dilution of Hela cells in PBS. (D) Western blot analysis of Rb expression in T39P, T117, and T127 samples and in the K562 and SAOS2 cell lines. The K562 cell line expresses phosphorylated Rb proteins (Rb-P). Rb is deleted in the SAOS2 cell line. Dilutions of K562 cells into PBS have been studied. Rb: unphosphorylated Rb protein.
Genomic configuration, MTS1 product expression and Rb phosphorylation in three cases with a short interstitial deletion. (A) Schematic representation of the 36-kb interstitial deletion occurring in the MTS locus in T39p, T117, and T127. Small vertical arrows indicate breakpoints. Large horizontal arrows indicate the transcriptional orientation. The DNA sequences involved in transcriptional activation of the promotor located 5′ to theMTS1 exon 1α in cells with physical or functional Rb inactivation24 are shown: I—-I. R: EcoRI; M:Mbo I, X: Xba I restriction sites. The Xba I site has been deleted by the V(D)J process in the T39p sample.7 (B) RT-PCR detection of ABL (30 PCR cycles) andMTS1α (26 PCR cycles) transcripts in T39p, T117, and T127 samples and in dilutions of Hela and PBMC RNAs into RS4;11 RNAs (results observed in this experiment for other T-ALL cases are shown in Fig 3A). PCR products were electrophoresed on agarose gels, transferred to filters and hybridized with specific radiolabeled oligoprobes. The Hela cell line expresses high level MTS1α; MTS1 is deleted on both alleles in the RS4;11 cell line. (C) Western blot analysis of p16INK4a and actin proteins in T39p, T117, T127, and in a dilution of Hela cells in PBS. (D) Western blot analysis of Rb expression in T39P, T117, and T127 samples and in the K562 and SAOS2 cell lines. The K562 cell line expresses phosphorylated Rb proteins (Rb-P). Rb is deleted in the SAOS2 cell line. Dilutions of K562 cells into PBS have been studied. Rb: unphosphorylated Rb protein.
Hematologic Features of the T39P, T117, and T127 T-ALLs
| . | Age (yr)/Sex . | WBC (109/L) . | TS . | TAL1 d . | Phenotype . | Clinical Outcome . | MTS1α Expression . | Rb Expression . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| RNA . | Protein (p16INK4a) . | Rb . | Rb-P . | |||||||
| T39 | 8/M | 140 | N | + | CD7−,2−,1−,4−,8−,3− | CR; relapse 10 mo later and death. | +++ | +++ | + | +++ |
| T117 | 22/M | 189 | N | − | CD7+,2+,1+,4+,8+,3− | CR; allogenic intrafamilial HLA matched bone marrow transplantation in 1st CR. Death by aspergillosis. | ++ | ++ | + | ++ |
| T127 | 16/F | 440 | N | + | CD7+,2+,1+/−,4+/−,8+,3+ | CR; relapse 9 mo later after autologous bone marrow transplantation and death. | + | + | + | + |
| . | Age (yr)/Sex . | WBC (109/L) . | TS . | TAL1 d . | Phenotype . | Clinical Outcome . | MTS1α Expression . | Rb Expression . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| RNA . | Protein (p16INK4a) . | Rb . | Rb-P . | |||||||
| T39 | 8/M | 140 | N | + | CD7−,2−,1−,4−,8−,3− | CR; relapse 10 mo later and death. | +++ | +++ | + | +++ |
| T117 | 22/M | 189 | N | − | CD7+,2+,1+,4+,8+,3− | CR; allogenic intrafamilial HLA matched bone marrow transplantation in 1st CR. Death by aspergillosis. | ++ | ++ | + | ++ |
| T127 | 16/F | 440 | N | + | CD7+,2+,1+/−,4+/−,8+,3+ | CR; relapse 9 mo later after autologous bone marrow transplantation and death. | + | + | + | + |
Abbreviations: WBC, white blood cell count; TS; tumoral syndrome; TAL1 d, TAL1 deletion (ie, SIL-TAL1 rearrangement); CR, complete remission; Rb-P, phosphorylated Rb.
Because the rearrangements have spared the MTS1 promoter located 5′ of the MTS1 exon 1α (see Fig 1 legend), we wondered if MTS1α transcripts were expressed and performed an RT-PCR analysis in T39p, T117, and T127 samples and in the Hela and RS4;11, positive and negative controls, respectively (Fig 1B). Clear expression was detected in the three primary leukemia blood samples, which contained more than 80% tumor cells. This expression reflects transcription from the rearranged allele, as the other is deleted7 and no expression was found in normal peripheral blood mononuclear cell (PBMC) under these experimental conditions (Fig1B).
To verify that the MTS1α transcripts encode proteins, p16INK4a protein expression was studied by Western blotting. As shown in Fig 1C, clear expression was found in the T39p and T117 cases, but was barely detectable in the third case (T127). This expression parallels that of the MTS1α transcripts. SSCP analysis of MTS1 exons 1α, 2, and 3 was performed in all three cases (data not shown). No mutations were detected.
It has previously been suggested that MTS1α transcript expression is enhanced when Rb is physically or functionally inactivated (eg, by phosphorylation).22 We analyzed whether this correlation had been modified by the recombinational event. Rb phosphorylation was studied by Western blotting in the T39p, T117, and T127 samples and in the K562 and SAOS2 cell lines used as positive and negative controls, respectively. As shown in Fig 1D, hyperphosphorylated forms were found in the T39p and T117 cases, but were not detectable in T127, correlating with MTS1α transcript expression.
These data show that the interstitial deletion had spared the possibility to express MTS1α and its protein product p16INK4a, suggesting that other proteins had been targeted by the chromosomal event. Because the rearrangement had disrupted the p19ARF encoding exons and deletedMTS2/p15INK4b, the sole gene identified in the 36-kb DNA region located between the two breakpoints, these results suggest that p15INK4b and/or p19ARFmight be the functional target(s) of the deletion in these cases.
The hypothesis that the gene encoding p19ARF could be inactivated by genetic events in other cases of T-ALL was further studied.
p19ARF encoding exons are deleted or disrupted by all chromosomal events involving the MTS locus.
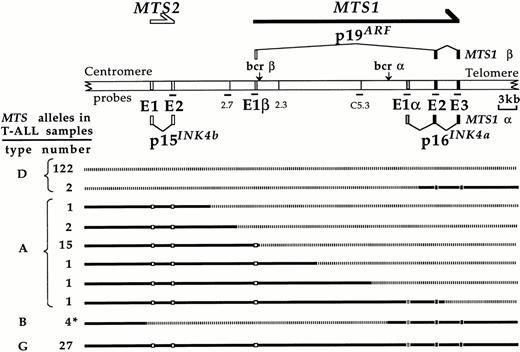
The MTS locus configuration, as defined by Southern blot analysis in 87 T-ALL samples and in the Molt4 cell line, was evaluated with respect to the integrity of p16INK4a, p19ARF, and p15INK4b encoding exons. A total of 176 alleles were analyzed.
The various allelic configurations are shown in Fig 2. Deletion and/or rearrangement had occurred in 149 alleles. Complete deletion/disruption of the locus was documented in 124 alleles (Type D alleles). The other rearrangements can be functionally classified into two types, A and B (Fig 2). In 21 cases, the recombinations have deleted the p16INK4a encoding exons and disrupted the p19ARF encoding exons, but spared the p15INK4bencoding exons, defining the type A alleles. In four other cases (Type B alleles), and as detailed above, a rearrangement sparing MTS1exon 1α, exon 2, and exon 3, but deleting MTS1 exon 1β and the p15INK4b encoding exons had occurred. Interestingly, the p19ARF encoding exons were deleted or disrupted in all cases (149/149), in which a deletion or a chromosomal rearrangement had occurred in the MTS locus, suggesting that the product of these exons might be a functional target of these recombinational events.
(Top) Schematic representation of the MTS locus according to Cayuela et al.5 7 The transcripts initiated from the MTS2 promotor and from the two MTS1 promotors (MTS1α and MTS1β) are represented. Their protein products are indicated. The principal probes used for Southern blot analysis of the MTS locus configuration in T-ALL samples are shown. Horizontal arrows indicate the direction of transcription. (Bottom) Representation of 176 alleles with respect to the configuration of the locus. *, the configuration shown corresponds to the three cases in which the two breakpoints have been precisely localized (T39p, T117, and T127). In the last case (T106), the telomeric breakpoint has not been localized.
(Top) Schematic representation of the MTS locus according to Cayuela et al.5 7 The transcripts initiated from the MTS2 promotor and from the two MTS1 promotors (MTS1α and MTS1β) are represented. Their protein products are indicated. The principal probes used for Southern blot analysis of the MTS locus configuration in T-ALL samples are shown. Horizontal arrows indicate the direction of transcription. (Bottom) Representation of 176 alleles with respect to the configuration of the locus. *, the configuration shown corresponds to the three cases in which the two breakpoints have been precisely localized (T39p, T117, and T127). In the last case (T106), the telomeric breakpoint has not been localized.
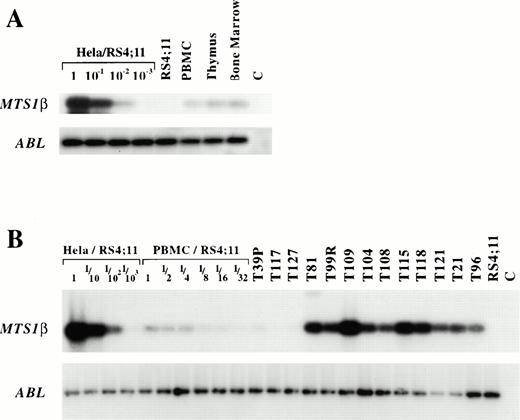
MTS1β transcripts are expressed in the thymus and in most T-ALL samples without genomic rearrangement of the corresponding exons.
The hypothesis that p19ARF inactivation is targeted by the recombinational events imply that normal and/or neoplastic immature T cells have the potential to express MTS1βtranscripts. Previous studies have shown that these RNAs are expressed in most murine tissues,11 but expression in the thymus has not been studied to date. MTS1β transcript expression was therefore analyzed by RT-PCR in one human thymus. A low level of expression was detected, which is similar to the level found in bone marrow (Fig 3A). MTS1βtranscript expression was also studied in 10 T-ALL cases in which no genomic alteration of the corresponding exons was found by Southern blotting on at least one of the two alleles. Clear expression was detected in all cases, contrasting with an expected lack of expression in the T39P, T117, and T127 cases in which the MTS1 exon1β had been deleted. SSCP analysis of MTS1 exon1β was performed in eight of the cases expressing the corresponding transcripts (T81, T99R, T108, T115, T118, T121, T21, and T96). Normal SSCP patterns were found in all cases (data not shown). These results show that immature T cells can express p19ARFencoding RNAs.
MTS1β expression in immature T cells. (A) RT-PCR detection of MTS1β and ABL transcripts. RT-PCR analysis of MTS1β (encoding p19ARF, 26 PCR cycles) and ABL transcript expression (30 PCR cycles) in leukemic samples and controls. PCR products were electrophoresed on agarose gels, transferred to filters, and hybridized with specific radiolabeled probes. c: control (no cDNA). (B) RT-PCR detection ofMTS1β transcripts in thymus, bone marrow, and PBMC. PCR products were electrophoresed on agarose gels, transferred to filters, and hybridized with specific radiolabeled probes. Hela and RS4;11 expression were used as positive and negative controls forMTS1/2 expression, respectively. MTS1β and Abl transcripts were studied using 27 and 30 PCR cycles, respectively.
MTS1β expression in immature T cells. (A) RT-PCR detection of MTS1β and ABL transcripts. RT-PCR analysis of MTS1β (encoding p19ARF, 26 PCR cycles) and ABL transcript expression (30 PCR cycles) in leukemic samples and controls. PCR products were electrophoresed on agarose gels, transferred to filters, and hybridized with specific radiolabeled probes. c: control (no cDNA). (B) RT-PCR detection ofMTS1β transcripts in thymus, bone marrow, and PBMC. PCR products were electrophoresed on agarose gels, transferred to filters, and hybridized with specific radiolabeled probes. Hela and RS4;11 expression were used as positive and negative controls forMTS1/2 expression, respectively. MTS1β and Abl transcripts were studied using 27 and 30 PCR cycles, respectively.
p19ARF, a candidate target of genetic inactivating events in T-ALL.
To date, no convincing data supporting the view that p19ARFmight be a tumor suppressor protein have been published and p16INK4a has been considered to be a more likely candidate, based on two arguments: (1) mutations have not been found in MTS1exon 1β (p19ARF specific) whereas MTS1 exon 1α (p16INK4a specific) mutations have been documented (reviewed in Quelle et al23); (2) cancer-associated inactivating mutations of MTS1 target p16INK4a and not p19ARF.23 Our data, however suggest that the possibility that the p19ARF inactivation may be important in oncogenesis should be reconsidered. Indeed, we have shown that p19ARF encoding exons are always deleted or disrupted when recombinational events occur in the MTS locus in T-ALL. Although we cannot exclude that these data may be due to the particular structure of the locus and to the localization of the V(D)J recognition signal sequences, which target the chromosomal rearrangements,7 we favor the hypothesis that p19ARF may be one of the functional targets of the genetic events, which involve the MTS locus in the majority of T-ALLs and that its inactivation may be relevant to the pathogenesis of the disease.
ACKNOWLEDGMENT
We thank E. Macintyre for helpful comments. We thank the Laboratoire de photographie de l'Institut d'Hématologiéfor photographs. We also thank all G.B.B.M. members for discussions.
Supported by grants from Comité de Paris de la Ligue contre le Cancer, Fondation Saint-Louis, and Délégation à la Recherche Clinique AP-HP, Paris, France.
Address reprint requests to François Sigaux, MD, Laboratory of Molecular Hematology, Centre Hayem, Hôpital Saint-Louis, 1 av. Claude Vellefaux, 75475, Paris, Cedex 10, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal