Abstract
The importance of lipoproteins in the etiology of atherosclerosis is well established. Evidence is now accumulating to implicate thrombin in the pathogenesis of atherosclerosis. We have investigated whether atherogenic lipoproteins can support thrombin generation. In the absence of platelets or endothelial cells, both very low-density lipoprotein (VLDL) and oxidized low-density lipoprotein (LDL) support assembly of the prothrombinase complex and generation of thrombin. Thrombin generation (per μg of apolipoprotein) supported by VLDL was 19.4-fold greater than that supported by high-density lipoprotein (HDL), P < .00001, and 11.7-fold greater than that supported by LDL, P < .00001. Oxidation of LDL increased lipoprotein-supported thrombin generation 12-fold compared to unmodified LDL, P < .0001. We have shown that the phenomenon of lipoprotein-supported thrombin generation is mediated predominantly by specific phospholipids and is enhanced by oxidation of these phospholipids. The addition of vitamin E (α-tocopherol) markedly reduced the increase in thrombin generation observed after oxidation of LDL (822 ± 57 v 138 ± 47 nmol/L;P < .0001). These effects suggest that lipoproteins are important in the production of thrombin and that vitamin E may confer protection from the detrimental effects of lipoprotein oxidation by limiting thrombin formation. These results suggest that atherogenic lipoproteins are linked to the development of atherosclerosis in part by their capacity to support thrombin generation.
THERE IS INCREASING evidence to support the hypothesis that thrombin generation may influence the development of atherosclerosis.1-7 Thrombin generation is dependent on assembly of the prothrombinase complex on a phospholipid surface.8,9 Whether platelets provide the only important phospholipid surface for prothrombinase assembly is uncertain. Thrombin can also be generated in vitro in the presence of phospholipid bilayers and micelles; and it is therefore possible that cell-free phospholipid may be able to support assembly of the prothrombinase complex in vivo. Various cell-free phospholipids appear capable of binding the individual components of the prothrombinase complex. Liposome vesicles containing phosphatidyl choline were able to support thrombin generation in the presence of factor Va.10 Very low-density lipoprotein (VLDL) has also been shown to bind prothrombin11,12 and it has been suggested that intact VLDL and VLDL phospholipid extracts were able to accelerate prothrombin conversion, although contamination of prothrombin by factor V may have contributed to this process.13
It is well established that hyperlipidemia is a risk factor for coronary heart disease (CHD) but the relationship between increased plasma lipoprotein concentrations and CHD is poorly defined. Given the role of phospholipids in thrombin generation we hypothesized that lipoprotein-supported thrombin generation provides a link between increased concentrations of atherogenic lipoproteins and CHD. There is indirect evidence supporting this notion because subjects with hyperlipidemia have increased concentrations of markers of thrombin generation14,15 and treatment of hyperlipidemia with lipid-lowering drugs reduced levels of these markers14-16 without necessarily affecting platelet function.17 Thus, there is in vitro, pathological, and clinical evidence suggesting that cell-free plasma lipids and lipoproteins may be capable of supporting assembly of the prothrombinase complex and thrombin production. We have tested the ability of lipoprotein fractions to support thrombin generation and examined whether modification of lipoproteins by oxidation, nonenzymatic glycosylation, or vitamin E alters this function.
MATERIALS AND METHODS
Preparation of lipoprotein subclasses.
Lipoprotein preparation was undertaken as described previously.18 Initially, experiments were undertaken in the presence of protease inhibitors, antibiotics, and an antioxidant to determine whether lipoprotein-supported thrombin generation was affected by these reagents. Omission of protease inhibitors and antioxidant did not alter lipoprotein-supported thrombin generation and therefore subsequent experiments were undertaken immediately after preparation of lipoproteins without addition of any of these reagents. Preparation of each lipoprotein class was undertaken by collection of venous blood from 12 healthy volunteers into EDTA (1.5 mg/mL of blood). Plasma was separated by centrifugation at 1,750×g for 20 minutes and the density adjusted to 1.215 g/mL by adding KBr. After ultracentrifugation at 235,000×g for 36 hours at 4°C, the lipoprotein fraction was removed. Purified lipoprotein fractions were obtained by separation through a Sepharose CL-6B gel filtration column (Sigma, St. Louis, MO), in phosphate-buffered saline (PBS), pH 7.2. Analysis of lipoprotein fractions collected from the gel filtration column by agarose gel electrophoresis (1% agarose, 0.4% bovine serum albumin (BSA), 50 mol/L sodium barbitone, 1.9 mol/L EDTA, pH 8.6, at 220 V, 150 mA for 30 minutes) was undertaken to ensure that each fraction used in the thrombin generation assay contained only a single class of lipoprotein. Agarose gels were fixed by incubation with 5% trichloroacetic acid and dried at 90°C for 15 minutes. Lipoproteins were visualized by incubation with Sudan Black in 60% ethanol. Using the above technique, pure fractions of chylomicrons, VLDL, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) were obtained. The protein content of each lipoprotein fraction was measured by a modified Lowry method.19 Cholesterol and triglyceride concentrations were measured by a standard enzymatic method and apo B concentrations measured by enzyme-linked immunosorbent assay (ELISA) with a polyclonal antibody apo B and apo B standard from The Binding Site (Birmingham, UK).20
Quantitation of lipoprotein-supported thrombin generation.
A chromogenic assay to quantify thrombin generation by lipoproteins and platelets was developed based on established work.21 22Prothrombin, factor V, and factor Xa purified from human plasma were obtained from Diagnostica, Stago (Asnières, France). Each factor was reconstituted in 0.05% human albumin in phosphate-buffered saline (PBS), pH 7.2. Lipoprotein [5 μL of 100 μg/mL (apolipo)protein] was added to 1 μmol/L prothrombin, 1.5 nmol/L factor V, and 1 nmol/L factor Xa (final concentrations). After incubation for 15 minutes at 37°C, 2.5 mmol/L CaCl2 in 6.25 mmol/L Tris HCl, 12.5 mmol/L NaCl, 0.0625% fatty acid-free BSA, pH 7.5 was added. After a further incubation for 25 minutes at 37°C, 10 μL of this solution was added to tubes containing 465 μL of buffer (50 mmol/L Tris HCl, 20 mmol/L EDTA, 0.1 mol/L NaCl, 0.5% fatty acid-free BSA at pH 7.5) and 25 μL of 4 mmol/L S2238, a thrombin-specific chromogenic substrate (Kabi Diagnostica, Kabi Vitrum, Stockholm, Sweden). After 4 minutes the reaction was stopped by the addition of 300 μL of 1 mol/L citric acid. Three hundred μL of the final reaction mixture was transferred to flat-bottomed 96-well microtitre plates (Nunc Maxisorp, Roskilde, Denmark) and the absorbance determined at 405 nm in an Anthos HTII, Anthos Labtec HT2 version 1.05 microtitre plate reader. The ability of each lipoprotein class to support thrombin generation was quantified separately for individual subjects and the results (mean ± standard error of the mean [SEM]) were expressed as nmol/L thrombin, calculated from a standard curve generated with α thrombin. The thrombin generation assay was also used to measure thrombin generation from unactivated platelets. Venous blood was collected into EDTA (1.5 mg/mL of blood) and centrifuged at 800×g for 8 minutes at room temperature. Platelets were prepared by gel filtration (Sepharose CL-2B [Sigma] in PBS, pH 7.2) of platelet-rich plasma after the platelet count was measured with a Coulter STKS counter (Coulter, Hiale, FL). Thrombin generation of 1×106 platelets was quantified immediately as described for lipoproteins.
Inhibition by annexin V.
Lipoproteins (5 μL of 100 μg/mL) were incubated with 5 μmol/L annexin V at 37°C for 30 minutes. The thrombin generation assay was then undertaken as described above. Control experiments were undertaken by adding equal volumes of PBS instead of annexin V. The small volume of PBS used did not produce a calcium precipitate when added to the solution of coagulation factors and calcium chloride.
Quantitation of phospholipid concentration and identification of lipoprotein phospholipids.
Phospholipid concentrations were measured using a commercial kit (Randox Laboratories Ltd, Crumlin, Co Antrim, UK). Phospholipids were analyzed by high performance thin-layer chromatography (HPTLC). Two hundred μg by total weight of each lipoprotein class was dried down under nitrogen. Lipoproteins were resuspended in 30 μL chloroform and methanol (2:1) and analyzed by HPTLC (silica gel, 60 precoated plates; MERCK, Darmstadt, Germany); in methyl acetate:isopropanol:chloroform:methanol:0.25% KCl (25:25:25:10:9) polar solvent. Commercial phospholipid standards were obtained from Sigma. Lipids were stained with a solution containing 3% cupric acetate and 8% phosphoric acid. Phospholipid bands were photographed using an Eagle Eye II Still Video System (Stratagene, La Jolla, CA). The image was exported in a TIFF file and phospholipid bands were quantified using NIH Image 1.55 software for Macintosh23(Apple Computer, Cupertino, CA).
Quantitation of phospholipid-supported thrombin generation.
To test each component of lipoproteins for thrombin generating capacity, the thrombin generation assay was modified and optimized for use in 96-well microtitre plates. Lipid or apolipoprotein was dried onto microtitre wells (PolySorp [lipid], MaxiSorp [protein] Nunc), blocked in 1% fatty acid-free BSA in PBS for 1 hour, and washed in PBS. The assay was performed in the microtitre plate with the same ratio of prothrombin, factor V, and factor Xa as described above. The factors were incubated for 15 minutes at 37°C before adding CaCl2. The plate was then incubated for a further 60 minutes before subsampling into the substrate solution. After 8 minutes incubation the reaction was terminated and assessed as described above. The results are expressed as nmol/L thrombin, calculated from a standard curve generated with α thrombin with the above conditions. In addition, the inhibitory action of annexin V on phospholipids was examined with 10 μmol/L annexin V.
Oxidation, glycosylation, and vitamin E treatment of lipoproteins and phospholipids.
Lipoproteins were modified by oxidation and nonenzymatic glycosylation. Oxidation of lipoprotein fractions was undertaken by incubation with 2.5 μmol/L CuSO4 (final concentration; Sigma) at 37°C for 17 to 20 hours.24 Lipoprotein oxidation was checked by comparison of diene conjugates before and after incubation with CuSO425 and by agarose gel electrophoresis. Oxidation was stopped by addition of 0.1 mol/L EDTA and refrigeration at 4°C. Lipoprotein fractions were also modified by nonenzymatic glycosylation with 5 mmol/L, 20 mmol/L, 50 mmol/L, and 100 mmol/L glucose by incubation for 3 days at 37°C.26After modification, the lipoprotein fractions were washed with PBS by centrifugation with Centricon 30 concentrators (Amicon, Beverly, MA) and were resuspended at 100 μg/mL protein concentration. The effect of vitamin E on thrombin generation produced after copper oxidation of LDL was examined. LDL from 9 of the subjects was mixed and incubated with 850 IU vitamin E/mL [(+)-α-tocopherol, mixed isomers; Sigma] for 30 minutes. The samples were then copper oxidized as described above. Vitamin E and the copper-containing solution were removed by washing in centricons with several volumes of PBS.
Thrombin generation was also determined after copper-induced oxidation of the phospholipids. The phospholipids were dried onto the microtitre wells, then incubated for 17 to 20 hours with 2.5 μmol/L CuSO4. The thrombin generation assay was then undertaken as described above.
Statistics.
Lipoprotein and phospholipid-supported thrombin generation was normally distributed. Presented data of means ±SEM and comparison of means was undertaken by unpaired Student's t-test.
RESULTS
Subject Characteristics
Twelve healthy Caucasian subjects (6 men and 6 women) were recruited, mean (±SD) age of subjects was 30.3 (±5.4) years and mean body mass index [BMI] (±SD) was 22.0 (±2.4) kg/m2. All subjects gave informed consent and underwent a fasting full lipid profile. Mean (±SD) values were total cholesterol 5.1 (±0.9) mmol/L, triglyceride 1.0 (±0.4) mmol/L, HDLc 1.5 (±0.3) mmol/L and LDL 3.1 (±0.9) mmol/L. On a different day, 60mL of blood were drawn from an antecubital vein at a standardized time, 2 hours after a light meal, to ensure that adequate amounts of chylomicrons and VLDL could be obtained. Plasma was immediately prepared by centrifugation, and chylomicrons, VLDL, LDL, and HDL separated by ultracentrifugation followed by gel filtration. Analysis of lipoprotein fractions collected from the gel filtration column by agarose gel electrophoresis was undertaken to ensure that each fraction used in the thrombin generation assay contained only a single class of lipoprotein. Fractions were also examined for platelet microparticle contamination with an ELISA for GPIIIa and no evidence of microparticle contamination was detectable in any of the fractions. Lipoproteins from the same subjects were isolated from a second plasma sample 6 months later and retested for their capacity to support thrombin generation. The results were consistent on both occasions.
Optimization of Thrombin Generation Assay
The thrombin generating properties of chylomicrons, VLDL, LDL, and HDL lipoproteins were investigated. Purified components of the prothrombinase complex were used to quantify the ability of lipoproteins to support thrombin generation. Omission of any of these components abolished thrombin generation. The assay was designed so that thrombin generation was limited only by the components of the lipoprotein rather than any other constituent of the assay. The factors were added in excess, as was the substrate. In addition, prolonged incubation periods showed that the activity of the factors was not diminished during the assay. The assay was also optimized to allow near maximal thrombin generation for VLDL, whereas allowing the optical density to be read off the linear section of the thrombin standard curve. Thrombin generation was linear with time during incubation of factors with lipoproteins and calcium, and during substrate development and proportional to the amount of lipoprotein or phospholipid in the assay. The thrombin generation assay was standardized using human α thrombin that had been active-site titrated. The standard curve was linear from 25 nmol/L to 1000 nmol/L thrombin generation. The limit of detection of the assay was 25 nmol/L. The interassay coefficient of variance (cv) was 14%, n = 10 and the intra-assay cv was 14%, n = 10. The assay for phospholipids was optimized separately. The difference between drying the phospholipids in nitrogen gas or air was found to be negligible so all subsequent experiments were carried out in air. The specificity of prothrombinase complex formation on phospholipid components was studied. It has been shown previously that up to 59 annexin V molecules are able to bind to a single phospholipid molecule,27-29 however it was anticipated that not all phospholipid bound to microtitre wells would be available for binding to annexin V, and therefore experiments were undertaken with 10 μmol/L annexin V. We have shown that the chromogenic substrate is specific for thrombin by using the specific thrombin inhibitor hirudin, derived from Hirudo medicinalis, the medicinal leech. Hirudin is the most potent and specific inhibitor of thrombin known and incubation with hirudin reduced absorbances to those of the detection limit of the assay. Thus, a specific and sensitive method for thrombin generation quantitation was developed.
Thrombin Generating Capacity of Lipoproteins and Effect of Annexin V Binding
Thrombin generation [per μg of (apolipo)protein] supported by VLDL was 19.4-fold greater than that supported by HDL, P< .00001 (and 11.7-fold greater than that supported by LDL,P < .00001). Levels of thrombin generation supported by LDL and HDL were not significantly different from each other. Annexin V reduced lipoprotein-supported thrombin generation and platelet-specific thrombin generation by similar amounts (Table 1) (45.7%, P = .002 and 47.4%, P = .015, respectively). Annexin V is a member of a family of Ca2+–dependent phospholipid-binding proteins. The exact prerequisites for binding are not fully established but binding is known to be strongly dependent on the presence of an sn-3 phosphate group and hydrophobic interaction with the sn-2 acyl chain of the phospholipid.30 Therefore, the reduction in lipoprotein-supported thrombin generation caused by annexin V strongly suggested that phospholipids are an important mediator of this phenomenon.
Effects of Annexin V and Modification by Oxidation and Glycosylation on Lipoprotein-supported Thrombin Generation
| Lipoprotein/ Platelets . | Unmodified . | Annexin V . | Oxidation . | Glycosylation . |
|---|---|---|---|---|
| Thrombin (nmol/L) (mean ± SEM) | ||||
| Chylomicrons | 292 ± 60* | 224 ± 115 | ||
| VLDL | 503 ± 58* | 273 ± 73‡ | 542 ± 91 | 499 ± 95 |
| LDL | 43 ± 12 | 526 ± 87† | 94 ± 40 | |
| HDL | 26 ± 11 | 77 ± 46 | ||
| Platelets | 338 ± 50 | 178 ± 18§ |
| Lipoprotein/ Platelets . | Unmodified . | Annexin V . | Oxidation . | Glycosylation . |
|---|---|---|---|---|
| Thrombin (nmol/L) (mean ± SEM) | ||||
| Chylomicrons | 292 ± 60* | 224 ± 115 | ||
| VLDL | 503 ± 58* | 273 ± 73‡ | 542 ± 91 | 499 ± 95 |
| LDL | 43 ± 12 | 526 ± 87† | 94 ± 40 | |
| HDL | 26 ± 11 | 77 ± 46 | ||
| Platelets | 338 ± 50 | 178 ± 18§ |
Thrombin generation supported by unmodified lipoproteins, lipoproteins modified by oxidation and nonenzymatic glycosylation, and lipoproteins incubated with annexin V. Experiments were undertaken with lipoproteins from 12 subjects (mean ± SEM) containing 1 μg of total (apolipo)protein or 1 × 106 platelets in the absence and presence of annexin V. Lipoproteins were oxidized by incubation with 2.5 μmol/L cupric sulfate. Of the lipoproteins, only VLDL was incubated with annexin V (5 μmol/L). Results for nonenzymatic glycosylation of lipoproteins were obtained after incubation with 20 mmol/L glucose (nonenzymatic glycosylation was only undertaken with VLDL and LDL). Specific comparisons between mean thrombin concentrations were undertaken by Student'st-test.
P < .00001 for chylomicrons or VLDL versus HDL.
P < .0001 for oxidized LDL versus unmodified LDL.
P = .002 for VLDL with annexin V versus unmodified VLDL.
P = .015 for platelets with annexin V compared to platelets alone.
Because lipoproteins differ in both type and content of lipid and protein between classes, we explored whether observed differences in lipoprotein-supported thrombin generation could be explained by standardizing expression of thrombin generation to a different denominator [rather than per μg of total (apolipo)protein]. Because our results strongly suggested that phospholipids were an important mediator of this phenomenon we investigated whether variation in total amount of phospholipids within lipoproteins could account for the differences in lipoprotein-supported thrombin generation between lipoprotein classes. The concentration of phospholipid in each lipoprotein class was measured. Differences in amount of phospholipid did not explain the marked differences in lipoprotein-supported thrombin generation observed between lipoprotein classes. In a lipoprotein solution containing 100 μg/mL (apolipo)protein, mean (±SD) concentrations of phospholipid were 117 (±33)mg/dl for HDL, 98 (±32)mg/dl for LDL, 152 (±62)mg/dl for VLDL, 307 (±131)mg/dl for chylomicrons. By using mg of phospholipid rather than μg of (apolipo)protein as the denominator in calculating thrombin generation for each lipoprotein class, VLDL-supported thrombin generation remained markedly different from both LDL- and HDL-supported thrombin generation. There was a 21-fold increase in thrombin generation supported by VLDL compared to HDL (P = 0.006) and an 18-fold increase in thrombin generation supported by VLDL compared to LDL (P = .004). In contrast to VLDL, the difference between chylomicron and HDL-supported thrombin generation became less marked and was reduced to fivefold greater thrombin generation supported by chylomicrons (P = .034). As a further test of whether the concentration of phospholipid determined the level of lipoprotein-supported thrombin generation, the amount of HDL in the thrombin generation assay was increased, by concentrating HDL threefold and sevenfold. No significant increase in thrombin generation was found.
Phospholipid Composition of Lipoproteins
Next, we analyzed the phospholipid components of each lipoprotein by HPTLC to determine if the presence of different phospholipids between lipoprotein classes might be responsible for the marked difference in lipoprotein-supported thrombin generation between classes. Similar percentages of each type of phospholipid were observed across lipoprotein classes. Typical values of individual phospholipids expressed as a percentage of total phospholipid extracted from the lipid core of a lipoprotein were: phosphatidyl choline, 46%; sphingomyelin, 12%; phosphatidyl serine, 5%; phosphatidyl ethanolamine, 12%; phosphatidyl inositol, 11%; and lysophosphatidyl choline, 14%. Sphingomyelin varied most in percentage content (±5%) between lipoprotein classes.
Thrombin Generating Capacity of VLDL and LDL Lipoproteins Calculated With apo B100 Concentration as Denominator
Because neither the total amount nor the type of phospholipid could explain the marked differences in lipoprotein-supported thrombin generation between VLDL and HDL (or LDL), we excluded the possibility that the observed differences with VLDL were attributable to different numbers of VLDL and LDL particles in the thrombin generation assay. Therefore, we determined lipoprotein-supported thrombin generation results per ng of apo B100, for VLDL and LDL, because this represented an indirect measure of the amount of thrombin generated per lipoprotein particle (VLDL and LDL contain a single apo B100 molecule per particle). VLDL-supported thrombin generation was 32-fold greater than LDL-supported thrombin generation per ng of apo B100 (P < .001). These data provided further support for the suggestion that the differences between lipoprotein classes were not an artifact resulting from the choice of denominator for expression of thrombin generation results.
Thrombin Generation Supported by Phospholipids
The magnitude of the difference in lipoprotein-supported thrombin generation between lipoprotein classes suggested that our findings had not occurred by chance. Confounding by amount and type of phospholipid or lipoprotein particle number was also excluded and therefore we investigated the mechanism of lipoprotein-supported thrombin generation by analyzing individual components of lipoproteins for thrombin generating capacity. Of the phospholipids present in each lipoprotein class, phosphatidyl inositol, phosphatidyl ethanolamine, and phosphatidyl serine provided the greatest support for assembly of the prothrombinase complex and thrombin generation and this phenomenon could be inhibited partially by annexin V (Table 2). Although sphingomyelin, lysophosphatidyl choline, and phosphatidyl choline were present in highest concentration in each lipoprotein class, thrombin generation supported by these phospholipids was at levels close to the limit of detection of the assay. Thrombin generation supported by sphingomyelin (685 μmol/L) was ≤25 nmol/L, lysophosphatidyl choline (1008 μmol/L) was 55 nmol/L, and phosphatidyl choline (654 μmol/L) was 38 nmol/L (results are means of two separate experiments). Other components of lipoproteins including cholesterol, triglyceride, apo B, and apo E were also examined for their capacity to support thrombin generation, but none of these components supported thrombin generation beyond the lower limit of detection of the assay. Thrombin generation supported by cholesterol (1292 μmol/L), triglyceride (1293 μmol/L), apo B (0.15 μmol/L), and apo E (0.2 μmol/L) was ≤25 nmol/L. A threefold increase in concentration of sphingomyelin, lysophosphatidyl choline, phosphatidyl choline, cholesterol, triglyceride, apo B, or apo E did not significantly increase thrombin generation.
Effects of Annexin V and Oxidation on Phospholipid-supported Thrombin Generation
| Lipid . | Unmodified . | Annexin V . | Oxidation . |
|---|---|---|---|
| Thrombin (nmol/L) (mean ± SEM) | |||
| Phosphatidyl inositol (125 μmol/L) | 741 ± 130 | 259 ± 85† | |
| Phosphatidyl inositol (72 μmol/L) | 204 ± 86 | 382 ± 131† | |
| Phosphatidyl ethanolamine (677 μmol/L) | 965 ± 61 | 460 ± 136‡ | |
| Phosphatidyl ethanolamine (163 μmol/L) | 257 ± 108 | 478 ± 117‡ | |
| Phosphatidyl serine (412 μmol/L) | 973 ± 72 | 425 ± 147‡ | |
| Phosphatidyl serine (270 μmol/L) | 221 ± 71 | 447 ± 158* | |
| Phosphatidyl glycerol (487 μmol/L) | 806 ± 189 | 141 ± 56* | |
| Phosphatidyl glycerol (488 μmol/L) | 821 ± 95 | 552 ± 294 |
| Lipid . | Unmodified . | Annexin V . | Oxidation . |
|---|---|---|---|
| Thrombin (nmol/L) (mean ± SEM) | |||
| Phosphatidyl inositol (125 μmol/L) | 741 ± 130 | 259 ± 85† | |
| Phosphatidyl inositol (72 μmol/L) | 204 ± 86 | 382 ± 131† | |
| Phosphatidyl ethanolamine (677 μmol/L) | 965 ± 61 | 460 ± 136‡ | |
| Phosphatidyl ethanolamine (163 μmol/L) | 257 ± 108 | 478 ± 117‡ | |
| Phosphatidyl serine (412 μmol/L) | 973 ± 72 | 425 ± 147‡ | |
| Phosphatidyl serine (270 μmol/L) | 221 ± 71 | 447 ± 158* | |
| Phosphatidyl glycerol (487 μmol/L) | 806 ± 189 | 141 ± 56* | |
| Phosphatidyl glycerol (488 μmol/L) | 821 ± 95 | 552 ± 294 |
Thrombin generation supported by unmodified phospholipids, phospholipids incubated with annexin V, and phospholipids modified by oxidation. Phospholipid concentrations were chosen to ensure that either reductions (with annexin V) or increases (with oxidation) would be measurable on the thrombin generation standard curve. The presented results were obtained after incubation with 10 μmol/L annexin V (similar results were obtained with 5 μmol/L annexin V) and 2.5 μmol/L cupric sulfate. Comparisons of mean thrombin concentrations, by Student's t-test, were undertaken for phospholipids: (1) incubated with or without annexin V or (2) incubated with and without cupric sulfate.
P = .015.
P = .02.
P = .03 (mean ± SEM, all experiments were undertaken on three to eight separate occasions).
Effect of Oxidation and Nonenzymatic Glycosylation on Lipoprotein-supported Thrombin Generation
The effect of modification of lipoproteins by oxidation and nonenzymatic glycosylation on lipoprotein-supported thrombin generation was then examined. Oxidation of LDL produced a 12-fold increase in lipoprotein-supported thrombin generation, compared to thrombin generation by unmodified LDL (P < .0001). No significant increase in lipoprotein-supported thrombin generation was observed with oxidation of any of the other lipoprotein classes (Table 1). LDL and VLDL lipoproteins were then studied after modification by nonenzymatic glycosylation. A range of glucose concentrations were chosen from physiological (5 mmol/L) to pathological (100 mmol/L). Incubation of VLDL and LDL with glucose concentrations over this range of concentrations did not increase lipoprotein-supported thrombin generation.
Effect of Oxidation on Thrombin Generating Capacity of Specific Phospholipids
In view of the effect of oxidation on LDL-supported thrombin generation, we examined the effect of oxidation of individual phospholipids on phospholipid-supported thrombin generation. Phospholipids contained within lipoproteins, identified by HPTLC, were tested before and after modification by oxidation. A variety of phospholipids were examined for their capacity to support thrombin generation. Oxidation increased phospholipid-supported thrombin generation with phosphatidyl inositol, phosphatidyl ethanolamine, and phosphatidyl serine (Table 2). Another phospholipid, phosphatidyl glycerol, that was not detected in lipoproteins by HPTLC was also tested and oxidation did not increase phosphatidyl glycerol-supported thrombin generation. Both oxidation and nonenzymatic glycosylation of apo B100, apo E, cholesterol, and triglyceride did not significantly increase thrombin generation, with thrombin levels remaining close to the lower limit of detection of the assay. The ability of both lipoproteins and phospholipids to support thrombin generation was Ca2+-dependent, and omission of any of the three coagulation factors reduced the signal to background levels confirming that all three coagulation factors are necessary for prothrombinase complex assembly.
Effect of Vitamin E on Thrombin Generation Capacity of Oxidized Lipoproteins
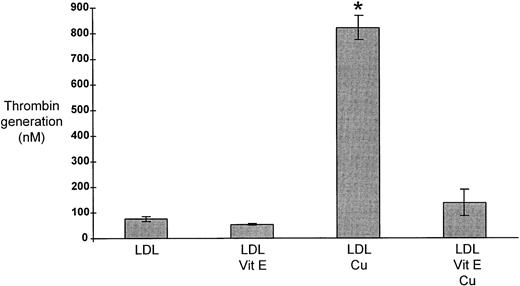
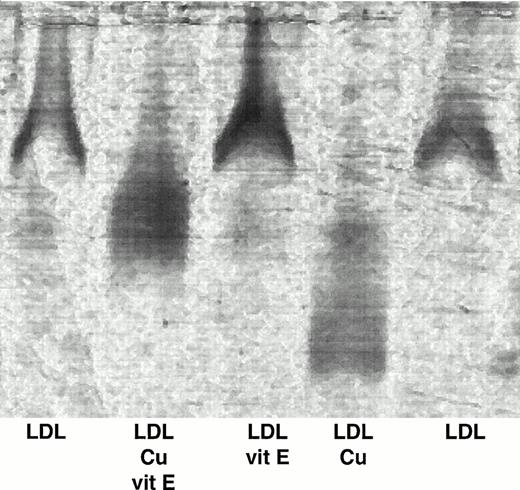
On a separate occasion, LDL from nine subjects was preincubated with vitamin E then subjected to copper oxidation. The LDL was washed and thrombin generating capacity was tested (Fig 1). In accordance with the results noted above, there was a dramatic increase in the thrombin generation potential when the LDL had been oxidized (unmodified LDL = 76 ± 12 mmol/L v oxidized LDL = 822 ± 57 nmol/L; P < .0001). The effect of copper oxidation alone on lipoprotein-supported thrombin generation was markedly reduced by preincubating LDL with vitamin E before oxidation (138 ± 47 nmol/L v 822±57;P < .0001). Thrombin generation supported by LDL that had been incubated with vitamin E only was 53 ± 2 nmol/L, whereas incubation with vitamin E without lipoprotein was below the limit of detection of the assay, suggesting that vitamin E and its solvent had little effect on baseline thrombin generation. The LDL samples were subsequently analyzed by agarose gel electrophoresis (Fig 2). Native LDL and LDL incubated with vitamin E migrated the least from the wells. In comparison, oxidized LDL migrated further down the gel. LDL that had been preincubated with vitamin E before oxidation migrated an intermediate distance. This result suggests a difference in charge between these forms of LDL with oxidation increasing net negative charge. In addition, the poorly defined bands in both the copper oxidized and copper oxidized after vitamin E preincubation fractions suggests heterogeneity within the oxidized species of LDL formed. Results obtained with the conjugate diene assay supported the observation that vitamin E diminished LDL oxidation. Thus, these results suggest that vitamin E prevents the copper-mediated increase in LDL-supported thrombin generation by decreasing LDL oxidation.
The effect of vitamin E on thrombin generation supported by LDL. LDL from 9 subjects was incubated with or without vitamin E for 30 minutes before oxidation for 17 to 20 hours at 37°C. LDL with no additions and LDL incubated only with vitamin E were also prepared. Thrombin generation potential was quantified as described previously. The results are expressed as nmol/L thrombin, calculated from the standard curve generated with α thrombin. *Thrombin generation supported by oxidized LDL was increased compared to each of the other three groups (LDL + copper v LDL, P < .0001; LDL + copper v LDL and vitamin E, P < .0001; LDL + copper v LDL + vitamin E + copper,P < .0001).
The effect of vitamin E on thrombin generation supported by LDL. LDL from 9 subjects was incubated with or without vitamin E for 30 minutes before oxidation for 17 to 20 hours at 37°C. LDL with no additions and LDL incubated only with vitamin E were also prepared. Thrombin generation potential was quantified as described previously. The results are expressed as nmol/L thrombin, calculated from the standard curve generated with α thrombin. *Thrombin generation supported by oxidized LDL was increased compared to each of the other three groups (LDL + copper v LDL, P < .0001; LDL + copper v LDL and vitamin E, P < .0001; LDL + copper v LDL + vitamin E + copper,P < .0001).
Effect of vitamin E on the oxidation state of LDL. LDL from 9 subjects was incubated with or without vitamin E before copper oxidation. The samples were subjected to gel electrophoresis and stained with Sudan Black as described previously. This is a representative photograph showing unmodified LDL, LDL preincubated with vitamin E before copper oxidation, LDL incubated with vitamin E, oxidized LDL, and unmodified LDL.
Effect of vitamin E on the oxidation state of LDL. LDL from 9 subjects was incubated with or without vitamin E before copper oxidation. The samples were subjected to gel electrophoresis and stained with Sudan Black as described previously. This is a representative photograph showing unmodified LDL, LDL preincubated with vitamin E before copper oxidation, LDL incubated with vitamin E, oxidized LDL, and unmodified LDL.
DISCUSSION
The novel and potentially important findings of our study are that atherogenic lipoproteins have the capacity to support assembly of the prothrombinase complex and to generate thrombin. Thrombin generation supported by VLDL is significantly greater than for any other unmodified lipoprotein species and oxidation specifically increases the capacity of LDL to support thrombin generation. Vitamin E is able to attenuate the increased capacity of oxidized LDL to support thrombin generation and this result suggests a unique mechanism by which vitamin E may protect against the development of CHD.
Although it is well established that oxidized LDL is incorporated into an atheromatous plaque via the macrophage scavenger receptor,31,32 the mechanisms linking VLDL and plaque formation are poorly understood. Our results suggest a unique association between VLDL, coagulation factors, and atherosclerosis. Furthermore, evidence is accumulating from in vitro, clinical, and epidemiological studies to support the notion that VLDL is an atherogenic lipoprotein particle. VLDL is retained in the intimal lining of arteries in humans33 and in the arteries of genetically hyperlipidemic rabbits.34 Subjects with type III hyperlipidemia (Frederickson classification) have increased plasma VLDL concentrations and an increased risk of CHD. Epidemiological studies show that fasting triglyceride levels are an important CHD risk factor35 36 and in the fasting state VLDL is the major triglyceride-containing lipoprotein.
At present the explanation for the differences in thrombin generating capacity between lipoprotein classes is unknown. In this study, lipoprotein-supported thrombin generation was mediated predominantly by the presence of certain phospholipids. Phosphatidyl inositol, phosphatidyl ethanolamine, and phosphatidyl serine supported thrombin generation, whereas thrombin generation supported by sphingomyelin, phosphatidyl choline, and lysophosphatidyl choline was close to the limit of detection of the assay. Recently the relative contribution of factor Va, prothrombin, and factor Xa in the generation of thrombin supported by phospholipid bilayers has been determined.37Thrombin generation increased with the amount of phosphatidyl serine in the phospholipid bilayer, with the concentration of factor Va, and with the prothrombin concentration. Incorporation of phosphatidyl ethanolamine into the phospholipid bilayer also increased the steady-state rate of thrombin production. Our results, however, show that the presence of phospholipid alone is not sufficient to explain the differences in thrombin generation supported by different lipoproteins. It is possible that specific phospholipids within these lipoprotein particles may be inaccessible and therefore are unable to bind components of the prothrombinase complex. Steric hindrance by apolipoproteins on the lipoprotein surface may prevent assembly of the prothrombinase complex on phospholipid surfaces in HDL and unmodified LDL. Alternatively, the differences in thrombin generating capacity between lipoproteins may be mediated by the presence of a certain protein(s) associated with the lipoproteins, such as tissue factor pathway inhibitor (TFPI), which is known to inhibit factor Xa activity.38 39
It is widely accepted that oxidized LDL is an atherogenic lipoprotein and is internalized into a developing atherosclerotic plaque via the macrophage scavenger receptor, creating a foam cell.31,32Oxidation may be caused by a number of initiators including copper or iron ions found in high concentrations in the plaque, perhaps associated with microthrombi.40 Our data suggest a novel mechanism by which oxidized LDL participates in atherogenesis. Oxidation of LDL markedly increased thrombin generation (compared with native LDL) and it is possible that local production of thrombin in the vicinity of a developing atherosclerotic plaque increases accumulation of fibrin from proteolytic cleavage of fibrinogen. Interestingly, with respect to these results it has been shown that the lipid-rich region of the atheromatous plaque is also the most thrombogenic. This region of the plaque was approximately sixfold more thrombogenic than the sclerotic collagen-rich component.41
The mechanism by which oxidation of LDL caused such a marked increase in lipoprotein-supported thrombin generation is uncertain. It is possible that the increased negative charge of oxidized surface phospholipids mediates more efficient binding of the prothrombinase complex to these phospholipids with a subsequent increase in thrombin generation. Thus, phospholipids that are known to bind components of the prothrombinase complex relatively poorly, such as phosphatidyl ethanolamine, may be converted on oxidation into phospholipids with similar binding characteristics to anionic phospholipids, such as phosphatidyl serine, that are known to bind components of the prothrombinase complex more avidly. Alternatively, it is known that modification of LDL by oxidation produces fusion of lipoprotein particles,42 similar to those found in atherosclerotic lesions, and fusion of several LDL particles may modulate the surface of accessible phospholipids for assembly of the prothrombinase complex.
Vitamin E, or α-tocopherol, is the most abundant antioxidant in LDL and is probably located within the phospholipid bilayer. It converts the highly reactive peroxyl free radical to the less damaging hydroperoxide, and thus presumably protects tissues and lipoproteins from oxidative damage. It is known that vitamin E delays oxidation of LDL in vitro and while taking oral supplements.43 In addition, large prospective epidemiological studies44 45and a randomized control trial in subjects with CHD46suggest that vitamin E is protective against CHD, although it is not known whether this effect is solely attributable to a reduction in macrophage uptake of LDL. Our results show that vitamin E dramatically reduced the vastly enhanced thrombin generation potential associated with oxidized LDL and suggest a novel mechanism by which vitamin E protects against development of CHD. This result suggests that the charge of a lipoprotein may be important in mediating the capacity to support thrombin generation because preincubation with vitamin E not only markedly reduced the capacity of LDL to support thrombin generation, but also partially protected the lipoprotein from oxidation.
This study was designed to define the thrombin generating capacity of different lipoprotein classes. Although the number of volunteers studied was too small to show significant differences between men and women or between subgroups of volunteers, there were indications that lipoprotein-supported thrombin generation differed in certain subjects compared to the mean thrombin generating capacity of the group as a whole. At present it is uncertain whether atherogenic lipoproteins from individuals with CHD, or individuals at increased risk of CHD, are capable of supporting increased thrombin generation.
Our results have raised several questions that now require addressing in other experimental models. Does the phenomenon of lipoprotein-supported thrombin generation:
(1) Explain the association between increased plasma triglyceride concentrations and a procoagulant state?
(2) Promote retention of both oxidized LDL and VLDL within atheromatous plaques; possibly mediated by thrombin providing the ligand for binding of both of these lipoproteins to either atherosclerotic lesions5 or neointima6 expressing the thrombin receptor?
(3) Explain some of the advantageous effects of vitamin E in the treatment of CHD?
In addition, it is necessary to elucidate the mechanisms by which VLDL and oxidized LDL support vastly increased amounts of thrombin generation compared with unmodified LDL and HDL. These results are potentially important because they may give insight into a novel mechanism by which lipoproteins and coagulation factors interact to cause atherosclerosis.
ACKNOWLEDGMENT
We gratefully thank Dr P. Flynn (University of Cambridge, UK) for assistance with ultracentrifugation of plasma samples; Dr J. Tait (University of Washington, Seattle, WA) for the kind gift of annexin V; Dr S. Stone (University of Cambridge, UK) for the kind gift of a thrombin; and Dr S.H. Wild for critical reading of the manuscript.
S.R. and N.A.M. contributed equally to this work.
Supported by the Medical Research Council, UK and the Anglia and Oxford Regional Health Authority, UK. S.R was supported by a fellowship from The Scientific and Technical Resource Council of Turkey.
Address correspondence to Christopher D. Byrne, MB, PhD, University Department of Clinical Biochemistry, Level 4, Addenbrooke's Hospital, Hills Road, Cambridge, CB2 2QR, UK.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal